Introduction
Melanocytes play pivotal roles in skin and hair
pigmentation by producing melanin (1,2).
They originate from neural crest-derived melanoblasts and migrate
into the epidermis and hair follicles during embryogenesis
(3,4). Melanocytes synthesize melanin in
melanosomes and transfer the melanin granules to the adjacent
keratinocytes, where melanins are accumulated to generate pigmented
skin or hairs. High melanin content protects the skin from harmful
ultraviolet rays owing to the ability to absorb UV radiation and
quench the UV-induced intracellular free radicals (1,5,6).
Defects in or a lack of melanocytes can lead to pigment disorders,
such as piebaldism, albinism, vitiligo, and hair graying (7–9).
Wnts are a large family of secreted glycoproteins
that act as ligands to activate receptor-mediated signaling
pathways that play important roles in cell fate, proliferation,
differentiation and migration (10–13). Wnt signaling can be divided into
at least two distinct pathways: canonical Wnt/β-catenin signaling
and noncanonical signaling. In the best characterized canonical
pathway, Wnt ligands bind to seven-pass transmembrane receptors of
the Frizzled (Fzd) family and co-receptors, low density
lipoprotein-related protein (LRP) 5 and 6, leading to the
inhibition of the APC/Axin/CK1/GSK3b destruction complex and
stabilization and translocation of β-catenin to the nucleus where
it interacts with TCF/Lef family transcription factors to regulate
the transcription of target genes (11,12). Contrary to canonical Wnt
signaling, noncanonical Wnt signaling is transduced independently
of β-catenin. Noncanonical Wnt signaling pathways are diverse and
less well characterized, and have been termed the Wnt/calcium,
Wnt/JNK pathway and Wnt/planar cell polarity pathway (PCP)
(11).
Previous studies revealed that Wnt signaling plays a
critical role in melanocyte development, specifically Wnt1 and
Wnt3a. Wnt1 and Wnt3a promote the development of neural crest cells
into pigment cells (14,15). Neural crest cells depleted of
these two proteins become neuronal rather than melanocytes
(14). Wnt1 acts on melanoblasts
to increase melanocyte numbers, while Wnt3a and β-catenin can
specify neural crest cells to become melanocytes (16,17). Mutant mice deficient in Wnt1 and
Wnt3a are almost completely devoid of pigment cells (18). Furthermore, Wnt3a acts on
melanoblasts to maintain microphthalmia-associated transcription
factor (MITF) expression and promote melanoblasts to differentiate
to become melanocytes (16). In
humans, high levels of DKK1 (an inhibitor of the canonical Wnt
signaling pathway) inhibits melanocyte growth, pigmentation and
induces a less pigmented skin on the palms (19,20).
Although the importance of Wnt3a in melanocyte
development has been well recognized, the role of Wnt3a in mature
melanocytes remains undetermined. To address this issue, adenoviral
gene delivery of Wnt3a was adopted to investigate the effects of
Wnt3a on melan-a melanocyte proliferation and melanogenesis, and to
elucidate the possible mechanisms involved.
Materials and methods
Cell culture
An immortal line of melanocytes, melan-a, were a
kind gift of Dr D.C. Bennett (21). The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS (Gibco, USA), 2 mM
L-glutamine, 200 nM 12-o-tetradecanoyl phorbol-13-acetate (Sigma,
USA), 100 IU/ml penicillin, 50 μg/ml streptomycin, and grown in a
humidified atmosphere containing 10% CO2 in air at
37°C.
Adenovirus (Ad) amplification and
infection
The adenoviruses expressing green fluorescent
protein (AdGFP), Wnt3a protein (AdWnt3a, also expressing GFP), and
SimMITF (AdSimMITF, a small interfering RNA of MITF mediated by
adenovirus, also expressing RFP) were kindly provided by Dr T.C. He
(Chicago University). The adenoviruses were propagated and purified
as previously described (22).
Briefly, the adenoviruses were propagated in HEK293 cells, which
were collected upon detection of viral cytopathic effect-successful
infections were verified by observable GFP incorporation. Cell
pellets were resuspended in PBS and lysed by four
freeze-thaw-vortex cycles. After being purified by cesium chloride
(Amresco, USA) density gradient centrifugation, adenoviruses were
dialyzed into storage buffer, then their titers were determined and
diluted with storage buffer to the ultimate titer of 108
plaque-forming unit (PFU)/ml. For infection, melan-a cells were
plated onto 6- or 24-well plates at a density of 2×104
cells/cm2 in the growth medium for 12 h, the cells were
then grown in medium supplemented with adenoviruses for 72 h.
Isolation of total-RNA and RT-PCR
Total cellular RNA was isolated at the indicated
time-points using TRIzol reagent (Invitrogen, USA). Single-stranded
cDNA was synthesized by using ReverTra Ace reverse transcriptase
(Toyobo, Japan) and oligo(dt) primers according to the
manufacturer’s protocol. Semi-quantitative PCR was performed using
primers for Fzd1-10, LRP5, LRP6, MITF, tyrosinase-related protein
(TRP)1, TRP2, tyrosinase (Table I
lists the primer sequences and amplicon size). PCR reaction was
performed by using a touchdown protocol previously described
(23). Briefly, touchdown PCR was
performed with the following program: 1 cycle at 94°C for 2 min, 12
cycles at 92°C for 20 sec, 68°C for 30 sec, and 70°C for 45 sec
with a decrease of one degree per cycle, and 25 cycles at 92°C for
20 sec, 55°C for 30 sec, and 70°C for 45 sec. PCR products were
analyzed by gel electrophoresis and stained with ethidium
bromide.
 | Table I.Primer sequences for RT-PCR
analysis. |
Table I.
Primer sequences for RT-PCR
analysis.
| Primer | Sequence
(5′→3′) | GenBank ID |
|---|
| LRP5 | F:
GGTCACCTGGACTTCGTCAT | NM_008513.3 |
| R:
TCCAGCGTGTAGTGTGAAGC | |
| LRP6 | F:
ACAGAGCCCTGACATCATCC | NM_008514.4 |
| R:
TGATTTGCGACTGAGTTTGC | |
| Fzd1 | F:
CAAGGTTTACGGGCTCATGT | NM_021457.3 |
| R:
GTAACAGCCGGACAGGAAAA | |
| Fzd2 | F:
TTAGCGGCCTGAGAGATGTT | NM_020510.2 |
| R:
CAGGAGAGACGGTTGAGAGC | |
| Fzd3 | F:
GCTCCAGGAACCTGACTTTG | NM_021458.2 |
| R:
GACACTCCCTGCTTTGCTTC | |
| Fzd4 | F:
AACCTCGGCTACAACGTGAC | NM_008055.4 |
| R:
TGGCACATAAACCGAACAAA | |
| Fzd5 | F:
AGGCATCCCGATTTTCTTTT | NM_022721.3 |
| R:
TGAGCGAGGGCAGAGTATTT | |
| Fzd6 | F:
TCCGACGCTTGAAGAAAACT | NM_001162494.1 |
| R:
CAACCCCAGGTCCTCAAGTA | |
| Fzd7 | F:
ATCATCTTCCTGTCGGGTTG | NM_008057.3 |
| R:
AAGCACCATGAAGAGGATGG | |
| Fzd8 | F:
CTGTTCCGAATCCGTTCAGT | NM_008058.2 |
| R:
CGGTTGTGCTGCTCATAGAA | |
| Fzd9 | F:
TTATGGTTGCTCCCTCCTTG | NM_010246.1 |
| R:
CACTCCCTGCATGAGACAGA | |
| Fzd10 | F:
TCCTCACCCTCACTTGGTTC | NM_175284.3 |
| R:
GCTGCCCACATAACACACAC | |
TOP/FOP-flash luciferase reporter
assay
Melan-a cells were seeded onto 24-well plates
overnight and subsequently infected with AdWnt3a or AdGFP. After 12
h, the cells were transfected with TOP/FOP-flash luciferase
reporter plasmid. For each well, 3 μg TOP- or FOP-Flash Firefly
Luciferase reporter plasmid, which contain several TCF4-binding
elements (TOP) or mutant sequences (FOP), respectively, were
transfected together with 0.03 μg phRG-TK Renilla Luciferase
standard plasmid (Promega, USA). The transfection reagent,
Lipofectamine 2000 (Invitrogen), was used according to the standard
protocol. Cell lysates were harvested 24 h after transfection and
the Dual-Luciferase Reporter Assay System (E1910; Promega) was used
to detect luciferase activity according to the manufacturer’s
protocol. The experiments were performed in triplicate.
Cytometry and MTT assays
To study the effect of Wnt3a on melan-a
proliferation, 2×104 melan-a cells were plated onto
24-well plates overnight and grown in culture medium supplemented
with AdWnt3a at various doses (1, 2, 3 μl) or 2 μl AdGFP as the
vehicle control. After 72 h, the cells were detached with trypsin
and counted in a hemocytometer. For the MTT assay, melan-a cells
were plated onto 96-well plates and treated with AdWnt3a at various
doses (0.2, 0.5, 0.8 μl) or 0.5 μl AdGFP for 72 h. Then MTT (Sigma)
was added, and the cells were incubated at 37°C for 4 h. The medium
was removed and dimethyl sulfoxide (DMSO) was added to dissolve the
formazan crystals. The absorbance was then measured at 490 nm with
a common ELISA reader.
5-Bromodeoxyuridine incorporation
assay
Melan-a cells were plated onto glass coverslips and
were treated with AdWnt3a or AdGFP. After 72 h, 5-bromodeoxyuridine
(BrdU, Sigma; stock of 10 mM in PBS) was added at 10 μM. The cells
were then incubated at 37°C for 2 h and fixed in cold acetone for
10 min. After washing three times in PBS, the cells were incubated
in 2 M HCl for 45 min to denature the DNA and then neutralized with
0.1 M Na2B4O7 (pH 8.5) for 30 min.
The detection of BrdU was performed with a mouse anti-BrdU antibody
(1:100; Zhongshan, China) at 4°C overnight. Then, cells were
incubated with goat anti-mouse FITC-conjugated secondary antibody
(1:100; Zhongshan) at 37°C for 1 h. After washing, the cells were
stained with DAPI for 10 min at room temperature. Six areas/well
were randomly selected and counted with an upright microscope BH2
(Olympus, Japan).
Flow cytometric analysis
Melan-a cells were plated onto 6-well plates and
treated with 2 μl AdWnt3a or 2 μl AdGFP for 72 h. Afterward, cells
were dissociated with trypsin, washed in PBS, and fixed in 1 ml of
70% methanol at 4°C for at least 24 h. The fixed cells were washed
twice in PBS, treated with 100 μg/ml RNase and 50 μg/ml propidium
iodide (PI) in PBS for 30 min at 37°C, and the cell cycle was
detected with a flow cytometer. All proliferation assays were
performed in triplicate.
Tyrosinase enzymatic assay
Tyrosinase activity assays were performed according
to the method previously reported (24). Melan-a cells in 6-well plates were
infected with AdWnt3a or AdGFP for 72 h, then trypsinized and
counted. Cells (1×105) were treated with 200 μl of 1%
Triton X-100/PBS at −70°C for 30 min and thawed at 37°C. Then, the
extracts were clarified by centrifugation, 50 μl of the supernatant
were transferred into 96-well plates and 10 μl of 2 mg/ml L-DOPA
(Sigma) were added. After incubation for 2 h at 37°C, absorbance
was measured at 490 nm. The experiments were performed at least
three times.
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyontime,
China), determined by the Enhanced BCA Protein Assay kit
(Beyontime) and denatured by boiling. Protein of 80 μg per lane was
loaded on 10% SDS-PAGE and then transferred onto a PVDF membrane.
Membranes were blocked with 5% fat-free milk in Tris-buffered
saline-Tween-20 (TBST; 20 mM Tris/HCl, pH 7.6, 137 mM NaCl, 0.1%
Tween-20) for 2 h, then membranes were probed with rabbit
anti-Wnt3a antibody (1:1,000; Abcam, USA), goat anti-TRP1 antibody
(1:1,000), rabbit anti-TRP2 antibody (1:1,000), goat
anti-tyrosinase antibody (1:1,000; Santa Cruz Biotechnology, Inc.,
USA), and mouse anti-MITF antibody (1:500, Millipore, USA) at 4°C
overnight. Blots were then incubated with HRP-conjugated secondary
antibody. Peroxidase activity on the membrane was visualized on
X-ray film by means of the ECL western blotting detection
system.
Statistical analysis
Data were presented as means ± SD for the three
independent experiments. Statistical differences were evaluated by
the t-test, and P<0.05 was considered to be statistically
significant.
Results
Wnt3a activates Wnt/β-catenin signaling
in melan-a cells
To explore the potential role of Wnt3a in mouse
mature melanocytes, we used a mouse melanocyte cell line, melan-a
(21), as an in vitro cell
model. DOPA staining was used to confirm that the cells were indeed
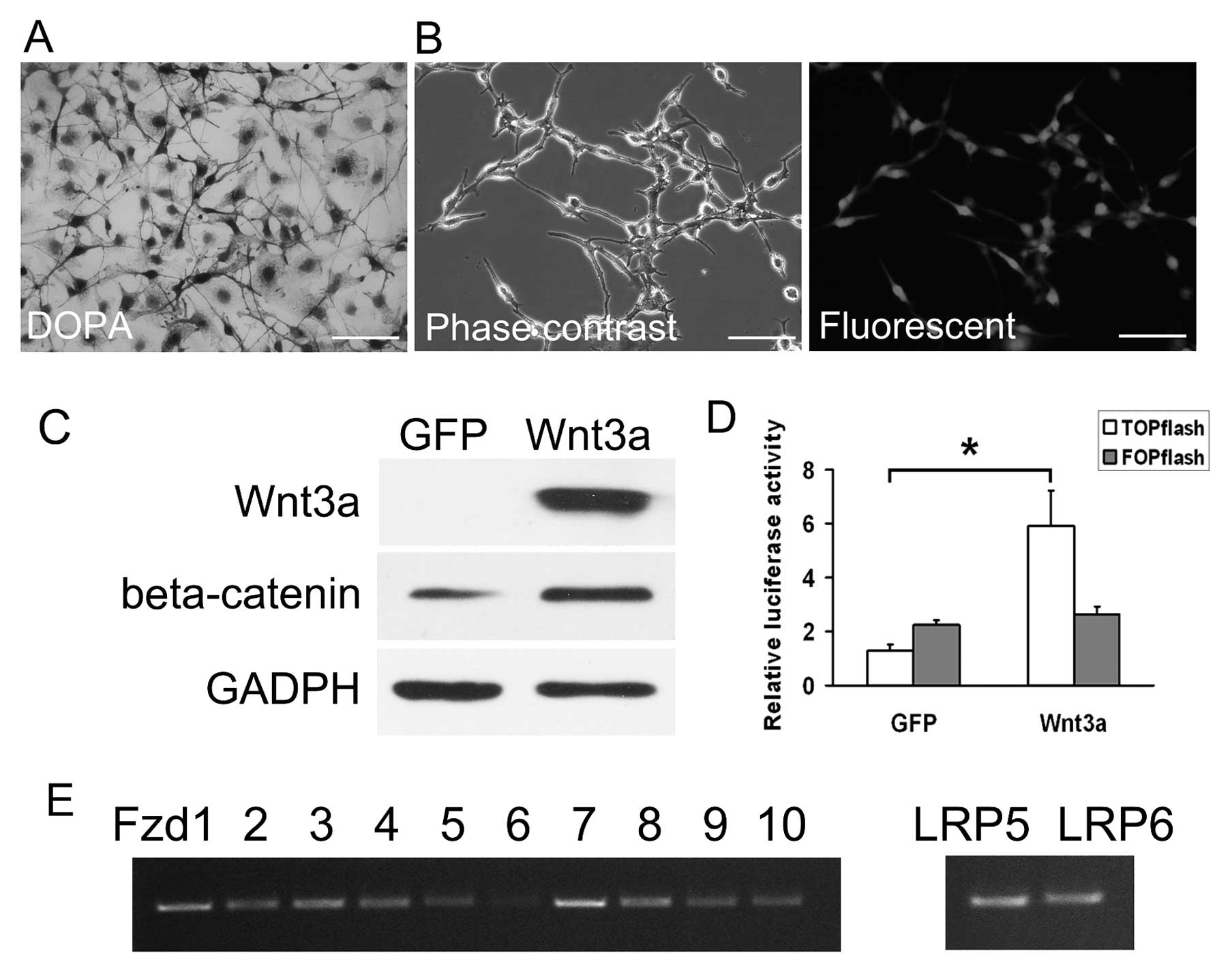
melanocytes (Fig. 1A). RT-PCR
analysis showed that the Wnt receptors Fzd1 to Fzd10 and the
co-receptors LRP5 and LRP6 were all expressed in melan-a cells,
indicating that melan-a cells could receive and transduce Wnt
signals (Fig. 1E). We infected
melan-a cells with AdWnt3a at a predetermined optimal titer. As
observed in Fig. 1B, AdWnt3a
mediated efficient gene transfer in melan-a cells. The expression
of Wnt3a was confirmed by western blot analysis in AdWnt3a-infected
cells but not in AdGFP-infected cells (Fig. 1C).
To evaluate the ability of AdWnt3a to activate
Wnt/β-catenin signaling, western blot analysis and the
TOP/FOP-flash luciferase reporter assay were performed. As shown in
Fig. 1C, AdWnt3a upregulated the
expression of β-catenin at the protein level. We infected melan-a
cells with AdWnt3a or AdGFP and co-transfected with either the
β-catenin responsive TCF4 reporter plasmid (TOP-flash) or with
negative control reporter plasmids (FOP-flash). The luciferase
activities showed that AdWnt3a-infected cells displayed higher
ratios of TOP-flash/FOP-flash compared to AdGFP-infected cells
(Fig. 1D). The data demonstrate
that AdWnt3a efficiently activates Wnt/β-catenin signaling in
melan-a cells.
Wnt3a inhibits the proliferation of
melan-a cells
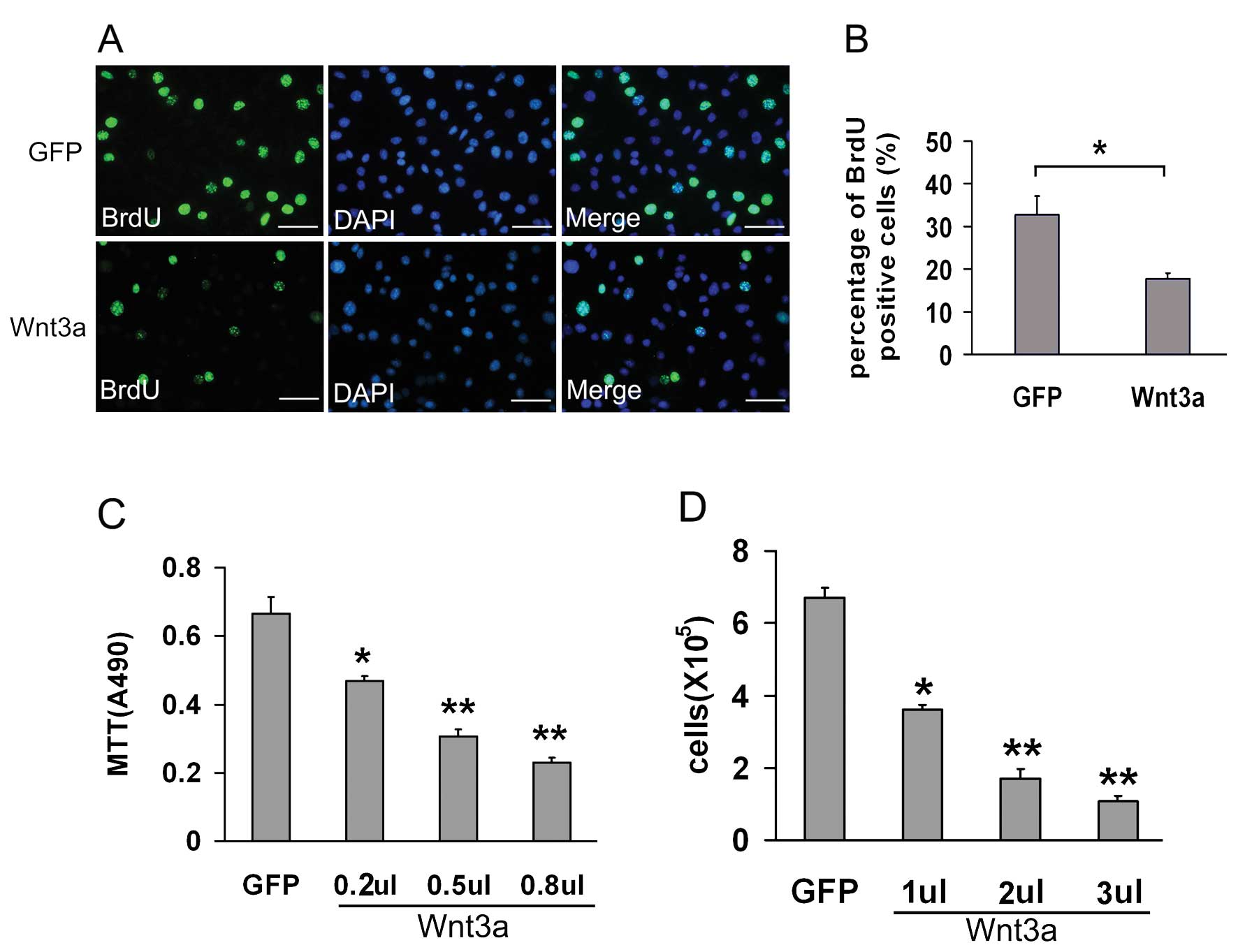
To analyze the effect of Wnt3a on melan-a cell
proliferation, we infected melan-a cells with different doses of
AdWnt3a or AdGFP as control. After 72 h, the MTT cell proliferation
assay and manual cell count both showed that Wnt3a inhibited the
proliferation of melan-a cell in a dose-dependent manner compared
to GFP (Fig. 2C and D).
BrdU is a synthetic thymidine analog that gets
incorporated into a DNA cell when the cell is dividing, so it is
commonly used for the detection of proliferating cells. We measured
the BrdU incorporation of AdWnt3a-infected melan-a cells and
AdGFP-infected cells. The incorporated BrdU was detected by using
the anti-BrdU antibody (Fig. 2A).
The percentage of BrdU positive cells in AdWnt3a-infected cells was
decreased by 14.9% (from 32.7±4.45 to 17.8±1.32%, P<0.05)
(Fig. 2B), which implied that
Wnt3a inhibited the proliferation of melan-a cells.
We also performed cell cycle analysis and found that
AdWnt3a-infected cells exhibited an increased population in the
G1 phase and a decreased population in the S phase
compared to AdGFP-infected cells (Fig. 3).
Taken together, these results showed that Wnt3a
inhibited melan-a cell proliferation and this was associated with
decreased population of cells in the S phase and an increase in the
G1 phase.
Wnt3a promotes the melanogenesis of
melan-a cells
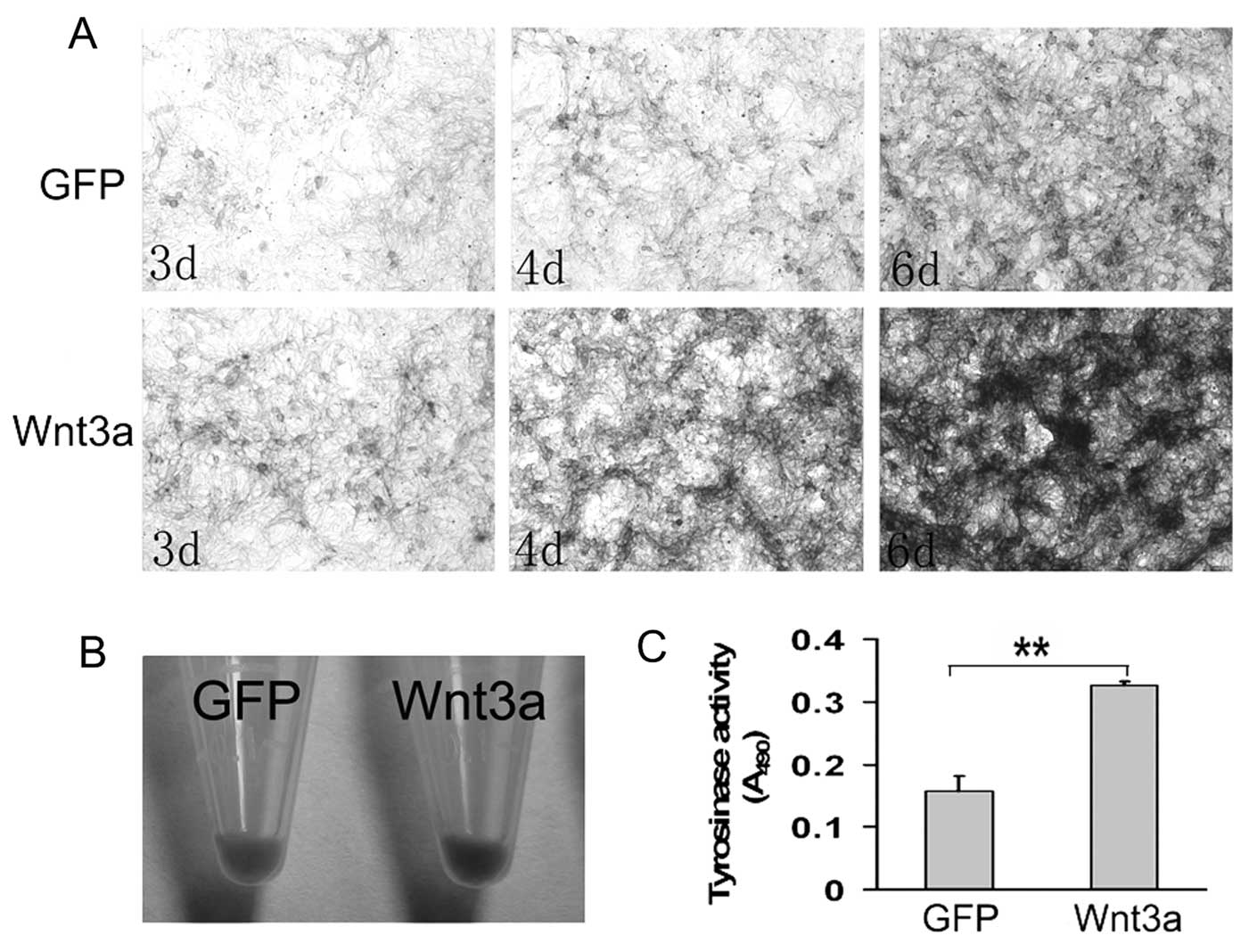
We analyzed the effect of Wnt3a on the melanogenesis
of melan-a cells by assessing melanin production and tyrosinase
activity. Although almost all the cells contained melanin pigment,
AdWnt3a-infected cells showed obvious higher level of melanin
accumulation compared to AdGFP-infected cells (Fig. 4A and B).
To analyze whether melanin synthesis is activated
via a direct effect on tyrosinase, tyrosinase activity assay was
adopted to analyze tyrosinase activity in melan-a cells. As shown
in Fig. 4C, a greatly significant
increase of tyrosinase activity was detected in AdWnt3a-infected
cells compared with AdGFP-infected cells.
To investigate how Wnt3a promotes melanin synthesis,
we studied the expression of microphthalmia-associated
transcription factor (MITF), the transcriptional master regulator
of melanocytes, and its downstream target genes, including
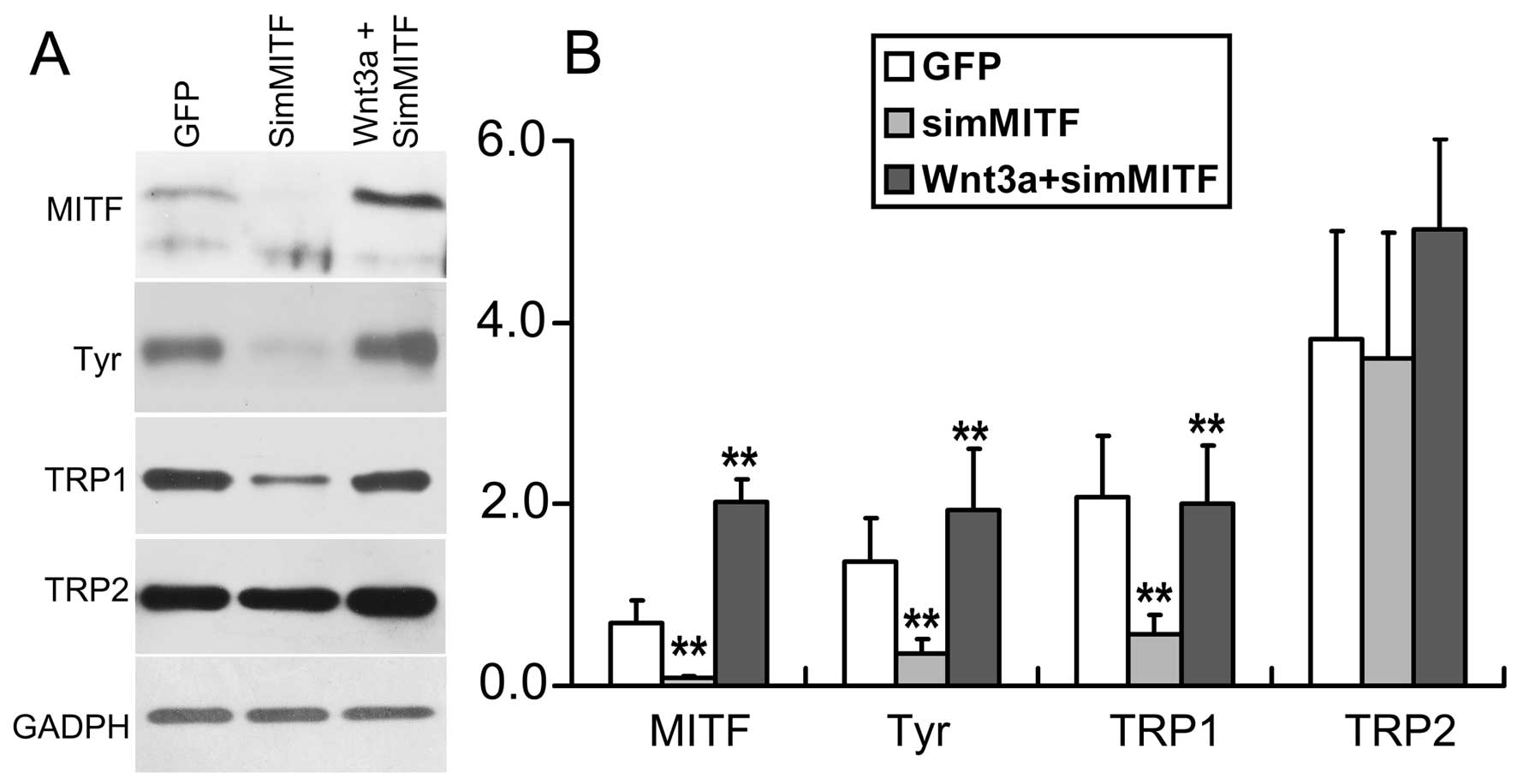
tyrosinase, TRP1, and TRP2 (25–27). Western blot analyses showed that
Wnt3a significantly increased the expression levels of MITF,
tyrosinase, and TRP1 in melan-a cells, but did not affect the
expression of TRP2 (Fig. 5).
Subsequently, we infected melan-a cells with AdSimMITF to knockdown
the endogenous MITF. According to western blot analysis, the
expression of MITF, and its downstream target genes, tyrosinase and
TRP1, were dramatically decreased in the cells infected with
AdSimMITF (Fig. 6). Co-infection
of melan-a cells with AdSimMITF and AdWnt3a demonstrated that Wnt3a
rescued the expression of MITF, tyrosinase and TRP1 (Fig. 6).
These results suggested that Wnt3a contributed to
increase melanin synthesis through upregulation of the expression
of MITF and its downstream genes, tyrosinase and TRP1.
Discussion
The Wnt signaling pathway is critical for regulating
multiple aspects of basic cell functions, including proliferation,
polarity, differentiation, and migration of cells (10–12). Despite the well-known role of
Wnt3a in the development of melanocytes, the role of Wnt3a in
mature melanocytes remains unknown. Therefore, this study was
conducted to address this question.
The melan-a melanocytes were originally derived from
normal epidermal melanoblasts from embryos of inbred C57BL/6J mice.
When the cell line was established, the primary culture was
unpigmented melanoblasts, which then matured to pigmented
melanocytes in the incubation (21). We thus used melan-a as an in
vitro cell model to investigate the effect of Wnt3a in mature
melanocytes.
Wnt/β-catenin signaling is mediated by binding to
Frizzled (Fzd) receptors and low density lipoprotein-related
protein (LRP)5/6, and induces various cellular events. Fzd1, Fzd3,
Fzd8 and LRP6 have been demonstrated to act as receptors for Wnt3a
(28–32). Our RT-PCR result showed that
melan-a cells expressed Wnt receptors, Fzd (Fzd1-10), and the
co-receptors LRP5 and LRP6, suggesting that melan-a cells could
respond to Wnt3a. In this study, we infected melan-a cells with
AdWnt3a and demonstrated that AdWnt3a expressed Wnt3a protein and
efficiently activated Wnt/β-catenin signaling in melan-a cells.
Rabbani et al (33)
co-cultured Wnt10b-transfected epithelial cells with melan-a cells
and found that Wnt10b secreted by epithelial cells could activate
Wnt/β-catenin signaling in melan-a cells. We treated melan-a cells
with the conditioned medium from JB6 mouse keratinocytes infected
with AdWnt3a for 2 days. Wnt/β-catenin signaling in melan-a cells
was also activated by testing TOP-flash system (data not shown).
These results suggest that melan-a cells may respond to Wnt3a
secreted by itself in an autocrine manner or secreted by
keratinocytes in a paracrine manner in vitro.
Wnt signaling has been associated with proliferation
of cells. Previous studies showed that Wnt3a promoted the
proliferation of epidermal stem cells, mesenchymal stem cells, and
fibroblast cells, and inhibited the proliferation of B-ALL cell
lines (33–37). As for the melanocyte cell lineage,
Dunn et al (16) reported
that Wnt3a did not stimulate proliferation of melanoblasts in
vitro, and Delmas et al (38) reported that stabilized β-catenin
reduced the number of melanoblasts in vivo. Similar to
findings in melanoblasts, Wnt3a failed to induce and, in fact,
inhibited mature melanocyte proliferation in this study. It is
important to bear in mind that Wnt3a may play distinct roles in
different cell types, developmental stages, and organismal
origin.
In mammalian melanocytes, melanin biosynthesis is
catalyzed by three melanocyte-specific enzymes: tyrosinase,
tyrosinase-related protein-1 (TRP1) and TRP2 (6). Tyrosinase is the rate-limiting
enzyme in melanogenesis while TRP1 and TRP2 function as downstream
of enzymes in the melanin biosynthetic pathway. To investigate the
influence of Wnt3a on melanogenesis of mouse melanocytes, we
analyzed tyrosinase activity and melanin content in melan-a cells.
The data showed that Wnt3a significantly increased tyrosinase
activity and melanin synthesis in melan-a cells. In support of our
findings, previous studies have reported that inhibition of
Wnt/β-catenin signaling strongly inhibited melanin synthesis
(20,39).
Melanin synthesis is stimulated by a large number of
effectors, including cAMP-elevating agents, cholera toxin, UV light
and so on. MITF is the master regulator of melanogenesis that
regulates the expression of the melanogenic enzymes, tyrosinase,
TRP1, and TRP2 as well as other pigmentation factors (25,40). In this study, we detected the
expression of MITF and its downstream target genes, tyrosinase,
TRP1 and TRP2, and demonstrated that Wnt3a upregulated the
expression of MITF, tyrosinase, and TRP1 in melan-a cells at the
protein level. Furthermore, Wnt3a rescued the expression of MITF
and its downstream target genes following reduction of endogenous
MITF in melan-a cells by AdSimMITF. With the same melan-a cells,
Takeda et al (41) found
that Wnt3a protein induced MITF mRNA expression and activated the
MITF promoter by recruiting LEF-1 and β-catenin to the
LEF-1-binding site. Our data were consistent with the result of
Takeda et al (41) which
demonstrated that MITF is a target gene of Wnt3a signaling.
In conclusion, the study demonstrates that Wnt3a
reduces the proliferation of melan-a cells while simultaneously
increases the melanin synthesis through the upregulation of MITF
and its downstream genes, tyrosinase and TRP1.
Abbreviations:
|
BrdU
|
5-bromodeoxyuridine;
|
|
Fzd
|
Frizzled;
|
|
LRP
|
low-density lipoprotein-related
protein;
|
|
Ad
|
adenovirus;
|
|
MITF
|
microphthalmia-associated
transcription factor;
|
|
TRP2
|
tyrosinase-related protein 2;
|
|
TRP1
|
tyrosinase-related protein 1
|
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 30872711). We
thank Dr T.C. He (Chicago University) for the adenovirus production
and technical assistance. We thank Professor Dorothy C. Bennett
(University of London) and Dr Zhixiu Lin (Chinese University of
Hong Kong) for providing melan-a cells and for helpful advice.
References
|
1.
|
Y YamaguchiM BrennerVJ HearingThe
regulation of skin pigmentationJ Biol
Chem2822755727561200710.1074/jbc.R70002620017635904
|
|
2.
|
A SlominskiJ WortsmanPM PlonkaKU
SchallreuterR PausDJ TobinHair follicle pigmentationJ Invest
Dermatol1241321200510.1111/j.0022-202X.2004.23528.x
|
|
3.
|
A UongLI ZonMelanocytes in development and
cancerJ Cell Physiol2223841201010.1002/jcp.2193519795394
|
|
4.
|
RM WhiteLI ZonMelanocytes in development,
regeneration, and cancerCell Stem
Cell3242252200810.1016/j.stem.2008.08.00518786412
|
|
5.
|
HY ParkM KosmadakiM YaarBA
GilchrestCellular mechanisms regulating human melanogenesisCell Mol
Life Sci6614931506200910.1007/s00018-009-8703-819153661
|
|
6.
|
A SlominskiDJ TobinS ShibaharaJ
WortsmanMelanin pigmentation in mammalian skin and its hormonal
regulationPhysiol
Rev8411551228200410.1152/physrev.00044.200315383650
|
|
7.
|
J CuiLY ShenGC WangRole of hair follicles
in the repigmentation of vitiligoJ Invest
Dermatol97410416199110.1111/1523-1747.ep124809971714927
|
|
8.
|
EK NishimuraSR GranterDE FisherMechanisms
of hair graying: incomplete melanocyte stem cell maintenance in the
nicheScience307720724200510.1126/science.109959315618488
|
|
9.
|
T PasseronF MantouxJP OrtonneGenetic
disorders of pigmentationClin
Dermatol235667200510.1016/j.clindermatol.2004.09.013
|
|
10.
|
JR MillerThe WntsGenome
Biol3review30012002
|
|
11.
|
DM EisenmannWnt
signalingWormBook1172005(www.wormbook.org/toc_signaltrans.html)
|
|
12.
|
JK SethiA Vidal-PuigWnt signalling and the
control of cellular metabolismBiochem
J427117201010.1042/BJ2009186620226003
|
|
13.
|
RT MoonJD BrownM TorresWNTs modulate cell
fate and behavior during vertebrate developmentTrends
Genet13157162199710.1016/S0168-9525(97)01093-79097727
|
|
14.
|
RI DorskyRT MoonDW RaibleControl of neural
crest cell fate by the Wnt signalling
pathwayNature396370373199810.1038/246209845073
|
|
15.
|
RI DorskyRT MoonDW RaibleNeural
crest-directed gene transfer demonstrates Wnt1 role in melanocyte
expansion and differentiation during mouse developmentProc Natl
Acad Sci USA971005010055200010.1073/pnas.97.18.1005010963668
|
|
16.
|
KJ DunnM BradyCO JamborS SnyderA IncaoWJ
PavanWNT1 and WNT3a promote expansion of melanocytes through
distinct modes of actionPigment Cell
Res18167180200510.1111/j.1600-0749.2005.00226.x15892713
|
|
17.
|
EJ JinCA EricksonS TakadaLW BurrusWnt and
BMP signaling govern lineage segregation of melanocytes in the
avian embryoDev Biol2332237200110.1006/dbio.2001.022211319855
|
|
18.
|
M IkeyaSM LeeJE JohnsonAP McMahonS
TakadaWnt signalling required for expansion of neural crest and CNS
progenitorsNature389966970199710.1038/401469353119
|
|
19.
|
Y YamaguchiS ItamiH
WatabeMesenchymal-epithelial interactions in the skin: increased
expression of dickkopf1 by palmoplantar fibroblasts inhibits
melanocyte growth and differentiationJ Cell
Biol165275285200410.1083/jcb.200311122
|
|
20.
|
Y YamaguchiT PasseronT HoashiDickkopf 1
(DKK1) regulates skin pigmentation and thickness by affecting
Wnt/beta-catenin signaling in keratinocytesFASEB
J2210091020200810.1096/fj.07-9475com17984176
|
|
21.
|
DC BennettPJ CooperIR HartA line of
non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma
and requiring a tumour promoter for growthInt J
Cancer39414418198710.1002/ijc.29103903243102392
|
|
22.
|
J LuoZL DengX LuoA protocol for rapid
generation of recombinant adenoviruses using the AdEasy systemNat
Protoc212361247200710.1038/nprot.2007.13517546019
|
|
23.
|
Y PengQ KangH ChengTranscriptional
characterization of bone morphogenetic proteins (BMPs)-mediated
osteogenic signalingJ Cell
Biochem9011491165200310.1002/jcb.1074414635189
|
|
24.
|
EK NishimuraM SuzukiV IgrasKey roles for
transforming growth factor beta in melanocyte stem cell
maintenanceCell Stem
Cell6130140201010.1016/j.stem.2009.12.01020144786
|
|
25.
|
HR WidlundDE
FisherMicrophthalamia-associated transcription factor: a critical
regulator of pigment cell development and
survivalOncogene2230353041200310.1038/sj.onc.120644312789278
|
|
26.
|
E SteingrimssonNG CopelandNA
JenkinsMelanocytes and the microphthalmia transcription factor
networkAnnu Rev
Genet38365411200410.1146/annurev.genet.38.072902.092717
|
|
27.
|
S ShibaharaK YasumotoS AmaeRegulation of
pigment cell-specific gene expression by MITFPigment Cell
Res13Suppl 8S98S102200010.1034/j.1600-0749.13.s8.18.x
|
|
28.
|
MA ChaconL Varela-NallarNC
InestrosaFrizzled-1 is involved in the neuroprotective effect of
Wnt3a against Abeta oligomersJ Cell
Physiol217215227200810.1002/jcp.2149718521822
|
|
29.
|
H YamamotoH KomekadoA KikuchiCaveolin is
necessary for Wnt-3a-dependent internalization of LRP6 and
accumulation of beta-cateninDev
Cell11213223200610.1016/j.devcel.2006.07.00316890161
|
|
30.
|
H YamamotoH SakaneH YamamotoT MichiueA
KikuchiWnt3a and Dkk1 regulate distinct internalization pathways of
LRP6 to tune the activation of beta-catenin signalingDev
Cell53748200810.1016/j.devcel.2008.04.01518606139
|
|
31.
|
Y EndoE BeauchampD WoodsWnt-3a and
Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a
Frizzled3-and c-Jun N-terminal kinase-dependent mechanismMol Cell
Biol2823682379200810.1128/MCB.01780-0718212053
|
|
32.
|
H KomekadoH YamamotoT ChibaA
KikuchiGlycosylation and palmitoylation of Wnt-3a are coupled to
produce an active form of Wnt-3aGenes
Cells12521534200710.1111/j.1365-2443.2007.01068.x17397399
|
|
33.
|
P RabbaniM TakeoW ChouCoordinated
activation of wnt in epithelial and melanocyte stem cells initiates
pigmented hair
regenerationCell145941955201110.1016/j.cell.2011.05.00421663796
|
|
34.
|
YC ShangSH WangF XiongWnt3a signaling
promotes proliferation, myogenic differentiation, and migration of
rat bone marrow mesenchymal stem cellsActa Pharmacol
Sin2817611774200710.1111/j.1745-7254.2007.00671.x17959027
|
|
35.
|
L JiaJ ZhouS PengJ LiY CaoE DuanEffects of
Wnt3a on proliferation and differentiation of human epidermal stem
cellsBiochem Biophys Res
Commun368483488200810.1016/j.bbrc.2008.01.09718242164
|
|
36.
|
GM BolandG PerkinsDJ HallRS TuanWnt 3a
promotes proliferation and suppresses osteogenic differentiation of
adult human mesenchymal stem cellsJ Cell
Biochem9312101230200410.1002/jcb.2028415486964
|
|
37.
|
MK NygrenG DosenME HystadH StubberudS
FunderudE RianWnt3A activates canonical Wnt signalling in acute
lymphoblastic leukaemia (ALL) cells and inhibits the proliferation
of B-ALL cell linesBr J
Haematol136400413200710.1111/j.1365-2141.2006.06442.x17156404
|
|
38.
|
V DelmasF BeermannS MartinozziBeta-catenin
induces immortalization of melanocytes by suppressing p16INK4a
expression and cooperates with N-Ras in melanoma developmentGenes
Dev2129232935200710.1101/gad.45010718006687
|
|
39.
|
M ChoM RyuY JeongCardamonin suppresses
melanogenesis by inhibition of Wnt/beta-catenin signalingBiochem
Biophys Res
Commun390500505200910.1016/j.bbrc.2009.09.12419800318
|
|
40.
|
I AksanCR GodingTargeting the
microphthalmia basic helix-loop-helix-leucine zipper transcription
factor to a subset of E-box elements in vitro and in vivoMol Cell
Biol186930693819989819381
|
|
41.
|
K TakedaK YasumotoR TakadaInduction of
melanocyte-specific microphthalmia-associated transcription factor
by Wnt-3aJ Biol
Chem2751401314016200010.1074/jbc.C00011320010747853
|