Introduction
Cartilage defects are difficult to heal
spontaneously, as cartilage tissue lacks blood vessels, nerves and
lymph supplies, and the cartilage lesions do not usually reach the
progenitor cells of the bone marrow (1). Severe cartilage damage caused by
degeneration or excessive use may lead to osteoarthritis (OA),
which causes pain, compromises mobility and poses a significant
disease burden across the world (2). At present, cartilage tissue
engineering is considered to be one of the most promising
therapeutic approaches for the treatment of cartilage defect. The
mechanism of this therapy is based on the fact that bone marrow
mesenchymal stem cells (BMSCs) have the potential for multilineage
differentiation, including osteogenesis, chondrogenesis and
adipogenesis, as well as extensive proliferation.
Growth factors play a crucial role in the regulation
of BMSC differentiation. A number of studies have demonstrated that
transforming growth factor-β (TGF-β), bone morphogenetic protein
(BMP) and insulin-like growth factor are able to induce
chondrogenic differentiation in vitro, and promote the
formation of cartilage-like tissue in vivo (3–6).
However, growth factors not only upregulate the expression of
hyaline cartilage-specific markers, such as collagen II, but also
inevitably lead to further hypertrophic differentiation and
contribute to the development of fibrous cartilage (7–10).
Furthermore, the high cost, rapid degradation and easily-lost
activity of growth factors limit their widespread use, particularly
in clinical practice (11–13). In order to promote chondrogenesis
and maintain the stable chondrogenic phenotype without hypertrophy,
there is an urgent requirement to develop safe and low-cost drugs
that can act as a substitute for or cooperate with growth factors
(11,12).
Herba Epimedii (HEP) is a widely used traditional
Chinese herb to treat osteoporosis in China, Japan and Korea
(13,14). Icariin (ICA;
C33H40O15; molecular weight,
676.65), the main pharmacologically active compound of HEP, has
been suggested to be a potential accelerator for cartilage tissue
engineering and a substitute for growth factors. However, these
results were based on the use of chondrocytes (11,12,15).
Although the application of chondrocytes in cartilage tissue
engineering is relatively prevalent, several major challenges
exist, including chondrocyte dedifferentiation, donor site
morbidity and limited sources for harvesting cartilage tissue
(1). Therefore, the present study
investigated whether ICA had the potential to promote stable
chondrogenesis of BMSCs without hypertrophic differentiation on the
basis that the same chondrogenic medium containing TGF-β3 was
added.
Materials and methods
Cell culture
Rat BMSCs were purchased from Cyagen Biosciences
(Guangzhou, China) and characterized by specific cell surface
markers, including cluster of differentiation (CD)29, CD34, CD44,
CD45, CD11b and CD90. The cells were highly positive for CD29
(83.99%), CD44 (99.69%) and CD90 (95.05%), and negative for CD34
(0.62%), CD45 (0.28%) and CD11b (4.25%), and were able to
differentiate into osteoblasts, chondrocytes and adipocytes. The
cells were cultured in low-glucose Dulbecco’s modified Eagle’s
medium (LG-DMEM; HyClone Laboratories, Inc., Logan, UT, USA)
containing 10% fetal bovine serum (Hyclone Laboratories, Inc.), 10
U/ml penicillin G and 10 mg/ml streptomycin (Hyclone Laboratories,
Inc.) in a 5% CO2 incubator at 37°C.
Cell differentiation
To establish BMSC chondrogenesis in monolayer
culture, a procedure was carried out as previously described
(16). In brief, cells at passage
six were seeded onto 24-well plates at a density of
1×104 cells/well and cultured in LG-DMEM without
chondrogenic supplements. The medium was replaced with chondrogenic
medium after one day, which was then changed every two days. The
chondrogenic medium contained 0.1 μM dexamethasone, 50 μg/ml
ascorbate, 1% insulin-transferrin-selenium, 100 μg/ml sodium
pyruvate, 40 μg/ml proline and 10 ng/ml TGF-β3 (Cyagen
Biosciences). The cells were divided into three groups: i) Control
(cultured with serum-free LG-DMEM only); ii) TGF-β3 (cultured with
chondrogenic medium containing 10 ng/mlTGF-β3); and iii) TGF-β3 +
ICA (cultured with chondrogenic medium containing 10 ng/ml TGF-β3
and 1×10−6 M ICA). ICA was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
of China (Beijing, China). The morphology of the seeded BMSCs was
observed using an inverted microscope (CKX41; Olympus, Tokyo,
Japan).
Immunofluorescence
At day 14, cultured cells were washed three times
with phosphate-buffered saline (PBS) and fixed for 10 min with 4%
paraformaldehyde. Specimens were blocked with 5% bovine serum
albumin for 1 h and incubated at 4°C overnight with the following
primary antibodies: Anti-collagen II (1:100; GeneTex, Irvine, CA,
USA), anti-aggrecan (1:200; Millipore, Billerica, MA, USA) and
anti-SRY (sex determining region Y)-box 9 (SOX9) (1:200; Abcam,
Cambridge, UK). Subsequent to washing three times with PBS, the
cells were incubated with fluorescent secondary antibodies
(Beyotime Institute of Biotechnology, Shanghai, China) for 2 h. For
nuclear staining, DAPI (Beyotime Institute of Biotechnology) was
applied for 3 min and subsequently observed under a fluorescence
microscope (Leica DM 4000 B; Leica Microsystems, Wetzlar, Germany).
Cell counting of 1,000 cells in five randomly selected fields was
performed by Image-Pro Plus 6.0 software (Media Cybernetics, Inc.
Rockville, MD, USA) and the percentage of positively stained cells
was calculated as the ratio of the number of positive cells to the
total number of DAPI-positive cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using RNAiso Plus (Takara
Bio, Inc., Dalian, China), following the manufacturer’s
instructions, and was quantified by absorbance analysis at 260 nm.
cDNA was synthesized using a Primescript™ RT reagent kit
with gDNA Eraser (Takara Bio, Inc.). The RT-qPCR reactions were
performed with an ABI PRISM® 7500 Real-Time PCR System
(Applied Biosystems®, Invitrogen Life Technologies,
Carlsbad, CA, USA) with SYBR Premix Ex Taq™ (Takara Bio,
Inc.). Subsequent to adding equal amounts of cDNA and specific
primers to the mix, initial denaturation was carried out at 94°C
for 30 sec, followed by 40 cycles of denaturation at 94°C for 5
sec, annealing at 60°C for 15 sec and extension at 72°C for 10 sec.
The primers used in RT-qPCR analysis were as follows: Collagen,
type II, α1 (Col2a1) forward, 5′-CGCCACGGTCCTACAATGTC-3′ and
reverse, 5′-GTCACCTCTGGGTCCTTGTTCAC-3′; collagen, type I, α1
(Col1a1) forward, 5′-GCCTCCCAGAACATC ACCTA-3′ and reverse,
5′-GCAGGGACTTCTTGAGGTTG-3′; aggrecan forward,
5′-TGGCATTGAGGACAGCGAAG-3′ and reverse,
5′-TCCAGTGTGTAGCGTGTGGAAATAG-3′; SOX9 forward,
5′-GCAGAGACTGAAGACCCTACACAGA-3′ and reverse,
5′-GAGGCAACTTCACGCTGCAA-3′; GAPDH forward,
5′-TATGACTCTACCCACGGCAA-3′ and reverse,
5′-ATACTCAGCACCAGCATCACC-3′. The levels of mRNA expression were
analyzed by the 2−ΔΔCt method using GAPDH as a
control.
Western blot analysis
Cell extracts were prepared using a Protein
Extraction kit (Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China),
and protein concentrations were measured with a bicinchonic acid
protein assay kit (Boster Biological Technology Co., Wuhan, China).
The protein samples were denatured at 100°C for 5 min and separated
by SDS-PAGE. The proteins were subsequently transferred to
polyvinylidene difluoride membranes and incubated at 4°C overnight
with the primary antibodies: Anti-collagen II (1:1,000; GeneTex),
anti-aggrecan (1:1,000; Millipore), anti-SOX9 (1:500; Abcam) and
anti-GAPDH (1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). Subsequent to being washed three times with PBS, the
membranes were incubated with anti-mouse or anti-rabbit secondary
antibodies (1:1,000; ZSGB-BIO, Beijing, China) for 2 h and
visualized with a BeyoECL Plus kit (Beyotime Institute of
Biotechnology).
Alkaline phosphatase (ALP) activity
ALP activity was measured in the culture
supernatants as previously described (7). Briefly, the media were replaced with
the phenol red free equivalent at day 12. The culture supernatants
were collected after two days and centrifuged at 1,400 × g for 10
min. Soluble ALP activity was detected with an alkaline phosphatase
kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA). The differences between the multiple group comparisons
were evaluated by one-way analysis of variance followed by Tukey’s
test. P<0.05 was considered to indicate a statistically
significant difference, and P<0.01 was considered to indicate a
marked statistically significant difference.
Results
ICA affects BMSC morphology during
chondrogenesis
Throughout the culture period, the BMSCs cultured in
LG-DMEM grew as monolayers and exhibited a characteristic
spindle-like, fibroblastic morphology (Fig. 1A). By contrast, cells induced with
the chondrogenic medium began to lose the typical morphology at day
3 and compacted to form a few mono-layered aggregates and small
multi-layered aggregates at days 7 and 14, respectively (Fig. 1B). Notably, a number of
mono-layered aggregates and large multi-layered aggregates were
visible at days 7 and 14 in the presence of 1×10−6 M ICA
(Fig. 1C).
ICA promotes the chondrogenic
differentiation of BMSCs
The effects of ICA on cartilage-specific markers are
shown in Fig. 2. Collagen II and
aggrecan are the major structural components of articular cartilage
extracellular matrix (ECM). Therefore, collagen II and aggrecan
were observed in the chondrogenic medium with and without ICA. They
were mainly present at the center of the aggregates but were barely
detectable in the control group. When treated with ICA, the cells
synthesized more collagen II and aggrecan compared with the TGF-β3
group (Fig. 2A and B). A
semi-quantitative assessment also verified this finding (Fig. 2D). In the control group, only
1.31±0.89 and 8.77±1.21% of the cells stained positively for
collagen II and aggrecan, respectively, while the percentage of
positively stained cells in the TGF-β3 group was significantly
increased to 34.62±6.34 and 47.37±8.39%, respectively. Furthermore,
treatment with ICA markedly increased the positive staining
percentages to 66.70±13.12 and 86.96±7.68% for collagen II and
aggrecan, respectively.
SOX9 is considered to be an early chondrogenic
marker that induces the synthesis of collagen II and aggrecan. The
present study further investigated whether ICA was able to promote
the expression of SOX9 and subsequently induce chondrogenesis. As
shown in Fig. 2C and D, the TGF-β3
+ ICA group exhibited more intense staining of SOX9 and an
increased percentage of positively stained cells (47.87±13.32%)
compared with the TGF-β3 group (32.02±9.42%). Notably, the majority
of cells stained positively within the aggregates, while less
staining was detected in the single cells, suggesting that it is
necessary for BMSCs undergoing chondrogenic induction to form
aggregates and that ICA can induce the chondrogenesis.
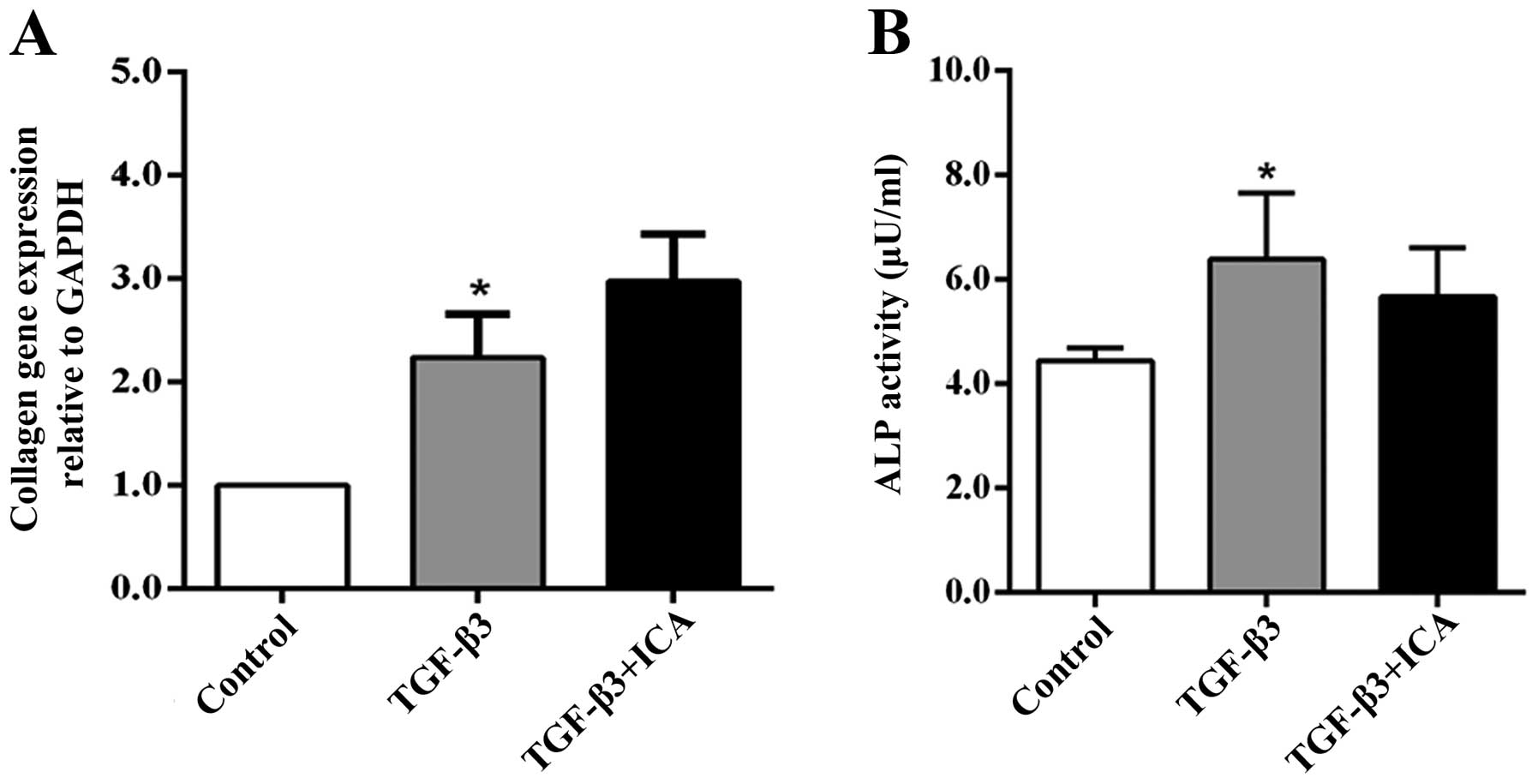
As shown in Fig.
3A, the effect of ICA on the gene expression levels of Col2a1
(the collagen II-encoding gene), aggrecan and SOX9 following BMSC
culture for 14 days was also investigated. RT-qPCR analysis
demonstrated that treatment with ICA produced a significant
1.38-fold increase in Col2a1 expression, a 2.06-fold increase in
aggrecan expression and a 1.85-fold increase in SOX9 expression, in
comparison with the TGF-β3 group.
It was also observed that BMSCs cultured in the
absence of chondrogenic medium did not synthesize collagen II,
aggrecan and SOX9; however, the protein expression levels were
markedly increased in the TGF-β3 group. Furthermore, ICA treatment
significantly promoted the synthesis of these cartilage-specific
markers during the chondrogenic induction, which was consistent
with the results of immunofluorescence analysis and RT-qPCR
(Fig. 3B–D).
ICA does not promote hypertrophic
differentiation of BMSCs
In order to demonstrate the effect of ICA on the
hypertrophic differentiation of BMSCs, Col1a1 gene expression and
ALP activity, markers of hypertrophy or dedifferentiation of
chondrocytes, were examined following 14 days of culture. RT-qPCR
analysis revealed that the expression levels of Col1a1 in the
TGF-β3 group were increased compared with those in the control
group. However, treatment with ICA did not significantly upregulate
the expression of Col1a1 compared with the TGF-β3 group (Fig. 4A). Similarly, only the TGF-β3 group
exhibited significantly higher ALP activity than the control group,
while a reduction in ALP activity was detected in the ICA treatment
group compared with the TGF-β3 group, although the difference was
not significant (Fig. 4B). These
results indicated that ICA did not potentiate BMSC hypertrophic
differentiation concomitantly with promoting chondrogenesis.
Discussion
ICA has been effectively used in the treatment of
osteoporosis, brain injury and cardiovascular disease (14,17,18).
In particular, the long-term development of the drug and extensive
case record of its safe usage have made it an attractive treatment
option (12). When chondrocytes
were cultured as the source cells, ICA was revealed to promote
cartilage ECM synthesis and the expression levels of chondrogenic
genes. ICA also improved the restoration efficiency of
supercritical-sized osteochondral defects and enhanced the
integration of newly formed cartilage with subchondral bone in a
rabbit model (11,12). Several studies have shown that ICA
is a safe and strong chondrocyte anabolic agent, which can enhance
chondrocyte proliferation, attenuate lipopolysaccharide-induced
inflammatory responses and reduce ECM degradation through the
inhibition of nitric oxide, matrix metalloproteinase synthesis and
cathepsin K activity (15,19). Furthermore, Zhang et al
(20) reported that ICA reduced
the activity of the transcription factor nuclear factor-κB in an
inflammatory model induced by tumor necrosis factor-α and also
protected chondrocytes from damage due to OA. There are certain
potential molecular mechanisms that explain these effects. For
instance, ICA not only enhances the expression and secretion of
various growth factors, including BMP-2 and TGF-β1, but also
upregulates the expression levels of Drosophila mothers against
decapentaplegic (Smad) proteins, including Smad1, Smad4 and Smad5,
which are key regulators specific to TGF-β1 activation affecting
chondrogenic genes (21,22). There has been considerable evidence
to suggest that BMP and TGF-β signals play a pivotal role in
chondrogenic differentiation (3,5).
Hypoxia-enhanced chondrogenesis of BMSCs occurs via the activation
of the mitogen-activated protein kinase (MAPK) P38 pathway
(23). Furthermore, ICA stimulates
P38-MAPK activation in cardiomyocyte differentiation or neuronal
protection (17,18). Thus, we hypothesized that ICA may
promote the directed chondrogenic differentiation of BMSCs.
Glennon-Alty et al (16) reported that MSCs initially grew as
a monolayer and subsequently compacted to form mono-layered and
multi-layered aggregates; collagen II tended to be expressed at the
center of the aggregates. A number of other studies also
demonstrated that BMSCs induced with chondrogenic medium developed
into a round phenotype and aggregated spontaneously into spheroid-
or rod-like cell agglomerates. The formation of the aggregates
exhibited a more intense staining for chondrogenic matrix
deposition (24,25). Consistent with these studies, the
present data revealed that treatment with ICA led to the formation
of an increased number of and larger aggregates. More intense
staining for collagen II, aggrecan and SOX9 was also visible within
the aggregates. Notably, less staining for SOX9 was detectable in
the single cells outside the aggregates, which may be due to the
fact that chondrogenic differentiation requires the cell-cell and
cell-matrix contacts created within the aggregates (25).
Previous studies have revealed that ICA
significantly affects the expression levels of cartilage-specific
genes (Col2a1, aggrecan and SOX9) and leads to higher levels of
aggrecan production (11,12). Similarly, it was observed in the
present study that ICA notably upregulated the expression levels of
these genes, increased the staining intensity and protein levels of
collagen II, aggrecan and SOX9. Hattori et al (26) demonstrated that SOX9 was highly
expressed in chondrocytes of the prehypertrophic zone and was able
to directly suppress hypertrophic differentiation. In the present
study, upregulation of the expression of the SOX9 gene and protein
indicated that ICA either delayed or prevented the BMSCs from
dedifferentiating into a hypertrophic phenotype, although the same
chondrogenic medium containing TGF-β3 was added. Aggrecan in the
ECM maintained the structural integrity of the articular cartilage
and allowed the normal biological function of the chondrocytes,
including adhesion, migration, proliferation and differentiation,
to be retained. In particular, aggrecan expression and synthesis
was greatly enhanced in the ICA treatment group, consistent with
another study (11).
ALP is a marker of hypertrophic differentiation and
collagen I is a the marker of dedifferentiated chondrocytes. A
number of studies have demonstrated that the use of growth factors,
including TGF-β3, produces cartilage-specific matrix but also
causes hypertrophy through the upregulation of hypertrophic markers
(7–10). In the present study, it was
observed that chondrogenic medium alone led to higher collagen I
expression and increased ALP activity. However, the presence of ICA
did not potentiate the effect of the growth factors on hypertrophic
differentiation while producing stronger chondrogenic
differentiating effects. Similarly, other studies found that ICA
downregulated Col1a1 gene expression. Collagen type X expression,
another marker of hypertrophic differentiation, was barely
detectable in the culture medium (11,12).
In conclusion, the present study investigated the
effects of ICA on BMSC phenotypes, including cell morphology and
ECM synthesis, and the expression levels of cartilage-specific
genes in vitro. It was demonstrated that ICA not only
promoted the formation of larger aggregates but also enhanced ECM
synthesis and increased the expression levels of cartilage-specific
genes. However, ICA exhibited no effect on hypertrophic
differentiation, suggesting that ICA may be a potential promoting
compound for cartilage tissue engineering and may reduce the effect
of growth factors that contribute to further hypertrophic
differentiation.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (nos. 81273508 and
81350017) and the Natural Science Fund of the Science and
Technology Bureau of the Dalian Government (nos. 2011E12SF035 and
2012E15SF164).
References
|
1
|
Lubis AM and Lubis VK: Adult bone marrow
stem cells in cartilage therapy. Acta Med Indones. 44:62–68.
2012.PubMed/NCBI
|
|
2
|
Litwic A, Edwards MH, Dennison EM and
Cooper C: Epidemiology and burden of osteoarthritis. Br Med Bull.
105:185–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Li B, Yang J, et al: The
restoration of full-thickness cartilage defects with BMSCs and
TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials.
31:8964–8973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolambkar YM, Peister A, Soker S, Atala A
and Guldberg RE: Chondrogenic differentiation of amniotic
fluid-derived stem cells. J Mol Histol. 38:405–413. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung MR, Shim IK, Chung HJ, et al: Local
BMP-7 release from a PLGA scaffolding-matrix for the repair of
osteochondral defects in rabbits. J Control Release. 162:485–491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levi B, James AW, Wan DC, et al:
Regulation of human adipose-derived stromal cell osteogenic
differentiation by insulin-like growth factor-1 and
platelet-derived growth factor-alpha. Plast Reconstr Surg.
126:41–52. 2010.
|
|
7
|
Giovannini S, Diaz-Romero J, Aigner T, et
al: Micromass co-culture of human articular chondrocytes and human
bone marrow mesenchymal stem cells to investigate stable
neocartilage tissue formation in vitro. Eur Cell Mater. 20:245–259.
2010.
|
|
8
|
Aung A, Gupta G, Majid G and Varghese S:
Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic
differentiation of human mesenchymal stem cells. Arthritis Rheum.
63:148–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pelttari K, Winter A, Steck E, et al:
Premature induction of hypertrophy during in vitro chondrogenesis
of human mesenchymal stem cells correlates with calcification and
vascular invasion after ectopic transplantation in SCID mice.
Arthritis Rheum. 54:3254–3266. 2006. View Article : Google Scholar
|
|
10
|
Mueller MB, Fischer M, Zellner J, et al:
Effect of parathyroid hormone-related protein in an in vitro
hypertrophy model for mesenchymal stem cell chondrogenesis. Int
Orthop. 37:945–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Zhang X, Li KF, et al: Icariin
promotes extracellular matrix synthesis and gene expression of
chondrocytes in vitro. Phytother Res. 26:1385–1392. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Yuan T, Zhang X, et al: Icariin: a
potential promoting compound for cartilage tissue engineering.
Osteoarthritis Cartilage. 20:1647–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan JJ, Cao LG, Wu T, et al: The
dose-effect of icariin on the proliferation and osteogenic
differentiation of human bone mesenchymal stem cells. Molecules.
16:10123–10133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng D, Peng S, Yang SH, et al: The
beneficial effect of Icariin on bone is diminished in
osteoprotegerin-deficient mice. Bone. 51:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MH, Sun JS, Tsai SW, Sheu SY and Chen
MH: Icariin protects murine chondrocytes from
lipopolysaccharide-induced inflammatory responses and extracellular
matrix degradation. Nutr Res. 30:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glennon-Alty L, Williams R, Dixon S and
Murray P: Induction of mesenchymal stem cell chondrogenesis by
polyacrylate substrates. Acta Biomater. 9:6041–6051. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding L, Liang XG, Hu Y, Zhu DY and Lou YJ:
Involvement of p38MAPK and reactive oxygen species in
icariin-induced cardiomyocyte differentiation of murine embryonic
stem cells in vitro. Stem Cells Dev. 17:751–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Zhang L, Chen ZB, et al: Icariin
enhances neuronal survival after oxygen and glucose deprivation by
increasing SIRT1. Eur J Pharmacol. 609:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun P, Liu Y, Deng X, et al: An inhibitor
of cathepsin K, icariin suppresses cartilage and bone degradation
in mice of collagen-induced arthritis. Phytomedicine. 20:975–979.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Li R, Wang S, Mu F and Jia P:
Effect of chinese traditional herb Epimedium grandiflorum C.
Morren and its extract Icariin on osteoarthritis via suppressing
NF-kappaB pathway. Indian J Exp Biol. 51:313–321. 2013.
|
|
21
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates
osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010.
|
|
22
|
Liang W, Lin M, Li X, et al: Icariin
promotes bone formation via the BMP-2/Smad4 signal transduction
pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol
Med. 30:889–895. 2012.PubMed/NCBI
|
|
23
|
Hirao M, Tamai N, Tsumaki N, Yoshikawa H
and Myoui A: Oxygen tension regulates chondrocyte differentiation
and function during endochondral ossification. J Biol Chem.
281:31079–31092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winter A, Breit S, Parsch D, et al:
Cartilage-like gene expression in differentiated human stem cell
spheroids: a comparison of bone marrow-derived and adipose
tissue-derived stromal cells. Arthritis Rheum. 48:418–429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Phillips JE, Petrie TA, Creighton FP and
García AJ: Human mesenchymal stem cell differentiation on
self-assembled monolayers presenting different surface chemistries.
Acta Biomater. 6:12–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hattori T, Müller C, Gebhard S, et al:
SOX9 is a major negative regulator of cartilage vascularization,
bone marrow formation and endochondral ossification. Development.
137:901–911. 2010. View Article : Google Scholar : PubMed/NCBI
|