Introduction
Coronary heart disease (CHD) is a major preventable
cause of morbidity and mortality in the United States. It was
previously demonstrated that, despite an increased prevalence of
smoking and consumption of diets containing significant amounts of
saturated fats, the incidence of cardiovascular disease is actually
lower in the French compared to that in the American population
(1). The prophylactic and
therapeutic effect of several plant foods and extracts in reducing
cardiovascular disease has been investigated (2). Numerous studies have focused on
experiments using natural antioxidants to alleviate the
atherosclerosis induced by lipaemic oxidative stress. The dietary
intake of phenolic compounds in red wine (3), green tea (4) and olive oil (5) may inhibit the oxidation of
low-density lipoprotein cholesterol (LDL-C), thereby reducing the
risk factors for cardiovascular disease. The presence of
polyphenols in green tea may contribute to its antioxidant effect
by inhibiting reactive oxygen species (ROS)-generating enzymes
(6). Green tea contains a number
of biologically active polyphenolic flavonoids, commonly known as
catechins, including epicatechin, epicatechin-3-gallate,
epigallocatechin and epigallocatechin-3-gallate (EGCG).
EGCG is a polyphenol and a well-characterized
antioxidant that constitutes ~30% of the solids in the green tea
leaf (Camellia sinensis) (7). In previous epidemiological studies,
green tea consumption has been associated with a dose-dependent
decrease in the incidence of diabetes, hypertension and
cardiovascular morbidity and mortality (8,9). It
was recently reported that EGCG may protect the heart from ischemic
injury (5). However, in actual
clinical cases, acute myocardial infarction patients have already
developed cardiac ischemic injury when they are admitted to the
hospital and it is not possible to administer therapeutic drugs
prior to the occurrence of an unexpected acute myocardial
infarction. Therefore, the treatment of acute myocardial infarction
with EGCG would include administration following acute coronary
artery occlusion or during reperfusion. The administration of EGCG
during reperfusion has been reported to reduce cardiac reperfusion
injury (6); however, there have
not yet been any studies on the extent of reduction of myocardial
necrosis, an indicator of reperfusion injury, with EGCG
treatment.
The present study was designed to investigate the
cardioprotective effect of EGCG against high-fat diet in an animal
model, with special emphasis to myocardial infarction.
Materials and methods
Experimental animals and grouping
Male Wistar rats (n=24; weight, 150–200 g; age, 12
weeks) were housed in room temperature with a regular 12-h
day/night cycle. The animals had access to food and water ad
libitum. The experimental animals were grouped as follows: i)
Control group (n=6), in which the animals were fed a standard diet
for 45 days; ii) positive control group (EGCG, n=6), in which the
animals were fed a standard diet throughout the experimental
period, with intraperitoneal (i.p.) injection of EGCG (100 mg/kg
body weight) for the last 15 days; iii) high cholesterol group (HC,
n=6), in which the animals were fed an HC diet for 30 days,
followed by standard diet for 15 days; and iv) treatment group
(HC+EGCG, n=6), in which the animals were fed an HC diet for 30
days, followed by standard diet with i.p. injection of EGCG for 15
days.
The animals were used in accordance with the
Institutional Guidelines and the experimental protocols were
approved by the Animal Ethics Committee.
HC diet formulation
The diet consisted of a mixture of equal quantities
of powdered commercial rat feed and high-fat constituents, such as
5% cholesterol, 20% sucrose, 20% hydrogenated vegetable oil, 2%
sodium cholate, 20% lactose, 0.4% choline chloride and 0.15%
thiouracil. Pellets were prepared from this mixture, which were
then shade-dried and fed to the rats.
Sample preparation
At the end of the experiment, the animals were
sacrificed by cervical decapitation, blood samples were collected,
serum was separated and a haemolysate was prepared according to the
procedure described by Quist (10). A lipid profile analysis was
performed on the serum samples, while the antioxidant levels were
analysed in the haemolysate. All the samples were stored at −80°C
until analysis. Prior to the biochemical analysis, cardiac tissue
(100 mg tissue/ml buffer) was homogenized in 50 mM phosphate buffer
(pH 7.2; Sigma-Aldrich, St. Louis, MO, USA); the homogenate was
then centrifuged at 1,200 × g for 15 min and the supernatant was
used for biochemical analysis. The protein concentration in each
fraction was determined with the method described by Bradford
(11), using crystalline bovine
serum albumin as a standard.
Evaluation of serum lipid profile
The lipid profile commonly includes total
cholesterol (TC), triglycerides (TG), LDL-C, very low-density
lipoprotein cholesterol (VLDL-C) and high-density lipoprotein
cholesterol (HDL-C). The serum lipid levels were measured using
standard assay kits (DiaSys, Holzheim, Germany). The units are
expressed as mg/dl.
Determination of lipid peroxidation
(LPO)
LPO was evaluated in the tissue homogenate and
haemolysate samples. To evaluate the level of LPO, the mean
concentration of malondialdehyde (MDA) was assayed in the form of
thiobarbituric acid-reacting substances (TBARS) with the method
described by Ohkawa et al (12).
Enzymatic antioxidant activity
The activity of enzymes in the antioxidant system
was evaluated in the tissue homogenate and haemolysate samples
following previously reported methods. Catalase (CAT) activity was
determined using the method of Sinha (13) and expressed as U/mg protein (μmol
of H2O2 consumed/min/mg protein). Superoxide
dismutase (SOD) activity was determined using the method of
Marklund and Marklund (14) and
expressed as U/mg protein. Glutathione peroxidase (GPx) was
determined as described by Rotruck et al (15) and expressed in terms of μg of
reduced glutathione (GSH) consumed/min/mg protein. The enzyme
activity was expressed as lmol of 1-chloro-2,4-dinitrobenzene
formed/min/mg protein.
Non-enzymatic antioxidant levels
The levels of non-enzymatic antioxidants in cardiac
tissue homogenate samples were determined by following previously
reported methods. The GSH content was estimated by the method of
Moron et al (16).
Ascorbate (vitamin C) was measured using the method of Omaye et
al (17). α-tocopherol
(vitamin E) was estimated by the method of Desai (18). The results of all the experiments
are expressed as μg/mg protein.
Western blot analysis
The cells were washed with Hanks’ buffer (Thermo
Fisher Scientific Inc., Waltham, MA, USA), scraped in 50–100 ml of
lysis buffer (with protease inhibitors), centrifuged and the
supernatant was collected. The protein content was determined by
the bicinchonic acid protein assay (Sigma-Aldrich). Total cell
extracts containing 16–20 mg of protein were prepared in SDS sample
buffer (Sigma-Aldrich) and subjected to SDS-PAGE and western blot
analysis. The proteins were transferred to nitrocellulose membranes
prior to immunodetection. The antibodies against sirtuin 1 (SIRT1;
donkey anti-mouse monoclonal; 1:1,000; cat. #8469), phosphorylated
AMP-activated protein kinase α (p-AMPKα; donkey anti-mouse
monoclonal; 1:1,000; cat. #2793; Thr172) and endothelial nitric
oxide synthase (eNOS; goat anti-rabbit polyclonal; 1:1,000; cat.
#9572) were purchased from Cell Signaling (Beverly, MA, USA) and
were used to detect protein levels in the heart tissues.
Glyceraldehyde 3-phosphate dehydrogenase (GADPH; donkey anti-mouse
monoclonal; 1:1,000; cat. #Ab8245; Abcam, Cambridge, MA, USA) was
used as control.
Assessment for markers of myocardial
tissue damage
The levels of markers of myocardial tissue damage,
such as lactate dehydrogenase (LDH), alkaline phosphatase (ALP),
aspartate transaminase (AST) and alanine transaminase (ALT), were
determined according to the method described by King (19).
Histopathological examination
Conventional techniques of paraffin wax sectioning
and haematoxylin-eosin (HE) staining were used in this study.
Specimens of fresh thoracic aorta were cut and fixed in buffered
neutral formalin for 24 h. Following fixation, the tissue specimens
were washed and processed through an ascending series of alcohol
(30, 50, 70, 90 and 100%), cleared in methyl salicylate and
infiltrated with paraffin wax at 57°C. Microtome sections (4–6 μm)
were cut, stained by aqueous haematoxylin and alcoholic eosin and
examined under a bright-field microscope (Axioskop 2 plus; Carl
Zeiss Jena, Gera, Germany).
Statistical analysis
The values are expressed as mean ± standard
deviation for 6 animals per group. Differences between groups were
assessed by one-way analysis of variance using SPSS software
package for Windows, version 11.5 (SPSS Inc., Chicago, IL, USA).
Post hoc testing was performed for intergroup comparisons using the
least significance difference test.
Results
EGCG improves serum lipid profile
The HC rats exhibited a significant (P<0.001)
increase in the serum TC, TG, LDL-C and VLDL-C levels and the
cardiac risk ratio, when compared to other groups. However, HC rats
treated with EGCG exhibited a significant (P<0.001) improvement
in their serum lipid profiles to near-normal levels. Of note, the
positive control group rats exhibited a well-maintained lipid
profile compared to that of control group rats (Table I).
 | Table IAdministration of EGCG improves serum
lipid profile. |
Table I
Administration of EGCG improves serum
lipid profile.
| Groups |
|---|
|
|
|---|
| Lipid profile | Control | EGCG | HC | HC+EGCG |
|---|
| LDL, mg/dl | 19±2.2 | 16±2.8 | 204±2.1a,c | 59±2.6b,c |
| TC, mg/dl | 49±3.76 | 39±5.07 | 423±7.34a,c | 122±5.38b,c |
| TG, mg/dl | 79±3.4 | 74±3.5 | 181±5.6a,c | 114±3.8b,c |
| HDL, mg/dl | 70±2.3 | 75±2.5 | 37±3.1a,c | 51±3.2b,c |
| VLDL, mg/dl | 21±1.5 | 16±1.2 | 51±1.9a,c | 24±1.3b,c |
| Cholesterol
ratio | 6±0.2 | 6±0.1 | 18±1.3a,c | 7±0.2b,c |
EGCG prevents LPO in the cardiac tissue
and haemolysate
LPO was determined by the mean concentration of MDA
assayed in the form of TBARS. The cardiac tissue and haemolysate
samples from HC rats exhibited a significant (P<0.001) increase
in the levels of MDA compared to those from the control group
(Table II). By contrast, in the
HC+EGCG group, LPO was significantly (P<0.05) inhibited in the
cardiac and haemolysate samples. Similarly, the positive control
group exhibited notably improved protection against LPO compared to
the control rats (Table II).
 | Table IIEGCG prevents lipid peroxidation
(LPO). |
Table II
EGCG prevents lipid peroxidation
(LPO).
| Groups |
|---|
|
|
|---|
| LPO | Control | EGCG | HC | HC+EGCG |
|---|
| Tissue (mg/g
tissue) | 0.7 | 0.5 | 1.4a,c | 0.9b,c |
| Haemolysate
(mg/ml) | 1.6 | 1.4 | 3.1a,c | 2.0b,c |
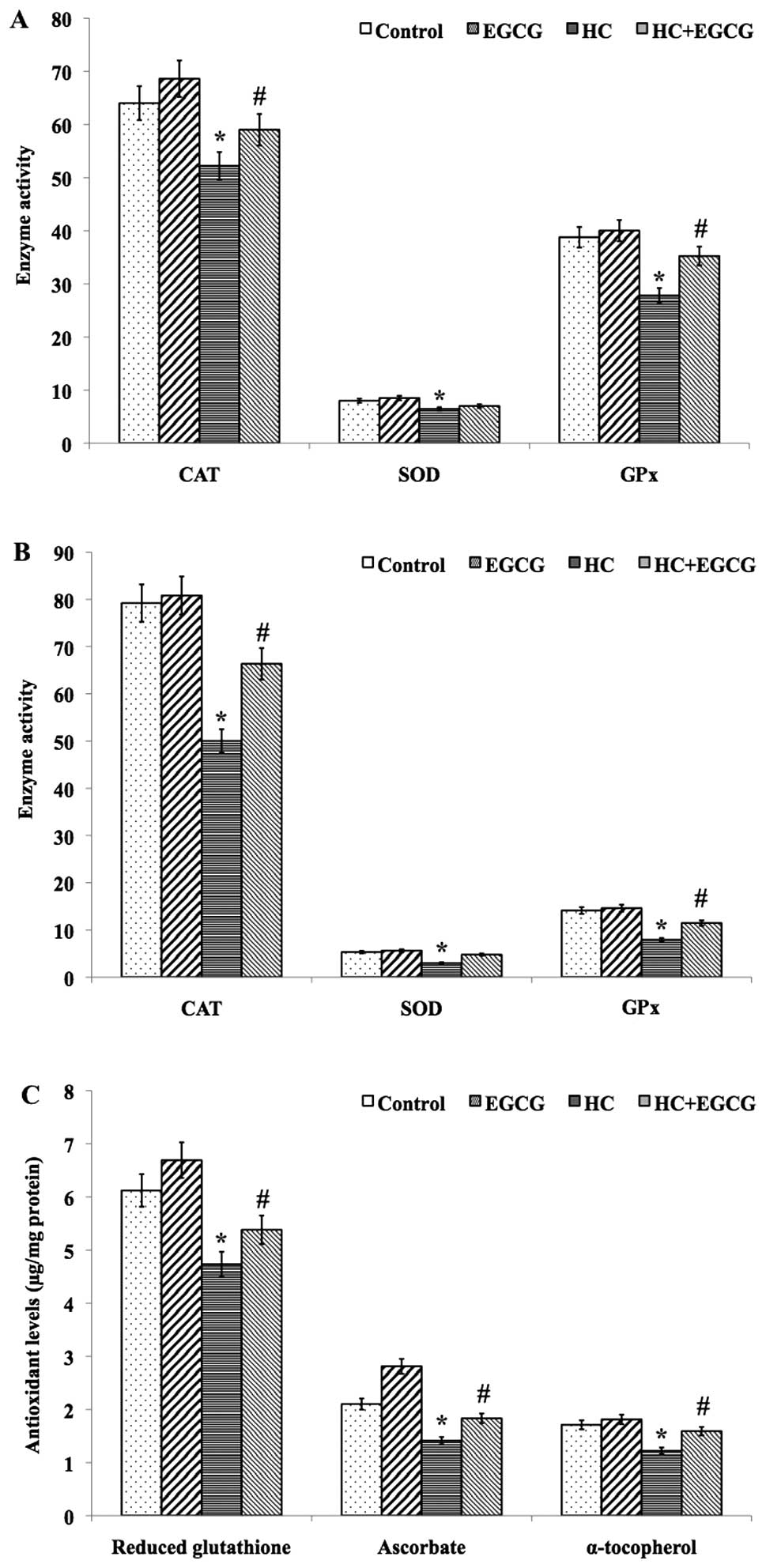
EGCG improves enzymatic antioxidant
activity and non-enzymatic antioxidants levels
A significant (P<0.01) decrease in the mean
activity of the enzymatic antioxidants CAT, SOD and GPx was
detected in cardiac tissue (Fig.
1A) and haemolysate (Fig. 1B)
samples of HC rats when compared to control rats, whereas HC+EGCG
rats exhibited improved antioxidant activities (Fig. 1A and B). Similarly, the mean levels
of non-enzymatic antioxidants GSH, ascorbate and α-tocopherol in
the cardiac tissues of HC rats showed a significant decrease when
compared with the control (P<0.001) and HC+EGCG (P<0.05)
treated groups (Fig. 1C), whereas
the mean concentration of ascorbate in the cardiac tissues of
HC+EGCG rats was significantly (P<0.05) increased to near-normal
levels. However, no significant difference was observed in the mean
levels of GSH and α-tocopherol in the cardiac tissue samples of
HC+EGCG rats (Fig. 1C).
EGCG regulates lipid metabolism
We detected the key proteins involved in lipid
metabolism using western blot analysis. Of note, HC+EGCG rats
exhibited significantly increased protein levels of SIRT1 and eNOS
and decreased p-AMPKα in cardiac tissue samples (Fig. 2); however, HC rats exhibited a
reverse effect. These results may constitute key evidence for the
elucidation of the mechanism of action of EGCG against lipid
deposition in cardiac tissues.
EGCG lowers the levels of cardiac marker
enzymes
Cardiac enzymes, such as LDH, CPK, ALP, AST and ALT,
were found to be significantly (P<0.01) elevated in HC rats
compared to the control group (Table
III). Of note, HC+EGCG rats exhibited a significant
(P<0.001) decrease in cardiac enzymes to near-normal levels
(Table III).
 | Table IIIEGCG prevents cardiac tissue
damage. |
Table III
EGCG prevents cardiac tissue
damage.
| Cardiac
markers | Control | EGCG | HC | HC+EGCG |
|---|
| LDH | 32.98±4.7 | 31.2±6.7a,c | 58.2±6.7a,c | 33.55±4.5b,c |
| CPK | 478±78.3 | 518±14 | 216±18a,c | 405±17b,c |
| ALP | 0.11±0.01 | 0.08±0.018a,c | 0.18±0.018a,c | 0.12±0.10b,c |
| ALT | 0.09±0.007 | 0.08±0.010a,c | 0.12±0.010a,c | 0.09±0.09b,c |
| AST | 0.22±0.02 | 0.20±0.04a,c | 0.34±0.04a,c | 0.24±0.03b,c |
EGCG maintains the structural integrity
of cardiac muscle
To evaluate the effect of EGCG in maintaining the
morphology of the myocardium, we performed a histopathological
examination. The HC-induced myocardial fiber disruption, edema and
neutrophil infiltration were prevented by the administration of
EGCG (Fig. 3). Of note, the
cardiac tissue of the positive control group exhibited a healthier
morphology compared to that of the control group.
Discussion
In this study, we investigated EGCG as a therapeutic
agent for myocardial infarction, with particular focus on its
effects on the antioxidant system and cholesterol metabolism. HC
diet enhances the deposition of cholesterol in the aorta and other
tissues in the form of cholesterol esters (20). Our study demonstrated that rats fed
with HC diet exhibited increased lipid levels in the serum and
cardiac tissues. The deposited cholesterol esters in the tissue
undergo hydrolysis to release free cholesterol. One of the
hydrolysing factors is HDL, since the HDL-C level was found to be
decreased in atherogenic diet-fed rats (21) and insufficient HDL level may lead
to increased free cholesterol in the plasma, enhancing
atherogenesis. Lipoproteins are the vehicle for transporting plasma
lipids to the blood. The increased levels of VLDL-C LDL-C observed
in animals fed a high-cholesterol diet may be due to decreased
LDL-receptor activity that reduces LDL catabolism (22). Yu et al (23) reported that serum TC and TG
increased significantly in rabbits receiving a high-fat and
-cholesterol diet, but decreased in rabbits receiving the same diet
supplemented with ellagic acid.
Oxidative stress is one of the causative factors
that link hypercholesterolemia with atherogenesis and myocardial
infarction. LPO is a chain event that enhances MDA production
(24). There is also an
association between LPO and hypercholesterolemia. Our results
demonstrated an enhanced LPO or MDA level, thereby inducing free
radical production in HC rats. Our results are consistent with
those of a previous study reporting that increased LPO was found in
the tissues, aorta and serum of hypercholesterolemic rabbits
(25). Another study also reported
that L-carnitine exerted potent inhibitory effects on the levels of
LPO in heart and liver tissue samples from atherosclerotic rats
(26). EGCG treatment effectively
prevented the HC-induced LPO.
Hypercholesterolemia increases the overproduction of
free radicals, increases mitochondrial respiration and lowers the
antioxidant status (27). The
antioxidant enzymes CAT, SOD and GPx are involved in free radical
scavenging, disposal of superoxide anions and hydrogen peroxide.
These activities constitute the first line of cellular defense
against oxidative injury. CAT specifically enables disposal of
H2O2 by the erythrocyte, thereby protecting
against ROS (28). Our results
demonstrated a significant decrease in the mean activities of CAT,
SOD, GPx and glutathione S-transferase in the haemolysate and
cardiac tissues of HC rats. A recent study demonstrated an
improvement in the function of the antioxidant system following
administration of lupeol and lupeol linoleate in
hypercholesterolemic rats (29).
Similarly, EGCG possibly acts by regulating the activities of these
antioxidant enzymes. Earlier studies reported that EGCG is an
effective scavenger of superoxide, hydroxyl and peroxynitrite
radicals (30,31). Similar to enzymatic antioxidants,
non-enzymatic antioxidants also protect cells from oxidative
damage. Ascorbic acid prevents the oxidative damage of the cell
membrane that is induced by aqueous radicals; it also reduces and
regenerates oxidized α-tocopherol and lipid peroxides (30). In the present study, EGCG was found
to be effective in improving the non-enzymatic antioxidant status
in HC rats.
Lipid metabolism in macrophages is an important
process in the context of hypercholesterolemia. Uptake of excessive
amounts of native and modified lipoproteins leads to their
conversion into foam cells, which accumulate to create fatty
streaks, a central characteristic of the early phase of
atherosclerotic lesion development. Of note, our present study
demonstrated that EGCG activated SIRT1 and eNOS and regulated the
phosphorylation of AMPK against the effects of the atherogenic
diet. SIRT1 exerts several effects associated with protection
against the development of cardiovascular disease. SIRT1 is an
important signaling molecule in the endothelium, improving its
function. SIRT1 binds directly to eNOS and has been shown to target
eNOS for deacetylation, thereby stimulating nitric oxide (NO)
production and promoting vascular relaxation (31). Endothelial-derived NO controls
vascular tone and exerts atheroprotective effects. AMPK is a sensor
of cellular energy status and a key controller in the regulation of
whole-body energy homeostasis (32); it plays an integral role in lipid
metabolism by switching on the oxidative process for fatty acids
and by inhibiting the synthesis of lipids (33). AMPK also aids in endothelial
relaxation and dilation.
We next investigated the tissue damage induced by HC
diet. The cardiac enzymes were measured and we observed that the HC
diet had increased the levels of cardiac markers such as LDH, ALP,
AST and ALT, due to leakage of these markers in the plasma
following tissue damage (34).
Administration of EGCG prevented the adverse effects of HC diet.
Similarly, our histopathological examination of myocardium revealed
abnormal morphology in HC rats. However these changes were
prevented in rats treated with EGCG. Our findings were consistent
with those of similar studies investigating treatment with
fluvastatin and methanol extract of Sorbus cortex (34,35).
In addition, EGCG proved to be potentially clinically useful in
preventing the onset and/or progression of atherosclerotic
cardiovascular disease. However, despite significant preclinical
evidence, data on the cardiovascular effects on humans are
currently limited (35).
In conclusion, the results of the present study
demonstrated the advantages of the administration of EGCG for the
prevention of cardiac abnormalities induced by HC diet. The
cardioprotective effect of EGCG was demonstrated by the
improvements in the the serum lipid profile, antioxidant system,
lipid metabolism and myocardial fiber morphology. These preliminary
findings support the regular consumption of EGCG-rich dietary
sources, such as green tea, grape seeds and pomegranate. However,
the molecular mechanism underlying the cardioprotective effects of
EGCG requires further investigation.
References
|
1
|
Ferrières J: The French paradox: lessons
for other countries. Heart. 90:107–111. 2004. View Article : Google Scholar
|
|
2
|
Walker AF: Of hearts and herbs. Biologist.
43:177–180. 1996.
|
|
3
|
Frankel EN, Waterhouse AL and Teissedre
PL: Principal phenolic phytochemicals in selected Californian wines
and their antioxidant activity in inhibiting oxidation of human
low-density lipoproteins. J Agric Food Chem. 43:890–894. 1995.
View Article : Google Scholar
|
|
4
|
Vinson JA, Teufel K and Wu N: Green and
black teas inhibit atherosclerosis by lipid, antioxidant, and
fibrinolytic mechanisms. J Agric Food Chem. 52:3661–3665. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aviram M and Eias K: Dietary olive oil
reduces the susceptibility of low-density lipoprotein to lipid
peroxidation and inhibits lipoprotein uptake by macrophages. Ann
Nutr Metab. 37:75–84. 1993. View Article : Google Scholar
|
|
6
|
Stangl V, Dreger H, Stangl K and Lorenz M:
Molecular targets of tea polyphenols in the cardiovascular system.
Cardiovasc Res. 73:348–358. 2007. View Article : Google Scholar
|
|
7
|
Chan K, Lu R, et al: NRF2, a member of the
NFE2 family of transcription factors, is not essential for murine
erythropoiesis, growth, and development. Proc Natl Acad Sci USA.
93:13943–13948. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Araujo JA, Barajas B, Kleinman M, et al:
Ambient particulate pollutants in the ultrafine range promote early
atherosclerosis and systemic oxidative stress. Circ Res.
102:589–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duarte MM, Rocha JB, Moresco RN, et al:
Association between ischemia-modified albumin, lipids and
inflammation biomarkers in patients with hypercholesterolemia. Clin
Biochem. 42:666–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quist EE: Regulation of erythrocyte
membrane shape by Ca2+. Biochem Biophys Res Commun.
92:631–637. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohkawa H, Ohishi N and Yagi K: Assay of
lipid peroxides in animal tissue by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha AK: Colorimetric assay of catalase.
Anal Biochem. 47:389–394. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autooxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rotruck JT, Pope AL, Ganther HE, Swanson
AB, Hafeman DG and Hoekstra WG: Selenium: biochemical role as a
component of glutathione peroxidase. Science. 179:588–590. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moron MS, Depierre JW and Mannervik B:
Levels of glutathione, glutathione reductase and glutathione
S-transferase activities in rat lung and liver. Biochim Biophys
Acta. 582:67–78. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omaye ST, Turnbull JD and Sauberlich HE:
Selected methods for the determination of ascorbic acid in animal
cells, tissues, and fluids. Methods Enzymol. 62:3–11. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Desai ID: Vitamin E analysis methods for
animal tissues. Methods Enzymol. 105:138–147. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King J: The dehydrogenases or
oxidoreductases - lactate dehydrogenase. Practical Clinical
Enzymology. Van Nostrand Company Ltd; London: pp. 83–93. 1965
|
|
20
|
Hodis HN, Crawford DW and Sevanian A:
Cholesterol feeding increases plasma and aortic tissue cholesterol
oxide levels in parallel: further evidence for the role of
cholesterol oxidation in atherosclerosis. Atherosclerosis.
89:117–126. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown MS, Ho YK and Goldstein JL: The
cholesterol ester cycle in macrophage foam cells. J Biol Chem.
225:9344–9352. 1980.
|
|
22
|
Applebaum BD, Haffner SM, Hartsook E, Luk
KH, Albers JJ and Hazzard WR: Down-regulation of the low-density
lipoprotein receptor by dietary cholesterol. Am J Clin Nutr.
39:360–367. 1984.
|
|
23
|
Yu YM, Chang WC, Wu CH and Chiang SY:
Reduction of oxidative stress and apoptosis in hyperlipidemic
rabbits by ellagic acid. J Nutr Biochem. 16:675–681. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee MK, Park YB, Moon SS, et al:
Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl)
propanoic acid derivatives in high-cholesterol fed rats. Chem Biol
Interact. 170:9–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gökkusu C, Ademoğlu E, Türkoğlu UM, Oz H
and Oz F: Thymosin alpha 1 protects liver and aorta from oxidative
damage in atherosclerotic rabbits. Life Sci. 59:1059–1067. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dayanandan A, Kumar P and Panneerselvam C:
Protective role of L-carnitine on liver and heart lipid
peroxidation in atherosclerotic rats. J Nutr Biochem. 12:254–257.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thiruchenduran M, Vijayan NA, Sawaminathan
JK and Devaraj SN: Protective effectof grape seed proanthocyanidins
against cholesterol cholic acid diet-induced hypercholesterolemia
in rats. Cardiovasc Pathol. 20:361–368. 2011. View Article : Google Scholar
|
|
28
|
Agar NS, Sadrzadeh SM, Hallaway PE and
Eaton JW: Erythrocyte catalase. A somatic oxidant defense? J Clin.
77:319–321. 1986.
|
|
29
|
Sudhahar V, Ashok Kumar S and Varalakshmi
P: Role of lupeol and lupeol linoleate on lipemic-oxidative stress
in experimental hypercholesterolemia. Life Sci. 78:1329–1335. 2006.
View Article : Google Scholar
|
|
30
|
Wang WF, Luo J, et al: Interaction of
phenolic antioxidants and hydroxyl radicals. Radiat Phys Chem.
42:985–987. 1993. View Article : Google Scholar
|
|
31
|
Stein S and Matter CM: Protective roles of
SIRT1 in atherosclerosi. Cell Cycle. 10:640–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinberg GR and Kemp BE: AMPK in health
and disease. Physiol Rev. 89:1025–1078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Misra P: AMP activated protein kinase; a
next generation target for total metabolic control. Expert Opin
Ther Targets. 12:91–100. 2008.
|
|
34
|
Mitani H, Egashira K and Kimura M: HMG-CoA
reductase inhibitor, fluvastatin, has cholesterol-lowering
independent ‘direct’ effects on atherosclerotic vessels in high
cholesterol diet-fed rabbits. Pharmacol Res. 48:417–427. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turan B, Tuncay E and Vassort G:
Resveratrol and diabetic cardiac function: focus on recent in vitro
and in vivo studies. J Bioenerg Biomembr. 44:281–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|