Introduction
The canonical Wnt signaling pathway is an ancient
and conserved signaling cascade, which regulates cell
proliferation, fate and behavior in contexts ranging from embryonic
development to disease (1).
Secreted Wnt proteins exert these effects through a transcription
co-activator, β-catenin, which acts as a key mediator of the Wnt
signaling pathway. Wnt ligands bind to a receptor complex composed
of Frizzled (Fzd) and low-density lipoprotein receptor-related
protein 5 or 6 (LRP5/6) (2–5).
This complex leads to the accumulation of cytoplasmic β-catenin,
which translocates into the nucleus and activates the target genes
by binding to members of the TCF/LEF transcription factor family
(6). In the absence of Wnt
stimulation, the level of cytoplasmic β-catenin remains low as a
result of the ubiquitination/proteosome degradation (7).
The canonical Wnt signaling has a critical role in
the development of the ventral mesencephalic dopaminergic (DA)
neurons, whose selective loss in the substantia nigra (SNc) results
in Parkinson’s disease (PD) (8,9). For
example, loss of Wnt1 disrupts the development of mesencephalon
(10) and Wnt1 expression
increases the number of rat midbrain DA neurons in vitro
(11). Analysis of LRP6 mutant
mice has revealed a delay in the onset of DA precursor
differentiation (12). In
addition, β-catenin controls DA neurogenesis by maintaining the
integrity of the neurogenic niche and the progression from
progenitors to DA neurons (13).
Recently, it has been demonstrated that the key components of the
canonical Wnt signaling link to the affected genes in familial PD
(14). In particular, the E3
ubiquitin ligase parkin, encoded by PARK2, has been reported to
repress β-catenin by inducing β-catenin ubiquitination and
degradation (15). Furthermore,
Wnt/β-catenin signaling is also involved in certain PD animal
models induced by DA neuron-specific toxins, including
6-hydroxydopamine (6-OHDA) (16)
and 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) (17).
However, the exact role of LRP5, LRP6 and β-catenin,
key components of canonical Wnt signaling, in adult DA neurons of
normal and MPTP-lesioned mice remain unclear. In order to
investigate this, in the present study, DA neuron-specific knockout
mice with the deletion of LRP5, LRP6 or β-catenin genes were
established. These mice, together with wild-type littermates, were
subjected to saline or MPTP injection. Using tyrosine hydroxylase
(TH)-immunohistochemical staining, the DA neurons in the compact
part of the SNc and the density of TH-immunoreactive (TH-ir) axonal
terminals in the striatum were quantified, and the neuroprotective
effects of the inactivation of LRP5, LRP6 or β-catenin against MPTP
exposure in the midbrain DA neurons were thereby investigated.
Materials and methods
Genotyping and maintenance of
animals
TH-Cre mice were generated and genotyped as
previously described (18). For
inactivation of LRP5, LRP6 or β-catenin expression in midbrain
dopamine-synthesizing neurons, TH-Cre mice were crossed with
LRP5flox/flox (19),
LRP6flox/flox (20),
and β-cateninflox/flox (21) mice, respectively. The
TH-Cre;LRP5flox/+ offspring were mated with their
littermates to generate TH-Cre;LRP5flox/flox mice. In a
similar way, TH-Cre;LRP6flox/flox and
TH-Cre;β-cateninflox/flox mice were generated. Hereafter
these mice are referred to as LRP5 CKO, LRP6 CKO and β-catenin CKO
mice, respectively. Animal experiments were reviewed and approved
by the Animal Studies Committee at the Tongji University School of
Medicine (Shanghai, China).
MPTP treatment
MPTP (25 mg/kg; Sigma-Aldrich, St. Louis, MO, USA)
dissolved in saline was administered via intraperitoneal injection
to five-month old wild-type and CKO mice once a day for five
consecutive weeks, as reported previously (22–24).
Mice used as controls were treated in the same way with injection
of an equivalent volume of saline instead of MPTP.
Immunohistochemistry
Mice were deeply anaesthetized with sodium
pentobarbital (100 mg/kg body weight), and perfused transcardially
with 0.01 M phosphate-buffered saline (PBS; pH 7.4), followed by 4%
paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) following the
five weeks of injections. Brains were dissected out, post-fixed
overnight and cryoprotected with 30% sucrose in PBS overnight at
4°C. Transverse sections (40 μm) were cut on a cryostat (CM1950;
Leica, Mannheim, Germany), and every sixth section was collected as
one set of serial sections that were processed for TH
immunohistochemistry. Brain sections were pretreated with citrate
buffer (pH 6.0) at 95°C for 6 min and then incubated overnight at
4°C with mouse anti-TH antibody (1:40,000; Sigma-Aldrich) diluted
in PBS containing 0.3% Triton X-100 and 1% bovine serum albumin
(BSA). Sections were then incubated with biotinylated horse
anti-mouse immunoglobulin G (1:500; Vector Laboratories,
Burlingame, CA, USA) in the aforementioned PBS/Triton X-100/BSA
buffer for 3 h, and then for 1 h with an avidin-biotin-peroxidase
complex (1:200; Vector Laboratories) in PBS at room temperature. TH
immunoreactivity was visualized using 0.05% 3,3′-diaminobenzidine,
as well as 0.04% hydrogen peroxide in PBS.
Microscopy and imaging
Images were captured on a microscope (Eclipse 80i;
Nikon, Tokyo, Japan) equipped with a digital camera (DS-Ri1;
Nikon). All images were imported into Photoshop software (Adobe
Systems, Inc., San Jose, CA, USA) and minor adjustments to the
contrast and brightness were applied if necessary.
Cell counting
The number of TH-positive neurons was counted in
every sixth 40-μm-thick transverse section. TH-positive cells in
the SNc were counted for quantitative comparison between wild-type
and CKO mice. Statistical significance was determined using a
one-way analysis of variance (ANOVA), followed by a post-hoc least
significant difference (LSD) test. Error bars represent the
standard error of the mean (SEM) and P<0.05 was considered to
indicate a statistically significant difference.
Striatal densitometry
The density of striatal DA terminals was measured as
the optical density of the striatal TH-ir using ImageJ software
(National Institutes of Health, Bethesda, MA, USA). Four sections
were randomly selected from those containing the striatum at the
approximate level of Bregma −1.10 to 0.22 mm (25), and the optical density in the
central striatum of each section was measured on each side. In each
section, the optical densities were corrected by subtraction of
background staining in the corpus callosum. Statistical analysis
was performed using a one-way ANOVA with post hoc LSD test. Data
are presented as the mean ± SEM and P<0.05 was considered to
indicate a statistically significant difference.
Results
LRP5 CKO
In wild-type and LRP5 CKO mice treated with saline,
no significant difference in the number of DA neurons in the SNc
was observed (Fig. 1A, B and I),
and the density of TH-ir axonal terminals in the striatum was
comparable between the two genotypes (Fig. 1E, F and J). Following MPTP
administration once per day for five weeks, the number of nigral TH
neurons in wild-type mice was reduced to ~55.3% of that in
wild-type mice treated with saline (Fig. 1A, C and J). By contrast, although
the MPTP treatment also resulted in a marked reduction in the
number of TH-ir neurons in the SNc of LRP5 CKO mice compared with
that in the saline-treated LRP5 CKO mice, an increase in the number
of TH-ir neurons was observed compared with that in the
MPTP-treated wild-type mice. In the MPTP-treated LRP5 CKO mice, the
TH-ir neuronal number was decreased to ~73.9% of that in LRP5 CKO
mice treated with saline (Fig. 1B, D
and I). Consistently, while the MPTP treatment led to
significant reductions in the density of striatal TH-ir axon
terminals in wild-type mice and LRP5 CKO mice, there was a greater
density of TH-ir axon terminals present in the striatum of the
MPTP-treated LRP5 CKO mice (Fig. 1G, H
and J). These results indicate that in the absence of LRP5
expression, a greater number of midbrain DA neurons survived
following exposure to MPTP.
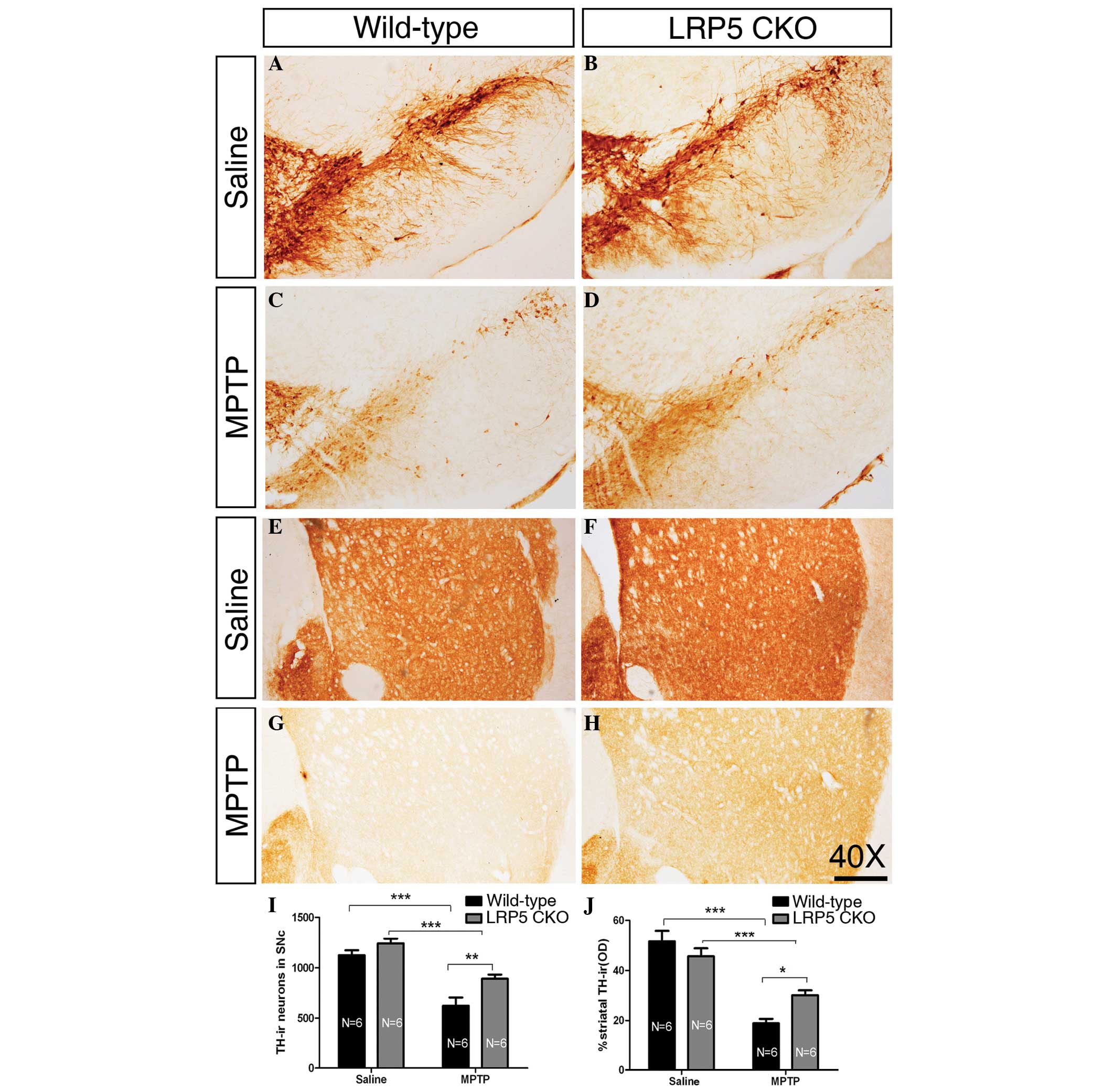 | Figure 1LRP5 deletion attenuates MPTP
toxicity. (A–D) Representative images showing TH-ir neurons of the
SNc in wild-type and LRP5 CKO mice treated with saline or MPTP.
(E–H) Representative images of TH-ir axonal terminals in the
striatum of wild-type and LRP5 CKO mice treated with saline or
MPTP. (I) Quantification of TH-ir cells in the SNc in the wild-type
and LRP5 CKO mice treated with saline or MPTP. A significant
difference is found between the two genotypes following MPTP
treatment. (J) Statistical data of the optical density of TH-ir
striatal terminals in the wild-type and LRP5 CKO mice following
treatment with saline or MPTP. There is a significant difference
between the two genotypes following MPTP injection. Sample sizes
are indicated. Error bars represent the standard error of the mean
and asterisks indicate significant differences
(*P<0.05, **P<0.01 and
***P<0.001). Scale bar, 250 μm; magnification, ×40.
LRP5, lipoprotein receptor-related protein 5; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH-ir, tyrosine
hydroxylase-immunoreactive; SNc, substantia nigra. |
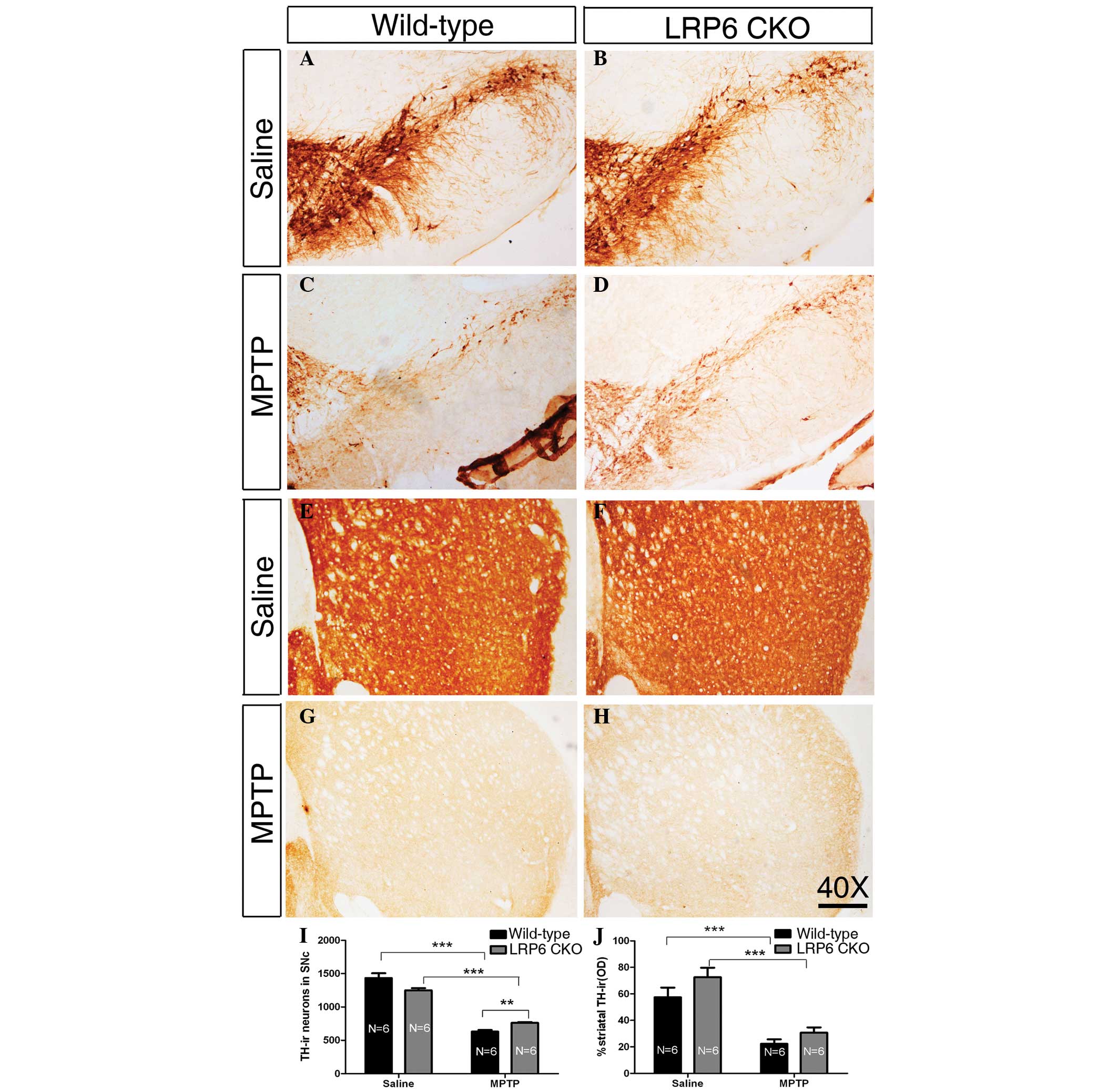
LRP6 CKO
In wild-type and LRP6 CKO mice treated with saline
injections for five weeks, the TH-ir neurons in the SNc were
intensely immunostained, and TH-ir axons were densely and evenly
distributed throughout the striatum, without a detectable
difference between the two genotypes (Fig. 2A, B, E, F, I and J), indicating
that the midbrain DA neurons and their nigrostriatal projection are
morphologically normal in the absence of LRP6 expression in
adulthood. MPTP administration induced a marked loss of TH-ir
neurons in the SNc of wild-type mice, and this reduction was
significantly higher compared with that in the MPTP-treated LRP6
CKO mice (Fig. 2C, D and I),
reflecting the possibility that the loss of LRP6 is beneficial for
the midbrain DA neurons exposed to MPTP. However, the nigrostriatal
projection shown by TH-ir axons in the striatum was similar between
wild-type and LRP6 CKO mice in terms of the density following the
MPTP treatment (Fig. 2G, H and J).
Thus, MPTP treatment leads to a decreased loss of midbrain DA
neurons in LRP6 CKO mice compared with that in wild-type mice;
however, this reduction is not reflected by the density of TH-ir
striatal axons.
β-catenin CKO
In saline-treated mice, the number of TH-ir neurons
in the SNc was lower in the β-catenin CKO mice relative to that in
the wild-type mice, with an approximate ratio of 76.7% (Fig. 3A, B and I; P<0.001).
Consistently, the density of TH-ir axons in the striatum was higher
in the wild-type mice compared with that in the β-catenin CKO mice
(Fig. 3E, F and J). The decreased
number of TH-ir neurons in adult β-catenin CKO mice suggests that
β-catenin may be required in the development and/or maintenance of
the midbrain DA neurons.
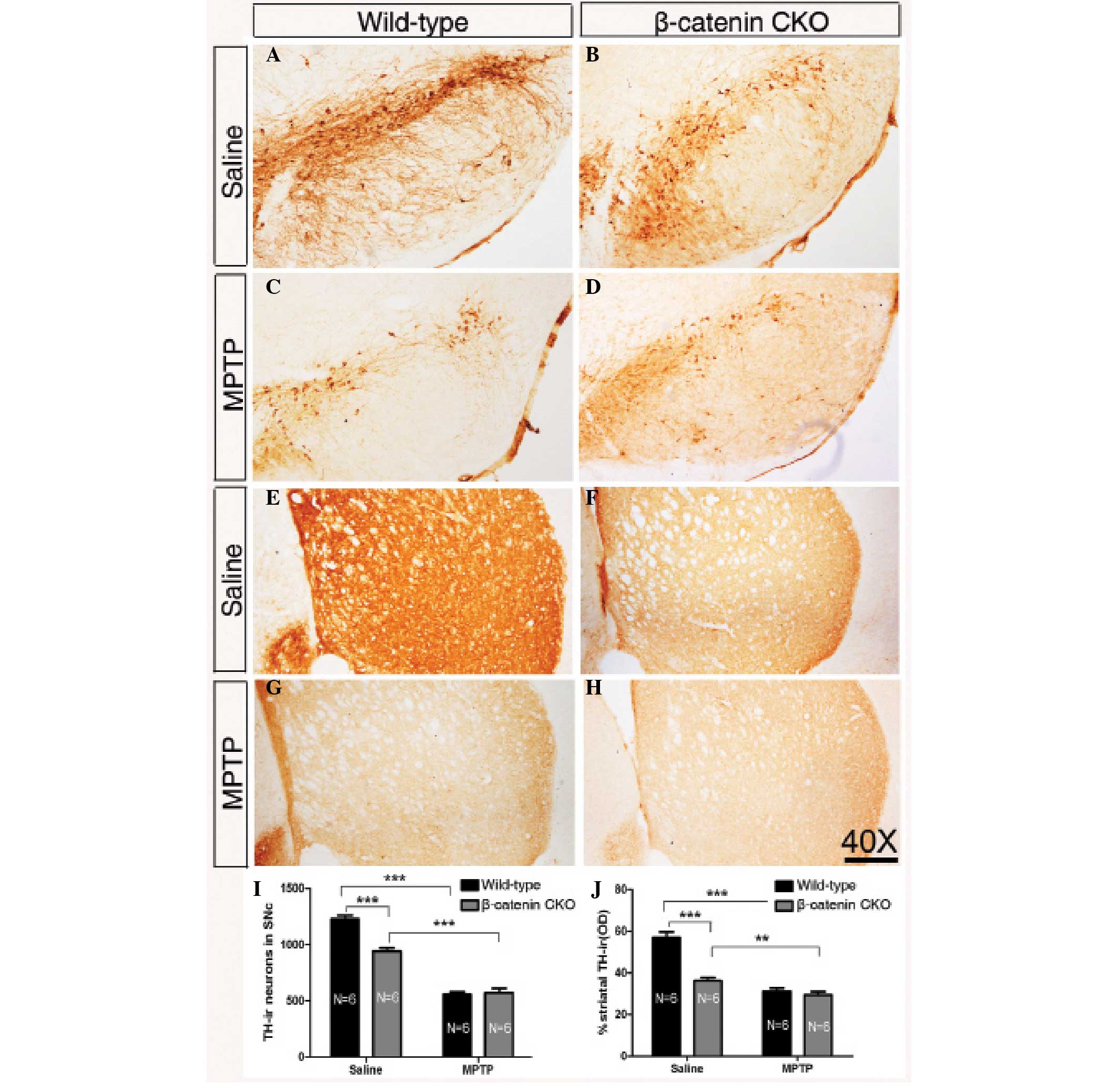 | Figure 3Inactivation of β-catenin reduces the
number of DA neurons and protects them from exposure to MPTP to a
cetain extent. (A–D) Representative images showing nigral TH-ir
cells in wild-type and β-catenin CKO mice treated with saline or
MPTP injection. (E–H) Representative images of striatal TH-ir
axonal terminals of wild-type and β-catenin CKO mice treated with
saline or MPTP. (I) Quantification of TH-ir neurons in the SNc of
wild-type and β-catenin CKO mice following saline or MPTP
injection. The number of TH-ir neurons is decreased in β-catenin
CKO mice compared with that in wild-type mice folowing treatment
with saline. Following MPTP treatment, no significant difference is
observed between the two genotypes. (J) Statistical data of the
optical density of TH-ir striatal terminals in the wild-type and
β-catenin CKO mice treated with saline or MPTP. A similar
significant difference between the two genotypes following saline
treatment is detected, while no change is found following MPTP
treatment. Sample sizes are indicated. Error bars represent the
standard of the mean and asterisks indicate significant differences
(**P<0.01 and ***P<0.001). Scale bar:
250 μm; magnification, ×40. DA, dopaminergic; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH-ir, tyrosine
hydroxylase-immunoreactive; SNc, substantia nigra. |
Following the five-week MPTP treatment, although the
TH-ir neurons in the SNc were significantly reduced quantitatively
in wild-type and β-catenin CKO mice, no marked difference was
observed between the two genotypes in terms of the number of TH-ir
neurons in the SNc and the density of the TH-ir axons in the
striatum (Fig. 3C, D and G–J).
Considering the fact that fewer TH-ir neurons remained in β-catenin
CKO mice than in the wild-type mice following saline treatment,
these results suggest that midbrain DA neurons lacking β-catenin
expression appear to be resistant to MPTP toxicity to a certain
extent.
Discussion
The canonical Wnt signaling pathway is critical for
many cellular processes. LRP5/6, co-receptors for Wnt ligands, are
highly homologous proteins with key functions in this signaling
pathway, including development, as well as disease (26,27).
However, the role of LRP5/6 in the development of midbrain DA
neurons and their association with PD remain unclear. In addition,
the function of β-catenin, the key mediator of canonical Wnt
signaling, is not yet clearly understood in mouse PD models.
Therefore, in the present study the expression of LRP5, LRP6 or
β-catenin was inactivated in the midbrain DA neurons of the LRP6,
LRP6 or β-catenin CKO mice, and the alterations in the numbers of
TH-ir neurons in the SNc and striatum of the MPTP-PD model were
then investigated.
LRP5/6 have previously been demonstrated to be
critical co-receptors by binding to Wnt-Fzd to form a trimeric
complex (28); however, the
analysis of genetically engineered mice has revealed their
different functions during embryonic development (27). Generally, the LRP6 loss-of-function
phenotypes are more severe compared with the LRP5 loss-of-function
phenotypes (5), indicating that
LRP6 has a more crucial role during embryogenesis. In accordance
with this, in LRP6 mutant mice a marked reduction in the number of
TH-positive neurons and a defect in midbrain morphogenesis were
observed at embryonic day (E) 11.5 (12). However, in the present study, no
change in the number of DA neurons in saline-treated LRP6 CKO mice
(Fig. 2A, B, E, F, I and J), as
well as in LRP5 CKO mice (Fig. 1A, B,
E, F, I and J), compared with that in saline-treated wild-type
mice, was observed. Given that a global knockout mouse model was
used in the previous study, this may suggest that i) loss of LRP6
in cell types other than in DA neurons of the midbrain causes the
developmental delay of DA neurons, while the selective knockout of
LRP6 in midbrain DA neurons has no apparent impact; and ii) the Cre
recombinase in TH-Cre mice is firstly expressed in postmitotic
midbrain DA neurons after E12, so the initial process of DA
neurogenesis is not interrupted in the LRP6 CKO mice.
β-catenin is an obligate component of the Wnt
signaling cascade, and recent studies have shown that it is
critical for midbrain DA neuron specification and neurogenesis
(29). In particular, β-catenin
loss-of-function experiments showed that key DA progenitor genes,
including Otx2, Lmx1a, Msx1 and Ngn2 are downregulated and fewer DA
neurons are generated (13,30).
In accordance with these results, in the present study, it was
found that there is a reduction in the number of midbrain DA
neurons and striatal DA terminals in β-catenin CKO mice compared
with that in wild-type mice when injected with saline (Fig. 3A, B, E, F, I and J), suggesting a
cell-autonomous function of β-catenin in DA neurons.
Symptoms of PD may be induced in mouse by DA
neuron-specific toxins, including 6-OHDA, rotenone and MPTP
(31). In the present study,
chronic MPTP treatment was performed, which has previously been
shown to be more effective compared with acute or sub-acute
protocols (32). In wild-type
mice, MPTP led to the loss of approximately half of the nigral DA
neurons and the striatal DA terminals, compared with those in the
saline-injected mice (Fig.
1–3), indicating that the
mouse model for PD was successfully generated.
Following MPTP treatment for five weeks, the numbers
of TH-ir DA neurons in the midbrain and of TH-ir axonal terminals
in the striatum were decreased in LRP5/6 CKO mice in a similar
manner to that in control mice; however, the number of surviving DA
cells in the CKO mice was increased compared with that in the
wild-type mice (Figs. 1A, B and I
and 2A, B and I). These data
suggest that specific ablation of LRP5/6 in midbrain DA neurons has
a neuroprotective role in MPTP-treated mice. Although the DA
neurons were decreased in number in the β-catenin CKO mice without
MPTP exposure, the numbers of surviving DA neurons were comparable
between wild-type and β-catenin CKO mice following chronic MPTP
injection (Fig. 1A, B and I),
indicating a similar protective effect in β-catenin depleted mice
and LRP5/6 CKO mice. However, recent studies have suggested that
canonical Wnt signaling contributes to the protection of midbrain
DA neurons (14). For example, the
Wnt signaling antagonist Dickkopf-1 aggravates the DA neuron damage
of SNc in 6-OHDA-lesioned rats (16) and counteracts astrocyte-induced
neuroprotection against MPTP toxicity in primary mesencephalic
astrocyte-neuron cultures (17).
In addition, an in vitro study showed that exogenous Wnt1
protects primary mesencephalic DA neurons against cell death
induced by 6-OHDA or MPTP, and this neuroprotection is abolished by
the knockdown of β-catenin or Fzd (33). These results are based on in
vitro cultures or pharmacological studies in vivo; thus,
a genetically engineered mouse model involved in Wnt signaling is
lacking. In the present study, the Cre-loxP strategy (34) was used to conditionally knockout
key components of the canonical Wnt signaling pathway and
MPTP-induced PD mouse model, which may be more reliable and reflect
the physiological condition. However, inconsistent results were
obtained (16,17,33).
Therefore, further investigations are required to clearly elucidate
the role of the canonical Wnt signaling pathway in PD.
In conclusion, in the present study, LRP5, LRP6 or
β-catenin were selectively knocked out in the midbrain TH-positive
neurons. The results indicated that the loss of β-catenin affects
the survival and/or maintenance of the midbrain DA neurons, while
the loss of LRP5/6 does not. When exposed to MPTP, the LRP5, LRP6
or β-catenin CKO mice showed that the depletion of these Wnt
signaling components has a neuroprotective effect on the midbrain
DA neurons. These data provide a novel perspective for the role of
canonical Wnt signaling components in the pathogenesis of PD.
Acknowledgements
The authors would like to thank Jia-Yin Chen,
Yu-Ling Sun and Chen-Hong Qin for their technical assistance. This
study was supported by grants from the National Basic Research
Program of China (2011CB51005), the Natural Science Foundation of
Zhejiang Province (LQ13C090004), the National Natural Science
Foundation of China (81200933, 81200692, 31100788 and 81101026),
Science and Technology Commission of Shanghai Municipality
(12XD1404800) and the Fundamental Research Funds for the Central
Universities (Tongji University).
References
|
1
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinson KI, Brennan J, Monkley S, Avery BJ
and Skarnes WC: An LDL-receptor-related protein mediates Wnt
signalling in mice. Nature. 407:535–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamai K, Semenov M, Kato Y, et al:
LDL-receptor-related proteins in Wnt signal transduction. Nature.
407:530–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wehrli M, Dougan ST, Caldwell K, et al:
arrow encodes an LDL-receptor-related protein essential for
Wingless signalling. Nature. 407:527–530. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tolwinski NS and Wieschaus E: A nuclear
function for armadillo/beta-catenin. PLoS Biol. 2:E952004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toledo EM, Colombres M and Inestrosa NC:
Wnt signaling in neuroprotection and stem cell differentiation.
Prog Neurobiol. 86:281–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inestrosa NC and Arenas E: Emerging roles
of Wnts in the adult nervous system. Nat Rev Neurosci. 11:77–86.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas KR and Capecchi MR: Targeted
disruption of the murine int-1 proto-oncogene resulting in severe
abnormalities in midbrain and cerebellar development. Nature.
346:847–850. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castelo-Branco G, Wagner J, Rodriguez FJ,
et al: Differential regulation of midbrain dopaminergic neuron
development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA.
100:12747–12752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castelo-Branco G, Andersson ER, Minina E,
et al: Delayed dopaminergic neuron differentiation in Lrp6 mutant
mice. Dev Dyn. 239:211–221. 2010.PubMed/NCBI
|
|
13
|
Tang M, Miyamoto Y and Huang EJ: Multiple
roles of beta-catenin in controlling the neurogenic niche for
midbrain dopamine neurons. Development. 136:2027–2038. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berwick DC and Harvey K: The importance of
Wnt signalling for neurodegeneration in Parkinson’s disease.
Biochem Soc Trans. 40:1123–1128. 2012.PubMed/NCBI
|
|
15
|
Rawal N, Corti O, Sacchetti P, et al:
Parkin protects dopaminergic neurons from excessive
Wnt/beta-catenin signaling. Biochem Biophys Res Commun.
388:473–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dun Y, Li G, Yang Y, et al: Inhibition of
the canonical Wnt pathway by Dickkopf-1 contributes to the
neurodegeneration in 6-OHDA-lesioned rats. Neurosci Lett.
525:83–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
L’Episcopo F, Tirolo C, Testa N, et al:
Reactive astrocytes and Wnt/beta-catenin signaling link
nigrostriatal injury to repair in
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s
disease. Neurobiol Dis. 41:508–527. 2011.PubMed/NCBI
|
|
18
|
Gelman DM, Noain D, Avale ME, Otero V, Low
MJ and Rubinstein M: Transgenic mice engineered to target
Cre/loxP-mediated DNA recombination into catecholaminergic neurons.
Genesis. 36:196–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Z, Baker JJ, Zylstra-Diegel CR and
Williams BO: Lrp5 and Lrp6 play compensatory roles in mouse
intestinal development. J Cell Biochem. 113:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joeng KS, Schumacher CA, Zylstra-Diegel
CR, Long F and Williams BO: Lrp5 and Lrp6 redundantly control
skeletal development in the mouse embryo. Dev Biol. 359:222–229.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brault V, Moore R, Kutsch S, et al:
Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion
results in dramatic brain malformation and failure of craniofacial
development. Development. 128:1253–1264. 2001.PubMed/NCBI
|
|
22
|
Bezard E, Dovero S, Bioulac B and Gross
CE: Kinetics of nigral degeneration in a chronic model of
MPTP-treated mice. Neurosci Lett. 234:47–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-Fischer D, Fuchs J, Castagner F,
et al: Engrailed protects mouse midbrain dopaminergic neurons
against mitochondrial complex I insults. Nat Neurosci.
14:1260–1266. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fornai F, Schlüter OM, Lenzi P, et al:
Parkinson-like syndrome induced by continuous MPTP infusion:
convergent roles of the ubiquitin-proteasome system and
alpha-synuclein. Proc Natl Acad Sci USA. 102:3413–3418. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paxinos G and Franklin KBJ: The Mouse
Brain in Stereotaxic Coordinates. 2nd edition. Academic Press; San
Diego: 2001
|
|
26
|
Li Y and Bu G: LRP5/6 in Wnt signaling and
tumorigenesis. Future Oncol. 1:673–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joiner DM, Ke J, Zhong Z, Xu HE and
Williams BO: LRP5 and LRP6 in development and disease. Trends
Endocrinol Metab. 24:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4:a0078802012.
|
|
29
|
Joksimovic M and Awatramani R:
Wnt/beta-catenin signaling in midbrain dopaminergic neuron
specification and neurogenesis. J Mol Cell Biol. 6:27–33. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joksimovic M, Yun BA, Kittappa R, et al:
Wnt antagonism of Shh facilitates midbrain floor plate
neurogenesis. Nat Neurosci. 12:125–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terzioglu M and Galter D: Parkinson’s
disease: genetic versus toxin-induced rodent models. FEBS J.
275:1384–1391. 2008.
|
|
32
|
Petroske E, Meredith GE, Callen S,
Totterdell S and Lau YS: Mouse model of Parkinsonism: a comparison
between subacute MPTP and chronic MPTP/probenecid treatment.
Neuroscience. 106:589–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
L’Episcopo F, Serapide MF, Tirolo C, et
al: A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a
candidate regulatory circuit controlling mesencephalic dopaminergic
neuron-astrocyte crosstalk: Therapeutical relevance for neuron
survival and neuroprotection. Mol Neurodegener. 6:492011.
|
|
34
|
Feil R, Brocard J, Mascrez B, LeMeur M,
Metzger D and Chambon P: Ligand-activated site-specific
recombination in mice. Proc Natl Acad Sci U S A. 93:10887–10890.
1996. View Article : Google Scholar : PubMed/NCBI
|