Introduction
Fibrosis is one of major complications in Crohn’s
disease (CD), which results from chronic inflammation. Severe
fibrosis could result in critical narrowing of the lumen stenosis,
or stricture, commonly leading to obstruction that requires
surgical or mechanical intervention (1–3).
At present, there are no approved or effective medical therapies
aimed specifically at fibrosis or stricture in CD.
Anti-inflammatory therapies do not prevent fibrosis nor do they
reverse established strictures, which may present years after
remission of active inflammation. Although this study is still
limited, it can expand our understanding of cellular and molecular
mechanisms leading to fibrosis in CD.
microRNAs (miRNAs) are noncoding RNAs that
negatively regulate target genes expression at the
post-transcriptional level. There is growing evidence to suggest
that members of miR-200 family are implicated in diverse biological
and pathological processes. Recent ground-breaking studies have
established functional associations between miR-200 and key
effectors of the epithelial-to-mesenchymal-transition (EMT)
occurring in the context of carcinogenesis and embryonic
development. Gregory et al (4) reported that miR-200 and miR-205
could inhibit the zinc finger E-box-binding homeobox 1 (ZEB1) and
ZEB2, ZEB1 (TCF8/EF1) and ZEB2 [SMAD-interacting protein 1
(SIP1)/ZFXH1B] and thus maintain the epithelial cell phenotype
(4). Oba et al (5) indicated that a miR-200b precursor
could ameliorate renal tubulointerstitial fibrosis. The purpose of
this study was to investigate the role of miR-200a and miR-200b in
the pathogenesis of intestinal fibrosis. Initial experiments
demonstrated that introduction of miR-200b in intestinal epithelial
cells could partially oppose fibrosis, which was induced by
transforming growth factor β1 (TGF-β1). Subsequent data showed the
aberrant expression of miR-200b in serum is a potential diagnostic
marker for CD patients with fibrosis complications.
Materials and methods
TGF-β1-induced fibrosis in vitro
DLD-1 was a colorectal adenocarcinoma epithelial
cell line, which was cultured in DMEM (Gibco) containing 10% fetal
bovine serum (FBS). DLD-1 cells were stimulated with 10 ng/ml
(TGF-β1, Sigma) for 24, 48 and 72 h. The total-RNA and protein were
harvested at the above-indicated times. To investigate the effect
of miRNAs on fibrosis, DLD-1 cells were transfected with 50 pM
miR-200a or miR-200b for 24 h using Lipofectamine RNAiMAX
(Invitrogen), then stimulated with 10 ng/ml TGF-β1. After 24 h, the
cells were harvested by extracting total-RNA and protein.
miRNA assays
Total-RNA was extracted from serum, tissues of
patients and DLD-1 cells by using the mirVana PARIS and miRVana
miRNA isolation kit (Ambion). TaqMan miRNA assay (Applied
Biosystems) was used to quantify the relative expression level of
miR-200a (assay ID. 000502), miR-200b (assay ID. 002251), and U6
(assay ID. 001093) was used as an internal control. cDNA was
synthesized using the TaqMan miRNA Reverse Transcription kit
(Applied Biosystems). The reaction was performed for 30 min at
16°C, 30 min at 42°C, and 5 min at 85°C. The
LightCycler® 480 Real-Time PCR System (Roche) was used
to detect miRNA expression. All reactions were run in
triplicate.
Real-time PCR
The total-RNA was extracted from DLD-1 cells with
TRIzol (Invitrogen) according to the protocol of manufacture.
Real-time PCR was performed to measure the expression of vimentin,
fibronectin, E-cadherin, fibronectin. Real-time PCR was performed
with the following PCR primers: GAPDH, forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′;
CDH1, forward, 5′-GGAGGAGAGCGGTGGTCAAA-3′ and reverse,
5′-TGTG CAGCTGGCTCAAGTCAA-3′; vimentin, forward, 5′-CCTC
CTACCGCAGGATGTT-3′ and reverse, 5′-CTGCCCAGG CTGTAGGTG-3′;
α-SMA, forward, 5′-CCGACCGAATGCA GAAGGA-3′ and reverse,
5′-ACAGAGTATTTGCGCTCCG AA-3′; fibronectin, forward,
5′AGACCATACCTGCCGAATG TAG-3′ and reverse,
5′-GAGAGCTTCCTGTCCTGTAGAG-3′; SYBR-Green Universal Master Mix kit
(ABI) and High Capacity cDNA Reverse Transcription kit (ABI) were
employed to detect the levels of these genes. All reactions were
repeated four times and GAPDH was used to normalize the target
genes.
Western blotting
Protein of 20 μg/well was separated on 4–12%
SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes (Invitrogen) using a dry blotting system (Invitrogen).
After blocking in 1X TBST, 5% nonfat dry milk, 0.2% Tween-20 at
room temperature for 30 min. The membranes were incubated with the
primary antibodies in blocking buffer (1X TBST, 3% nonfat dry milk,
0.2% Tween-20) overnight at 4°C. Antibodies were used at a dilution
of 1:1,000. The membranes were washed three times for 30 min with
1X TBST and then incubated with secondary antibodies at a final
dilution of 1:2,000. After final washes with 1X TBS, 0.2% Tween-20,
the signals were detected using ECL chemiluminescence reagents
(Pierce). Antibodies of N-cadherin, E-cadherin, vimentin and actin
α2 smooth muscle (α-SMA) were used in this assay. To confirm that
the same amount of protein was investigated, the expression of
GAPDH was investigated simultaneously.
Specimen preparation
A total of 20 tissue specimens from CD patients (10
fibrosis samples and 10 no-fibrosis samples) were obtained from the
Department of Pathology, Xinhua Hospital, Shanghai, China. The
blood samples were donated from the above patients (Table I). All of the patients or their
guardians provided written informed consent, and Faculty of
Medicine’s Ethics Committee of Xinhua Hospital approved all aspects
of this study.
 | Table ICharacteristics of Crohn’s disease
patients. |
Table I
Characteristics of Crohn’s disease
patients.
| Age | Gender | Diagnosis |
Tissue-staining | N-cadherin
staining | Vimentin
staining | α-SMA |
|---|
| Fibrosis | 31 | M | CD |
Intestine/serum | +++ | ++ | ++ |
| 35 | M | CD |
Intestine/serum | ++ | ++ | ++ |
| 47 | F | CD |
Intestine/serum | + | + | + |
| 73 | F | CD | Intestine,
colon/serum | ++ | ++ | ++ |
| 41 | F | CD | Intestine,
colon/serum | +++ | + | + |
| 39 | F | CD |
Intestine/serum | + | ++ | ++ |
| 54 | F | CD | Intestine,
sigmoid/serum | + | + | + |
| 74 | M | CD |
Intestine/serum | +++ | ++ | ++ |
| 54 | M | CD | Intestine,
sigmoid/serum | ++ | + | + |
| 68 | F | CD | Intestine,
sigmoid/serum | ++ | + | + |
| No-fibrosis | 37 | M | CD | Colon ascendens,
intestine/serum | − | + | − |
| 49 | M | CD |
Intestine/serum | + | − | − |
| 17 | M | CD | Intestine,
colon/serum | − | − | − |
| 24 | M | CD |
Intestine/serum | − | − | − |
| 27 | M | CD |
Intestine/serum | + | − | − |
| 29 | F | CD |
Intestine/serum | − | − | − |
| 16 | F | CD |
Intestine/serum | − | − | − |
| 22 | M | CD |
Intestine/serum | − | − | − |
| 27 | M | CD | Intestine,
colon/serum | − | − | − |
| 24 | M | CD |
Intestine/serum | − | − | − |
Immunohistochemistry
Immunohistochemical studies for the expression of
N-cadherin, E-cadherin, vimentin, and α-SMA were performed on 20 CD
samples by using an avidin-biotin peroxidase method with
diaminobenzidine (DAB) chromogen. After antigen retrieval
(microwave treatment of formalin-fixed, paraffin-embedded, 40 min
at 240 W in citrate buffer, pH 6.0), the tissues were blocked with
bovine serum albumin (BSA). As primary antibodies, mouse monoclonal
and rabbit polyclonal antibodies (N-cadherin, Novus, dilution
1:200; E-cadherin, Cell Signal Technology, dilution 1:200;
vimentin, Cell Signal Technology, 1:100; α-SMA, Novus, dilution
1:200) were used in this study. After incubation for 30 min at 37°C
the slides were rinsed in phosphate-buffered saline (PBS) and
incubated with the secondary antibody for 2 h at room temperature.
Antibody binding was visualized with 0.05% DAB and 0.01% hydrogen
peroxide. The material was rinsed in PBS and counterstained with
hematoxylin.
Statistical analysis
All data are presented as mean ± SD. When
comparisons were made between two different groups, statistical
significance was determined by the Student’s t-test using the
StatView software program.
Results
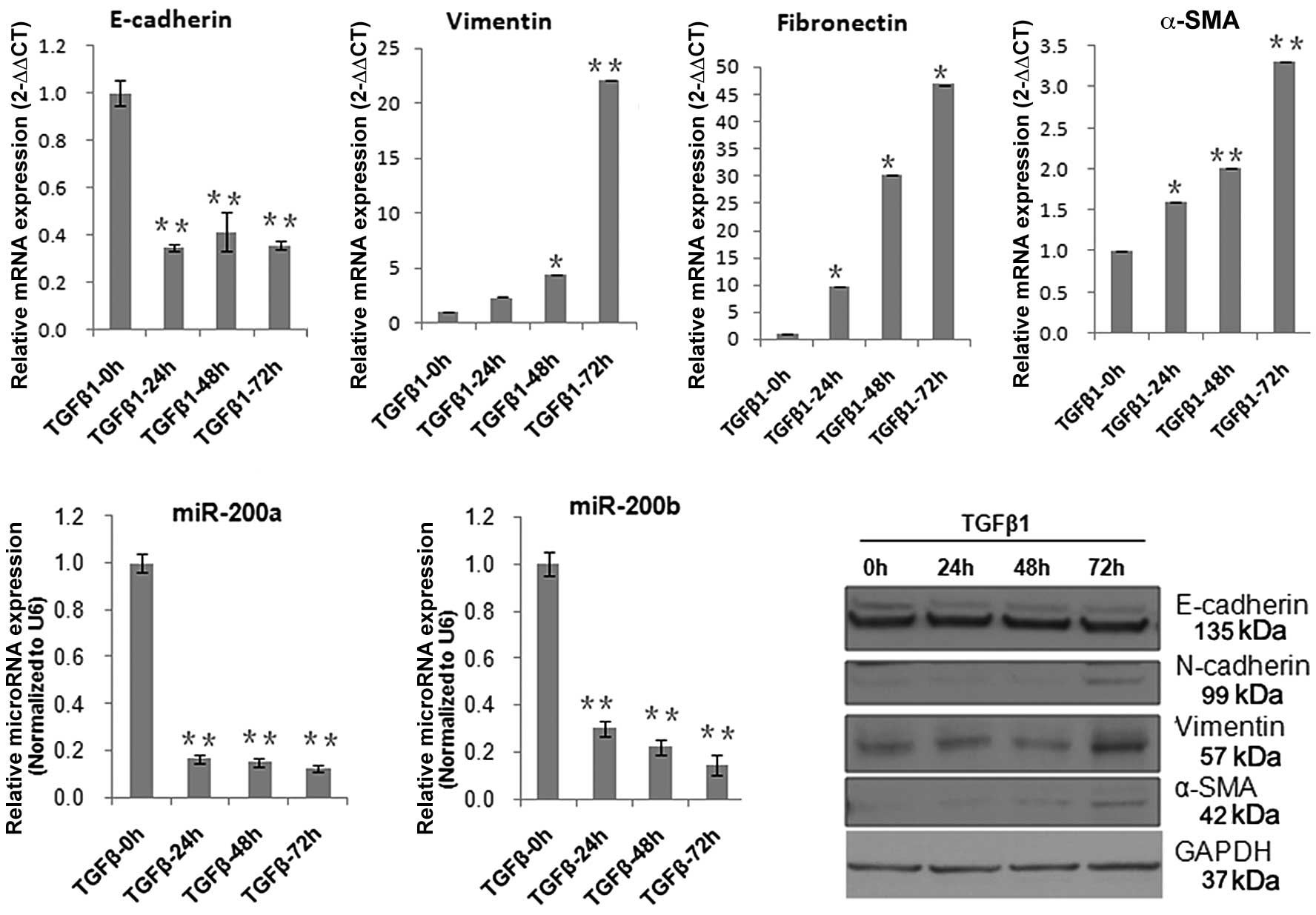
Established TGF-β1-induces fibrosis in
vitro
It is well accepted that TGF-β is a major inducer of
fibrosis and has different isoforms. High levels of TGF-β1 have
often been found in many fibrotic tissues, and have been implicated
as a mediator of fibrosis in many diseases. Furthermore, TGF-β1 was
firstly identified as an inducer of EMT in normal mammary
epithelial cells and has since been shown to mediate EMT in a
number of different epithelial cells, including renal proximal
tubular and lens cells (6–9).
Here, in order to clarify the effect of miR-200a and miR-200b on
intestinal fibrosis, we initially established a TGF-β1-induced
fibrotic model in vitro in a colorectal epithelial cell line
(DLD-1). In this model, the DLD-1 cells were stimulated with TGF-β1
(10 ng/ml) for 24, 48 and 72 h. Real-time PCR and western blot
analysis demonstrated that TGF-β1 mediated repression of
E-cadherin, and induction of N-cadherin, α-SMA, fibronectin, and
vimentin. E-cadherin was known to be involved in homophilic
interactions between epithelial cells and was necessary for the
formation of zonulae occludens. In the normal intestinal epithelial
cells, E-cadherin staining was strong at the lateral cell membrane
between cell contacting sites (10). However, E-cadherin expression was
lost or was significantly reduced during the process of EMT.
N-cadherin, which is usually expressed in mesenchymal cells and
fibroblasts, has often been used to monitor the progress of EMT and
fibrogenesis (11,12). Vimentin is a type III intermediate
filament protein that is expressed in mesenchymal cells and
fibroblasts (13,14). During the development of
intestinal fibrosis in responding to chronic pressure or volume
overload, fibroblasts could become activated to become
myofibroblasts, which strongly express α-SMA and secrete abundant,
disorganized collagen (15–17). The above data indicate that TGF-β1
not only induced fibrosis, but also the EMT process. At the same
time, we found that the expression of miR-200a and miR-200b were
significantly inhibited by TGF-β1 (Fig. 1).
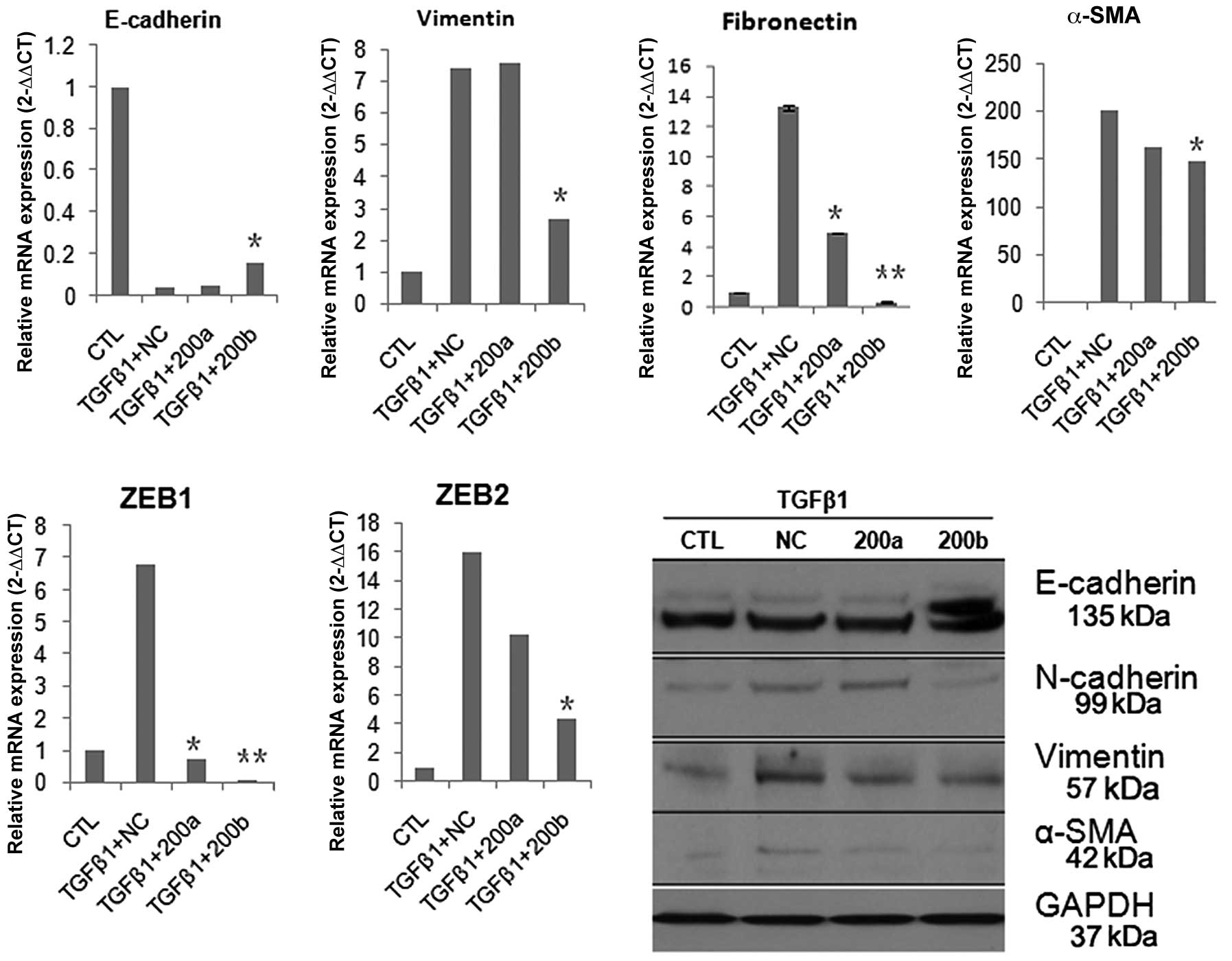
miR-200b ameliorates TGF-β1-induced
fibrosis
We next investigated the role of miR-200a and
miR-200b in intestinal fibrogenesis in vitro, with TGF-β1
stimulated DLD-1 cells for 24 h prior to introduction of miR-200a
or miR-200b. miR-200b, but not miR-200a, increased the expression
of E-cadherin and simultaneously reduced the expression of
vimentin, fibronectin and N-cadherin (Fig. 2). It was thus suggested that
miR-200b could ameliorate the fibrosis or EMT of intestinal
epithelial cells in vitro. Recent studies have indicated
that the transcriptional repressors ZEB1 (TCF8/EF1) and ZEB2
[SMAD-interacting protein 1 (SIP1)/ZFXH1B] are two direct targets
of members of the miR-200 family (4). Here, real time PCR analysis showed
that ZEB1 and ZEB2 were significantly inhibited by miR-200a or
miR-200b (Fig. 2). These results
support the notion that miR-200b could ameliorate intestinal
fibrosis through downregulation of ZEB1, ZEB2.
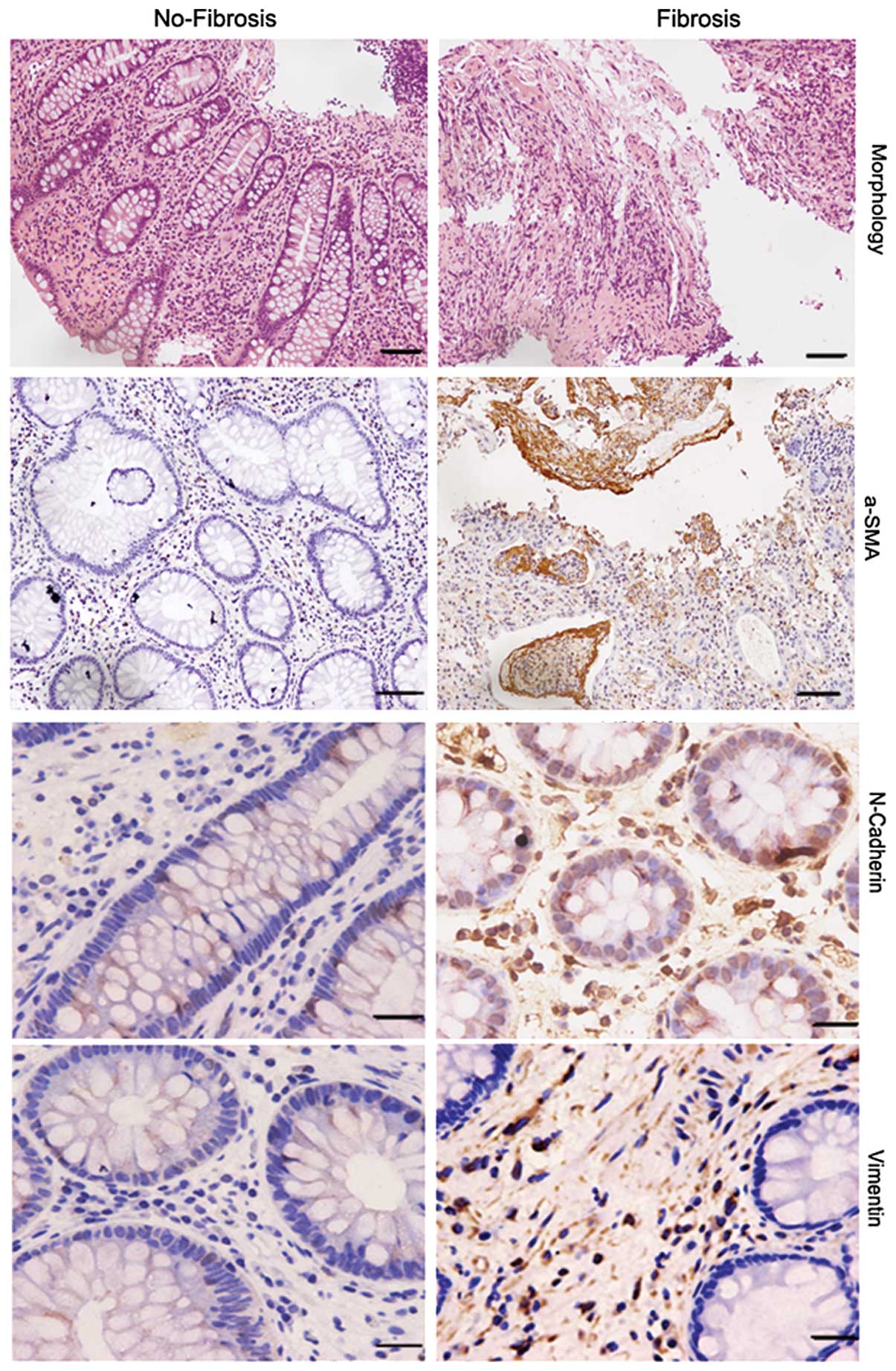
miR-200 is related to fibrogenesis of
CD
Intestinal fibrosis is a major complication of CD,
but the precise mechanism is only partially understood. As a
result, the exploration of specific therapies to halt fibrosis have
been hindered. Here, we evaluated the possible contribution of the
epithelial to mesenchymal transition (EMT) to intestinal fibrosis
associated with CD. We detected the expression of specific markers
of EMT and fibrosis in twenty CD specimens (10 fibrosis, and 10
no-fibrosis) by immunohistochemistry. Compared to the no-fibrosis
specimens, we found that staining of N-cadherin, α-SMA and vimentin
was very strong in fibrosis specimens (Fig. 3, Table I). These results prompted us to
assess whether miR-200a or miR-200b are related to fibrogenesis of
CD.
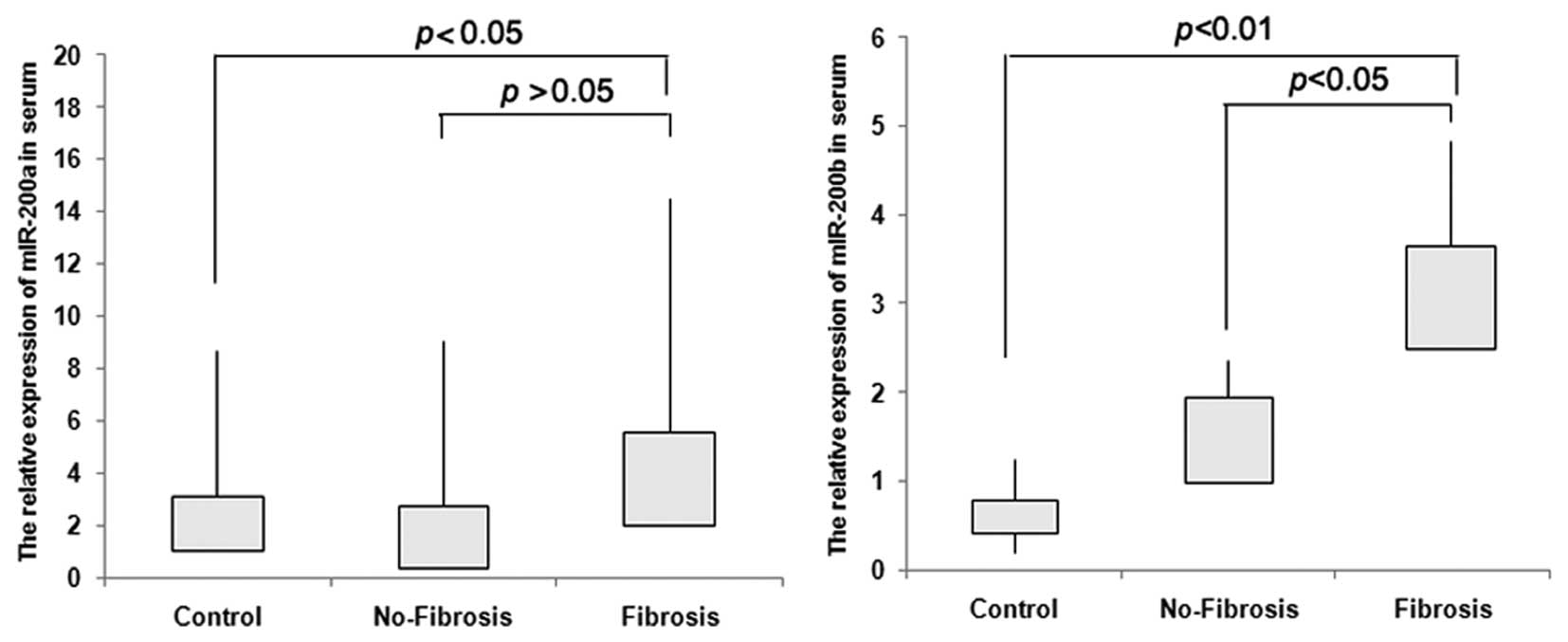
Serum miR-200b is a potential diagnostic
marker for CD with fibrosis complications
In previous studies, Murakami et al (18) showed that overexpression of
miR-200b could be connected to the progression of liver fibrosis.
In order to investigate whether miR-200a or miR-200b could serve as
diagnostic markers for CD fibrosis, we evaluated their expression
in CD serum. We collected 10 fibrosis and 10 no-fibrosis blood
samples and calculated the expression of miR-200a and miR-200b by
TaqMan real-time PCR. Sixteen healthy blood samples were used as
negative control. The results indicate that miR-200b increased
significantly in serum of the fibrosis group when compared to that
of the no-fibrosis group or from healthy individuals, (P<0.05,
P<0.01). For miR-200a, we could not find a significant
difference between the fibrosis and no-fibrosis groups (P>0.05)
(Fig. 4).
Discussion
Intestinal fibrosis is a common complication of CD
that may require surgical intervention if it became seriously
symptomatic. The traditional mechanisms underlying intestinal
fibrosis are associated to the presence of chronic inflammation.
However, it is also possible that novel mechanisms independent of
persistent immune activation exist in the gut (18,19). At present, the development of a
preventive and more effective management of intestinal fibrosis is
hampered by the lack of a in-depth understanding of the basic
pathophysiological factors involved in it. To provide novel
mechanisms for intestinal fibrosis, we revealed the association
between miR-200 and intestinal fibrosis. Initially, we analyzed the
role of miR-200a and miR-200b in the intestinal fibrosis in
vitro. The findings indicate that miR-200b could ameliorate
intestinal fibrosis that was induced by TGF-β1. Several studies
have shown that the members of the miR-200 family could target ZEB1
and ZEB2 (4) directly. Here, we
indicated that ZEB1 and ZEB2 decreased significantly in the
presence of miR-200a or miR-200b. It was suggested that miR-200b
inhibited the process of intestinal fibrosis through repressing
ZEB1 and ZEB2. However, more studies are needed to investigate the
exact mechanisms involved (Figs.
1 and 2).
To provide further evidence for intestinal fibrosis,
we revealed the association between miR-200 expression and CD
fibrosis. We divided CD samples into two groups: fibrosis and
no-fibrosis. Then, we evaluated the expression of miR-200a and
miR-200b in the serum of CD samples. The results showed that
miR-200b was significantly elevated in the group of CD fibrosis
compared to the no-fibrosis group or the healthy controls, but
surprisingly, miR-200a did not increase (Fig. 4). miRNAs are usually described as
highly tissue-specific biomarkers with potential clinical
applicability for defining the cancer origin of metastases. Recent
studies suggested that circulating miRNA has been a valuable
biomarker for several diseases, including different types of cancer
(20–39). In this study, it was shown that
expression of miR-200b increased significantly in the serum of the
CD fibrosis group. This led us to hypothesize that serum miR-200b
could be a candidate CD fibrosis diagnostic biomarker.
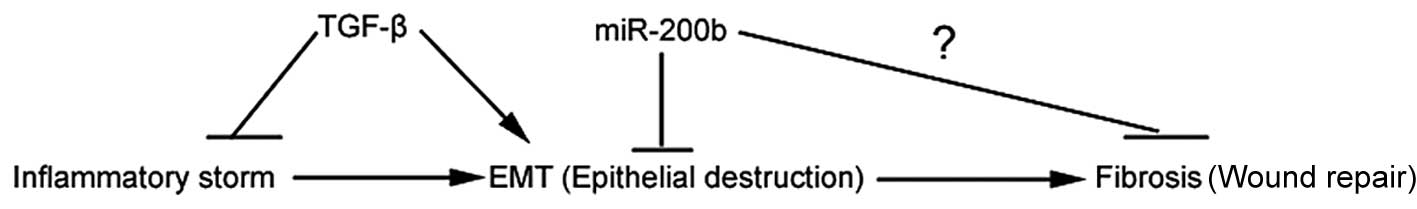
EMT is a process in which epithelial cells lose
their phenotypic and functional characteristics while acquiring
mesenchymal features. Although EMT is not a common event in adults,
this process has been implicated in such instances as wound healing
and fibrosis. The role of EMT in intestinal fibrosis has yet to be
investigated. Intestinal fibrosis is thought to occur as a result
of chronic inflammation and dysregulated wound healing (40,41). In this study, we demonstrated that
EMT may promote intestinal fibrogenesis, which was probably
inhibited by miR-200b (Figs. 3
and 5). In future studies, we
will attempt to provide additional evidence to prove this
hypothesis.
Acknowledgements
This study was supported by the Key Scientific
Research Program of the Shanghai Health Bureau (2010005), Program
for Innovative Research Team of the Shanghai Municipal Education
Commission and by funding of the Xinhua Hospital (11QYJ010) and
Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition
(11DZ2260500). We thank Professor W. Cai for his contribution.
References
|
1
|
JB PucilowskaKL WilliamsPK
LundFibrogenesis. IV Fibrosis and inflammatory bowel disease:
cellular mediators and animal modelsAm J Physiol Gastrointest Liver
Physiol279G653G659200011005750
|
|
2
|
F RiederC FiocchiIntestinal fibrosis in
inflammatory bowel disease - current knowledge and future
perspectivesJ Crohns
Colitis2279290200810.1016/j.crohns.2008.05.00921172225
|
|
3
|
F RiederC FiocchiIntestinal fibrosis in
inflammatory bowel disease: progress in basic and clinical
scienceCurr Opin
Gastroenterol24462468200810.1097/MOG.0b013e3282ff8b3618622160
|
|
4
|
PA GregoryAG BertEL PatersonThe miR-200
family and miR-205 regulate epithelial to mesenchymal transition by
targeting ZEB1 and SIP1Nat Cell
Biol10593601200810.1038/ncb172218376396
|
|
5
|
S ObaS KumanoE SuzukimiR-200b precursor
can ameliorate renal tubulointerstitial fibrosisPLoS
One5e13614201010.1371/journal.pone.001361421049046
|
|
6
|
S LamouilleR DerynckCell size and invasion
in TGF-beta-induced epithelial to mesenchymal transition is
regulated by activation of the mTOR pathwayJ Cell
Biol178437451200710.1083/jcb.20061114617646396
|
|
7
|
JJ HillTL TremblayC CantinM
O’Connor-McCourtJF KellyAE LenferinkGlycoproteomic analysis of two
mouse mammary cell lines during transforming growth factor
(TGF)-beta induced epithelial to mesenchymal transitionProteome
Sci72200910.1186/1477-5956-7-219128513
|
|
8
|
AB RobertsF TianSD ByfieldSmad3 is key to
TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis,
tumor suppression and metastasisCytokine Growth Factor
Rev171927200610.1016/j.cytogfr.2005.09.00816290023
|
|
9
|
J XuS LamouilleR DerynckTGF-beta-induced
epithelial to mesenchymal transitionCell
Res19156172200910.1038/cr.2009.519153598
|
|
10
|
K GravdalOJ HalvorsenSA HaukaasLA AkslenA
switch from E-cadherin to N-cadherin expression indicates
epithelial to mesenchymal transition and is of strong and
independent importance for the progress of prostate cancerClin
Cancer Res1370037011200710.1158/1078-0432.CCR-07-126318056176
|
|
11
|
S NakajimaR DoiE ToyodaN-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinomaClin Cancer
Res1041254133200410.1158/1078-0432.CCR-0578-0315217949
|
|
12
|
JB KimS IslamYJ KimN-Cadherin
extracellular repeat 4 mediates epithelial to mesenchymal
transition and increased motilityJ Cell
Biol15111931206200010.1083/jcb.151.6.119311121435
|
|
13
|
K VuoriluotoH HaugenS KiviluotoVimentin
regulates EMT induction by Slug and oncogenic H-Ras and migration
by governing Axl expression in breast
cancerOncogene3014361448201110.1038/onc.2010.50921057535
|
|
14
|
J IvaskaVimentin: central hub in EMT
induction?Small Gtpases25153201110.4161/sgtp.2.1.1511421686283
|
|
15
|
G CarpinoS MoriniS Ginanni
CorradiniAlpha-SMA expression in hepatic stellate cells and
quantitative analysis of hepatic fibrosis in cirrhosis and in
recurrent chronic hepatitis after liver transplantationDig Liver
Dis37349356200510.1016/j.dld.2004.11.009
|
|
16
|
K InaH KitamuraS TatsukawaY
FujikuraSignificance of alpha-SMA in myofibroblasts emerging in
renal tubulointerstitial fibrosisHistol
Histopathol26855866201121630215
|
|
17
|
N AkpolatS YahsiA GodekmerdanM YalnizK
DemirbagThe value of alpha-SMA in the evaluation of hepatic
fibrosis severity in hepatitis B infection and cirrhosis
development: a histopathological and immunohistochemical
studyHistopathology47276280200510.1111/j.1365-2559.2005.02226.x16115228
|
|
18
|
Y MurakamiH ToyodaM TanakaThe progression
of liver fibrosis is related with overexpression of the miR-199 and
200 familiesPLoS
One6e16081201110.1371/journal.pone.001608121283674
|
|
19
|
W Van MoerkerckeG DeboeverG LambrechtK
HertveldtSevere bridging fibrosis of the colon in a man with
inflammatory bowel diseaseEndoscopy39Suppl 1E294200717957610
|
|
20
|
J AiR ZhangY LiCirculating microRNA-1 as a
potential novel biomarker for acute myocardial infarctionBiochem
Biophys Res Commun3917377201010.1016/j.bbrc.2009.11.00519896465
|
|
21
|
N KosakaH IguchiT OchiyaCirculating
microRNA in body fluid: a new potential biomarker for cancer
diagnosis and prognosisCancer
Sci10120872092201010.1111/j.1349-7006.2010.01650.x20624164
|
|
22
|
AM ZahmM ThayuNJ HandA HornerMB LeonardJR
FriedmanCirculating microRNA is a biomarker of pediatric Crohn
diseaseJ Pediatr Gastroenterol
Nutr532633201110.1097/MPG.0b013e31822200cc21546856
|
|
23
|
GK WangJQ ZhuJT ZhangCirculating microRNA:
a novel potential biomarker for early diagnosis of acute myocardial
infarction in humansEur Heart
J31659666201010.1093/eurheartj/ehq01320159880
|
|
24
|
R MahnLC HeukampS RogenhoferA von
RueckerSC MullerJ EllingerCirculating microRNAs (miRNA) in serum of
patients with prostate
cancerUrology7712651266201110.1016/j.urology.2011.01.02021539977
|
|
25
|
Y D’AlessandraP DevannaF LimanaCirculating
microRNAs are new and sensitive biomarkers of myocardial
infarctionEur Heart J3127652773201020534597
|
|
26
|
SK GuptaC BangT ThumCirculating microRNAs
as biomarkers and potential paracrine mediators of cardiovascular
diseaseCirc Cardiovasc
Genet3484488201010.1161/CIRCGENETICS.110.95836320959591
|
|
27
|
KZ QuK ZhangH LiNH AfdhalM
AlbitarCirculating microRNAs as biomarkers for hepatocellular
carcinomaJ Clin
Gastroenterol45355360201110.1097/MCG.0b013e3181f18ac221278583
|
|
28
|
C RothB RackV MullerW JanniK PantelH
SchwarzenbachCirculating microRNAs as blood-based markers for
patients with primary and metastatic breast cancerBreast Cancer
Res12R90201010.1186/bcr276621047409
|
|
29
|
HM HeneghanN MillerAJ LoweryKJ SweeneyJ
NewellMJ KerinCirculating microRNAs as novel minimally invasive
biomarkers for breast cancerAnn
Surg251499505201010.1097/SLA.0b013e3181cc939f20134314
|
|
30
|
R ContuMV LatronicoG CondorelliCirculating
microRNAs as potential biomarkers of coronary artery disease: a
promise to be fulfilled?Circ
Res107573574201010.1161/CIRCRESAHA.110.22798320814026
|
|
31
|
PS MitchellRK ParkinEM KrohCirculating
microRNAs as stable blood-based markers for cancer detectionProc
Natl Acad Sci
USA1051051310518200810.1073/pnas.080454910518663219
|
|
32
|
W ZhuW QinU AtasoyER SauterCirculating
microRNAs in breast cancer and healthy subjectsBMC Res
Notes289200910.1186/1756-0500-2-8919454029
|
|
33
|
S FichtlschererS De RosaH FoxCirculating
microRNAs in patients with coronary artery diseaseCirc
Res107677684201010.1161/CIRCRESAHA.109.21556620595655
|
|
34
|
M TsujiuraD IchikawaS KomatsuCirculating
microRNAs in plasma of patients with gastric cancersBr J
Cancer10211741179201010.1038/sj.bjc.660560820234369
|
|
35
|
S KomatsuD IchikawaH TakeshitaCirculating
microRNAs in plasma of patients with oesophageal squamous cell
carcinomaBr J Cancer105104111201110.1038/bjc.2011.19821673684
|
|
36
|
Z ZuoGA CalinHM de PaulaCirculating
microRNAs let-7a and miR-16 predict progression-free survival and
overall survival in patients with myelodysplastic
syndromeBlood118413415201110.1182/blood-2011-01-33070421602527
|
|
37
|
J XuC WuX CheCirculating microRNAs,
miR-21, miR-122, and miR-223, in patients with hepatocellular
carcinoma or chronic hepatitisMol
Carcinog50136142201110.1002/mc.2071221229610
|
|
38
|
T SukataK SumidaM KushidaCirculating
microRNAs, possible indicators of progress of rat
hepatocarcinogenesis from early stagesToxicol
Lett2004652201110.1016/j.toxlet.2010.10.01321035526
|
|
39
|
K WangS ZhangB MarzolfCirculating
microRNAs, potential biomarkers for drug-induced liver injuryProc
Natl Acad Sci USA10644024407200910.1073/pnas.081337110619246379
|
|
40
|
JM Lopez-NovoaMA NietoInflammation and
EMT: an alliance towards organ fibrosis and cancer progressionEMBO
Mol Med1303314200910.1002/emmm.20090004320049734
|
|
41
|
PK LundCC ZunigaIntestinal fibrosis in
human and experimental inflammatory bowel diseaseCurr Opin
Gastroenterol17318323200110.1097/00001574-200107000-0000417031177
|