Introduction
Constipation is a common health problem with a
tendency to cause discomfort and affect patient quality of life
(1). This highly prevalent
functional gastrointestinal disorder affects 3–15% of the general
population (2,3). Constipation may also cause abdominal
distension, vomiting, restlessness, gut obstruction and
perforation, and may even be associated with aspiration or fatal
pulmonary embolism (4). At
present, constipation disproportionately affects older adults, with
prevalences of 50% among the community-dwelling elderly and 74% in
nursing-home residents (5).
Drugs that contain magnesium oxide or sennoside, the
main constituent of Senna, are frequently administered for the
treatment of constipation due to their powerful purgative/laxative
effects. However, these drugs also induce severe diarrhea as a
side-effect (1). Furthermore,
repeated use of Senna or other anthraquinone-containing drugs may
induce melanosis coli, which is a risk factor for colorectal
neoplasia (6).
Loperamide-induced delay in colonic transit is
accepted as spastic constipation due to the inhibition of stool
frequency and increased colonic contractions in humans (7). The drug inhibits intestinal water
secretion (8) and colonic
peristalsis (9), which extends the
fecal evacuation time and delays intestinal luminal transit
(10). Thus, loperamide-induced
constipation is considered to be a model of spastic constipation
(11).
Fermented rice extracts (FRes) have been shown to
possess various pharmacological effects, including antiosteoporotic
(12), hypoglycemic (13), antitumor (14), neuroprotective (15), antiatherosclerotic (16), antistress and antifatigue effects
(17), when compared with
non-fermented extracts.
A previous study revealed the growth-stimulating
effects of FRe on lactic acid bacteria and Bifidobacterium
spp. in vitro (18).
However, whether FRe is effective for treating constipation in
vivo remains unknown.
In the present study, changes in fecal (number,
weight and water content), peristalsis (gastrointestinal transit
ratio) and histological (fecal mucus content, colonic
mucus-producing cell number and mean colonic mucosa thickness)
parameters were observed in rats with loperamide-induced
constipation in order to analyze the laxative effects of FRe. The
laxative effects of FRe were compared with those of 5 mg/kg sodium
picosulfate (S. picosulfate), a cathartic stimulant that requires
activation by colonic bacteria (19,20),
as a reference drug (21).
Materials and methods
Materials
FRe preparations were supplied by Glucan Corporation
(Busan, Korea) as brown powders. Briefly, FRe production was
performed in three fermentation steps. In the first fermentation
step (saccharification), washed, non-glutinous rice (1 kg) was
soaked for 6 h, drained for 30 min and steamed for 15 min at 121°C.
Following rapid cooling, 10 g malt powder and 4 liters water were
added and the mixture was fermented in a 20-liter sterile glass
container at 55°C for 12 h. In the second fermentation step, 20 ml
Saccharomyces cerevisiae (ATCC® 9804™;
American Type Culture Collection, Manassas, VA, USA) suspension was
added to the first fermentate, mixed well and incubated at 30°C for
48 h. Finally, in the third fermentation step, 20 ml lactic acid
bacteria, Weissella paramesenteroides (KACC 91704; Korean
Agricultural Culture Collection, Suwon, Korea), was added to the
second fermentate, mixed well and incubated at 30°C for 48 h. This
final fermentate was sterilized using an auto-steam sterilizer
(VS-1321-80; Vision Scientific Co. Ltd., Daejeon, Korea) at 150°C
for 15 min and filtered through a 40-mesh sieve to obtain the final
filtrate, which was freeze-dried for two days until a moisture
content of ~0.8% was obtained. The freeze-dried FRe was ground in a
mill and passed through a 500-mesh sieve. The sieved material was
stored at −20°C until required for further use.
S. picosulfate was purchased from Crown Pharm. Co.
Ltd. (Seoul, Korea). FRe and S. picosulfate were stored at −20°C
and protected from light and moisture.
Animals
Animal experiments were performed in accordance with
the Korean Food and Drug Administration Guidelines for Good
Laboratory Practice (notification no. 2000-116, 2009). In total, 58
male Sprague-Dawley rats (age, 8 weeks; Japan SLC, Inc., Hamamatsu,
Japan) were used in the experiments following a 12-day
acclimatization period. Animals were housed, with three animals per
polycarbonate cage, in a temperature- (20–23°C) and humidity-
(40–50%) controlled room. The light/dark cycle was 12/12 h and food
(Samyang Foods Co., Ltd., Wonju, Korea) and water were supplied
ad libitum.
Constipation was induced in the animals through oral
administration of 3 mg/kg loperamide hydrochloride (Sigma-Aldrich,
St. Louis, MO, USA) daily for six days at 1 h prior to test
material administration, as described previously (22,23).
The control rats were administered saline.
FRe (100, 200 or 300 mg/kg) was dissolved in
distilled water (DW) and administered orally 1 h after loperamide
administration, daily for six days. S. picosulfate (5 mg/kg body
weight) was also dissolved in DW and administered orally as a
treatment reference (21). In the
vehicle and constipation groups, the control rats were administered
DW only instead of treatment.
Rats were selected based on body weight and divided
into six groups (six rats/group) at day 1 prior to the initiation
of test material administration. Briefly, excluding overweight and
underweight rats, the rats were arranged in order of weight, and
the heaviest six rats were randomly assigned to each of the six
groups. The six next heaviest rats were then randomly assigned to
each of the six groups; this division process continued until the
36 rats had been randomly assigned to the six groups. The vehicle
control group received DW administration, while the constipation
control group received loperamide treatment and DW administration.
The S. picosulfate group received loperamide treatment and S.
picosulfate administration. Finally, there were three FRe treatment
groups that received loperamide treatment followed by FRe at a
concentration of 100, 200 or 300 mg/kg. This study was approved by
the Ethics Committee of Daegu Haany University (Gyeongsan,
Korea)
Body weight measurements
Changes in body weight were measured once per day
from one day prior to the initiation of test material
administration (D0) to the termination (D6) of the experiment,
using an automatic electronic balance (Precisa Instruments,
Dietikon, Switzerland). At termination, all experimental animals
fasted overnight with unrestricted access to water in preparation
for intestinal charcoal transit ratio measurements. Body weight
gain (g) was calculated by subtracting the body weight on the first
day of administration (D1) from that at D6.
Fecal parameter measurements
Excreted fecal pellets of the individual rats over
24 h were collected on day 5 of administration (D5). The total
number, wet weight and water content of the fecal pellets were
determined. The water content was calculated as follows: Fecal
water content (%) = [(fecal wet weight - fecal dry weight)/fecal
wet weight] × 100.
Measurement of intestinal charcoal
transit ratio
Assessment of the gastrointestinal propulsion of a
charcoal meal was determined according to the method proposed by
Sagar et al (24), with
minor modifications. Animals fasted for 18 h prior to the
experiment, but consumed water ad libitum. At 10 min
following the last drug (FRe/S. picosulfate/DW) administration, the
animals were fed 1 ml charcoal meal (3% suspension of activated
charcoal in 0.5% aqueous methylcellulose; Sigma-Aldrich). At 30 min
after the charcoal meal administration, the animals were sacrificed
by cervical dislocation and the total intestine length (pyloric
sphincter to cecum) and charcoal meal transit distance were
measured. The intestinal charcoal transit ratio was calculated as
follows: Charcoal transit ratio (%) = [(total small intestine
length - transited distance of charcoal meal)/total small intestine
length] × 100.
Measurement of fecal pellets in the large
intestine
With the intestinal charcoal transit ratio
measurement, the total number and mean thickness (short axis) of
fecal pellets remaining in the colon lumen were measured.
Histological procedures
Assessments of the colonic mucosa and fecal pellets
remaining in the colon lumen were determined according to the
method described by Wu et al (25), with minor modifications. Briefly,
segments of the rat distal colon containing one fecal pellet were
isolated by ligatures, removed, and immediately fixed in 10%
formaldehyde. The fixed tissue was then embedded, serially
cross-sectioned (3–4 μm) and stained with alcian blue stain (pH
2.5) (05500; Sigma-Aldrich). The histological profiles were
subsequently interpreted by a histopathologist who was blinded to
the group distribution.
The mean thickness of the mucosal layer at the fecal
surface (μm/fecal pellets), number of mucus-producing cells
(cells/mm2 of colonic mucosa) and the colonic mucosa
thickness (μm/colon) were measured histomorphometrically using an
automated image analyzer (DMI-300; DMI, Daegu, Korea) under a
microscope (Eclipse 80i, Nikon Corp., Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Multiple comparison tests were conducted for the
different dose groups. Homogeneity of variance was examined using
Levene’s test. When Levene’s test indicated no significant
deviation from the homogeneity of variance, the obtained data were
analyzed by one-way analysis of variance followed by Fisher’s least
significant difference multi-comparison test to identify pairs of
group comparisons that differed significantly. When Levene’s test
indicated significant deviations from the homogeneity of variance,
a non-parametric comparison test, the Kruskal-Wallis H-test, was
used. When a statistically significant difference was observed in
the Kruskal-Wallis H-test, the Mann-Whitney U test was used to
identify pairs of group comparisons that differed significantly.
Statistical analyses were conducted using SPSS for Windows software
(Release 14K; SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects on body weight
A statistically significant (P<0.01 or 0.05)
decrease in body weight was detected in the S. picosulfate group,
when compared with the vehicle and loperamide control groups.
However, no significant change in body weight was detected when
comparing any of the FRe-treated groups with the vehicle and
loperamide control groups (Table
I).
 | Table IBody weight change following oral
treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation. |
Table I
Body weight change following oral
treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation.
| Body weight (g) | |
|---|
|
| |
|---|
| Groups | D1a | D5 | D6a | Difference [D6 -
D1] |
|---|
| Controls |
| Vehicle | 250.50±11.67 | 294.33±14.38 | 267.67±12.44 | 17.17±1.94 |
| Loperamide | 248.33±8.41 | 291.17±9.02 | 266.83±8.52 | 18.50±2.43 |
| S. picosulfate | 250.33±9.35 | 277.50±12.36b,c | 253.83±12.27b | 3.50±3.56d,e |
| FRe (mg/kg) |
| 100 | 252.17±5.42 | 292.00±5.97 | 270.50±7.06 | 18.33±6.74 |
| 200 | 250.17±9.06 | 290.83±13.48 | 268.33±13.02 | 18.17±6.88 |
| 300 | 247.33±10.27 | 286.00±10.64 | 264.67±12.16 | 17.33±5.65 |
Body weight gain increased by 7.75% in the
loperamide control group compared with the vehicle control group,
but decreased by 81.08, 0.90, 1.80 and 6.31% in the S. picosulfate
and FRe 100-, 200- and 300-mg/kg-treated groups, respectively, as
compared with the loperamide control group.
Effects on fecal parameters
Statistically significant (P<0.01) decreases in
the fecal number and water content collected during 24 h were
detected in the loperamide control group when compared with the
vehicle control group. By contrast, statistically significant
(P<0.01 or 0.05) increases in the fecal number and water content
were detected on D5 in the S. picosulfate and all FRe-treated
groups when compared with the loperamide control group (Table II).
 | Table IIFecal parameters following oral
treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation. |
Table II
Fecal parameters following oral
treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation.
| Fecal parameters on
D5 (collection for 24 h) |
|---|
|
|
|---|
| Groups | Pellets (n) | Wet weight (g/24
h/rat) | Dry weight (g/24
h/rat) | Water content
(%) |
|---|
| Controls |
| Vehicle | 59.17±3.19 | 10.006±0.954 | 5.855±0.496 | 41.311±4.106 |
| Loperamide | 46.17±6.40a | 7.673±1.110b | 5.560±0.270 |
26.320±10.426a |
| S.
picosulfate | 77.50±23.45c |
25.991±5.432a,c | 6.575±1.072 |
74.394±2,769a,c |
| FRe (mg/kg) |
| 100 | 57.67±9.18 | 9.964±1.976 | 5.392±0.748 |
45.307±4.113c |
| 200 | 62.00±7.90c |
13.134±2.721b,c | 6.265±1.153 |
52.042±1.885a,c |
| 300 | 73.00±5.87a,c |
13.651±1.791a,c | 6.725±0.597b,c |
50.332±4.768a,c |
The total number of fecal pellets collected over 24
h on D5 decreased by 21.97% in the loperamide control group when
compared with the vehicle control group, and increased by 67.86,
24.91, 34.29 and 58.11% in the S. picosulfate and FRe 100-, 200-
and 300-mg/kg-treated groups, respectively, as compared with the
loperamide control group.
The total water content of the fecal pellets
collected over 24 h on D5 decreased by 32.29% in the loperamide
control group when compared with the vehicle control group, and
increased by 182.65, 72.14, 97.73 and 91.23% in the S. picosulfate
and FRe 100-, 200- and 300-mg/kg-treated groups, respectively, as
compared with the loperamide control group.
Effects on fecal pellets remaining in the
colon lumen
Statistically significant (P<0.01) increases in
the fecal number and mean diameter of the pellets remaining in the
colon lumen were detected in the loperamide control group when
compared with the vehicle control group. By contrast, statistically
significant (P<0.01) decreases in the fecal number and mean
diameter of the pellets remaining in the colon lumen at sacrifice
were detected in the S. picosulfate and all FRe-treated groups when
compared with the loperamide control group (Table III).
 | Table IIIFecal pellets in the colon following
oral treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation. |
Table III
Fecal pellets in the colon following
oral treatment with FRe or S. picosulfate in rats with
loperamide-induced constipation.
| Fecal pellets in
the colon |
|---|
|
|
|---|
| Groups | Pellets (n) | Mean thickness
(μm) |
|---|
| Controls |
| Vehicle | 3.17±1.47 | 4.86±0.59 |
| Loperamide | 6.83±0.98a | 6.35±0.50d |
| S.
picosulfate | 1.33±1.21a,c | 3.80±0.47e,f |
| FRe (mg/kg) |
| 100 | 1.83±1.17b,c | 4.55±0.75f |
| 200 | 0.83±0.75a,c | 4.05±0.35b,f |
| 300 | 1.50±0.84b,c | 4.35±0.25f |
The total number of fecal pellets remaining in the
colon lumen increased by 115.79% in the loperamide control group
when compared with the vehicle control group, and decreased by
80.53, 73.21, 87.85 and 78.04% in the S. picosulfate and FRe 100-,
200- and 300-mg/kg-treated groups, respectively, when compared with
the loperamide control group.
The mean diameter of the fecal pellets remaining in
the colon lumen increased by 30.70% in the loperamide control group
when compared with vehicle control group, but decreased by 40.24,
28.40, 36.17 and 31.57% in the S. picosulfate and FRe 100-, 200-
and 300-mg/kg-treated groups, respectively, when compared with the
loperamide control group.
Effects on the intestinal charcoal
transit ratio
A statistically significant (P<0.01) decrease in
the intestinal charcoal transit ratio was detected in the
loperamide control group when compared with the vehicle control
group. By contrast, statistically significant (P<0.01 or
<0.05) increases in the intestinal charcoal transit ratio were
detected after six days of continuous oral treatment with S.
picosulfate and in all the FRe-treated groups when compared with
the vehicle control group (Table
IV).
 | Table IVGastrointestinal charcoal transit
ratio following oral treatment with FRe or S. picosulfate in rats
with loperamide-induced constipation. |
Table IV
Gastrointestinal charcoal transit
ratio following oral treatment with FRe or S. picosulfate in rats
with loperamide-induced constipation.
| Gastrointestinal
motility (during 30 min) |
|---|
|
|
|---|
| Groups | Total small
intestine length (cm) | Transit distance of
charcoal meal (cm) | Gastrointestinal
charcoal transit ratio (%) |
|---|
| Controls |
| Vehicle | 113.17±11.00 | 78.40±10.66 | 69.25±6.42 |
| Loperamide | 112.07±4.67 | 61.15±5.61a | 54.80±7.01a |
| S.
picosulfate | 111.83±5.45 | 70.58±9.41c | 63.07±7.27c |
| FRe (mg/kg) |
| 100 | 110.58±7.51 | 70.42±4.75c | 63.79±4.07c |
| 200 | 111.75±5.18 | 74.75±6.12a | 67.21±8.81b |
| 300 | 112.08±5.15 | 72.83±6.73c | 65.07±6.44c |
The intestinal charcoal transit ratio decreased by
20.87% in the loperamide control group when compared with the
vehicle control group, and increased by 15.10, 16.40, 22.65 and
18.74% in the S. picosulfate and FRe 100-, 200- and
300-mg/kg-treated groups, respectively, as compared with the
loperamide control group.
Effects on histopathology
Statistically significant (P<0.01) decreases in
the surface mucus thickness of the fecal pellets remaining in the
colon lumen, the mucosa thickness and the number of mucus-producing
cells were detected in the loperamide control group when compared
with the vehicle control group. By contrast, statistically
significant (P<0.01 or <0.05) increases in the surface mucus
thickness of the fecal pellets remaining in the colon lumen, the
mucosa thickness and the number of mucus-producing cells were
detected after six days of continuous oral treatment with S.
picosulfate and in all the FRe-treated groups when compared with
the loperamide control group (Table
V; Fig. 1).
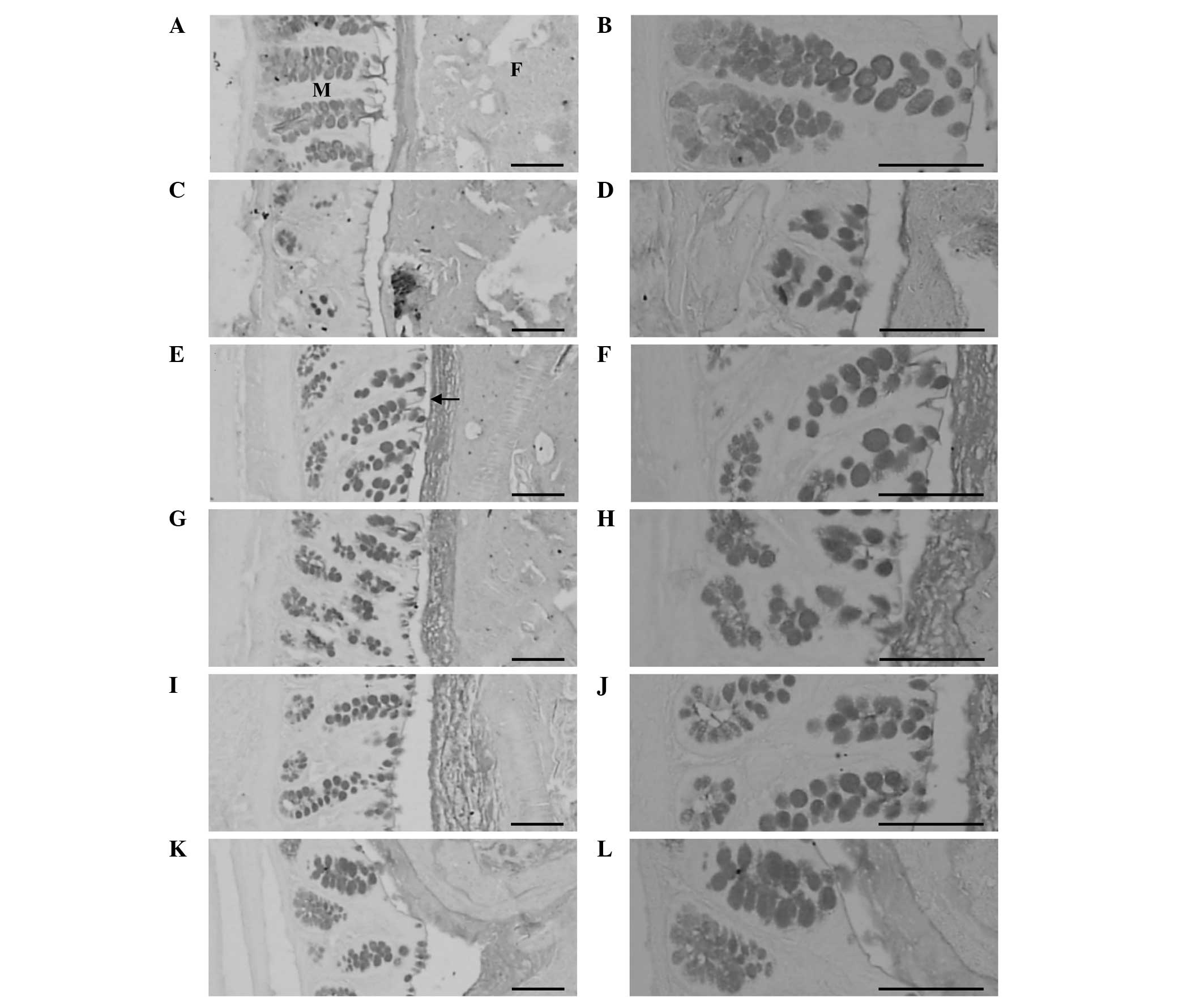 | Figure 1Histological profiles of colons
containing fecal pellets from the (A and B) vehicle control, (C and
D) loperamide control, (E and F) sodium picosulfate (S.
picosulfate), (G and H) fermented rice extract (FRe) 100- , (I and
J) 200- and (K and L) 300-mg/kg groups of rats with
loperamide-induced constipation. Marked decreases were observed in
the surface mucus thickness of the remnant fecal pellets in the
colon lumen, the mucosa thickness and the mucus-producing cell
number in the loperamide control group when compared with the
vehicle control group. By contrast, increases in the surface mucus
thickness of the fecal pellets remaining in the colon lumen, the
mucosa thickness and the number of mucus-producing cells were
detected after six days of continuous oral treatment with S.
picosulfate and the three doses of FRe, when compared with the
loperamide control group. Oral administration of FRe was performed
daily for six days following loperamide treatment. Oral
administration of S. picosulfate (5 mg/kg) was performed daily for
six days following loperamide treatment. Arrow indicates the
surface mucus thickness measurement. Alcian blue staining; Scale
bars = 150 μm. M, colonic mucosa; F, fecal pellets. |
 | Table VHistomorphometry of colon and
remaining fecal pellets following oral treatment with FRe or S.
picosulfate in rats with loperamide-induced constipation. |
Table V
Histomorphometry of colon and
remaining fecal pellets following oral treatment with FRe or S.
picosulfate in rats with loperamide-induced constipation.
| Histomorphometry
(at sacrifice) |
|---|
|
|
|---|
| Groups | Fecal pellet
surface mucus thickness (μm) | Mucus-producing
cell numbers (cells/mm2) | Colon mucosa
thickness (μm) |
|---|
| Controls |
| Vehicle | 64.71±14.09 | 643.20±117.17 | 499.08±102.84 |
| Loperamide | 17.27±2.97d |
194.00±14.73d |
231.16±42.32a |
| S.
picosulfate |
157.77±48.75d,f |
388.20±55.56d,f |
487.54±95.24b |
| FRe (mg/kg) |
| 100 | 99.85±12.08d,f |
276.00±51.35d,f |
347.64±54.21a,c |
| 200 |
134.34±44.04e,f |
372.60±89.34d,f |
432.08±74.44b |
| 300 |
104.72±14.41d,f |
337.80±60.41d,f |
428.53±102.52b |
The surface mucus thickness of the fecal pellets
remaining in the colon lumen decreased by 73.71% in the loperamide
control group when compared with the vehicle control group, and
increased by 813.55, 478.17, 677.88 and 506.37% in the S.
picosulfate and FRe 100-, 200- and 300-mg/kg-treated groups,
respectively, as compared with the loperamide control group.
The number of mucus-producing cells in the colonic
mucosa decreased by 69.84% in the loperamide control group when
compared with vehicle control group, and increased by 100.10,
42.27, 92.06 and 74.12% in the S. picosulfate and FRe 100-, 200-
and 300-mg/kg-treated groups, respectively, as compared with the
loperamide control group.
The thickness of the colonic mucosa decreased by
53.68% in the loperamide control group when compared with the
vehicle control group, and increased by 110.91, 50.39, 86.92 and
85.38% in the S. picosulfate and FRe 100-, 200- and
300-mg/kg-treated groups, respectively, as compared with the
loperamide control group.
Discussion
Constipation is a common health problem with a
tendency to cause discomfort and affect patient quality of life
(23). The occurrence of
constipation increases with age and may develop into a chronic
condition requiring the long-term use of laxatives. Constipation
may arise from a variety of causes, including the use of chemical
compounds, such as morphine, dietary habits and psychological
stress (1).
Various fermentations of rice have increased
bioavailabilities and pharmacological activities (12–18).
In the present study, the laxative effects of FRe were evaluated
based on changes in fecal parameters (numbers, weight and water
content), the gastrointestinal transit ratio (motility), fecal
mucus content, number of colonic mucus-producing cells and the mean
colonic mucosa thickness in rats with loperamide-induced
constipation, used as a model of spastic constipation (11). The laxative effects of FRe were
compared with those of S. picosulfate, a cathartic stimulant that
requires activation by colonic bacteria (19,20),
as a reference drug (21).
As a result of loperamide treatment, a marked
decrease was detected in the fecal pellet number and water content
discharged over 24 h, the surface mucus thickness in the colonic
lumen, the intestinal charcoal transit ratio, the thickness of the
colonic mucosa and the number of mucus-producing cells. By
contrast, an increase was observed in the number of fecal pellets
remaining in the colon and their mean diameters in the colonic
lumen, as compared with the normal vehicle control. Therefore, the
observations indicated a successful model of constipation was
established. However, marked increases were observed in the three
groups of FRe-treated rats with regard to the fecal pellet number
and water content discharged over 24 h, the surface mucus thickness
in the colonic lumen, the intestinal charcoal transit ratio,
thickness of the colonic mucosa and the number of mucus-producing
cells, while decreases were observed in the remaining fecal pellet
number and their mean diameters in the colonic lumen, when compared
with loperamide control. These changes were less significant
compared with those in the S. picosulfate group, with the exception
of the intestinal charcoal transit ratio. Similar effects in the
intestinal charcoal transit ratio were detected at all three doses
of FRe and with S. picosulfate. These results provide direct
evidence that compared with S. picosulfate, FRe exhibits a laxative
effect without causing diarrhea; thus, may be highly effective as a
complementary treatment for humans suffering from lifestyle-induced
constipation.
The optimal effective dose of FRe was considered to
be ~100 mg/kg since marked dose-dependent effects were detected
between 100 and 200 mg/kg, but not between 200 and 300 mg/kg in the
present study. FRe did not induce severe diarrhea as a side-effect,
possibly since milder and more favorable laxative effects were
demonstrated compared with S. picosulfate. In addition, FRe did not
influence body weight gain in the present study, whereas S.
picosulfate induced a marked reduction in body weight gain due to
its powerful purgative/laxative activity.
A marked decrease in fecal discharge was observed
during constipation, and the delay of the fecal pellets in the
large intestinal lumen induced the over-absorption of water from
the fecal pellets. Accordingly, the water content in the discharged
fecal pellets was markedly decreased. Changes in the fecal
parameters, including the number of discharged fecal pellets and
the water content, have been used as an index to detect the effects
of various laxative agents (23,25).
In the present study, increases in the number of discharged fecal
pellets and water content were detected with FRe treatment, and
these changes were considered to be direct evidence that FRe
exhibits favorable laxative effects. Furthermore, increased numbers
of fecal pellets remaining in the colonic lumen and a decrease in
the surface mucus content have been detected in individuals with
constipation (25,26). The increase in the surface mucus
content and decrease in the number of fecal pellets remaining in
the colonic lumen following treatment with FRe were considered as
direct evidence that FRe has favorable laxative effects.
The transit process of the entire gastrointestinal
tract reflects the overall gastrointestinal motor activity. Thus,
measuring the gastrointestinal charcoal transit ratio is useful in
the diagnosis of constipation (23). A decrease in the gastrointestinal
charcoal transit ratio indicates constipation (21,24).
In the present study, an increase in the gastrointestinal charcoal
transit ratio was observed in the rats treated with FRe, providing
indirect evidence that FRe exerts a favorable laxative effect.
Reduced mucus production in the colonic mucosa is
directly associated with constipation (26), and marked decreases in the
thickness of the colonic mucosal layer and the number of
mucus-producing cells have been detected by histopathology in
individuals with constipation (27). Thus, in the present study,
increases in the number of mucus-producing cells and the thickness
of the mucosal layer in the rats following FRe treatment was
considered as direct evidence that FRe exhibits a favorable
laxative effect.
A number of bifidogenic growth stimulators have been
identified, including 2-amino-3-carboxy-1,4-naphthoquinone,
1,4-dihydroxy-2-naphthoic acid (28) and panose (29). Panose has been demonstrated to
exert bifidogenic effects and significantly increase the growth of
Bifidobacterium sp. in vitro (29). Furthermore, the ingestion of
bifidogenic growth stimulators has been demonstrated to increase
the number of defecations in females with constipation (30) and mitigate inflammatory bowel
disease (31) via an improvement
of the intestinal microflora.
In a previous study (18), a FRe containing 6.7% panose was
found to stimulate the growth of lactic acid bacteria, including
Lactobacillus acidophilus, Streptococcus thermophilus
and Bifidobacterium lactis, when compared with the control
group. A 13.8-16 fold increase was observed in growth following
incubation for 12 and 18 h. Therefore, FRe as a bifidogenic growth
stimulator, was hypothesized to mediate its laxative effects in the
current study.
In conclusion, a number of factors, including diet,
malnutrition, metabolic or endocrine disorders and side effects of
medication, can lead to constipation. The results of the present
study indicate that FRe can exert a treatment effect on certain
types of constipation induced by the opioid drug loperamide. When
compared with S. picosulfate, FRe exhibited laxative effects
without causing diarrhea. Thus, FRe may be effective as a
complementary treatment for certain types of constipation in
humans. The optimal effective dosage of FRe is considered to be
~100 mg/kg since marked dose-dependent effects were detected
between 100 and 200 mg/kg in the current study. Further studies are
required to elucidate whether FRe causes a healing effect in
constipation induced by other factors.
Acknowledgements
The study was supported by grants from the Busan
Traditional Liquor Industry RIS (no. B0012284) and the Ministry of
Trade, Industry and Energy of the Republic of Korea (no.
R0002840).
References
|
1
|
Kakino M, Tazawa S, Maruyama H, et al:
Laxative effects of agarwood on low-fiber diet-induced constipation
in rats. BMC Complement Altern Med. 10:682010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones R and Lydeard S: Irritable bowel
syndrome in the general population. BMJ. 304:87–90. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talley NJ, Stanghellini V, Heading RC, et
al: Functional gastroduodenal disorders. Gut. 45:II37–II42. 1999.
View Article : Google Scholar
|
|
4
|
Mostafa SM, Bhandari S, Ritchie G, Gratton
N and Wenstone R: Constipation and its implications in the
critically ill patient. Br J Anaesth. 91:815–819. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao SS and Go JT: Update on the management
of constipation in the elderly: new treatment options. Clin Interv
Aging. 5:163–171. 2010.PubMed/NCBI
|
|
6
|
Siegers CP, von Hertzberg-Lottin E, Otte M
and Schneider B: Anthranoid laxative abuse - a risk for colorectal
cancer. Gut. 34:1099–1101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kojima R, Doihara H, Nozawa K, et al:
Characterization of two models of drug-induced constipation in mice
and evaluation of mustard oil in these models. Pharmacology.
84:227–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hughes S, Higgs NB and Turnberg LA:
Loperamide has antisecretory activity in the human jejunum in
vivo. Gut. 25:931–935. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sohji Y, Kawashima K and Shimizu M:
Pharmacological studies of loperamide, an anti-diarrheal agent. II.
Effects on peristalsis of the small intestine and colon in guinea
pigs (author’s transl). Nihon Yakurigaku Zasshi. 74:155–163.
1978.(In Japanese).
|
|
10
|
Yamada K and Onoda Y: Comparison of the
effects of T-1815, yohimbine and naloxone on mouse colonic
propulsion. J Smooth Muscle Res. 29:47–53. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takasaki K, Kishibayashi N, Ishii A and
Karasawa A: Effects of KW-5092, a novel gastroprokinetic agent, on
the delayed colonic propulsion in rats. Jpn J Pharmacol. 65:67–71.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho YE, Alcantara E, Kumaran S, et al: Red
yeast rice stimulates osteoblast proliferation and increases
alkaline phosphatase activity in MC3T3-E1 cells. Nutr Res.
30:501–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu HE, Jian CH, Chen SF, et al:
Hypoglycaemic effects of fermented mycelium of Paecilomyces
farinosus (G30801) on high-fat fed rats with
streptozotocin-induced diabetes. Indian J Med Res. 131:696–701.
2010.PubMed/NCBI
|
|
14
|
Ho BY, Wu YM, Hsu YW, et al: Effects of
Monascus-fermented rice extract on malignant cell-associated
neovascularization and intravasation determined using the chicken
embryo chorioallantoic membrane model. Integr Cancer Ther.
9:204–212. 2010. View Article : Google Scholar
|
|
15
|
Lee CL, Kuo TF, Wu CL, Wang JJ and Pan TM:
Red mold rice promotes neuroprotective sAPPalpha secretion instead
of Alzheimer’s risk factors and amyloid beta expression in
hyperlipidemic Abeta40-infused rats. J Agric Food Chem.
58:2230–2238. 2010.PubMed/NCBI
|
|
16
|
Setnikar I, Senin P and Rovati LC:
Antiatherosclerotic efficacy of policosanol, red yeast rice extract
and astaxanthin in the rabbit. Arzneimittelforschung. 55:312–317.
2005.PubMed/NCBI
|
|
17
|
Kim KM, Yu KW, Kang DH and Suh HJ:
Anti-stress and anti-fatigue effect of fermented rice bran.
Phytother Res. 16:700–702. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JK, Cho HR, Kim KY, et al: Lactic acid
bacteria and Bifidobacterium spp. growth stimulating effects
of fermented rice extract (FRe). Food Sci Technol Res. 20:479–483.
2014.
|
|
19
|
Forth W, Nell G, Rummel W and Andres H:
The hydragogue and laxative effect of the sulfuric acid ester and
the free diphenol of 4,4′-dihydroxydiphenyl-(pyridyl-2)-methane.
Naunyn Schmiedebergs Arch Pharmacol. 274:46–53. 1972.PubMed/NCBI
|
|
20
|
Jauch R, Hankwitz R, Beschke K and Pelzer
H: Bis-(p-hydroxyphenyl)-pyridyl-2-methane: the common laxative
principle of Bisacodyl and sodium picosulphate.
Arzneimittelforschung. 25:1796–1800. 1975.PubMed/NCBI
|
|
21
|
Méité S, Bahi C, Yéo D, et al: Laxative
activities of Mareya micrantha (Benth.) Müll Arg
(Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern
Med. 10:72010.
|
|
22
|
Bustos D, Ogawa K, Pons S, et al: Effect
of loperamide and bisacodyl on intestinal transit time, fecal
weight and short chain fatty acid excretion in the rat. Acta
Gastroenterol Latinoam. 21:3–9. 1991.PubMed/NCBI
|
|
23
|
Wintola OA, Sunmonu TO and Afolayan AJ:
The effect of Aloe ferox Mill. in the treatment of
loperamide-induced constipation in Wistar rats. BMC Gastroenterol.
10:952010.
|
|
24
|
Sagar L, Sehgal R and Ojha S: Evaluation
of antimotility effect of Lantana camara L. var. acuelata
constituents on neostigmine induced gastrointestinal transit in
mice. BMC Complement Altern Med. 5:182005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu D, Zhou J, Wang X, et al: Traditional
Chinese formula, lubricating gut pill, stimulates cAMP-dependent
Cl(−) secretion across rat distal colonic mucosa. J Ethnopharmacol.
134:406–413. 2011.PubMed/NCBI
|
|
26
|
Yang ZH, Yu HJ, Pan A, et al: Cellular
mechanisms underlying the laxative effect of flavonol naringenin on
rat constipation model. PLoS One. 3:e33482008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCullogh JS, Ratcliffe B, Mandir N, Carr
KE and Goodlad RA: Dietary fibre and intestinal microflora: effects
on intestinal morphometry and crypt branching. Gut. 42:799–806.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Isawa K, Hojo K, Yoda N, et al: Isolation
and identification of a new bifidogenic growth stimulator produced
by Propionibacterium freudenreichii ET-3. Biosci Biotechnol
Biochem. 66:679–681. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mäkeläinen H, Hasselwander O, Rautonen N
and Ouwehand AC: Panose, a new prebiotic candidate. Lett Appl
Microbiol. 49:666–672. 2009.
|
|
30
|
Hojo K, Yoda N, Tsuchita H, et al: Effect
of ingested culture of Propionibacterium freudenreichii ET-3
on fecal microflora and stool frequency in healthy females. Biosci
Microflora. 21:115–120. 2002.
|
|
31
|
Uchida M, Mogami O and Matsueda K:
Characteristic of milk whey culture with Propionibacterium
freudenreichii ET-3 and its application to the inflammatory
bowel disease therapy. Inflammopharmacology. 15:105–108.
2007.PubMed/NCBI
|















