Introduction
Diabetes mellitus (DM) is one of the most frequently
occurring chronic diseases worldwide. The condition comprises a
group of metabolic diseases characterized by hyperglycemia
resulting from aberrant insulin secretion and synthesis. Although
the etiology of the disease is not well defined, evidence suggests
that oxidative stress or reactive oxygen species (ROS) have a
central role in the onset of DM and its complications, which can
directly result in the injury of islet β cells and consequent
hyperglycemia (1). Hyperglycemia
further causes ROS overproduction and exacerbates the pancreatic
lesions (2); therefore,
antioxidant therapy is a promising treatment strategy for DM that
may ameliorate the injury to the function and structure of β cells
and regulate the level of blood glucose (3). At present, due to the adverse effects
of synthetic hypoglycemic drugs, the use of natural antioxidants,
particularly those of plant origin, is attracting considerable
focus.
Black bean peel extract (BBPE) has a high
concentration of phenolic compounds. The primary phenolic compounds
of BBPE are flavonoids, predominantly anthocyanins and
proanthocyanidins, which have shown antioxidant (4–6) and
antimutagenic activities (7–9)
in vitro as well as in vivo. Xu and Chang (10) found that the seed peel contributed
90% of the total antioxidant capacity of black bean. Pomegranate
peel extract (PPE) is also rich in polyphenols, including
ellagitannins, gallotannins, ellagic acids, gallagic acids,
catechins, anthocyanins, ferulic acids and quercetins (11). These polyphenols exhibit numerous
biological activities, such as eliminating free radicals,
inhibiting oxidation and the growth of microbes, and reducing the
risks of cardio- and cerebrovascular diseases and certain types of
cancer (11–14). Due to their strong antioxidant
effects, black bean peel and pomegranate peel have been common
herbal materials in oriental medicine for hundreds of years
(4–6,11).
Since diabetes is associated with ROS abnormalities and oxidative
stress, it is possible that BBPE or PPE may have a protective
effect in DM mice; however, this issue has not been fully
discussed.
In the present study, streptozotocin (STZ), an
antibiotic cytotoxic to pancreatic β cells due to ROS
overproduction-mediated oxidative stress (15–17),
was utilized in the production of a DM mouse model. The effects of
BBPE, PPE and a combination of the two (PPE + BBPE) on blood
glucose, antioxidant parameters, such as the total antioxidative
capability (T-AOC) and levels of glutathione (GSH), and the
morphological changes in the pancreas of DM mice were observed. The
aim of the study was to provide experimental evidence for the
clinical application of the two extracts in DM.
Materials and methods
Materials
BBPE (containing 40% anthocyanin) and PPE
(containing 40% total polyphenols) were supplied by Ningxia Kaiyuan
Biotechnology Co. Ltd (Ningxia, China). STZ was obtained from
Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, USA) and
dissolved in 1% carboxymethyl cellulose (CMC) solution. The T-AOC
and GSH kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The mouse insulin kit was purchased
from R&D Systems (Minneapolis, MN, USA). All other reagents
were of analytical grade.
Induction of diabetes in mice and study
protocol
Male Kunming mice weighing 20–22 g (Shandong Lukang
Animal Pharmaceutical Co. Ltd., Jining, China) were
intraperitoneally injected with 150 mg/kg STZ (dissolved in citrate
buffer at pH 4.5 immediately prior to injection) to establish a DM
model. The mice with a fasting blood glucose concentration of ≥11.1
mmol/l were recognized as successful DM models two days after STZ
administration; normal control mice (NS group) received citrate
buffer alone. The DM mice were further divided into four groups: i)
DM group (n=8; oral administration of 1% CMC solution); ii) PPE
group (n=6; oral administration of 400 mg/kg PPE); iii) BBPE group
(n=6; oral administration of 400 mg/kg BBPE); and iv) PPE + BBPE
group (n=6; oral administration of 200 mg/kg PPE plus 200 mg/kg
BBPE). The same volume of CMC solution was administered to the NS
group (n=9). The animals were allowed ad libitum access to
food and water. After four-week treatment via oral gavage, blood
samples were collected for the determination of T-AOC and GSH,
insulin and blood glucose levels. Following the sacrifice of the
animals under sodium pentobarbital anesthesia in order to minimize
their suffering, the fresh pancreases were weighed and stored in
formaldehyde solution for hematoxylin and eosin (HE) staining. This
study was carried out in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The protocol was approved by the
Committee on the Ethics of Laboratory Animals of Xuzhou Medical
College (Xuzhou, China). All surgeries were performed under sodium
pentobarbital anesthesia, and all efforts were made to minimize the
suffering of the mice.
Blood glucose and pancreas weight
index
Blood glucose was measured using the glucose oxidase
method with kits purchased from Rongsheng-Biotech Co. Ltd.
(Shanghai, China) in accordance with the manufacturer’s
instructions. The assay was based on the reaction of
4-aminoantipyrine and phenol with glucose to yield a red complex.
The absorbance was measured at 505 nm. The pancreas weight index
(mg/g) was the ratio of the weight of the pancreas to the total
body weight.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of insulin in the serum were determined
by ELISA. Insulin in the serum first combined with mouse insulin
monoclonal antibody, prior to combination with
streptavidin-horseradish peroxidase; the optical density of the
colored immune complex was then measured at 450 nm. The levels of
insulin were determined using a mouse insulin kit according to the
manufacturer’s instructions (R&D Systems).
GSH assay
The level of GSH was measured following the method
of Beutler with certain modification (18). The determination of GSH was based
on the ability of the -SH group to reduce
5,5′-dithiobis(2-nitrobenzoic acid) and form a yellow anionic
product whose optical density was measured at 412 nm. The levels of
GSH were determined using the GSH kit from Nanjing Jiancheng
Bioengineering Institute.
Determination of T-AOC
The plasma T-AOC was determined using a modification
of the ferric reducing ability of plasma (FRAP) assay reported by
Benzie and Strain (19). FRAP
reagent was prepared using acetate buffer, ferric chloride and
2,4,6-tripyridyl-S-triazine. The plasma was mixed with FRAP reagent
thoroughly prior to determination of the absorbance at 593 nm
according to the manufacturer’s instructions (Nanjing Jiancheng
Bioengineering Institute).
HE staining
Pancreatic tissues were harvested from the
sacrificed mice and fixed in 10% neutral buffered formalin
solution, prior to dehydration in ethanol and paraffin-embedding.
Sections measuring 5-μm thickness were prepared using a rotary
microtome and stained with HE dyes for microscopic observation.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using the paired t-test and
one-way analysis of variance with Dunnett’s test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of PPE and BBPE on body
weight
The experimental mice were weighed between weeks 0
and 4 following treatment with PPE, BBPE or PPE + BBPE. The
time-point when the development of DM was noted in the mice was
recognized as week 0. The body weights of all diabetic mice (the
DM, PPE, BBPE and PPE + BBPE groups) were decreased following STZ
induction compared with those in the NS group (P<0.01). The body
weights of the diabetic mice started to increase following three
weeks of treatment with BBPE or PPE + BBPE, but no statistical
significance was found (Fig.
1).
Effects of PPE and BBPE on pancreas
weight index and fasting blood glucose and insulin levels
In this study, a mouse model of DM was established
by a single intraperitoneal injection of STZ. The pancreas weight
index and fasting blood glucose and insulin levels were determined
once the mice had been sacrificed (Table I). The levels of fasting blood
glucose of all diabetic mice (the DM, PPE, BBPE and PPE + BBPE
groups) were significantly increased when compared with those in
the NS group. After four weeks of treatment with PPE, BBPE or PPE +
BBPE, the levels of fasting blood glucose in the DM mice were
reduced. The level of blood glucose in the PPE + BBPE group was
lower than that in the PPE group. However, no significant
differences were found in the levels of blood glucose, insulin and
pancreas weight index between the PPE+BBPE group and the BBPE group
(P>0.05). These results demonstrated that PPE and BBPE,
respectively, caused significantly inhibitory effects on fasting
blood glucose, while the effects subsequent to treatment with PPE +
BBPE were stronger than those following PPE treatment alone.
 | Table IEffects of PPE and BBPE on the blood
glucose, insulin levels and pancreas weight index of mice. |
Table I
Effects of PPE and BBPE on the blood
glucose, insulin levels and pancreas weight index of mice.
| Group | n | Blood glucose
(mmol/l) | Insulin (mIU/l) | Pancreas weight index
(mg/g) |
|---|
| NS | 9 | 6.69±1.06 | 6.87±0.69 | 6.03±0.49 |
| DM | 8 | 25.76±4.60a | 3.73±1.21a | 5.37±0.40a |
| PPE | 6 | 20.90±3.61a,b | 4.67±1.91a | 5.74±0.44 |
| BBPE | 6 | 19.55±3.10a,b | 4.96±1.27a | 5.48±1.30 |
| PPE+BBPE | 6 | 16.72±2.57a,c,d | 5.48±1.69c,e,f | 5.86±0.57b |
The results also indicated that the insulin levels
of all diabetic mice were notably decreased when compared with
those in the NS group. Following treatment with PPE, BBPE or PPE +
BBPE, the quantities of insulin were increased; however,
significant changes relative to the DM group were only found in the
PPE + BBPE group (P<0.01). In addition, the level of insulin was
significantly increased in the PPE + BBPE group compared with that
in the PPE group. These results demonstrated that the combination
of PPE and BBPE was more potent in stimulating the secretion of
insulin than treatment with PPE alone.
The pancreas weight index in the DM group was
decreased (P<0.01) in comparison with that in the NS group.
Following treatment with PPE, BBPE or PPE + BBPE, the pancreas
weight index was increased compared with that in the DM group.
However, statistical significance was only found in the PPE + BBPE
group (P<0.05). The combination of PPE and BBPE therefore
demonstrated the stronger capacity in increasing the weight of the
pancreas in DM mice.
Antioxidant properties of PPE and
BBPE
The levels of GSH and the T-AOC can reflex
antioxidant activity. The levels of GSH and the T-AOC in the DM
group were significantly reduced compared with those in the NS
group, indicating the lowered antioxidant activity of DM mice.
After the four-week treatment period, the values of the two indices
were increased in the three treatment groups compared with those in
the DM group. Furthermore, the two antioxidant indices in the PPE +
BBPE group were significantly higher than those in the PPE or BBPE
groups (Fig. 2). The results
indicated that PPE and BBPE significantly enhanced the antioxidant
capacity in DM mice, while the effect of PPE + BBPE was
superior.
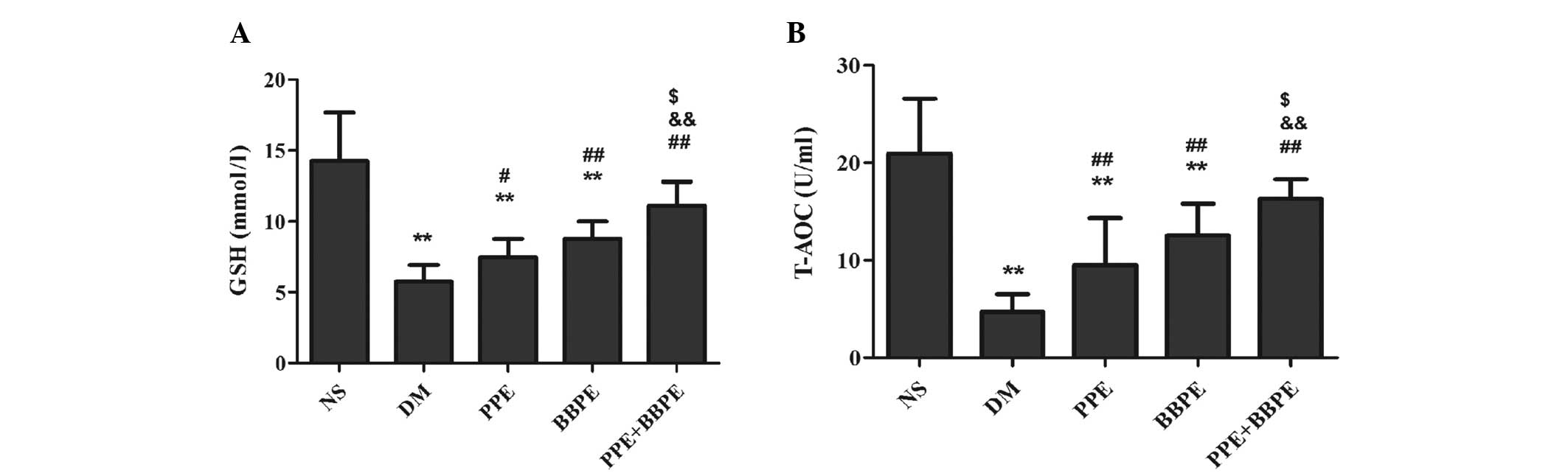 | Figure 2Changes in the (A) GSH levels and (B)
T-AOC in mice. The mice were grouped as follows: NS, normal mice
treated with 1% CMC solution; DM, DM mice treated with 1% CMC
solution; PPE, DM mice treated with 400 mg/kg PPE; BBPE, DM mice
treated with 400 mg/kg BBPE; and PPE + BBPE, DM mice treated with
200 mg/kg PPE plus 200 mg/kg BBPE. Data are presented as the mean ±
standard deviation; n=6–9. **P<0.01 vs. the NS group;
##P<0.01 and #P<0.05 vs. the DM group;
&&P<0.01 vs. the PPE group;
$P<0.05 vs. the BBPE group. CMC, carboxymethyl
cellulose; DM, diabetes mellitus; PPE, pomegranate peel extract;
BBPE, black bean peel extract; GSH, glutathione; T-AOC, total
antioxidative capability. |
Changes in the pancreatic islets
According to the HE staining images, the islets in
the NS group were regularly shaped. The cells in the islets were
well distributed and cell sizes were uniform. By contrast, the
islets in the DM group were small, with irregular outlines and
continuity. Vacuolar denaturation, nuclear concentration and
lymphocyte infiltration were observed. In addition, the cells in
the islets were irregularly shaped and exhibited a messy
arrangement, while the structures of the cells were indistinct.
Following treatment with PPE, BBPE or PPE + BBPE, the abnormalities
were partially alleviated (Fig.
3).
 | Figure 3Morphological changes of the
pancreatic islets by hematoxylin and eosin staining (original
magnification, ×200). The arrows indicate islets. The mice were
grouped as follows: NS, normal mice treated with 1% CMC solution;
DM, DM mice treated with 1% CMC solution; PPE, DM mice treated with
400 mg/kg PPE; BBPE, DM mice treated with 400 mg/kg BBPE; and PPE +
BBPE, DM mice treated with 200 mg/kg PPE plus 200 mg/kg BBPE. CMC,
carboxymethyl cellulose; DM, diabetes mellitus; PPE, pomegranate
peel extract; BBPE, black bean peel extract. |
Discussion
Diabetes is a chronic disease characterized by
disordered metabolism and abnormally high blood glucose levels. The
increase in ROS from the mitochondria is deleterious to cellular
functioning, and molecules such as hydrogen peroxide and
peroxynitrite may cross the mitochondrial membranes and damage
macromolecules in other cellular regions (3,20).
It has been found that ROS overproduction can directly lead to
pancreatic β-cell dysfunction and consequently result in the
impairment of insulin secretion in Type 1 diabetes (1). Mammalian cells have a complex network
of antioxidant enzymes, including glutathione peroxidases,
superoxide dismutase and catalase, and non-enzymatic antioxidants,
such as GSH, vitamin C, vitamin E and β-carotene, to scavenge ROS
(21). Disturbances in the balance
between ROS production and antioxidant defense mechanisms, termed
as oxidative stress, result in pancreatic damage in DM (1). GSH acts to protect normal cell
structure and function by maintaining the redox homeostasis,
quenching free radicals and participating in detoxification
reactions. As well as functioning as a direct scavenger of free
radicals, GSH is a co-substrate for peroxide detoxification by
glutathione peroxidases (16,21).
The T-AOC reflects the total antioxidant capacity in the body by
its effect of transforming Fe3+ into Fe2+
(21). In the present study, STZ,
an antibiotic produced by Streptomyces achromogenes, was
used to produce a mouse model of DM through the induction of ROS
overproduction and damage to pancreatic β cells (15–17).
The entry of STZ into β cells is facilitated via the glucose
transporter, type 2 due to the structural resemblance of STZ to
glucose (22). Once inside the β
cell, oxidative reactions occur with thiol-containing enzymes, such
as glucokinase and aconitase, leading to glucose sensing
impairments, mitochondrial dysfunction and necrotic cell death
(22). In addition, α cells and δ
cells in the pancreatic islets undergo necrosis through the
influence of STZ (15). The
present results showed that there was a fall in the levels of GSH
and the T-AOC accompanied by an increase in blood glucose in the DM
mice, which indicated the occurrence of oxidative stress and the
success of DM model establishment by STZ induction. Histological
findings showed that the islets were irregularly shaped and small
and that the structures of the cells were indistinct, which was
most likely due to oxidative stress induced-protein modification
(15).
The black bean contains phytochemicals, including
phenolic compounds, which can provide health benefits to the
consumers. It has been found that anthocyanins, which constitute a
major flavonoid group, can promote endothelial repair and prevent
atherogenesis in diabetic apolipoprotein E-deficient mice (23). Furthermore, a higher consumption of
anthocyanins and anthocyanin-rich fruit was found to be associated
with a lower risk of type 2 diabetes (24). Studies have shown that black seed
peel exhibits considerably higher total phenolic indices (including
anthocyanins) and antioxidant activities than whole or dehulled
black beans, despite the different cultivars of black bean
(10,24). The present study therefore used the
extracts from black bean peel in DM mice, in addition to a second
extract derived from pomegranate peel. Pomegranate peel is rich in
antioxidants of the polyphenolic class, which includes flavonoids
such as ellagitannins and anthocyanins (11). It has been suggested that
ellagitannins could be responsible for the promising antioxidant
and antimutagenic activities of PPE (25). Fawole et al (26) found that PPE exhibited strong
antibacterial and antioxidant activities. Furthermore,
epidemiological studies have demonstrated that the consumption of
foods rich in flavonoids (the primary polyphenolic compounds) could
protect against human diseases associated with oxidative stress,
such as coronary heart disease and cancer (11–14).
Little information, however, is available regarding the application
of PPE and BBPE in DM.
In the present study, the effects of polyphenol-rich
PPE and BBPE were observed in DM mice. The results showed that,
after four weeks of treatment with PPE, BBPE or PPE + BBPE, the DM
mice showed, to different degrees, a decrease in blood glucose,
increases in insulin secretion and the pancreas weight index, and
an increase in antioxidative activity. The results indicated that
PPE and BBPE, respectively, protected the pancreatic β cells from
STZ-mediated oxidative stress and thereby stimulated the recovery
of pancreatic β cells and the increased synthesis and secretion of
insulin, consequently leading to adjustments in the level of blood
glucose. It could be therefore be concluded that PPE and BBPE
possess significant antidiabetic and antioxidant potential in
STZ-induced diabetic mice. The histological observation of the
pancreatic tissues further proved the potential protective effects
of PPE and BBPE on pancreatic tissue and showed that the protective
effects were stronger in the DM mice that were subjected to the
joint intervention of PPE + BBPE. This may have been due to the
fact that the biological activity of flavonoids depends on the
types of phytochemical constituents, the complexity of their
structures and the composition of the flavonoid mixtures (27,28),
which produce an additive or synergistic effect (29,30).
There was, however, no significant change in the body weight
following the treatment with the two extracts and their mixture,
although an increasing trend was noted; this requires further
investigation.
In conclusion, the present study demonstrates that
PPE and BBPE, particularly the combination of the two, have the
ability to ameliorate hyperglycemia by inhibiting oxidative
stress-induced damage to the pancreas; as such, these extracts may
be useful in the prevention and treatment of DM. Further studies
are required to identify the molecular mechanism involved in the
protection of pancreatic tissue by PPE and BBPE in oxidative
stress-induced DM mice.
Acknowledgements
This study was funded by the National Innovative
Practice Training Program for Students of Higher Education
Institutions (no. 201310313028) and the Innovative Practice
Training Program for Students of Jiangsu Higher Education
Institutions (no. 201310313028Z). It was also a special project
supported by the Deans of Xuzhou Medical College (no. 2011KJZ19),
the Priority Academic Program Development of Jiangsu Higher
Education Institutions (PAPD), the National Natural Foundation of
China (no. 81173104), and the Jiangsu ‘Six Talent Peaks’ Foundation
of China (no. 2011-SWYY-0195).
References
|
1
|
Piconi L, Quagliaro L and Ceriello A:
Oxidative stress in diabetes. Clin Chem Lab Med. 41:1144–1149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyazaki Y, Kawano H, Yoshida T, et al:
Pancreatic B-cell function is altered by oxidative stress induced
by acute hyperglycaemia. Diabet Med. 24:154–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bandeira MS, da Fonseca LJ, da Guedes
SG, et al: Oxidative stress as an underlying contributor in the
development of chronic complications in diabetes mellitus. Int J
Mol Sci. 14:3265–3284. 2013. View Article : Google Scholar
|
|
4
|
Beninger CW and Hosfield GL: Antioxidant
activity of extracts, condensed tannin fractions, and pure
flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J
Agric Food Chem. 51:7879–7883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuda T, Ohshima K, Kawakishi S and Osawa
T: Antioxidative pigments isolated from the seeds of Phaseolus
vulgaris L. J Agric Food Chem. 42:248–251. 1994. View Article : Google Scholar
|
|
6
|
Cardador-Martínez A, Loarca-Piña G and
Oomah BD: Antioxidant activity in common beans (Phaseolus vulgaris
L.). J Agric Food Chem. 50:6975–6980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardador-Martínez A, Castaño-Tostado E and
Loarca-Piña G: Antimutagenic activity of natural phenolic compounds
in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food
Addit Contam. 19:62–69. 2002. View Article : Google Scholar
|
|
8
|
Aparicio-Fernández X, Manzo-Bonilla L and
Loarca-Piña G: Comparison of antimutagenic activity of phenolic
compounds in newly harvested and stored common beans Phaseolus
vulgaris against aflatoxin B1. J Food Sci. 70:S73–S78. 2005.
View Article : Google Scholar
|
|
9
|
Gu L, Kelm MA, Hammerstone JF, et al:
Liquid chromatographic/electrospray ionization mass spectrometric
studies of proanthocyanidins in foods. J Mass Spectrom.
38:1272–1280. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu B and Chang SK: Antioxidant capacity of
seed coat, dehulled bean, and whole black soybeans in relation to
their distributions of total phenolics, phenolic acids,
anthocyanins, and isoflavones. J Agric Food Chem. 56:8365–8373.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Jia D and Yao K: Antiliperoxidant
activity of pomegranate peel extracts on lard. Nat Prod Res.
21:211–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang C, Lee SY, Lin CL, et al:
Co-treatment with quercetin and
1,2,3,4,6-penta-O-galloyl-β-D-glucose causes cell cycle arrest and
apoptosis in human breast cancer MDA-MB-231 and AU565 cells. J
Agric Food Chem. 61:5558–5564. 2013. View Article : Google Scholar
|
|
13
|
Hou X, Liu Y, Niu L, et al: Enhancement of
voltage-gated K+ channels and depression of
voltage-gated Ca2+ channels are involved in
quercetin-induced vasorelaxation in rat coronary artery. Planta
Med. 80:465–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bondonno CP, Downey LA, Croft KD, et al:
The acute effect of flavonoid-rich apples and nitrate-rich spinach
on cognitive performance and mood in healthy men and women. Food
Funct. 5:849–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action of β-cells of the rat pancreas. Physiol Res.
50:537–546. 2001.
|
|
16
|
Sefi M, Fetoui H, Lachkar N, et al:
Centaurium erythrea (Gentianaceae) leaf extract alleviates
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. J Ethnopharmacol. 135:243–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grdović N, Dinić S, Arambašić J, et al:
The protective effect of a mix of Lactarius deterrimus and Castanea
sativa extracts on streptozotocin-induced oxidative stress and
pancreatic beta-cell death. Br J Nutr. 108:1163–1176. 2012.
View Article : Google Scholar
|
|
18
|
Beutler E, Duron O and Kelly BM: Improved
method for the determination of blood glutathione. J Lab Clin Med.
61:882–888. 1963.PubMed/NCBI
|
|
19
|
Benzie IF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of ‘antioxidant
power’: the FRAP assay. Anal Biochem. 239:70–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nickel A, Kohlhaas M and Maack C:
Mitochondrial reactive oxygen species production and elimination. J
Mol Cell Cardiol. 73:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bose KS, Vyas P and Singh M: Plasma
non-enzymatic antioxidants-vitamin C, E, beta-carotenes, reduced
glutathione levels and total antioxidant activity in oral sub
mucous fibrosis. Eur Rev Med Pharmacol Sci. 16:530–532.
2012.PubMed/NCBI
|
|
22
|
Lightfoot YL, Chen J and Mathews CE:
Oxidative stress and beta cell dysfunction. Methods Mol Biol.
900:347–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Wang X, Wang Y, et al:
Supplementation of cyanidin-3-O-β-glucoside promotes endothelial
repair and prevents enhanced atherogenesis in diabetic
apolipoprotein E-deficient mice. J Nutr. 143:1248–1253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oomah BD, Corbé A and Balasubramanian P:
Antioxidant and anti-inflammatory activities of bean (Phaseolus
vulgaris L.) hulls. J Agric Food Chem. 58:8225–8230. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aparicio-Fernández X, Yousef GG,
Loarca-Pina G, et al: Characterization of polyphenolics in the seed
coat of Black Jamapa bean (Phaseolus vulgaris L.). J Agric Food
Chem. 53:4615–4622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fawole OA, Makunga NP and Opara UL:
Antibacterial, antioxidant and tyrosinase-inhibition activities of
pomegranate fruit peel methanolic extract. BMC Complement Altern
Med. 12:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou DX, Kai K, Li JJ, et al:
Anthocyanidins inhibit activator protein 1 activity and cell
transformation: structure activity relationship and molecular
mechanism. Carcinogenesis. 25:29–36. 2004. View Article : Google Scholar
|
|
28
|
Lazzè MC, Savio M, Pizzala R, et al:
Anthocyanins induce cell cycle perturbations and apoptosis in
different human cell lines. Carcinogenesis. 25:1427–1433. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seeram NP, Adams LS, Hardy ML and Heber D:
Total cranberry extract versus its phytochemical constituents:
antiproliferative and synergistic effects against human tumor cell
lines. J Agric Food Chem. 52:2512–2517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shafiee M, Carbonneau M, d’Huart JB, et
al: Synergistic antioxidative properties of phenolics from natural
origin toward low-density lipoproteins depend on the oxidation
system. J Med Food. 5:69–78. 2002. View Article : Google Scholar : PubMed/NCBI
|

















