Introduction
Solitary fibrous tumors (SFTs) are a rare type of
mesenchymal tumor, which were first described by Klemperer and
Rabin in 1931 (1). Although they
are commonly found in the pleura, statistics show that 50–70% of
all SFTs are extrapleural (2).
However, few cases of SFTs in the urogenital organs have been
documented. To the best of our knowledge, only a small number of
these cases forming in the spermatic cord have been reported to
date (3–7).
Due to the rarity of these tumors, there was no
consensus on its standard treatment. However, treatment for SFTs
include surgical resection, radiotherapy and chemotherapy.
Resection of the tumor with a sufficient surgical margin was the
mainstay of treatment. However, a number of studies have
demonstrated that radiotherapy and chemotherapy, with ifosfamide
and adriamycin, is effective (5,8).
However, these treatments were not established yet. The present
study reports a case of SFT involving the spermatic cord.
Case report
The patient was a 31-year-old male with a six-year
history of left inguinoscrotal swelling, which had been gradually
increasing in size for the past three years. The size of the
swelling showed no association with increased intraperitoneal
pressure. Physical examination revealed no evident defects in the
appearance of the scrotum, with the exception of several hard,
botryoidal masses, located between the left testicle and the
inguinal region, with smooth surfaces and limited capacity of
mobility. Ultrasound examination revealed several solid,
well-demarcated, hypoechoic extratesticular masses arising from the
left spermatic cord, the largest of which was ~3 × 2 cm in size.
The masses were separated from the cutis and showed no connections
with the left testis and epididymis, and no vascularity was
observed. The patient’s family signed an informed consent prior to
the treatment.
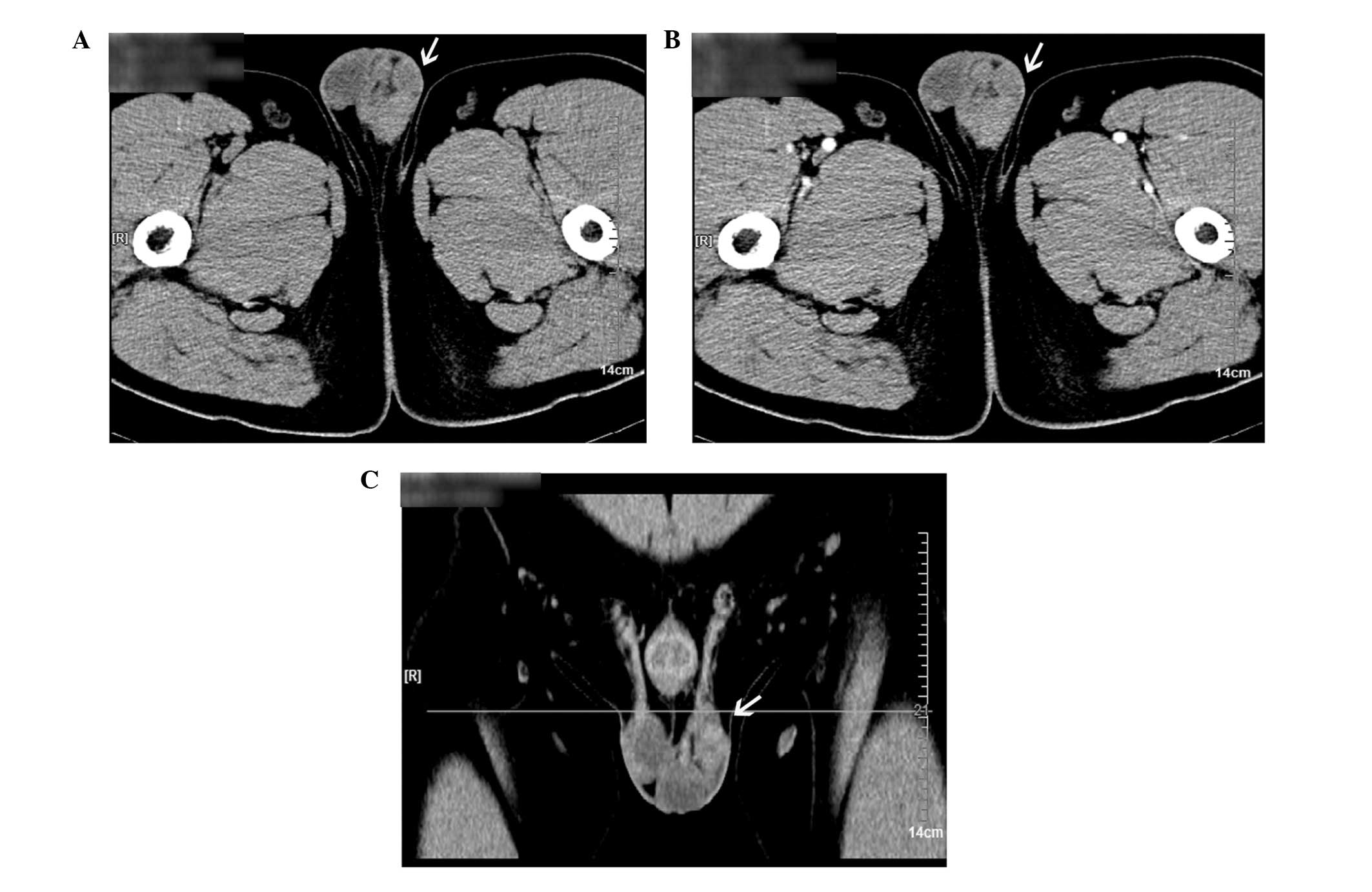
A computed tomography (CT) scan revealed multiple
high-density nodules associated with the left spermatic cord, which
showed partial fusion, uniform density and a clear boundary with
the testis (Fig. 1). In addition,
the density of the nodules was enhanced slightly and no bilateral
inguinal swollen lymph nodes were observed in the enhanced
scan.
The masses were removed by a left spermatic cord
tumor resection via a left inguinal approach, under epidural
anesthesia. Benign mesenchymal tissue was diagnosed by
intraoperative frozen pathological sections. Postoperative
pathological examination confirmed that the masses belonged to
multiple mesenchymal tumors with classic properties, including
fibroplasia, small vascular hyalinization, clear boundaries, less
tumor component and no nuclear division. Immunohistochemical
examination revealed the presence of numerous tumor markers,
including CD99+, Bcl-2+, partial
CD34+, focal S-100+, SMA+ and
CD68− (Fig. 2). Thus,
the final diagnosis was solitary fibrous tumors of the spermatic
cord. The patient remained healthy, with no local recurrence or
metastasis observed during the 25-month follow-up period.
Discussion
Although SFTs have been observed in male and female
patients of a wide range of ages, the underlying mechanism remains
unknown. Initially, SFTs were most commonly located in the pleura
(9). At present, only a limited
number of SFT cases have been identified in the urogenital organs,
including the kidney, renal pelvis, bladder, prostate and seminal
vesicles (4,6,7,9–12).
Moreover, SFTs involving the spermatic cord are extremely rare and
should be distinguished from other urogenital mesenchymal tumors,
which include inflammatory myofibromatous tumors,
hemangiopericytomas, malignant fibrohistiocytomas, fibrosarcomas,
leiomyosarcomas and benign and malignant nerve sheath tumors. In
addition, SFTs should be distinguished from other diseases that can
result from inguinal masses, similarly to an inguinal hernia
(11).
Clinical symptoms of urogenital SFTs, including a
painless mass, dysuria, hematospermia and abdominal pain, are
mainly determined by tumor properties, including the size,
location, invasion and the degree of mucosal integrity (11).
Ultrasonography and magnetic resonance imaging
(MRI), two common imaging examinations, play an important role in
the diagnosis of SFTs. Ultrasound examination is more effective in
the identification of solid and cystic tumors, while MRI is able to
illustrate anatomical associations in the tumor surroundings more
precisely (7). Isodensity with
patchy hypodensity for unenhanced CT images, and mild or marked
heterogeneous enhancement for contrast-enhanced CT images are the
major features of pleural SFTs (13,14).
A study by Zhang et al demonstrated that a well-defined,
ovoid or rounded mass with low signal intensity on MRI T2-weighted
images and different degrees of enhancement on CT and MRI scans may
indicate the presence of an SFT (14). Positron emission tomography-CT
scanning is able to preoperatively indicate malignancy and
recognize local recurrence or distant metastases; however, Lococo
et al deemed that it is controversial for the identification
of benign and malignant SFTs for PETCT. Thus, further evaluation is
required (15,16). In the present study, an unenhanced
CT scan demonstrated multiple high-density nodules, and the signal
only strengthened slightly in the enhanced CT scan. Therefore, the
diagnosis of an SFT by imaging only is inadvisable and further
pathological examinations are necessary.
Previous studies have confirmed that tumor markers,
including CD34+, Bcl-2+ and CD99+,
are generally positive in SFT cases, which has aided the diagnosis
of SFTs (17,18). Currently, the positive staining of
CD34+ is indispensable in the diagnosis of an SFT;
however, the expression of CD34 is not limited to SFTs (11). In the present study, the positive
staining of CD34+, Bcl-2+ and
CD99+ was demonstrated by immunohistochemistry
examinations, confirming the diagnosis of an SFT.
The conventional treatment for an SFT is the
complete resection of the tumor with negative margins (19,20)
or resection experience satisfactory health outcomes (21). However, the resection of tumors
with positive margins is associated with a higher rate of local
recurrence, and surgical excision remains the first choice for the
treatment of local recurrence (20). With regard to urogenital SFTs, the
selection of the surgical procedure should depend on the
characteristics of each individual tumor, including the location,
size and degree of malignancy. For tumors with infiltrative growth,
of a large size or aggressive malignancy, the recommended treatment
is the extended resection of the involved organs, including radical
nephrectomy and cystoprostatectomy. In the present study,
considering the clear demarcations between the tumors and
surrounding organs, complete resection of the tumors was performed
without removing the spermatic cord.
The prognosis of SFTs is obscure for rare cases.
Usually, an SFT is defined as a benign form of tumor due to its low
rates or even absence of local recurrence and metastasis, as
demonstrated by a number of previous studies (1,2,22,23).
However, an increasing number of recent studies have demonstrated
that a considerable number of SFTs are malignant (11,18,24).
In the case of an SFT which is unresectable, metastatic or has
positive surgical margins, patients are unlikely to benefit
significantly from radiotherapy or chemotherapy; the alternative
treatments for an SFT (2).
Therefore, a careful long-term follow-up period is advised in all
SFT cases due to the possibility of a late recurrence (21). The case reported in the present
study was considered to be a benign SFT due to its small size
(<10 cm), lack of growth and minimal cancer component. During
the 25-month follow-up period, the patient remained healthy and
exhibited no tumor recurrence.
In conclusion, pathological examination remains the
standard approach for the diagnosis of SFTs, while radical
resection of the tumor/s is the first choice for treatment.
Furthermore, patients should undergo careful follow-up examination
in order to identify tumor recurrence.
References
|
1
|
Klemperer P and Rabin LB: Primary
neoplasms of the pleura. A report of five cases Arch Pathol.
11:385–412. 1931.
|
|
2
|
van Houdt WJ, Westerveld CM, Vrijenhoek
JE, et al: Prognosis of solitary fibrous tumors: a multicenter
study. Ann Surg Oncol. 20:4090–4095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cafiero F, Gipponi M, Peressini A, et al:
Solitary fibrous tumor of the inguinal region: a
clinicopathological, light-microscopic, immunohistochemical,
electron microscopic and flow-cytometric DNA study. Anticancer Res.
21:4091–4094. 2001.
|
|
4
|
Fisher C and Bisceglia M: Solitary fibrous
tumour of the spermatic cord. Br J Urol. 74:798–799. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gold JS, Antonescu CR, Hajdu C, et al:
Clinicopathologic correlates of solitary fibrous tumors. Cancer.
94:1057–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Márquez Moreno AJ, Vicioso Recio L, Castro
Léon A, Casals Sánchez JL, Alcázar Ramírez J and Matilla Vicente A:
Paratesticular solitary fibrous tumor. Arch Esp Urol. 54:716–718.
2001.(In Spanish).
|
|
7
|
Ilica AT, Ors F, Bag M, Guvenc I, Duran C
and Buyukbayram H: An uncommon cause of inguinoscrotal swelling:
Solitary fibrous tumor of the spermatic cord. Eur J Radiol Extra.
67:25–28. 2008. View Article : Google Scholar
|
|
8
|
de Perrot M, Fischer S, Brundler MA, et
al: Solitary fibrous tumor of the pleura. Ann Thorac Surg.
74:285–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiessner D, Dittert DD, Manseck A and
Wirth MP: Large solitary fibrous tumor of the seminal vesicle.
Urology. 62:9412003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magro G, Cavallaro V, Torrisi A, Lopes M,
Dell’Albani M and Lanzafame S: Intrarenal solitary fibrous tumor of
the kidney report of a case with emphasis on the differential
diagnosis in the wide spectrum of monomorphous spindle cell tumors
of the kidney. Pathol Res Pract. 198:37–43. 2002.PubMed/NCBI
|
|
11
|
Westra WH, Grenko RT and Epstein J:
Solitary fibrous tumor of the lower urogenital tract: a report of
five cases involving the seminal vesicles, urinary bladder, and
prostate. Hum Pathol. 31:63–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yazaki T, Satoh S, Iizumi T, Umeda T and
Yamaguchi Y: Solitary fibrous tumor of renal pelvis. Int J Urol.
8:504–508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong S, Kim TS, Cho EY, Kim J and Kim H:
Benign localized fibrous tumour of the pleura: CT features with
histopathological correlations. Clin Radiol. 61:875–882. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang WD, Chen JY, Cao Y, Liu QY and Luo
RG: Computed tomography and magnetic resonance imaging findings of
solitary fibrous tumors in the pelvis: correlation with
histopathological findings. Eur J Radiol. 78:65–70. 2011.
View Article : Google Scholar
|
|
15
|
Lococo F, Cafarotti S and Treglia G: Is
18F-FDG-PET/CT really able to differentiate between
malignant and benign solitary fibrous tumor of the pleura? Clin
Imaging. 37:9762013. View Article : Google Scholar
|
|
16
|
Yan J, Ahl KL, Manning KA, Mann FA and
Lewis DH: Radiology-Pathology Conference: 18F FDG PET-CT
imaging of solitary fibrous tumor of the pleura. Clin Imaging.
37:598–601. 2013. View Article : Google Scholar
|
|
17
|
Hanau CA and Miettinen M: Solitary fibrous
tumor: histological and immunohistochemical spectrum of benign and
malignant variants presenting at different sites. Hum Pathol.
26:440–449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao N, Colby TV, Falconieri G, Cohen H,
Moran CA and Suster S: Intrapulmonary solitary fibrous tumors:
clinicopathologic and immunohistochemical study of 24 cases. Am J
Surg Pathol. 37:155–166. 2013. View Article : Google Scholar
|
|
19
|
Marak CP, Dorokhova O and Guddati AK:
Solitary fibrous tumor of the pleura. Med Oncol. 30:5732013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uemura K, Hayashi T and Matsuoka K:
Solitary fibrous tumor of the greater omentum in an inguinal hernia
sac. Intern Canc Conf J. 1:70–73. 2012. View Article : Google Scholar
|
|
21
|
Brunnemann RB, Ro JY, Ordonez NG, Mooney
J, El-Naggar AK and Ayala AG: Extrapleural solitary fibrous tumor:
a clinicopatho-logic study of 24 cases. Mod Pathol. 12:1034–1042.
1999.PubMed/NCBI
|
|
22
|
Lee SC, Tzao C, Ou SM, Hsu HH, Yu CP and
Cheng YL: Solitary fibrous tumors of the pleura: clinical,
radiological, surgical and pathological evaluation. Eur J Surg
Oncol. 31:84–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo W, Xiao HL, Jiang YG, Wang RW, Zhao
YP, Ma Z, et al: Retrospective analysis for thirty-nine patients
with solitary fibrous tumor of pleura and review of the literature.
World J Surg Oncol. 9:1342011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|