Introduction
Vertebral artery dissecting aneurysm (VADA)
represents the underlying aetiology in a significant number of
posterior circulation ischaemic strokes and subarachnoid
haemorrhages (SAHs). When the lesions rupture and cause SAH, the
percentage of rebleeding of these lesions is up to 30% (1,2).
Thus, early treatment is strongly recommended. The standard
treatment option is the complete occlusion of the dissected lesion
through surgical or endovascular procedures. As the surgical
approach is associated with a high incidence rate of
treatment-associated mortality and morbidity, endovascular
procedures are favoured in the treatment of VADA (3–5).
Endovascular treatment of VADA is complex and
proximal occlusion of the parent artery and embolisation of the
dissected segment have been proven to be optimal methods for VADA
treatment (6). However, a number
of more complex cases of VADA cannot be treated with proximal
occlusion due to parent vessel sacrifice. Thus, parent vessel
occlusion or stent-assisted coil embolisation has been utilised to
treat these lesions. Treatment with a combination of stents and
coils or with a stent or coil alone has been described in a number
of patients (7–9). This treatment requires different
therapeutic strategies due to the various states of the
contralateral vertebral arteries or the location of the aneurysm in
relation to the posterior inferior cerebellar artery (PICA).
However, to the best of our knowledge, the classification of VADA
on the basis of the state and location of bilateral vertebral
arteries is unavailable. Thus, the current study aimed to classify
VADAs by analysing the clinical features of the aneurysm,
particularly its location and the state of the contralateral
vertebral artery, in order to guide the optimal treatment strategy
for these lesions.
Materials and methods
Patients
Between January 2004 and December 2011, 31 patients
with VADA underwent endovascular treatment at the Department of
Neurosurgery of the Second Hospital of Shandong University (Jinan,
China). A total of 20 males and 11 females were included in the
study group, with ages ranging from 25 to 73 years (mean age, 42.3
years). A total of 25 patients had hypertension and four had head
or neck trauma. Approval was obtained from the Institutional Review
Boards of the Second Hospital of Shandong University and Qilu
Hospital of Shandong University (Qingdao, China) to study the use
of coiling or stent-assisted coiling to treat VADA. The current
study was conducted in accordance with the Declaration of Helsinki
and with approval from the Ethics Committee of Shandong University.
Written informed consent was obtained from all participants.
Clinical features
A total of 23 patients with ruptured aneurysm
presented with sudden headaches and computed tomography (CT) scans
(SOMATOM Sensation 64; Siemens Medical Systems, Erlangen, Germany)
revealed SAH. Among these patients, eight presented a coma and nine
had dysphagia or another cranial nerve manifestation. Eight
patients had headaches, vertigo and an unsteady gait, but no
haemorrhage was detected by the CT scan. Only one patient presented
brain stem infarction upon magnetic resonance imaging (MRI)
examination (Magnetom Avanto; Siemens Medical Systems).
Angiographic results and
classification
All patients agreed to undergo CT scans and digital
subtraction angiography (DSA; Innova 3100; GE Healthcare, Waukesha,
WI, USA) for diagnosis. Angiograms were assessed for size, shape
and location of VADA with respect to the PICA. The aneurysms were
classified into three types on the basis of the location of the
aneurysm in relation to the PICA: type I aneurysms, located
distally to the PICA; type II aneurysms, located at the PICA origin
and; type III aneurysms, located proximally to the PICA. Each type
of aneurysm was further divided into two subtypes according to the
developmental state of the contralateral vertebral artery. Subtype
a included well-developed contralateral vertebral arteries and a
guaranteed posterior circulation blood supply following the
occlusion of the ipsilateral vertebral artery. Subtype b included
contralateral vertebral arteries that were hypoplastic and would
provide an inadequate posterior circulation blood supply following
ipsilateral vertebral artery occlusion.
Endovascular interventional therapy
Patients were placed under a state of general
anaesthesia. Following a standard Seldinger puncture, a 6F
introducer sheath (Cordis, Bridgewater, NJ, USA) was placed in the
right femoral artery with heparinisation (by administrating heparin
2–3 mg/kg). Following the placement of a 6F guiding catheter
(Cordis) in the ipsilateral vertebral artery at the level of the
second cervical vertebrae, a roadmap was produced and an SL-10
microcatheter (Boston Scientific Corp, Natick, MA, USA) was
delivered into the aneurysm. The aneurysm was embolised by
appropriate micro-coils according to the shape and size of the
aneurysm.
A 6F introducer sheath was placed in the right
femoral artery and a 5F introducer sheath was placed in the left
femoral artery. Following placement of the 6F guiding catheter into
the ipsilateral vertebral artery, occlusion tests were carried out
with an occlusion balloon to analyse whether the blood supply was
adequate. The aneurysm was partially embolised with coiling after
the microcatheter was navigated. The ipsilateral parent vertebral
artery was subsequently occluded with coiling under the condition
that PICA could obtain a sufficient blood supply from the
ipsilateral or contralateral vertebral arteries.
Patients scheduled for stent-assisted coiling
received oral aspirin (300 mg daily) and oral clopidogrel (75 mg
daily) for three days to one week prior to the procedure. Patients
undergoing emergency procedures received combined antiplatelet
therapy (oral clopidogrel 300 mg and aspirin 300 mg) on the day of
surgery.
A 4.5×22 or 4.5×28 mm Enterprise stent (Codman &
Shurtleff, Raynham, Massachusetts, USA) was used to cover the neck
of the aneurysm following placement of a Prowler-plus microcatheter
(Codman) in the dissecting aneurysm. Coiling was subsequently
performed and the stent was finally released.
Post-operative treatment
Patients with SAH or intraventricular haemorrhage
were given a lumbar puncture or catheter following surgery.
External ventricular drainage was performed on patients in a coma
with intraventricular haemorrhage. Patients with stent-assisted
coiling were administered antiplatelet agents (clopidogrel 75 mg
and aspirin 300 mg daily) for three months. After three months, the
antiplatelet agents were adjusted according to the patients’
condition. All patients were reviewed for post-operative
angiography at one, three and six months, and one year.
Results
Primary classification
A total of 10 patients had a type I aneurysm (10/31
patients), of which six patients were type Ia and four patients
were type Ib. A total of 13 patients were type II (13/31 patients),
of which seven were type IIa and six patients were type IIb. A
total of eight patients were type III (8/31 patients), of which
five patients were type IIIa and three patients were type IIIb
(Table I).
 | Table IPrimary classification of vertebral
artery dissection aneurysm. |
Table I
Primary classification of vertebral
artery dissection aneurysm.
| Type | Subtype | Cases (n) | Proportion (%) |
|---|
| I | Ia | 5 | 16.1 |
| Ib | 5 | 16.1 |
| II | IIa | 7 | 22.6 |
| IIb | 6 | 19.4 |
| III | IIIa | 5 | 16.1 |
| IIIb | 3 | 9.7 |
Endovascular interventional therapy
Among the 31 patients, 18 patients underwent
stent-assisted coiling, two cases received coiling only, 10
received coiling with parent artery occlusion and one case was
treated conservatively. Among the 31 patients with VADA, 21 were
embolised completely, nine were partially embolised and one was not
embolised. One patient developed a coma following coiling and the
rest of the patients recovered well without any neurological
deficits. No mortalities were reported among the 31 patients.
Treatment approach according to
classification
The approaches used in the endovascular
interventional treatment were selected carefully according to the
classification of the VADA (Table
II).
 | Table IIClassification and treatment
strategy. |
Table II
Classification and treatment
strategy.
| Type | Coiling with parent
artery embolization (%) | Stent-assisted
coiling (%) | Coiling (%) | Conservative
treatment (%) |
|---|
| I type | 4 (40) | 4 (40) | 1 (10) | 1 (10) |
| Ia | 4 (80) | 1 (20) | | |
| Ib | | 3 (60) | 1 (20) | 1 (20) |
| II type | 2 (15.4) | 11 (84.6) | | |
| IIa | 2 (28.6) | 5 (71.4) | | |
| IIb | | 6 (100) | | |
| III type | 4 (50) | 3 (37.5) | 1 (12.5) | |
| IIIa | 4 (80) | 1 (20) | | |
| IIIb | | 2 (66.7) | 1 (33.3) | |
| Subtype a | 10 (58.8) | 7 (41.2) | | |
| Subtype b | | 11 (78.6) | 2 (14.3) | 1 (7.1) |
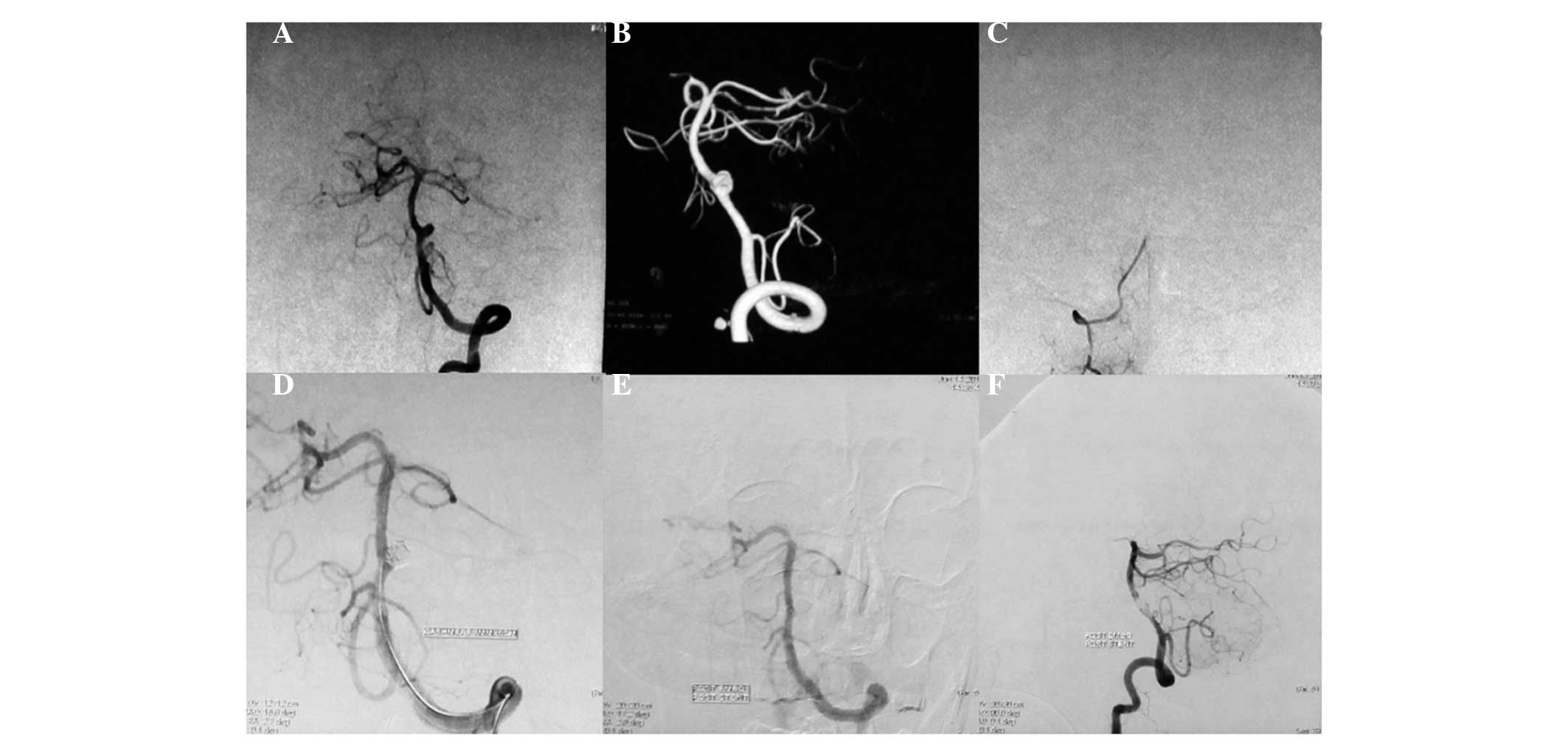
Among the 10 patients with type I aneurysms, four
patients with type Ia received coiling with parent artery occlusion
(Fig. 1), one patient with type Ia
received stent-assisted coiling, three patients with type Ib
received stent-assisted coiling (Fig.
2), one patient with type Ib received coiling only and one
patient with type Ib was treated conservatively. Among the 13
patients with type II aneurysms, two patients with type IIa
received coiling with parent artery occlusion (Fig. 3), five patients with type IIa
received stent-assisted coiling (Fig.
4) and all six patients with type IIb received stent-assisted
coiling. Among the eight patients with type III aneurysms, four
patients with type IIIa received coiling with parent artery
occlusion (Fig. 5), one patient
with type IIIa received stent-assisted coiling (Fig. 6), two patients with type IIIb
received stent-assisted coiling and one patient with type IIIb
received coiling only.
Of the 17 patients classified as subtype a, 10
patients received coiling with parent artery occlusion and seven
patients received stent-assisted coiling. Of the 14 patients
classified as subtype b, 11 patients received stent-assisted
coiling, two patients received coiling only and one patient was
treated conservatively.
Discussion
The incidence rate of VADA is relatively low and the
annual incidence rate has been reported to be 1/100,000 cases in
the USA and 1.5/100,000 cases in France (10–12).
VADA is common in adults, particularly those aged 40–50 years. The
onset manifestation is SAH or cerebral ischaemia. SAH usually
occurs in patients with intracranial aneurysm whereas ischaemic
attack occurs in patients with extracranial aneurysms (12,13).
A local mass effect with a giant aneurysm has also been detected.
SAH caused by VADA explains ~10% of all causes of non-traumatic SAH
with a reported mortality rate ranging from 19 to 83% (14).
VADA is frequently ruptured due to severe SAH with
catastrophic neurological outcomes and a high incidence rate of
recurrent haemorrhage (1,2,15).
Mizutani et al retrospectively analysed 42 patients with
recurrent haemorrhage caused by the rupture of VADA and revealed
that 40.5% of recurrent haemorrhages occurred within 24 h and that
57.1% occurred one week following the first haemorrhage (16). Yamada et al studied the
conservative management of 24 patients with VADA and determined
that 58% of these cases occurred with recurrent haemorrhage and 46%
succumbed due to catastrophic rebleeding (17).
DSA remains the gold standard in diagnostic imaging.
The classical manifestations of VADA in DSA are as follows: pearl
and string, double lumen, rosette or a simple fusiform dilation, as
well as a delayed clearance of dilatation or a false lumen
(18–20). The treatment of VADA is a technical
challenge due to the histopathological features and special
localisations of aneurysms. Endovascular intervention therapy,
rather than conventional surgery, is preferred in the treatment of
certain cases of VADA as surgical trauma is avoided in the former
(2,13). The dissecting aneurysm does not
have a real neck and conventional clipping does not manage the
aneurysm successfully. Endovascular intervention therapy is
becoming the primary option for VADA treatment (21). The vertebral artery should be
occluded or preserved when the aneurysm is treated with the
intervention technique. Occlusion of the vertebral artery may bring
about a low rate of recurrence, or prevent recurrence. However,
this occlusion affects the ipsilateral vertebral blood supply,
which may result in severe complications, including ischaemic
lesioning of the brainstem. Certain factors affecting contralateral
vertebral arteries, including the condition of the artery and the
age of the patient, should be carefully considered.
In the current study, 17 patients exhibited a
subtype a aneurysm. Of these, 10 patients received coiling with
parent artery occlusion (58.8%) and seven patients received
stent-assisted coiling (41.2%). All patients who were treated with
coiling combined with parent artery occlusion were not recurrent
and had no ischaemia in the posterior blood circulation in the
follow-up review. In the seven patients with stent-assisted
coiling, the vertebral artery was preserved due to the patients’
refusal to undergo vertebral occlusion in two cases and due to
intolerance to the occlusion test in two cases; the three remaining
three patients were younger patients in which preservation of the
artery was selected to avoid potential ischaemic events. However,
in a later review, one of the seven patients developed a mild
recurrence at the aneurysm neck and was subjected to repeated
embolism. A total of 14 patients exhibited subtype b aneurysm and
the vertebral artery was not occluded due to hypoplasty of the
contralateral vertebral artery. Eleven of the 14 patients accepted
stent-assisted coiling, of which eight were occluded completely and
three were partially embolised. In patients with complete
occlusion, one experienced recurrence during the follow-up period
and one patient developed a mild recurrence at the aneurysm neck.
In patients with partly occluded aneurysms, one patient was
observed to have an enlarged aneurysm in the follow-up angiography.
Two patients of subtype b were treated with coiling directly since
the neck of aneurysm was relatively narrow and one patient selected
conservative treatment for financial reasons. Stent-assisted
coiling was the preferred treatment for type II patients to avoid
affecting the PICA since the aneurysms were located at the origin
of the PICA.
Positive therapeutic effects for VADA have been
attained with the development of the intervention technique.
However, further studies are required to optimise the therapy
strategy in order to improve the selection of the most appropriate
therapies for individual patients (22–24).
The classification of VADA may enable the therapy to be optimized.
Coiling with parent artery embolisation is an effective choice for
patients classified as subtype a since this method is able to avoid
recurrence and potential ischaemic events. The vertebral artery
should be preserved in young patients and patients who cannot fully
tolerate an occlusion experiment prior to embolisation. For
patients classified as subtype b, preservation of the vertebral
artery is required and stent-assisted coiling is preferred despite
ready recurrence. Patients should be regularly reviewed and
embolism performed if required. For patients with a type II
aneurysm, the parent artery should be preserved to prevent affects
on the PICA. In the future, flow-diverting devices (dense
stent-mesh) are likely to be an effective choice for large
dissection aneurysms.
Thus, the classification of an aneurysm based on its
location and the developmental state of the contralateral vertebral
arteries appears to be an effective and safe approach for the
selection of appropriate endovascular interventional therapy
strategies.
Acknowledgements
This study was supported by a grant from the
Shandong Province Outstanding Young Scientists Fund (BS2012YY012).
The funding source had no role in the collection, analysis, or
interpretation of the data or in the decision to submit the study
for publication.
References
|
1
|
Aoki N and Sakai T: Rebleeding from
intracranial dissecting aneurysm in the vertebral artery. Stroke.
21:1628–1631. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MS: Endovascular coil trapping of a
ruptured dissecting aneurysm of the vertebral artery using
detachable coils and micro-tornado® coils. J Cerebrovasc
Endovasc Neurosurg. 15:96–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin SC, Kwon DH, Choi CG, Ahn JS and Kwun
BD: Endovascular strategies for vertebrobasilar dissecting
aneurysms. AJNR Am J Neuroradiol. 30:1518–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Sun Z, Bao J, Li Z, Bai D and Cao
S: Endovascular management of vertebrobasilar artery dissecting
aneurysms. Turk Neurosurg. 23:323–328. 2013.PubMed/NCBI
|
|
5
|
Briganti F, Cicala D, Tortora F, Leone G,
Napoli M and Maiuri F: Endovascular treatment of a giant dissecting
aneurysm of the posterior cerebral artery. A case report and
literature review. Neuroradiol J. 25:695–701. 2012.PubMed/NCBI
|
|
6
|
Ihn YK, Sung JH and Byun JH: Antegrade
recanalization of parent artery after internal trapping of ruptured
vertebral artery dissecting aneurysm. J Korean Neurosurg Soc.
51:301–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin YS, Kim HS and Kim SY: Stenting for
vertebrobasilar dissection: a possible treatment option for
nonhemorrhagic vertebrobasilar dissection. Neuroradiology.
49:149–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki S, Kurata A, Iwamoto K, et al:
Endovascular surgery using stents for vertebral artery dissecting
aneurysms and a review of the literature. Minim Invasive Neurosurg.
51:193–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kashiwazaki D, Ushikoshi S, Asano T, et
al: Long-term clinical and radiological results of endovascular
internal trapping in vertebral artery dissection. Neuroradiology.
55:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halbach VV, Higashida RT, Dowd CF, et al:
Endovascular treatment of vertebral artery dissections and
pseudoaneurysms. J Neurosurg. 79:183–191. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santos-Franco JA, Zenteno M and Lee A:
Dissecting aneurysms of the vertebrobasilar system. A comprehensive
review on natural history and treatment options. Neurosurg Rev.
31:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali MS, Amenta PS, Starke RM, et al:
Intracranial vertebral artery dissections: evolving perspectives.
Interv Neuroradiol. 18:469–483. 2012.PubMed/NCBI
|
|
13
|
Suma T, Shibuya T, Kutsuna N, et al:
Endovascular treatment for ruptured vertebral artery dissecting
aneurysms at the acute stage. Acta Neurochir Suppl. 118:273–276.
2013.PubMed/NCBI
|
|
14
|
Ramgren B, Cronqvist M, Romner B, Brandt
L, Holtås S and Larsson EM: Vertebrobasilar dissection with
subarachnoid hemorrhage: a retrospective study of 29 patients.
Neuroradiology. 47:97–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nashimoto T, Komata T, Honma J, et al:
Successful treatment of bilateral vertebral artery dissecting
aneurysms with subarachnoid hemorrhage: report of three cases. J
Stroke Cerebrovasc Dis. 21:422–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizutani T, Aruga T, Kirino T, Miki Y,
Saito I and Tsuchida T: Recurrent subarachnoid hemorrhage from
untreated ruptured vertebrobasilar dissecting aneurysms.
Neurosurgery. 36:905–911. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada I, Kitahara T, Kurata A, Fujii K
and Miyasaka Y: Intracranial vertebral artery dissection with
subarachnoid hemorrhage: clinical characteristics and outcomes in
conservatively treated patients. J Neurosurg. 101:25–30. 2004.
View Article : Google Scholar
|
|
18
|
Ahn JY, Han IB, Kim TG, et al:
Endovascular treatment of intracranial vertebral artery dissections
with stent placement or stent-assisted coiling. AJNR Am
Neuroradiol. 27:1514–1520. 2006.PubMed/NCBI
|
|
19
|
Joo JY, Ahn JY, Chung YS, et al: Treatment
of intra- and extracranial arterial dissections using stents and
embolization. Cardiovasc Intervent Radiol. 28:595–602. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamada J, Kai Y, Morioka M, Yano S, Todaka
T and Ushio Y: Multimodal treatment of ruptured dissecting
aneurysms of the vertebral artery during the acute stage. J
Neurosurg. 99:960–966. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taha MM, Sakaida H, Asakura F, et al:
Endovascular management of vertebral artery dissecting aneurysms:
review of 25 patients. Turk Neurosurg. 20:126–135. 2010.PubMed/NCBI
|
|
22
|
Lv X, Jiang C, Li Y and Wu Z: Clinical
outcomes of ruptured and unruptured vertebral artery-posterior
inferior cerebellar artery complex dissecting aneurysms after
endovascular embolization. AJNR Am J Neuroradiol. 31:1232–1235.
2010. View Article : Google Scholar
|
|
23
|
Albuquerque FC, Fiorella DJ, Han PP,
Deshmukh VR, Kim LJ and McDougall CG: Endovascular management of
intracranial vertebral artery dissecting aneurysms. Neurosurg
Focus. 18:E32005.PubMed/NCBI
|
|
24
|
Peluso JP, Van Rooij WJ, Sluzewski M,
Beute GN and Majoie CB: Endovascular treatment of symptomatic
intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol.
29:102–106. 2008. View Article : Google Scholar : PubMed/NCBI
|