Introduction
Ischemia/reperfusion injury (IRI) occurs in the
myocardium and is a negative factor for the morbidity and mortality
rates of inpatients, particularly for patients undergoing cardiac
coronary surgery or elderly patients undergoing major surgery
(1). In 1986, Murry et al
(2) established the technique of
ischemia preconditioning (IPC), in which periods of IRI were
applied to confer an intrinsic protective mechanism that in turn
decreased the extent of IRI. Subsequently in 1993, Przyklenk et
al (3) found that short periods
of ischemia in a distant organ were able to induce protection to
target organs against sustained IRI. The authors referred to this
process as remote IPC (RIPC). Several studies have demonstrated
that IPC is a complicated multi-mechanism process that involves
humoral and neural pathways (4,5).
In 2004, Ren et al (6) identified a novel cardioprotective
phenomenon, where the myocardium infarct size (IS), following IRI,
was decreased by a transverse abdominal incision. The term ʻremote
preconditioning of traumaʼ (RPCT) was devised for this non-ischemic
preconditioning phenomenon. In subsequent experiments, scientists
have proposed that nociceptive stimulation of peripheral sensory
nerves leads to the activation of the cardiac sympathetic nervous
system via spinal nerves (7).
The underlying mechanisms of RIPC and RPCT are
different. However, whether RIPC or RPCT exerts a stronger
cardioprotective function is yet to be elucidated. In addition,
whether a combination of RIPC with RPCT is able to enhance the
cardioprotective function is also unclear. Thus, the present study
utilized an in vivo rat model of myocardial IRI to testify
these questions.
Materials and methods
The study design was approved by the Institutional
Animal Care and Use Committee of Sichuan University (Chengdu,
China). All the experimental procedures were undertaken at the West
China Clinical Skills Training Center and the West China School of
Medicine/West China Hospital of Sichuan University.
Animal preparation
In total, 70 male Sprague Dawley rats (age, 7 weeks;
weight, 250–300 g) were provided by Sichuan Provincial Laboratory
Animal Public Service Center (Chengdu, China). The animals were fed
the same diet and were provided with water ad libitum. The
housing environment was maintained at 23°C and 60% humidity, with a
12-h day and night cycle. The rats were randomly divided into five
groups according to a random number table generated by a computer.
The animals were anesthetized by an intraperitoneal injection of 2%
sodium pentobarbitone (50 mg/kg; Sigma-Aldrich, St. Louis, MO,
USA), and anesthesia was maintained with repeated administration of
sodium pentobarbitone (25 mg/kg) every 60 min. The body temperature
of the rats was maintained at 37°C using a heating blanket
(Shanghai Yuyan Instruments Co., Ltd., Shanghai, China). A
tracheotomy was conducted with a 14-G catheter (Terumo Corporation,
Tokyo, Japan) for controlled ventilation, comprising a respiratory
rate of 60–70 breaths/min with 40% oxygen support, 2.5–3.5 ml tidal
volume and a 1:2 inspiration/expiration ratio. A standard limb lead
II electrocardiogram (Chengdu Taimeng Software Co., Ltd., Chengdu,
China) was used to monitor the heart rate (HR) and ST-segment
changes via subcutaneous needle electrodes. Furthermore, a 20-G
catheter (Terumo Corporation, Tokyo, Japan), filled with normal
saline containing heparin, was inserted into the right carotid
artery to monitor the mean arterial blood pressure (MABP). HR and
MABP measurements were recorded using a computer-based monitoring
system (Chengdu Taimeng Software Co., Ltd).
Surgical procedure
An anterolateral thoracotomy was conducted at the
left fourth intercostal space, which was subsequently accompanied
by a pericardiotomy to expose the heart. The left anterior
descending (LAD) branch of the left coronary artery was ligated
with a 6/0 silk suture (Shanghai Pudong Jinhuan Medical Products
Co., Ltd., Shanghai, China) at the midpoint between the base and
apex. Changes in the ST segment, emerging arrhythmia for a short
duration, regional color change at the distal ligation site and
reduced MABP were useful indicators for confirming the successful
establishment of myocardial ischemia. Rats were subjected to 30 min
regional ischemia followed by 180 min reperfusion to establish the
myocardium IRI model. Rats with hypotension (MABP of <60 mmHg)
that did not return to normal, or hemoglobin levels of <50 g/l
at the end of the experiment were excluded from the study.
Experimental protocols
Animals were divided into five groups (Fig. 1). The sham group underwent a
thoracotomy only, while the control group were subjected to
myocardial IRI only. The RIPC + RPCT treatment group received an
abdomen incision, followed by three cycles of 5 min bilateral
femoral artery occlusion and reperfusion, after which the incision
was sutured prior to the conduction of myocardial IRI. The RPCT
group underwent an abdomen incision, which was sutured 30 min later
prior to subjection to myocardial IRI. Finally, the RIPC group
received three cycles of 5 min bilateral femoral artery occlusion
and reperfusion, without abdomen incision, prior to the induction
of myocardial IRI.
Enzyme-linked immunosorbent assays
(ELISA)
Blood samples were collected at the end of the
experiment. Serum levels of cardiac troponin I (cTnI) were analyzed
using an ELISA kit (USCN Life Science, Inc., Wuhan, China), and the
data were measured on a microplate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA).
Cardiac IS determination
At the endpoint of reperfusion, the LAD artery was
ligated once more in order to identify the area at risk (AAR).
Evan's blue dye (2%, 2 ml; Sigma-Aldrich) was injected through the
right carotid artery. The rats were died from acute blood loss
caused by the injection and their hearts were obtained and frozen.
The heart samples were cut into 1-mm transverse sections from the
apex to the occlusion site, and the slices were immersed in 1%
2,3,5-triphenyltertrazolium chloride (Sigma-Aldrich) phosphate
buffer for 30 min (the buffer was adjusted to pH 7.4 at 37°C).
Next, the specimens were fixed with 4% paraformaldehyde for 24 h to
enhance the contrast of the stain. Every stained slice was scanned
into a computer (Shanghai Microtek Trade Co., Ltd., Shanghai,
China), and the infarct area and AAR were calculated using Image J
1.44p software (National Institutes of Health, Bethseda, MD, USA).
The IS was expressed as a percentage of the infarct area over the
AAR.
Apoptotic index (AI) measurements
A terminal deoxynucleotidyl transferase-mediated
dUTP nick-end labeling assay (TUNEL; Promega Corporation, Madison,
WI, USA) was used for the identification of apoptotic cells, while
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) staining was
applied to identify the normal cells. All the staining experimental
protocols were performed by a pathological technician who was
blinded to the experiment. Photographs were captured with a
fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany). A minimum of five fields of view under the microscope
were randomly selected for every slice. The number of normal and
apoptotic cells were analyzed by Image J 1.44p software, and the AI
was expressed as a percentage of the number of TUNEL-positive
nuclei over the total number of nuclei.
Statistical analysis
All data are expressed as the mean ± standard
deviation and were analyzed using one-way analysis of variance.
Statistical analysis was performed using SPSS 13.0 software for
Windows (SPSS, Inc., Chicago, IL, USA), where P<0.05 was
considered to indicate a statistically significant difference.
Results
HR and MABP measurements
Baseline HR and MABP values were not significantly
different among the five groups. Furthermore, the differences were
not statistically significant among the experimental groups at any
time point during the experiments (Tables I and II).
 | Table I.Mean arterial blood pressure (mmHg) of
the rats. |
Table I.
Mean arterial blood pressure (mmHg) of
the rats.
|
|
|
|
| Reperfusion
(min) |
|---|
|
|
|
|
|
|
|---|
| Group | Baseline | Washout | Ischemia | 60 | 120 | 180 |
|---|
| Sham | 126±15.7 | 126±15.8 | 121±17.6 | 125±14.2 | 119±16.9 | 120±24.3 |
| Control | 126±14.5 | 116±12.1 | 113±18.0 | 114±13.2 | 104±16.8 | 99±17.6 |
| RIPC + RPCT | 134±12.1 | 133±14.9 | 115±16.2 | 114±11.9 | 106±9.8 | 105±10.8 |
| RPCT | 136±11.8 | 123±11.7 | 123±11.7 | 114±11.1 | 107±14.8 | 101±19.1 |
| RIPC | 136±17.5 | 124±18.7 | 112±20.6 | 115±14.4 | 113±20.6 | 110±12.7 |
 | Table II.Heart rate (bpm) of the rats. |
Table II.
Heart rate (bpm) of the rats.
|
|
|
|
| Reperfusion
(min) |
|---|
|
|
|
|
|
|
|---|
| Group | Baseline | Washout | Ischemia | 60 | 120 | 180 |
|---|
| Sham | 314±12.6 | 315±11.4 | 345±13.6 | 325±10.5 | 375±10.2 | 365±16.1 |
| Control | 325±15.2 | 328±14.2 | 478±21.4 | 465±13.4 | 475±13.8 | 465±14.7 |
| RIPC + RPCT | 355±16.1 | 375±8.9 | 425±14.2 | 375±11.8 | 396±12.1 | 425±12.4 |
| RPCT | 333±14.7 | 356±11.4 | 455±16.7 | 396±12.5 | 424±15.4 | 436±16.8 |
| RIPC | 328±13.3 | 395±10.8 | 475±18.3 | 425±14.6 | 465±13.7 | 453±15.3 |
Serum cTnI levels are lower in the
experimental groups
Serum levels of cTnI can be used as an indicator of
myocardial damage. The three experimental groups exhibited
significantly lower serum cTnI levels when compared with the
control group at the end of the experiment (control, 58.59±12.50
pg/ml; RIPC + RPCT, 46.05±8.62 pg/ml; RPCT, 45.98±11.24 pg/ml;
RIPC, 43.46±5.05 pg/ml; P<0.05; Fig.
2). However, no statistically significant difference was
observed in the serum cTnI level among the three experimental
groups.
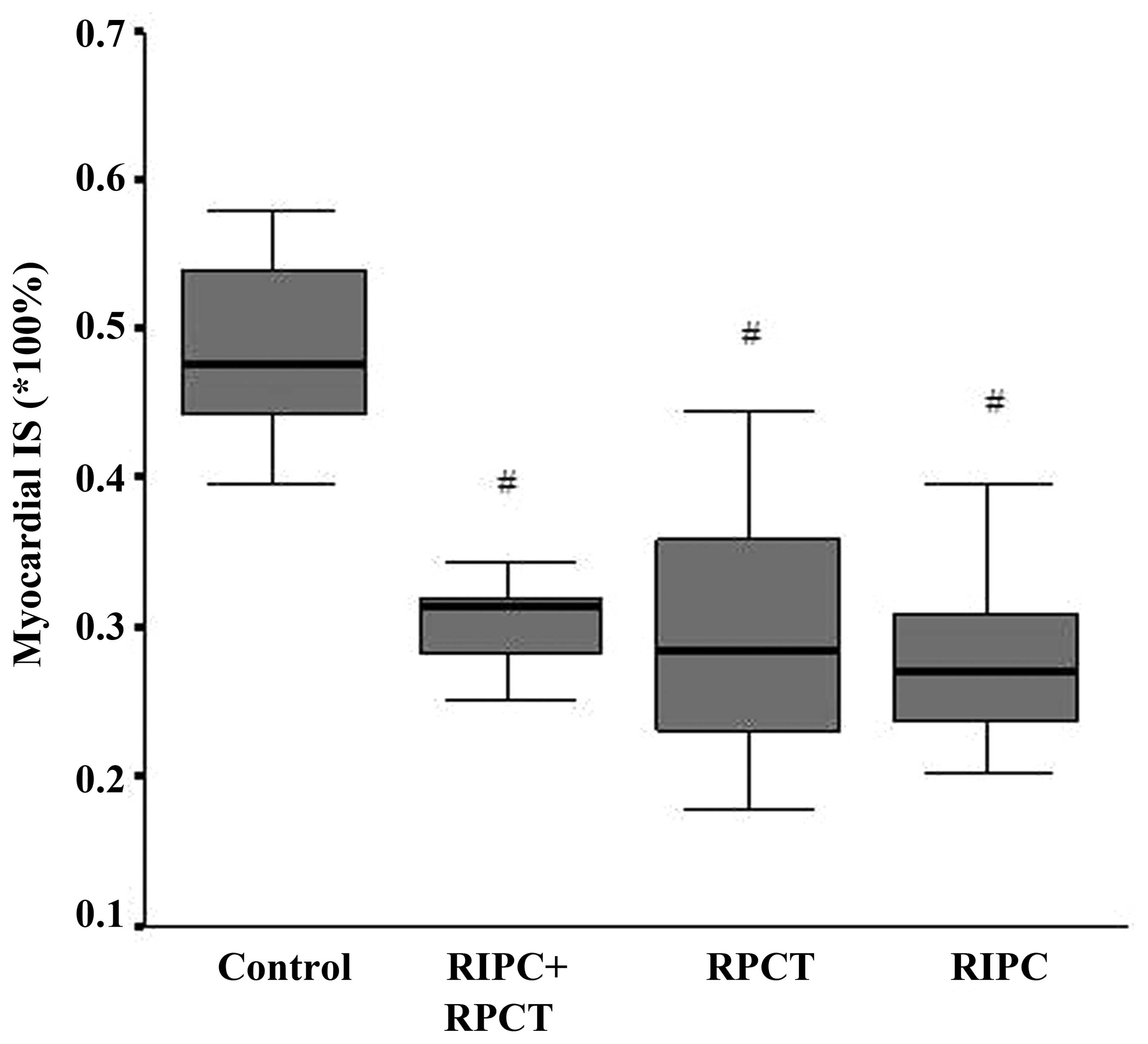
Preconditioning reduces the myocardial
IS
Myocardial IS was expressed as a percentage of the
infarct area out of the AAR. In the control group, the IS was
48.34±6.79%. By contrast, the IS was 29.64±4.51% in the RIPC + RPCT
group, 29.05±8.51% in the RPCT group and 27.72±6.27% in the RIPC
group. Statistically significant differences were observed when
comparing the control group with the experimental groups.
(P<0.001; Fig. 3). However, there
were no statistically significant differences when comparing the
RIPC + RPCT group with the RIPC and RPCT groups.
Fewer apoptotic cardiomyocytes are
observed in the experimental groups
The AI was expressed as a percentage of the
TUNEL-positive apoptotic nuclei out of the total number of nuclei.
The AI in the control group was determined to be 31.75±10.65%, and
statistically significant differences were observed when compared
with the RIPC + RPCT group (18.32±9.30%; P=0.016), the RPCT group
(18.51±9.26%; P=0.017) and the RIPC group (20.41±3.86%; P=0.038).
However, no statistically significant differences were identified
among the three experimental groups (Figs. 4 and 5).
Discussion
The results of the present study indicated that RIPC
and RPCT exert cardioprotective effects whether used alone or in
combination. However, a combination of RIPC with RPCT did not
exhibit a stronger cardioprotective effect when compared with
either technique applied alone.
A previous study demonstrated that the mechanism
underlying RIPC may be mediated by humoral mediators, neurogenic
pathways or a combination of the two (4). Following RIPC, the organ releases
humoral mediators that are transported to the target organ. These
mediators subsequently induce additional humoral mediators at the
target organ following transportation, which activate a local
neurogenic pathway in the target organ. The activation of afferent
nerves in the RIPC organ triggers the release of mediators in the
target organ. The RIPC stimulus may also activate a neurogenic
pathway in the target organ. A number of mediators may be involved
in this mechanism, including adenosine, bradykinin, heat shock
proteins, tumor necrosis factor-α, nitric oxide and opioids
(8–10). However, the mechanism underlying RPCT
may depend on the neurogenic pathway. Jones et al (7) proposed that nociceptive stimulation of
the peripheral sensory nerve activates cardiac sensory nerves via
the spinal nerves at the T9–10 vertebral level, causing cardiac
sympathetic nerves to release norepinephrine and bradykinin, which
underlies the cardioprotective function following RPCT. Thus, the
mechanisms of RIPC and RPCT differ. Nevertheless, the signaling
pathway underlying the cardioprotection of RIPC and RPCT at the
heart appears to be similar, with involvement of protein kinase C,
mitrogen-activated protein kinases and mitochondrial potassium ATP,
amongst others (7,8,11–13).
Therefore, whichever method used to transduce the stimulation of
preconditioning to the heart, the mechanism appears to be
functioning through the same signaling transduction pathways. Thus,
it was hypothesized that this may be the reason as to why RIPC and
RPCT, or their combination, did not manifest different
cardioprotective effects in the present study.
Currently, an increasing number of elderly
individuals with cardiovascular complications are undergoing major
surgeries. Perioperative myocardial infarction is one of the main
causes of morbidity and mortality in these patients. Therefore,
improving the tolerance of the heart to IRI is important for
improving the clinical outcome.
Based on the results of the present study, it was
hypothesized that for patients undergoing major abdominal
surgeries, RIPC is unnecessary since the individuals already
undergo RPCT via the abdominal incision at the beginning of the
surgery. By contrast, for patients undergoing other forms of
non-cardiac major surgery, RPCT is unable to be conducted; thus,
RIPC may be useful. However, for patients undergoing cardiac
surgery, previous studies performed on human subjects have yielded
controversial conclusions. In the study by Karuppasamy et al
(14), 54 patients underwent
elective coronary artery bypass grafting surgery. The levels of
cTnI were analyzed following surgery; however, there were no
differences between the RIPC group and the control group. Perrault
et al (15) concluded that a
number of cardioprotective methods, including hypothermia,
cardiopulmonary bypass, cold cardioplegia and intermittent
cross-clamp fibrillation, are used routinely in human cardiac
surgery, which is in contrast to animal surgery. The combined use
of these protective methods renders RIPC negligible. Although RIPC
appears to be unnecessary for open chest cardiac surgery, the
technique may be useful for types of minimally invasive
interventional surgery, such as percutaneous transluminal coronary
angioplasty.
By contrast, RIPC may be an effective treatment
strategy for a number of other diseases. Hoda et al
(16) conducted a study to
investigate the therapeutic potential of RIPC in a murine embolic
stroke model. The authors found that RIPC was effective alone, but
also had additive effects in combination with the administration of
intravenous tissue-type plasminogen activator following middle
cerebral artery occlusion in mice. The RIPC therapeutic method was
demonstrated to improve the cerebral blood flow and neurological
outcomes, while reducing the IS. According to the results of the
present study, RIPC may also be effective for use in other
thrombolytic therapies for embolism diseases.
In conclusion, RIPC and RPCT exhibit
cardioprotective effects; however, a combination of the two methods
does not exert a stronger cardioprotective effect compared with the
application of one method alone. Therefore, the results indicate
that for patients undergoing major abdominal surgery, RIPC is
unnecessary. However, for patient undergoing other types of
non-cardiac major surgery and minimally invasive interventional
surgery, RIPC may be useful. In addition, RIPC may be considered as
an effective procedure for the thrombolytic therapy of embolism
diseases.
Acknowledgements
The authors thank Xiaying Peng, Yanfang Chen and
Nanfu Luo for their assistance in the study. In addition, the
authors thank Chen Fei and Li Li for their assistance in the
pathological procedure.
References
|
1
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic ‘preconditioning’ protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SY, Yellon DM and Hausenloy DJ: The
neural and humoral pathways in remote limb ischemic
preconditioning. Basic Res Cardiol. 105:651–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kingma JG Jr, Simard D, Voisine P and
Rouleau JR: Role of the autonomic nervous system in
cardioprotection by remote preconditioning in
isoflurane-anaesthetized dogs. Cardiovasc Res. 89:384–391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren X, Wang Y and Jones WK: TNF-alpha is
required for late ischemic preconditioning but not for remote
preconditioning of trauma. J Surg Res. 121:120–129. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones WK, Fan GC, Liao S, et al:
Peripheral nociception associated with surgical incision elicits
remote nonischemic cardioprotection via neurogenic activation of
protein kinase C signaling. Circulation. 120:(Suppl 11). S1–S9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G
and Bolli R: Endothelial nitric oxide synthase plays an obligatory
role in the late phase of ischemic preconditioning by activating
the protein kinase C epsilon p44/42 mitogen-activated protein
kinase pSer-signal transducers and activators of transcription1/3
pathway. Circulation. 116:535–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong GT, Ling Ling J and Irwin MG:
Activation of central opioid receptors induces cardioprotection
against ischemia-reperfusion injury. Anesth Analg. 111:24–28.
2010.PubMed/NCBI
|
|
10
|
Kanoria S, Jalan R, Seifalian AM, Williams
R and Davidson BR: Protocols and mechanisms for remote ischemic
preconditioning: a novel method for reducing ischemia reperfusion
injury. Transplantation. 84:445–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sadat U: Signaling pathways of
cardioprotective ischemic preconditioning. Int J Surg. 7:490–498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das B and Sarkar C: Is preconditioning by
oxytocin administration mediated by iNOS and/or mitochondrial
K(ATP) channel activation in the in vivo anesthetized rabbit heart?
Life Sci. 90:763–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross GJ, Baker JE, Moore J, Falck JR and
Nithipatikom K: Abdominal surgical incision induces remote
preconditioning of trauma (RPCT) via activation of bradykinin
receptors (BK2R) and the cytochrome P450 epoxygenase pathway in
canine hearts. Cardiovasc Drugs Ther. 25:517–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karuppasamy P, Chaubey S, Dew T, et al:
Remote intermittent ischemia before coronary artery bypass graft
surgery: a strategy to reduce injury and inflammation? Basic Res
Cardiol. 106:511–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perrault LP, Menasché P, Bel A, et al:
Ischemic preconditioning in cardiac surgery: a word of caution. J
Thorac Cardiovasc Surg. 112:1378–1386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoda MN, Siddiqui S, Herberg S, et al:
Remote ischemic perconditioning is effective alone and in
combination with intravenous tissue-type plasminogen activator in
murine model of embolic stroke. Stroke. 43:2794–2799. 2012.
View Article : Google Scholar : PubMed/NCBI
|