Introduction
Encephalitis is an unusual manifestation of human
viral infection; only a few patients who have acquired systemic
viral infections develop symptomatic infection of the central
nervous system. Acute viral encephalitis is characterized by a
triad of fever, headache and an altered level of consciousness
(1). Other clinical
characteristics include behavior and speech disturbances,
disorientation, hemiparesis and seizures. The most common cause of
sporadic encephalitis is herpes simplex virus (HSV) type I, which
may be diagnosed by methods including serological testing for IgM
antibodies, polymerase chain reaction (PCR) analysis to search for
HSV DNA or brain biopsy (2).
Computed tomography (CT) scans and magnetic resonance imaging (MRI)
scans may also provide useful information.
An alternative situation, in which a gliomatosis
cerebri (GC) presents as an acute encephalitic illness, has rarely
been reported. To the best of our knowledge, only one case has
previously been reported (3). GC
is a rare tumor of the central nervous system, and the World Health
Organization (WHO) criteria define GC as a diffusely infiltrative
glioma that involves at least three cerebral lobes (4). In the present study, a patient with
GC, which mimicked the clinicoradiological course of acute viral
encephalitis, is presented.
Case report
The study was approved by the Ethics Committee of
Liaoning Cancer Hospital & Institute (Shenyang, China). Written
informed consent was obtained from the patient’s family. A
56-year-old man was admitted to the Department of Neurology,
Dandong Central Hospital (Dandong, China) in June 2013 after 3 days
of fever, dizziness, headache and numbness in the right
extremities. Physical examination showed a blood pressure of 140/85
mmHg and a body temperature of 38.2°C. Neurological examination
indicated mild memory deterioration and calculation impairment;
however, reading and writing were normal. Examinations of motor,
cerebellar function and gait were normal; however, the ability to
sense pain and temperature in the right upper and lower extremities
was impaired. On routine laboratory testing, the white blood cell
count was observed to be increased to 15,200/mm3 (90.4%
were neutrophilic leukocytes) and the C-reactive protein level was
1.0 mg/dl. The cerebrospinal fluid (CSF) obtained at admission
showed mild pleocytosis of 10.0×106/l leukocytes with 5%
neutrophilic cells, 75% lymphocytes and 20% monocytes. The CSF
protein concentration was 100 mg/dl, and the glucose level was 3.6
mmol/l. CSF gram stain, bacterial and viral cultures and HSV PCR
were negative. Brain CT scans revealed abnormally low density in
the left frontal, temporal, insular lobes and in the left thalamus
(Fig. 1A). The results from the
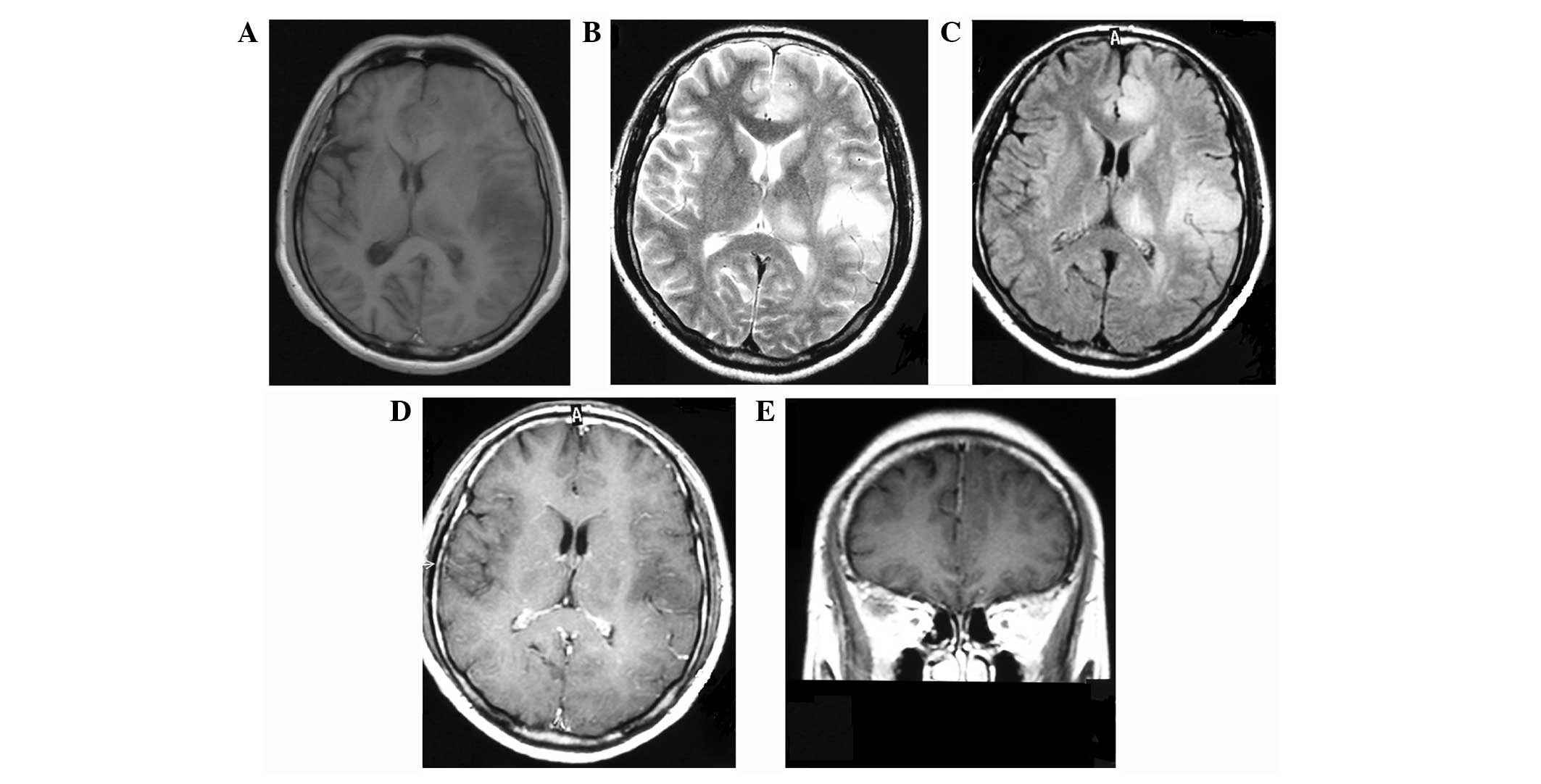
MRI demonstrated that all the lesions showed hypointensity on
T1-weighted images (Fig. 2A),
relatively homogeneous hyperintensity on T2-weighted images
(Fig. 2B) and on fluid-attenuated
inversion recovery (FLAIR) images (Fig. 2C). No signal-enhancement was
observed following gadolinium administration (Fig. 2D and E). The patient received a
10-day course of acyclovir, intravenously. The patient was then
discharged but the symptoms were not markedly relieved.
At the follow-up three months later, the patient
presented with personality changes and memory deterioration. The
follow-up MRI showed no remarkable changes. At the follow-up six
months after presentation, the patient had expressive aphasia and
severe headache. Subsequently, the patient had two tonic-clonic
seizure onsets and was admitted to the Department of Neurosurgery,
Liaoning Cancer Hospital & Institute (Shenyang, China).
Physical examination was normal; however, neurological examination
showed expressive aphasia, disorientation, memory deterioration,
calculation impairment and strength weakness in the right
extremities. The CSF was essentially normal. Brain CT scans
revealed the enlarged extent of the low density in the left frontal
lobe (Fig. 1B); however, the
lesions in the left temporal and insular lobes and in the left
thalamus were nearly unchanged. The MRI showed that the lesion had
increased in size with more edema around the lesion in the left
frontal lobe (Fig. 3A–C).
Irregular enhancement signals were observed following gadolinium
administration (Fig. 3D and E).
The results from the magnetic resonance spectroscopy (MRS) showed
elevated choline (Cho)/creatine (Cr) and
Cho/N-acetylaspartate (NAA) ratios, as well as decreased
NAA/Cr ratios in the lesions (Fig.
3F). Surgery was performed and the neuropathological diagnosis
of WHO grade III astrocytoma was confirmed.
Discussion
The term gliomatosis cerebri (GC) was first proposed
in 1938 by Nevin (5). GC is a rare
glial neoplasm, characterized by extensive diffuse brain
infiltration and relative preservation of the underlying
architecture. GC is an intriguing disease for several reasons.
Firstly, it is difficult to distinguish between GC and diffuse
gliomas. In this regard, GC may represent the most invasive form of
diffuse gliomas. Secondly, in terms of histological grading and
clinical course, GC is a heterogeneous disease, ranging from
rapidly evolving to slowly and somewhat indolent forms. Due to the
extensive spread of the disease, surgery, apart from biopsy for
diagnosis, is rarely performed for patients with GC (6). Pathologists have described two types
of primary GC. Type 1 is a classical form of GC, characterized by
diffuse overgrowth, with neoplastic glial elements and without a
focal mass presence. Type 2, which may stem from type 1, is
characterized by a diffuse brain infiltration and focal mass
presence, and is usually a high-grade glioma (7).
GC may occur at any age; however, the majority of
patients are 40–50 years old. Clinical manifestations include
headaches, seizures, visual disturbance, corticospinal tract
deficit, lethargy and dementia (8). Correct diagnosis may be acquired in
the majority of GC cases via clinical manifestations, neurologic
examination, MRI scans and MRS. MRI reveals widespread invasive
lesions with hypointensity on T1-weighted images and hyperintensity
on T2-weighted images and FLAIR sequences. Furthermore, the
abnormality is shown more clearly on FLAIR sequence images than on
T2-weighted images. MRS usually shows elevated Cho/Cr and Cho/NAA
ratios, as well as decreased NAA/Cr ratios in the lesions (9).
In general, the differential diagnosis of GC
includes multiple sclerosis, viral encephalitis,
adrenoleukodystrophy, metachromatic leukodystrophy and subacute
sclerosing panencephalitis since the cerebral lesions are confluent
and extensive on CT and MRI scans (3). Brain biopsy is used when differential
diagnosis via clinical manifestations, CSF testing, MRI and MRS is
very challenging. As a result of the diffuse infiltration of the
neoplasm, surgery is not suitable. However, it may be used when the
neoplasm grows into a large mass and high intracranial pressure is
induced.
In the present study, a patient with type 2 GC
mimicking acute viral encephalitis following clinical onset is
presented. In particular, the malignant transformation of lesions
into astrocytoma in the left frontal lobe was observed, while
lesions in other areas were nearly unchanged after six months. This
suggests that the neoplasm cells in the left frontal lobe may be
different from those in other areas. All the neoplasm cells may be
at distinct stages of malignant transformation; however, the cells
in the left frontal lobe may be more likely to undergo malignant
transformation than others. This confusing clinicoradiological
profile led to the initial misdiagnosis of acute viral
encephalitis. At the three-month follow-up, the patient had
personality changes and memory deterioration. In spite of this, the
MRI scan showed no remarkable changes. Therefore, a brain biopsy is
recommended for the diagnosis of GC so that the correct measures
may be taken for the treatment of this disease as early as
possible.
Acknowledgements
This study was supported by the Natural Science
Foundation of Liaoning Province (Grant no. 2013020205).
References
|
1
|
Whitley RJ and Gnann JW: Viral
encephalitis: familiar infections and emerging pathogens. Lancet.
359:507–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rees JH and Howard RS: High-grade glioma
mimicking acute viral encephalitis - three case reports. Postgrad
Med J. 75:727–730. 1999.PubMed/NCBI
|
|
3
|
Nagata R, Ikeda K, Nakamura Y, et al: A
case of gliomatosis cerebri mimicking limbic encephalitis:
malignant transformation to glioblastoma. Intern Med. 49:1307–1310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mattox AK, Lark AL and Adamson DC: Marked
response of gliomatosis cerebri to temozolomide and whole brain
radiotherapy. Clin Neurol Neurosurg. 114:299–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nevin S: Gliomatosis cerebri. Brain.
61:170–191. 1938. View Article : Google Scholar
|
|
6
|
Rudà R, Bertero L and Sanson M:
Gliomatosis cerebri: a review. Curr Treat Options Neurol.
16:2732014.
|
|
7
|
Mawrin C, Kirches E, Schneider-Stock R, et
al: Analysis of TP53 and PTEN in gliomatosis cerebri. Acta
Neuropathol. 105:529–536. 2003.PubMed/NCBI
|
|
8
|
Kim DG, Yang HJ, Park IA, et al:
Gliomatosis cerebri: clinical features, treatment, and prognosis.
Acta Neurochir (Wien). 140:755–762. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu A, Li K and Li H: Value of diagnosis
and differential diagnosis of MRI and MR spectroscopy in
gliomatosis cerebri. Eur J Radiol. 59:216–221. 2006. View Article : Google Scholar : PubMed/NCBI
|