Introduction
As the lifestyle of the population worldwide
changes, fatty liver disease is gradually increasing in incidence
(1) and has become the second most
severe type of liver disease after viral hepatitis (2). Although fatty liver disease is
considered to be a benign disease, without treatment it gradually
develops into inflammatory cell infiltration and necrosis. This
results in liver fibrosis and cirrhosis and may lead to a malignant
disease, such as hepatocellular carcinoma (3). Histopathological examination via a
liver puncture is considered to be the gold standard for evaluation
of the risk and the severity of fatty liver disease (4–7).
However, as it is an invasive examination, liver puncturing is not
readily accepted by patients; therefore, an appropriate imaging
examination is the preferred method for evaluating the severity of
fatty liver disease. Traditional ultrasound exhibits a strong
subjectivity in the quantitative diagnosis of fatty liver disease,
whereas computed tomography, magnetic resonance imaging and other
imaging techniques pose numerous limitations in the evaluation of
fatty liver disease. Thus, studies to identify a noninvasive
technology for the diagnosis of fatty liver disease are required
(3,8).
The ultrasound elastography method objectively
demonstrates elasticity information of tissue and reflects the
stiffness of the measured tissue using grayscale or color images
(9,10). The real-time shear wave
elastography (SWE) method that was developed on the basis of this,
rapidly, noninvasively, objectively and quantitatively detects the
degree of fibrosis of liver diseases, including fatty liver
disease, and has attracted increasing attention (10).
The present study investigated a rabbit model using
the real-time SWE technique and investigated the fibrosis
development process associated with the progression of
non-alcoholic and alcoholic fatty liver disease to provide
objective indices for clinical intervention and to facilitate the
evaluation of curative effects.
Materials and methods
Animal grouping
Thirty male Japanese white rabbits (age, 10–26 days;
body weight, 0.7–1.52 kg) were randomly divided into three groups
of 10 rabbits per group. One group was a rabbit model of simple
fatty liver disease obtained via high-fat diet feeding (formula; 2%
cholesterol, 10% butter, 5% white sugar, 8% egg yolk powder and 75%
conventional, basal feed) and water intake was ad libitum.
This process was conducted by the Laboratory Animal Research Center
of Kunming Medical University (Kunming, China). The second group
was a rabbit model of alcoholic fatty liver disease obtained via
conventional feeding (basal feed), a 10 ml infusion of Chinese
spirits [Chinese spirits (Beijing Red Star CO., Ltd., Beijing,
China)] twice a day and water intake ad libitum. The feeding
method was as follows: 10 ml spirit was injected into the pharynx
oralis of the rabbit with the mouth forced shut for 2 min until the
rabbit swallowed. Care was taken to ensure that there was no
leakage of the spirit from the mouth. The alcoholic beverage
comprised drinking water and alcohol at a concentration of 15%
ethanol, which was provided by the Animal Laboratory of Kunming
Medical University (Kunming, China). The third group served as a
normal control group with conventional feeding (basal feed). After
feeding for 12 weeks, the rabbits were fasted for 12 h, weighed and
sacrificed following performance of ultrasonography and
elastography. The animals were humanely sacrificed by air embolism
method. After obtaining the weight of the liver, it was
pathologically observed. The present study was conducted in strict
accordance with the recommendations set out in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (eighth edition, 2011). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
the Fourth Affiliated Hospital of Kunming Medical University
(Kunming, China).
Ultrasonography
Real-time SWE studies were performed using the
Aixplorer® ultrasound system (SuperSonic Imagine S.A.,
Aix-en-Provence, France) with a linear array probe (L15-4) and a
frequency of 4–15 MHz.
The rabbits were fixed in the supine position
without anesthesia. The skin was prepared from the fourth right rib
to the abdomen so as to fully expose the area to be examined. The
abdomen and liver were initially subjected to comprehensive and
systematic ultrasonography, including identification of the size,
echo, capsule and ascites of the liver. The mode was switched to
SWE, the probe was gently moved without exerting any pressure and
once the image was stable, the frame was determined. The elasticity
in the selected region of interest was measured using the method
provided by the ultrasonic apparatus (vessel structures were
avoided during sampling). For sampling, two segments of the hepatic
right lobe in the longitudinal section of the first hepatic portal
(including the junction of the portal vein, proper hepatic artery
and the common bile duct) and one segment of the left lobe in the
xiphoideusal horizontal section were selected; three samples were
taken from each segment. When the deviation of the values
determined for the three samples was <10%, the sampling was
considered to be successful. Mean values were calculated for each
segment, and the mean value of the means of all the segments was
calculated to obtain the required data. Data determined for each
group were the maximum elastic modulus (Max), the mean elastic
modulus (Mean), the minimum elastic modulus (Min) and the speed
dispersion (Sd). The data were measured automatically using an
Aixplorer ultrasound system, and the images and data were recorded
and saved on a computer.
Max reflected tissue structures with the highest
stiffness within a sampling area, including diseased tissue and
certain structures in the liver, such as ligaments, whereas Min
reflected tissue structures with the lowest stiffness in a sampling
area, including the lumens of the intrahepatic blood vessels and
the bile duct. Mean was the mean elastic modulus of the entire area
and the standard deviation reflected the uniformity of the tissue
stiffness in the sampling area, and the successful sampling
rate.
Liver sample collection and gross
observation
On completion of the ultrasonography, the rabbits
were sacrificed for removal of the liver, which was weighed and
observed for size, shape, color, surface smoothness, interlobar
fissure width, edge sharpness and composition. The liver tissue
samples were immobilized with 10% formalin, followed by routine
dehydration, vitrification, waxing, embedding, slicing (thickness,
4–6 μm), hematoxylin and eosin (H&E) staining, and picrosirius
red staining (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai,
China).
The degree of pathological change of fatty liver
disease in the rabbits was observed under a microscope (Nikon
Eclipse E200, Tokyo, Japan) and divided into five degree classes
according to the area of steatosis that was observed in the liver
cell specimen under the light microscope (11,12).
Normal liver F0, steatosis area <10%; slight steatosis F1,
steatosis area 10–33%; mild fatty liver F2, steatosis area 33–50%;
moderate fatty liver F3, steatosis area 50–66%; and severe fatty
liver F4, >66% steatosis of liver cells.
Picrosirius red and silver staining
A paraffin section was successively dewaxed using
xylene, and washed and stained in 0.5% picrosirius red for 5–30
min, washed with distilled water three times, differentiated with
absolute ethanol and dehydrated. Observation under a light
microscope demonstrated that the collagen fibers were red, the
nucleus was green and the other components were yellow.
The sections were dewaxed and then oxidized with
0.25% potassium permanganate solution. After washing with water,
the sections were bleached using 2% oxalic acid solution and washed
with water again. The sections were then mordanted with a 2%
aqueous solution of ammonium sulfate and washed with water, prior
to staining with Gomori silver ammonia solution, washing with
water, deoxidizing with 4% neutral formalin solution and washing
with water. After toning with 0.2% gold chloride and washing with
water, the sections were fixed with 5% sodium thiosulfate,
conventionally dehydrated until transparent, and sealed and
cemented with neutral vegetable glue.
Staging
The staging was determined according to the
formation of fibrous tissue (13)
as follows: Stage (S) 0, normal liver without fibrosis; S1,
fibrosis formed in a small region, predominantly including the
fibers in and around the portal area; S2, a plurality of fibrous
septa, with the lobular structure being roughly retained; S3, a
large quantity of fibrous septa, causing the lobular structure to
be disordered but without cirrhosis; S4, early cirrhosis.
Statistical analysis
Student’s t-test of the various indices of the liver
in the three groups was performed using SPSS statistical software,
version 13 (SPSS Inc., Chicago, IL, USA) with all the data
displayed as means ± standard deviation. A correlation analysis
between the Mean and the area of pathological steatosis was
performed. P<0.05 was considered to indicate a statistically
significant result.
Results
General data
In the alcohol feeding group, three rabbits died at
10 weeks old. The anatomical results showed apparent ascites due to
cirrhosis and serious liver damage, and the deaths were ascribed to
liver damage and malnutrition. In the normal group, one rabbit died
at nine weeks due to diarrhea, rather than loss of hepatic
function; liver samples were collected from the rabbit and the
results showed normal liver. By contrast, the rabbits in the
high-fat diet feeding group all survived. Ten rabbits with a
non-alcoholic fatty liver and seven rabbits with an alcoholic fatty
liver were successfully established as models 12 weeks after
ultrasonography.
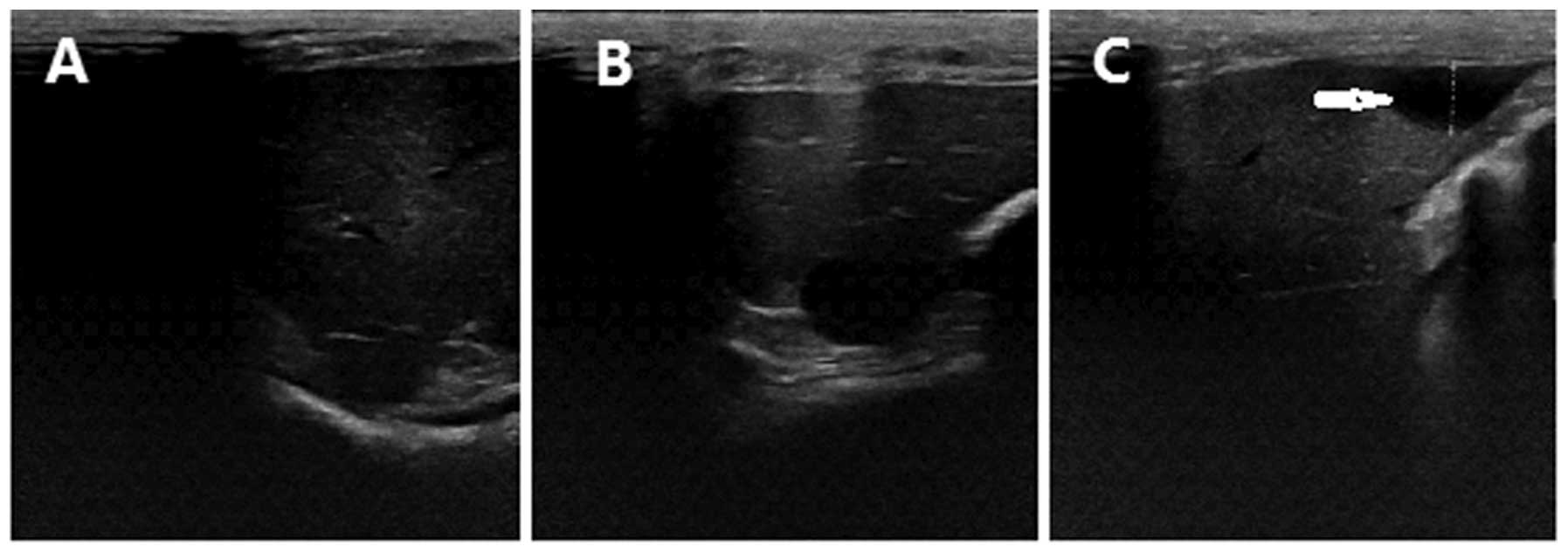
Ultrasonography of the liver in the three
groups
The livers of the nine normal rabbits exhibited an
echo marginally lower than a moderate level. The internal echo was
identified to be uniform, the light spots were moderately intense,
the vessel structure was clear and the echo of the vessel wall was
stronger than that of the liver parenchyma. Furthermore, the
capsule was regular and smooth without exhibiting ascites.
The 10 rabbit models with simple fatty liver
exhibited an enlarged liver with an increased echo, a significant
attenuation in the frontal region, partial attenuation in the
posterior region and fine light spots. The vessel structure was
less clear than that of the normal group. The echo of the vessel
wall was enhanced, although not as markedly as that of the liver
parenchyma, and the capsule was predominantly smooth. One rabbit
presented with ascites, which were indicated by a dark area of 1–3
cm diameter in the space between the liver and the kidney.
The seven rabbit models with alcoholic fatty liver
exhibited narrowing of the liver. The ultrasonography results were
comparable to those of the fatty liver group, however, their degree
of variation was more apparent. Two rabbits demonstrated a less
regular liver capsule and exhibited ascites to different degrees
and the ultrasonography demonstrated cirrhosis (Fig. 1 and Table I).
 | Table IComparison of the measured elasticity
of the rabbit livers in the three groups. |
Table I
Comparison of the measured elasticity
of the rabbit livers in the three groups.
| Liver type | Elastic modulus
(kPa) | Diameter (mm) |
|---|
|
|---|
| Rabbits (n) | Age (days) | Max | Min | Mean |
|---|
| Normal | 9 | 96.1±4.4 | 16.21±4.79 | 1.97±1.37 | 5.78±0.66 | 9.48±0.96 |
| Simple fatty | 10 | 97.2±4.9 | 25.73±5.21a | 3.81±1.49a | 10.60±2.06a | 9.24±1.24 |
| Alcoholic fatty | 7 | 95.1±4.5 | 41.11±4.67a,b | 8.42±1.76a,b | 19.43±2.13a,b | 9.90±1.37 |
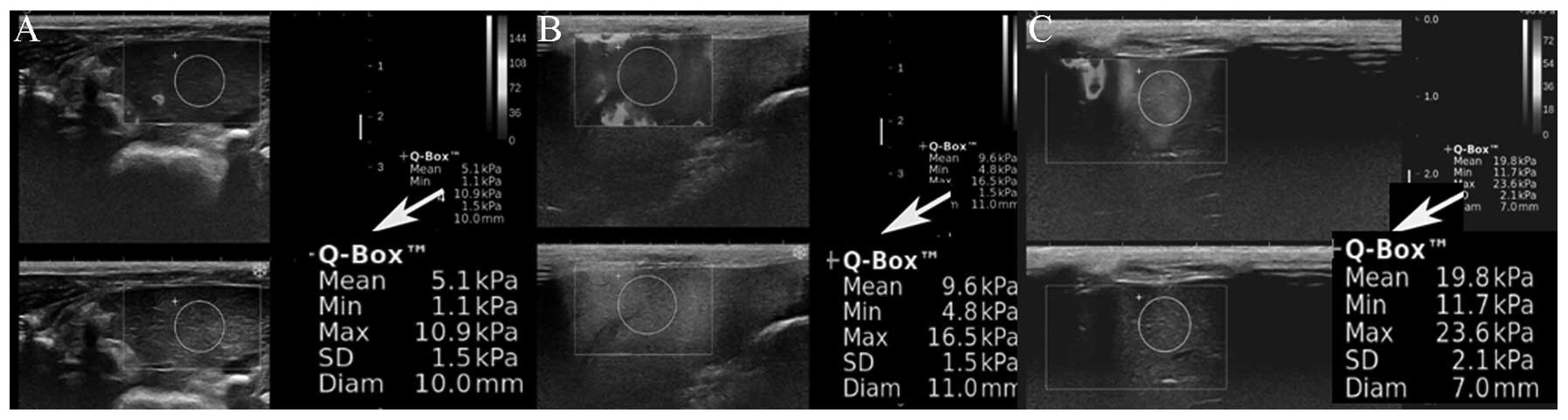
Elastic modulus of the three groups and a
comparison of the related data
The age of the rabbits in the three groups was not
significantly different (P>0.05). Liver elasticity was measured
and demonstrated that the sampling volume of the region of interest
in the liver of the three groups of rabbits was not significantly
different (P>0.05). The Mean, Max and Min reflected the liver
stiffness and showed the following: The values for the alcoholic
fatty liver group were higher than those in the simple fatty liver
group and the normal group, and the values in the simple fatty
liver group were higher than those in the normal group (P<0.05;
Fig. 2 and Table I).
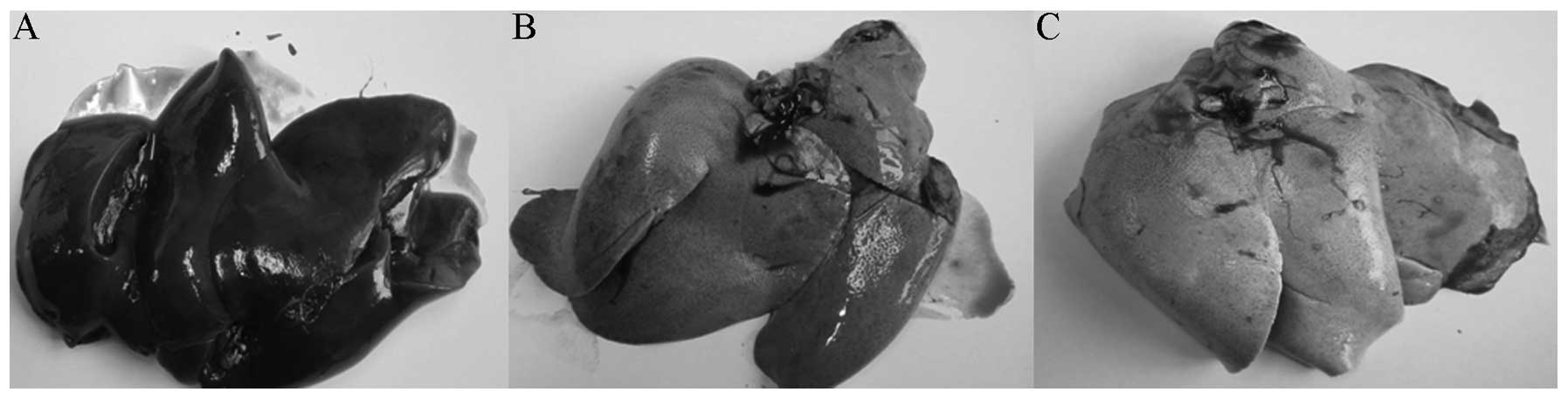
Observation of the gross liver samples of
the rabbits in the three groups
After the rabbits were sacrificed, the gross liver
samples of the rabbits were observed. The color of the livers in
the nine normal rabbits was dark red and the left lobe was
significantly larger than the right lobe. Compared with the normal
group, the fatty liver groups exhibited stiffer livers, with a
moderately hard, elastic texture. The liver had good elasticity and
the interlobar fissure was clear with a sharp edge and smooth
section. The livers of the 10 rabbits with non-alcoholic fatty
liver disease were identified to be uniformly enlarged with full
hepatic lobes. The right lobe was enlarged and the ratio of left
lobe size to right lobe size was reduced; furthermore, the
interlobar fissure became smaller and the liver edge became blunt.
The livers were a gray-red color and were stiffer than those of the
normal rabbits with a moderately stiff and elastic texture. Of the
seven rabbits, five exhibited enlarged livers, with a morphological
structure that was comparable to that observed in the simple fatty
liver group. Two of the livers were significantly reduced in
volume, with an uneven surface and exhibited an indistinct or
concealed interlobar fissure. The size of the left lobe was
identified to be equal to that of the right one. Compared with the
other groups, the alcoholic fatty liver group displayed noticeably
stiffened livers that were hard in texture. The livers were
gray-white in color with a granular section and when the liver was
being sectioned, it was stiff and sounded as though fibrous tissue
was being cut (Fig. 3).
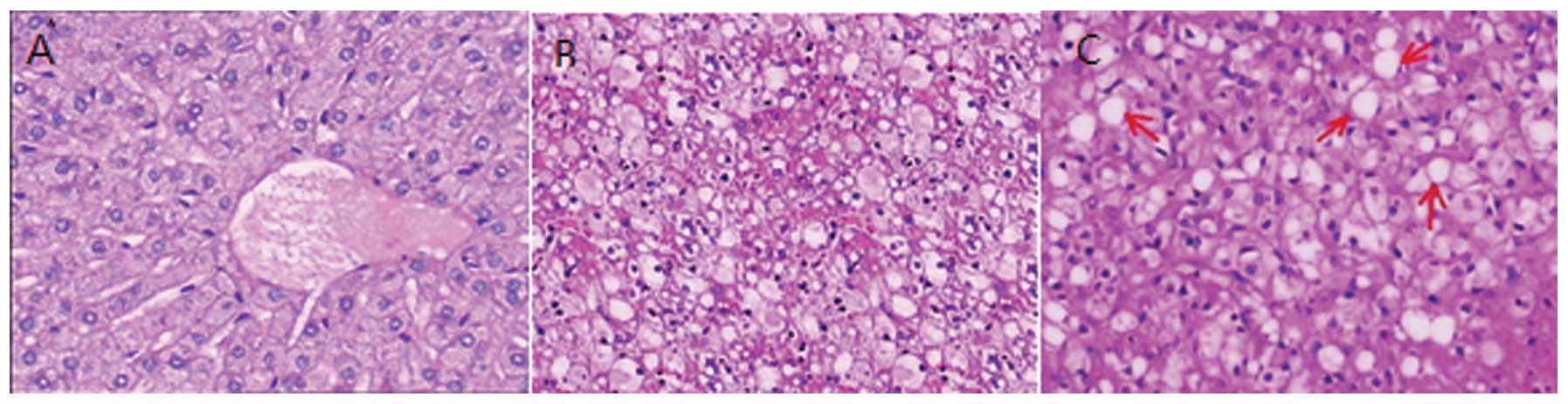
Observation of the livers from the three
groups of rabbits using a microscope
The lobules of the liver that were obtained from the
rabbits in the normal group displayed a complete structure and the
hepatic cords were normal. Lipid droplets were occasionally
observed in the liver cells and 5.3–8.9% of the area of the liver
displayed steatosis. Lipid droplets of a uniform size (diameter,
3–5 μm) were scattered in the liver cells of the rabbits with
simple fatty liver. These lipid droplets may have fused and
undergone nuclear deviation or centered around the nucleus, and the
area of steatosis was identified to constitute 25–55% of the liver.
The livers of the rabbits in the alcoholic fatty liver group were
suffused with lipid droplets in a panlobular manner. The liver
cells were swollen, the sinus space was narrow, and the nuclei and
organelles moved to the borders of cells due to the extrusion of
the lipid droplets; in addition, foam-like cells were observed. The
liver cells were 4–5 times larger than the normal liver cells, the
intracellular structure was not able to be identified and the area
of steatosis accounted for 60–80% of the total area of the liver
(Fig. 4).
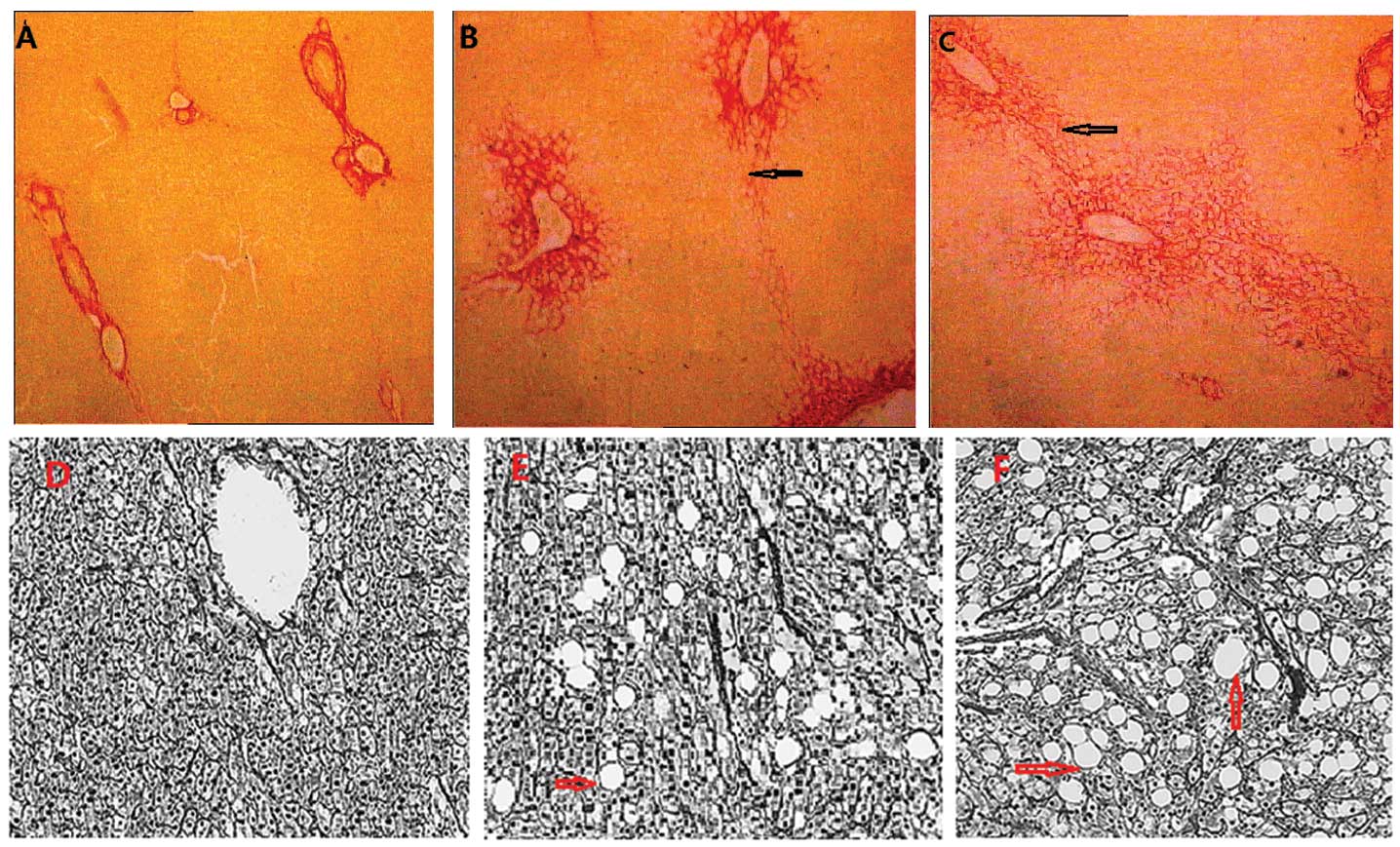
Picrosirius red staining indicated the following:
There was no evident fibrosis in the livers of the rabbits in the
normal group (all nine rabbits were S0); the non-alcoholic fatty
liver group predominantly showed inflammation and fibrosis
formation in the portal area, including three rabbits at S1, six
rabbits at S2 and one rabbit at S3; the alcoholic fatty liver group
predominantly exhibited fibrous septa with lobular structure
disorders, including one rabbit at S1, two rabbits at S2, two
rabbits at S3 and two rabbits at S4 (the cirrhosis stage; Fig. 5 and Table II).
 | Table IIComparison of the pathological
observations of the rabbit livers in the three groups. |
Table II
Comparison of the pathological
observations of the rabbit livers in the three groups.
| Liver type | Rabbits (n) | BW (kg) | LW (kg) | Steatosis area
(%) | S1 (n) | S2 (n) | S3 (n) | S4 (n) |
|---|
| Normal | 9 | 2.8±0.54 | 0.123±0.021 | 6.5±2.5 | 0 | 0 | 0 | 0 |
| Simple fatty | 10 | 3.2±0.48a | 0.197±0.027a | 40.2±8.3a | 3 | 6 | 1 | 0 |
| Alcoholic fatty | 7 | 2.5±0.67a,b | 0.108±0.029b | 75.0±10.6a,b | 1 | 2 | 2 | 2 |
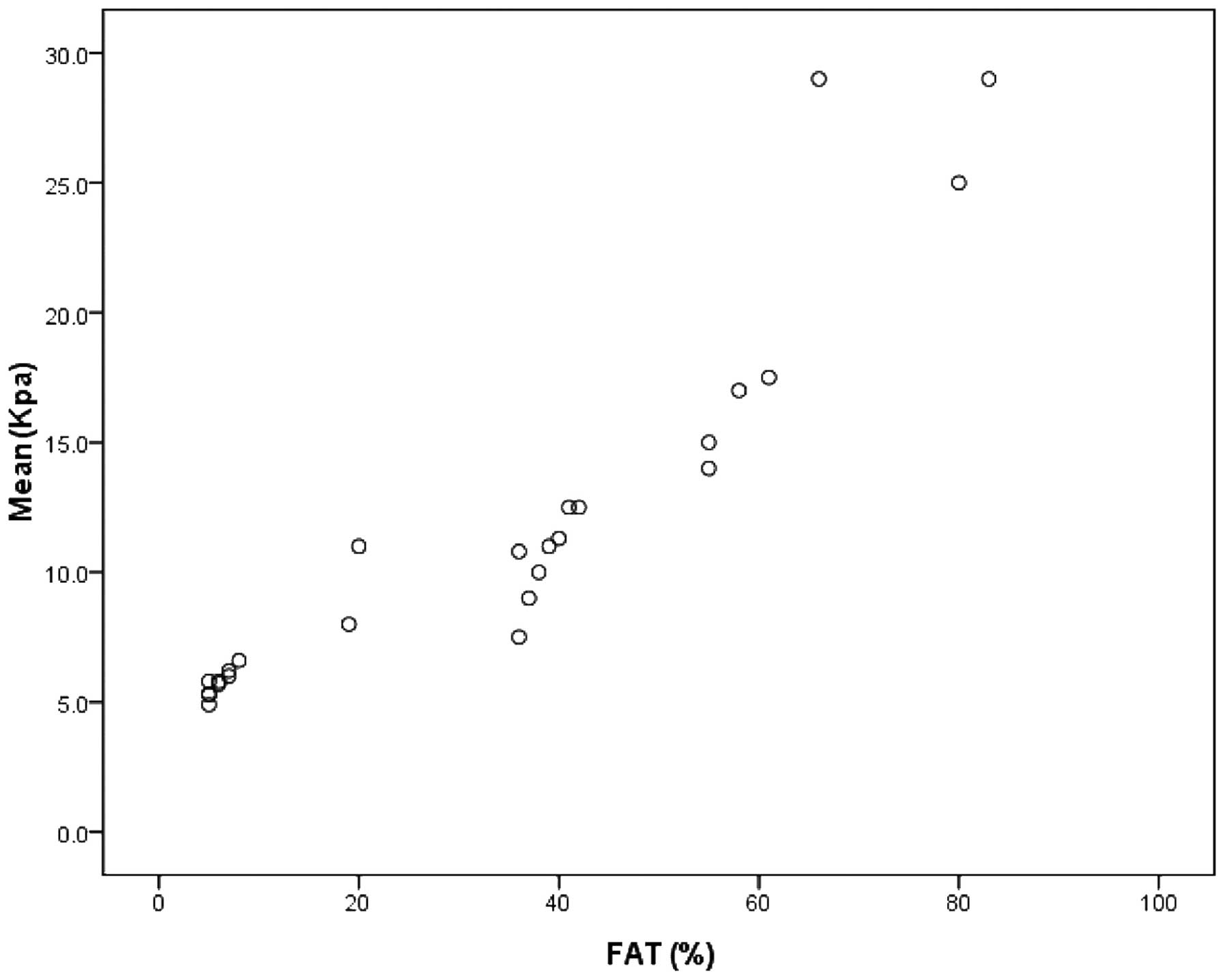
The correlation analysis indicated that the Mean of
the liver in the 26 rabbits (all three groups) was positively
correlated with the pathological steatosis area (r=0.92,
P<0.01), as was the Max (r=0.67, P<0.05). Furthermore, Mean
and Max were identified to be positively correlated with the
steatosis area between S1 and S3 (P<0.05; Table III and Fig. 6). The analysis of variance results
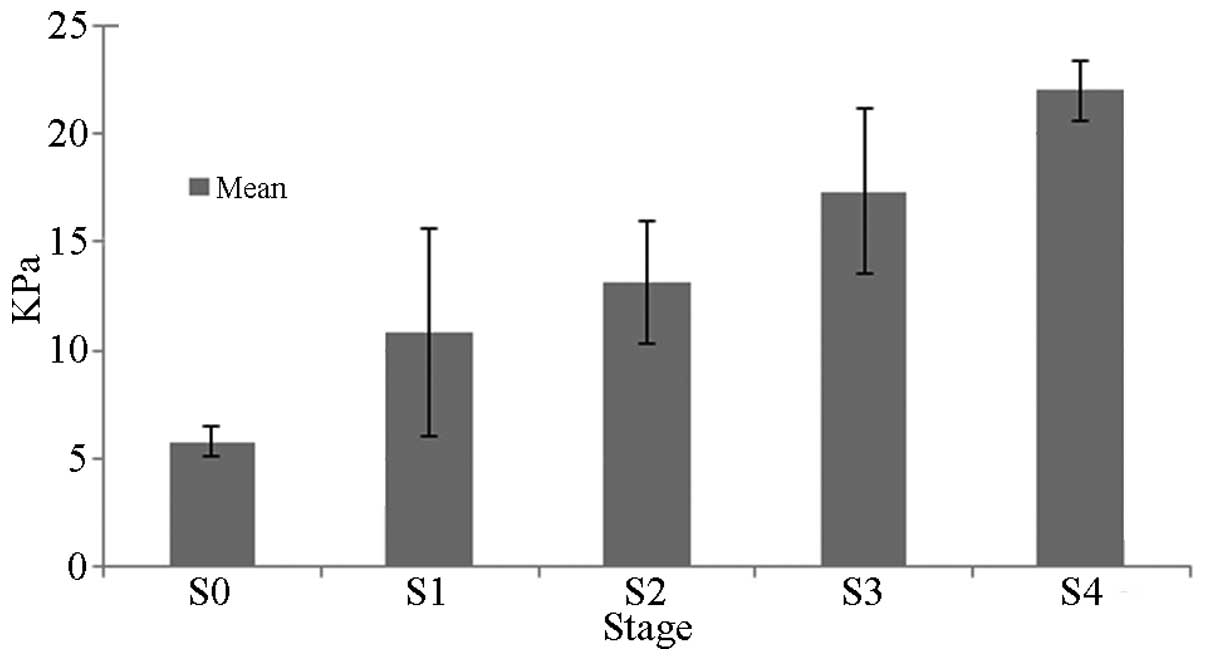
of the 26 rabbits at the different fibrosis stages (S0–4) indicated
statistically significant differences among the Mean values, and
also among the Max values, for the different stages. The Mean
values were as follows: S0, 5.78±0.66 kPa; S1, 10.83±4.81 kPa; S2,
13.15±2.82 kPa; S3, 17.33±3.79 kPa; and S4, 22.0±1.41 kPa (Table III and Fig. 7). The results indicated that a
higher stage implied a greater elastic modulus (stiffness).
 | Table IIIAssociation between the elastic
modulus and steatosis area of the rabbit liver according to
fibrosis stage. |
Table III
Association between the elastic
modulus and steatosis area of the rabbit liver according to
fibrosis stage.
| Fibrosis stage | Rabbits (n) | Steatosis area
(%) | Elastic modulus
(kPa) |
|---|
|
|---|
| Mean | Max | Min |
|---|
| 0 | 9 | 6.5±2.5 | 5.78±0.66 (r=0.34,
P>0.05) | 6.21±4.79 (r=0.37,
P>0.05) | 1.97±1.37 (r=0.167,
P>0.05) |
| 1 | 4 | 32.3±7.4 | 10.83±4.81 (r=0.69,
P<0.05) | 15.62±4.31 (r=0.65,
P<0.05) | 3.81±1.49 (r=0.27,
P>0.05) |
| 2 | 8 | 45.8±8.1 | 13.15±2.82 (r=0.78,
P<0.05) | 25.14±5.28 (r=0.71,
P<0.05) | 4.42±1.76 (r=0.35,
P>0.05) |
| 3 | 3 | 64.5±9.8 | 17.33±3.79 (r=0.75,
P<0.05) | 35.23±4.99 (r=0.66,
P<0.05) | 5.97±1.37 (r=0.25,
P>0.05) |
| 4 | 2 | 80±8.9 | 22.0±1.41 (r=0.41,
P>0.05) | 43.73±6.56 (r=0.39,
P>0.05) | 4.81±1.49 (r=0.16,
P>0.05) |
| Total | 26 | 40.9±10.3 | 11.40±5.65a
(r=0.92, P<0.01) | 24.36±5.34a
(r=0.67, P<0.05) | 4.53±1.65 (r=0.16,
P>0.05) |
Discussion
The application of ultrasound elastography in the
screening of patients with fatty liver disease is able to identify
at an early stage whether the liver is exhibiting fibrosis, which
facilitates the monitoring of fatty liver disease and aids
clarification of the pathological type.
Ultrasound elastography is a technique that reflects
the tissue hardness via digital imaging (14–16)
and SWE, which is derived from this technique, directly measures
the Young’s modulus (elastic modulus) of tissues (8,17).
The ultrasonic diagnostic apparatus widely used for
instantaneous clinical elastography in the early stages of disease
includes the French FibroScan®, which has been used for
studies of viral hepatitis (B and C) as well as for the
quantitative diagnosis of liver fibrosis. However, it is a
one-dimensional instantaneous elastography system and adipose
tissue markedly attenuates the low frequency shear and ultrasonic
waves; therefore, the application of this apparatus is limited
during observation of fatty liver disease (18).
The use of Aixplorer (10,19,20)
real-time SWE achieves two-dimensional color imaging with a maximum
imaging depth of 14 cm, overcomes the attenuation of the adipose
tissue on sound velocity and is, therefore, used to observe fatty
liver stiffness. A certain study has demonstrated that liver
stiffness is well correlated with the stage of liver fibrosis
(21).
Thus, the present study simulated the current poor
diet of humans and established an animal model of fatty liver
disease by administering a high-fat diet and alcoholic drinks,
respectively and studied the models using conventional
ultrasonography and real-time SWE using Aixplorer ultrasound
system.
The present study indicated, via continuous
ultrasonic observation, that all the rabbits that were fed a
high-fat diet exhibited fatty liver disease 12 weeks later,
confirming that the model was successfully established.
Furthermore, the present study investigated the disease features
and pathological quantitative diagnosis indices of fatty liver
disease. Generally, fatty liver disease develops from a simple
fatty liver to steatohepatitis and eventually to fatty cirrhosis
(3,20). However, there are exceptions, for
example, alcoholic fatty liver may not exhibit clear
steatohepatitis, but directly evolve to cirrhosis (3). The animal models of fatty liver
disease (non-alcoholic and alcoholic) that were established in the
present study via different feeding methods also followed the
abovementioned processes. However, as rabbits are grazing animals
and have a poor lipid metabolic capability, a high-fat diet may
have resulted in a rapid progression of fatty liver disease and
severe injury. They rapidly entered the stages of steatohepatitis,
fibrosis and cirrhosis and similarly developed inflammatory cell
infiltration and fibrogenesis.
Ultrasonography demonstrated that the majority of
the rabbits in the high-fat diet group developed moderate fatty
liver disease and seldom developed cirrhosis. By contrast, the
rabbits in the alcoholic diet group exhibited noticeable liver
lesions; the majority developed severe fatty liver disease and the
number of cases of cirrhosis increased. These results were further
evidenced via a pathological examination; compared with the
high-fat diet group, the alcoholic diet group indicated greater
areas of hepatic steatosis (25–55% versus 60–80%; P<0.05) and
more severe fibroplasia. Furthermore, the three groups of rabbits
were of similar ages, however, their body weights were markedly
different (high fat>normal>alcohol group), which indicated
that the growth of the rabbits in the alcohol group was restricted
and significantly associated with the liver injury.
In addition, the present study investigated the Mean
and Max of the livers of the rabbits in the normal, high-fat and
alcohol feeding group using real-time SWE. The results indicated
that the liver stiffness in the alcoholic fatty liver group was
significantly higher than that observed in the simple fatty and
normal groups, and the liver stiffness in the simple fatty liver
group was higher than that observed in the normal group; this
coincided with the trend of the pathological changes.
All of the rabbit models underwent ultrasonography
and pathological comparison, in addition to H&E and picrosirius
red staining, respectively. Following H&E staining, the lipid
droplets that were observed in the liver cells were examined under
an optical microscope. The fatty liver was categorized into stages
F0–4 according to the proportion of liver cells that exhibited
steatosis in the liver tissue sections.
Sirius red is a long, unfolded molecule that
undergoes an extremely stable adsorption reaction with collagen
molecules, does not readily fade following staining and has strong
specificity. Therefore, it is currently the optimal staining method
for collagen fibers, enabling clear observation of the fibrous
tissue in the liver for qualitative or quantitative diagnosis of
liver fibrosis. Liver fibrosis is divided into stages S1–4
according to the deposition site and scope of the collagen fibers,
the damage to the liver structure and its influence on the hepatic
microcirculation (8).
Although the present study indicated that the
hepatic steatosis area and the fibrosis grade positively correlated
with the elastic modulus of the liver, the authors of the present
study hypothesize that the latter was the key influential factor of
liver stiffness, which was consistent with previous studies
(18,22). The development of fatty liver
disease proceeds from a simple fatty liver to steatohepatitis,
fibrosis and liver cirrhosis (22). Accordingly, the liver stiffness
demonstrates different variation trends at each individual stage;
at the simple fatty liver stage, the liver is comparatively soft
due to the lipoid degeneration of regions within the cells. At the
steatohepatitis stage, the liver hardness value is normal or
marginally increased as a result of cell lipoid degeneration and
inflammation. At the fibrosis and liver cirrhosis stages, liver
stiffness increases due to fibroplasia. In the present study, the
fatty liver models were obtained through adverse factor
intervention and when compared with naturally developing fatty
liver disease, these models showed rapid progression. As a result
of this, the majority of the models had entered the fibrosis or
cirrhosis stage when the pathological examination was performed and
the simple fatty liver, and occasionally the steatohepatitis stage,
was not observed. Therefore, the models used in the present study
failed to represent all the stages in the progression and the
complex variations of human fatty liver disease. Hepatocellular
fatty degeneration closely correlates with the development and
severity of fatty liver disease. It becomes increasingly apparent
at the advanced stages (fibrosis and cirrhosis), which indicates
serious fatty liver pathological changes, a progressively
noticeable fibrosis trend and a higher liver stiffness value. In
addition, the present study showed that the hepatic steatosis area
and liver stiffness were associated with the severity of fibrosis,
identifying a positive correlation between them (r=0.92,
P<0.01).
The present study had certain limitations. The
assessment of the liver elasticity and pathology of fatty liver
disease in the rabbits was conducted following formation of the
fatty livers. Therefore, the present study lacks data regarding the
formation and development of fatty liver disease. In addition, the
present study simulated liver changes that were observed in rabbits
resulting from bad dietary habits. As rabbits are herbivorous
animals, they exhibit a different capability to humans regarding
high lipid and alcohol metabolism. Therefore, the established fatty
liver models in the present study only generally, rather than
completely, reflect the trend of the development of fatty liver
disease in humans. Thus, studies on human-related diseases are
required.
In conclusion, real-time SWE exhibits satisfactory
repeatability and stability in quantitatively determining liver
elasticity. Furthermore, the technology is noninvasive. These
advantages indicate that real-time SWE is a method worthy of wider
use in clinical practice.
Acknowledgements
This study was supported by the High-Level Health
Technical Personnel Training Special Fund of Yunnan Province (grant
no. D-201204), in addition to the Joint Fund of Kunming Medical
University and the Yunnan Provincial Science and Technology
Department (grant no. 2012FB081).
References
|
1
|
Adams LA and Angulo P: Treatment of
non-alcoholic fatty liver disease. Postgrad Med J. 82:315–322.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paschos P and Paletas K: Non alcoholic
fatty liver disease and metabolic syndrome. Hippokratia. 13:9–19.
2009.PubMed/NCBI
|
|
3
|
Brunt EM: Nonalcohlic steatohepatitis.
Semin Liver Dis. 24:3–20. 2004. View Article : Google Scholar
|
|
4
|
Diehl AM: Nonalcoholic steatohepatitis.
Semin Liver Dis. 19:221–229. 1999. View Article : Google Scholar
|
|
5
|
Chalasani N, Younossi Z, Lavine JE, et al;
American Association for the Study of Liver Diseases; American
College of Gastroenterology; American Gastroenterological
Association. The diagnosis and management of non-alcoholic fatty
liver disease: Practice guideline by the American Association for
the Study of Liver Diseases, and the American Gastroenterological
Association. Am J Gastroenterol. 107:811–826. 2012. View Article : Google Scholar
|
|
6
|
Afdhal NH: Diagnosis fibrosis in hepatitis
C: is the pendulum swinging from biopsy to blood tests? Hepatology.
37:972–974. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bravo AA, Sheth SG and Chopra S: Liver
biopsy. N Engl J Med. 344:495–500. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gulizia R, Ferraioli G and Filice C: Open
questions in the assessment of liver fibrosis using real-time
elastography. AJR Am J Roentgenol. 190:W370–W373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greenleaf JF, Fatemi M and Insana M:
Selected methods for imaging elastic properties of biological
tissues. Annu Rev Biomed Eng. 5:57–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bercoff J, Tanter M and Fink M: Supersonic
shear imaging: a new technique for soft tissue elasticity mapping.
IEEE Trans Ultrason Ferroelectr Freq Control. 51:396–409. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: a
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burt AD, Mutton A and Day CP: Diagnosis
and interpretation of steatosis and steatohepatitis. Semin Diagn
Pathol. 15:246–258. 1998.PubMed/NCBI
|
|
13
|
Knodell RG, Ishak KG, Black WC, et al:
Formulation and application of a numerical scoring system for
assessing histological activity in asymptomatic chronic active
hepatitis. Hepatology. 1:431–435. 1981. View Article : Google Scholar
|
|
14
|
Khalil AS, Chan RC, Chau AH, Bouma BE and
Mofrad MR: Tissue elasticity estimation with optical coherence
elastography: toward mechanical characterization of in vivo soft
tissue. Ann Biomed Eng. 33:1631–1639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han L, Noble JA and Burcher M: A novel
ultrasound indentation system for measuring biomechanical
properties of in vivo soft tissue. Ultrasound Med Biol. 29:813–823.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beaugrand M: How to assess liver fibrosis
and for what purpose? J Hepatol. 44:444–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedrich-Rust M, Ong MF, Herrman E, et
al: Real-time elastography for noninvasive assessment of liver
fibrosis in chronic viral hepatitis. AJR Am J Roentgenol.
188:758–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bavu E, Gennisson JL, Couade M, et al:
Noninvasive in vivo liver fibrosis evaluation using supersonic
shear imaging: a clinical study on 113 hepatitis C virus patients.
Ultrasound Med Biol. 37:1361–1373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deffieux T, Montaldo G, Tanter M and Fink
M: Shear wave spectroscopy for in vivo quantification of human soft
tissues visco-elasticity. IEEE Trans Med Imaging. 28:313–322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehl AM: Liver disease in alcohol
abusers: clinical perspective. Alcohol. 27:7–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoneda M, Yoneda M, Mawatari H, et al:
Noninvasive assessment of liver fibrosis by measurement of
stiffness in patients with nonalcoholic fatty liver disease
(NAFLD). Dig Liver Dis. 40:371–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferraioli G, Tinelli C, Dal Bello B,
Zicchetti M, Filice G and Filice C; Liver Fibrosis Study Group.
Accuracy of real-time shear wave elastography for assessing liver
fibrosis in chronic hepatitis C: a pilot study. Hepatology.
56:2125–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|