Introduction
Ischemic stroke is one of the most common causes of
mortality and complex disability in adults worldwide (1–3).
Despite improvements in the interventional techniques and
pharmacological agents, there are currently no exact effective
drugs and techniques to manage post-stroke rehabilitation or
recovery. Acupuncture originated in ancient China and is among the
oldest healing methodologies in the world. As part of traditional
Chinese medicine (TCM), acupuncture has been used for thousands of
years as a complementary and alternative therapy practice that
supplements conventional medicine in many oriental countries as
well as recently in Western society (4). A large number of studies have
demonstrated the clinical efficacy of acupuncture in stroke
rehabilitation (5–9). Recently, we reported that
electroacupuncture alleviates neurological deficits possibly by
promoting the proliferation and differentiation of nerve stem cells
(10). Additionally, based on a
number of documents and materials, the Zusanli (ST36) and Quchi
(LI11) acupoints were commonly used in China to clinically treat
stroke (11,12). However, the precise mechanism of
its neuroprotective effect remains largely unknown. Inflammatory
response has been shown to play a critical role in brain damage
after cerebral ischemia-reperfusion (I/R) injury (13,14), which is tightly regulated by
Toll-like receptors (TLRs). The transmembrane TLRs are a family of
pattern-recognition receptors (PRRs) which enable the innate and
adaptive immune systems to recognize pathogen-associated molecular
patterns (PAMPs). TLRs contain an intracellular Toll-interleukin 1
receptor (TIR) domain and exert their functions by interacting with
TIR domain-containing adaptor proteins such as MyD88, Mal, TRIF and
TRAM (15,16). To date, over 13 members of the TLR
family have been identified in mammals (17), of which TLR4 is the best studied.
TLR4 recognizes LPS from Gram-negative bacteria and various
host-derived molecules, such as heat-shock proteins, fibronectin,
hyaluronic acid and heparan sulfate (18–20). Upon ligand binding, TLR4 undergoes
a conformational change and dimerizes, which then recruits TIR
domain-containing adaptor proteins, transducing the immune-related
signals to the nucleus via transcription factors such as nuclear
factor-κB (NF-κB). As one of the most important nuclear
transcription factors, NF-κB is involved in the control of many
critical physiological processes, such as cell proliferation,
apoptosis, and especially in pathways of the immune and
inflammatory responses. In unstimulated cells, NF-κB is sequestered
in the cytosol via interaction with inhibitory IκB proteins.
However, when cells receive pathological stimuli, IκB proteins
could be phosphorylated by IκB kinase (IKK) that is activated by
various mechanisms including TLR4-mediated immune signaling.
Phosphorylation of IκB proteins results in their ubiquitination and
degradation, which in turn releases sequestered NF-κB, leading to
its translocation to the nucleus where it induces the expression of
various pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6.
Therefore, suppression of the TLR4/NF-κB pathway has become a
promising target for the anti-inflammatory treatment in ischemic
stroke.
Using a focal cerebral I/R injured rat model, in the
present study we evaluated the neuroprotective and
anti-inflammatory activities of electroacupuncture at Quchi and
Zusanli, and investigated the underlying molecular mechanisms.
Materials and methods
Materials and reagents
TRIzol reagent was purchased from Invitrogen
(Carlsbad, CA, USA). TLR4, NF-κB p65, p-IκB and β-actin antibodies,
horseradish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling Technology (Beverly, MA, USA). Rat
TNF-α, IL-1 and IL-6 ELISA kits were purchased from Shanghai XiTang
Biological Technology Co., Ltd. (Shanghai, China). All the other
chemicals used, unless otherwise stated, were obtained from Sigma
Chemicals (St. Louis, MO, USA).
Animals
Adult male Sprague-Dawley rats (with an initial body
weight of 200-250 g) were obtained from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and housed in a temperature- and
humidity-controlled room with a 12-h light/dark cycle and free
access to food and water. All animal procedures were strictly in
accordance with international ethical guidelines and the National
Institutes of Health Guide concerning the Care and Use of
Laboratory Animals, and the experiments were approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine.
Establishment of the cerebral I/R injured
rat model and animal grouping
The I/R injured model was generated by occlusion of
the middle cerebral artery (MCA). Prior to surgery, rats were
allowed access to water but fasted for 24 h and the surgical
procedures were performed as previously described by Longa et
al (21) with slight
modifications. Briefly, after a rat was anesthetized with 10%
chloral hydrate by intraperitoneal injection (300 mg/kg), the left
common carotid artery (CCA), the left external carotid artery (ECA)
and the internal carotid artery (ICA) were exposed and isolated
through a midline cervical incision. An embolus was advanced
through the ICA to the MCA until mild resistance was encountered
(20±2 mm), thereby occluding the origin of the MCA. The cervical
incision was closed with a silk suture. Reperfusion was achieved by
withdrawing the intraluminal occlusive embolus to restore blood
supply to the MCA area after 2 h of ischemia. For the rats in the
sham group, the left CCA, ECA and ICA were exposed, but no
ligations and occlusions were performed. The rectal temperature of
rats was maintained at 37˚C throughout the surgical procedures.
Animals were randomly divided into three groups
(n=8): the sham operation control group (SC), the ischemia control
group (IC) and the electroacupuncture group (EA). All rats were
studied for behavioral parameters at 2 and 24 h after I/R, and then
sacrificed for subsequent experiments.
EA stimulation
EA stimulation was applied to the acupuncture points
of Quchi (LI11) and Zusanli (ST36) on the right paralyzed limb by
using an EA stimulator instrument (Model G6805; SMIF, Shanghai,
China) after recovery from operation (2 h after I/R treatment). Two
stainless steel acupuncture needles 0.3 mm in diameter, which were
connected with the output terminals, were inserted at a depth of
2–3 mm into the above-mentioned acupuncture points. The stimulation
with 1- or 20-Hz frequencies was generated at an intensity of the
muscle twitch threshold (the muscle twitch threshold was
approximately 0.01 mA).
Neurological assessment
Neurological function was evaluated at 2 and 24 h
after I/R in a blinded fashion according to a standard scoring
system on a four-point scale (21): score 0, no apparent deficits;
score 1, failure to extend the right forepaw fully; score 2,
circling to the right; score 3, falling to the right; score 4, loss
of walking. In brief, rats scoring 1–3 points indicated successful
model establishment.
Measurement of the infarct volumes
Following cerebral I/R injury for 24 h, rats were
euthanized under deep anesthesia using 10% chloral hydrate and
perfused transcardiacally with 0.9% NaCl. The brains of all rats
were quickly removed for dissecting into five coronal blocks at a
thickness of 2 mm/section. The fresh slices were incubated in 2%
2,3,5-triphenyltetrazolium chloride (Sigma, St. Louis, MO, USA)
solution in phosphate-buffered saline (PBS) (Hyclone, Beijing,
China) at 37˚C for 20 min. The normal area of brain was stained
dark red based on intact mitochondrial function, whereas the
infarct area remained unstained. Stained slices were photographed
by a high-resolution digital camera (Cannon sx20). Using a
computerized image analysis system (Motic Med 6.0 System), the
total volume of infarction was determined by integration of the
areas from the sections.
Hematoxylin and eosin (H&E) staining
of the brain
Rats were anesthetized and perfused transcardiacally
with 0.9% NaCl and 4% paraformaldehyde through the left ventricle.
After the brain was removed, it was fixed in cold 4%
paraformaldehyde at 4˚C for 24 h. After dehydration, the specimens
were embedded in paraffin, cut into 5-μm sections and stained with
H&E. The histological morphology of each brain specimen was
examined under optical microscopy.
Direct immunofluorescence analysis of
NF-κB p65 nuclear translocation
The paraffin sections of brain tissues were treated
with microwave heat-induced epitope retrieval. After the specimens
were washed three times in PBS (pH 7.4), they were incubated for 1
h at 37˚C with a 150 dilution of rabbit anti-rat NF-κB p65 antibody
(green). The wash step was repeated. Nuclei of all cells were
counterstained with DAPI. After three washes with PBS, the tissues
were mounted in Prolong Gold Antifade reagent. Images were
collected by a confocal fluorescence microscope (Leiss LSM710) with
a magnification of ×200.
Western blot analysis
Total proteins were extracted from the infarct
cortex and protein concentrations were determined by BCA assay.
Samples, containing 50 μg proteins, were separated by
electrophoresis on 12% SDS-polyacrylamide gels. Subsequently,
proteins were transferred onto PVDF membranes in a Tris-glycine
transfer buffer. The membranes were blocked for 2 h with 5% nonfat
dry milk at room temperature and detected with rabbit anti-TLR4,
anti-NF-κB p65, anti-p-IκB and anti-β-actin antibodies (at a
dilution of 1:1,000) at 4˚C overnight, followed by incubation with
the appropriate HRP-conjugated secondary antibody for 50 min. The
bands were visualized with enhanced chemiluminescence, and images
were captured using a Bio-Image Analysis System (Bio-Rad, Hercules,
CA, USA).
RNA extraction and RT-PCR
Total-RNA from cerebral tissues were isolated with
the TRIzol reagent according to the manufacturer’s instructions.
Oligo(dT)-primed RNA (1 μg) was reverse transcribed into cDNA,
which was then used to determine the amount of TLR4, NF-κB p65 mRNA
by PCR. The primer sequences and the sizes of the amplification
were, for β-actin: forward, 5′-ACT GGC ATT GTG ATG GAC TC-3′ and
reverse, 5′-CAG CAC TGT GTT GGC ATA GA-3′; TLR4: forward, 5′-GGA
CTC TGC CCT GCC ACC ATT TA-3′ and reverse, 5′-CTT GTG C CCT GTG AGG
TCG TTG A-3′; NF-κB p65: forward, 5′-GTG CAG AAA GAA GAC ATTG AGG
TG-3′ and reverse, 5′-AGG CTA GGG TCA GCG TAT GG-3′. Samples were
analyzed by gel electrophoresis (1.5% agarose). The DNA bands were
examined using a Gel Documentation system (Model Gel Doc 2000;
Bio-Rad).
Determination of the serum level of
TNF-α, IL-1β and IL-6 by ELISA
Animal blood was obtained aseptically from abdominal
aorta. Blood-containing tubes were allowed to stand at room
temperature for 2 h, and sera were obtained by centrifugation at
3,000 × g for 20 min in 4˚C. The serum was assayed for levels of
TNF-α, IL-1β and IL-6 by ELISA kits (Shanghai XiTang Biological
Technology Co., Ltd.) according to the manufacture’s instructions.
Briefly, the wells were coated with 100 μl capture antibody at 4˚C.
After three washes, the wells were blocked with 200 μl assay
diluents at room temperature for 1 h, followed by another three
washes. Then, 100 μl diluted TNF-α, IL-1β or IL-6 standard and test
samples were added and incubated for 1 h at 37˚C. After repeated
washes, the substrate was added and incubated for 20 min at room
temperature. Absorbance at 450 nm wavelength was measured, and
protein concentration was determined by interpolation onto
absorbance curves generated by recombinant TNF-α, IL-1β or IL-6
protein standards using an ELISA reader (Model ELX800; BioTek,
USA).
Statistical analysis
Statistical data are expressed as the means ± SD.
Statistical analysis was performed with the Student’s t-test and
ANOVA using the SPSS package for Windows (Version 16.0).
Differences with P<0.05 were considered statistically
significant.
Results
Electroacupuncture at the acupoints of
Zusanli (ST36) and Quchi (LI11) displays neuroprotective activity
in I/R injured rats
The neuroprotective effect of electroacupuncture was
examined by neurological assessment and evaluation of cerebral
infarct volume. Rats in the IC and EA groups displayed obvious
manifestation of neurological deficits and cerebral infarction,
whereas rats in the SC group did not show any signs of cerebral
injury (P<0.05 vs. the SC group), indicating the success of the
model construction (Table I and
Fig. 1). In addition, prior to
electric stimulation, the rats in the EA group did not show any
remarkable difference in clinical evaluation compared with the IC
group rats. However, 24 h after electroacupuncture at the acupoints
of Zusanli (ST36) and Quchi (LI11) the neurological function was
improved and the cerebral infarct volumes were reduced
significantly (P<0.05 vs. the IC group), demonstrating the
therapeutic efficacy of electroacupuncture against cerebral I/R
injury.
 | Table INeurological assessment of rats. |
Table I
Neurological assessment of rats.
| Group (n=8) | 2 h after IR | 24 h after IR |
|---|
| SC | 0 | 0 |
| IC | 2.38±0.74 | 2.13±0.83 |
| EA | 2.50±0.53 | 1.38±0.52a |
Electroacupuncture alleviates cerebral
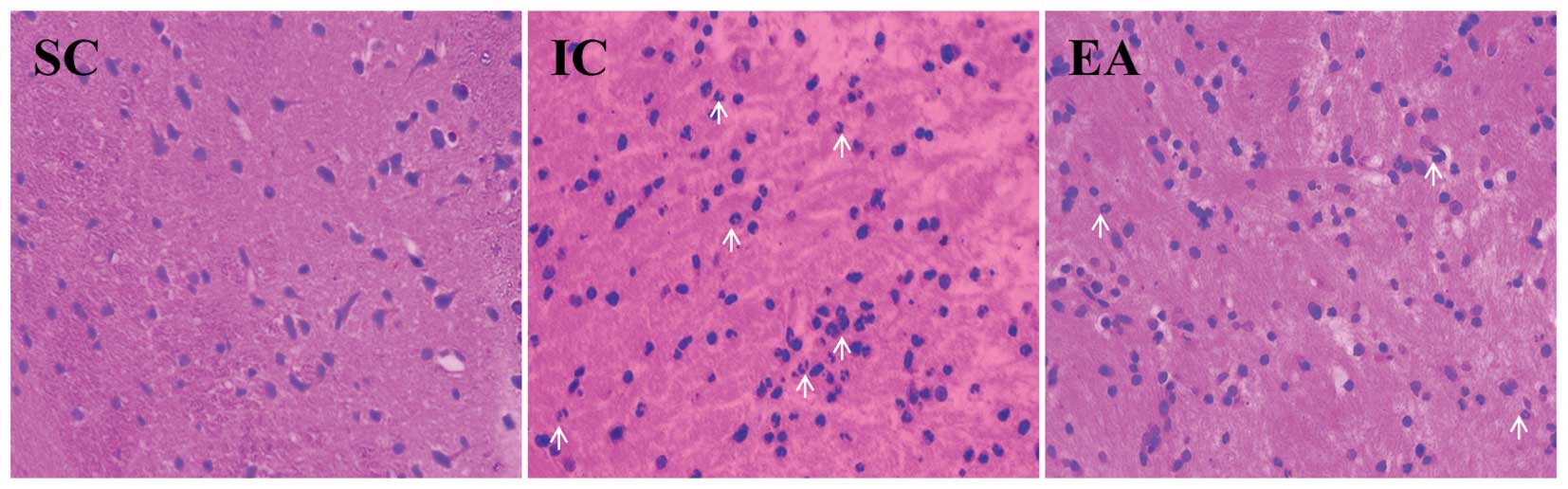
inflammation in cerebral I/R injured rats
Brain damage caused by cerebral I/R is partly
attributed to secondary injury from inflammation for a short time
after the onset of cerebral ischemia, therefore developing
anti-inflammation therapies at the early stage of ischemia has
become an attractive strategy to combat cerebral lesion. To
determine the anti-inflammatory efficacy of acupuncture in ischemic
cerebral tissues, the I/R-induced cerebral histopathological
changes and the degree of inflammatory cell infiltration in infarct
areas were observed under optical microscope after H&E
staining. As expected, no histopathological abnormalities and
inflammatory cells were observed in the SC group rats. By contrast,
in the infarct core zone of the IC group rats the glial and neuron
cells appeared shrunken and showed condensed nuclei, which,
however, was ameliorated by electroacupuncture (Fig. 2). Moreover, compared to the IC
group, much fewer inflammatory cells were infiltrated into the
cerebral infarct areas in the EA group, suggesting that
electroacupuncture alleviates I/R-mediated cerebral
inflammation.
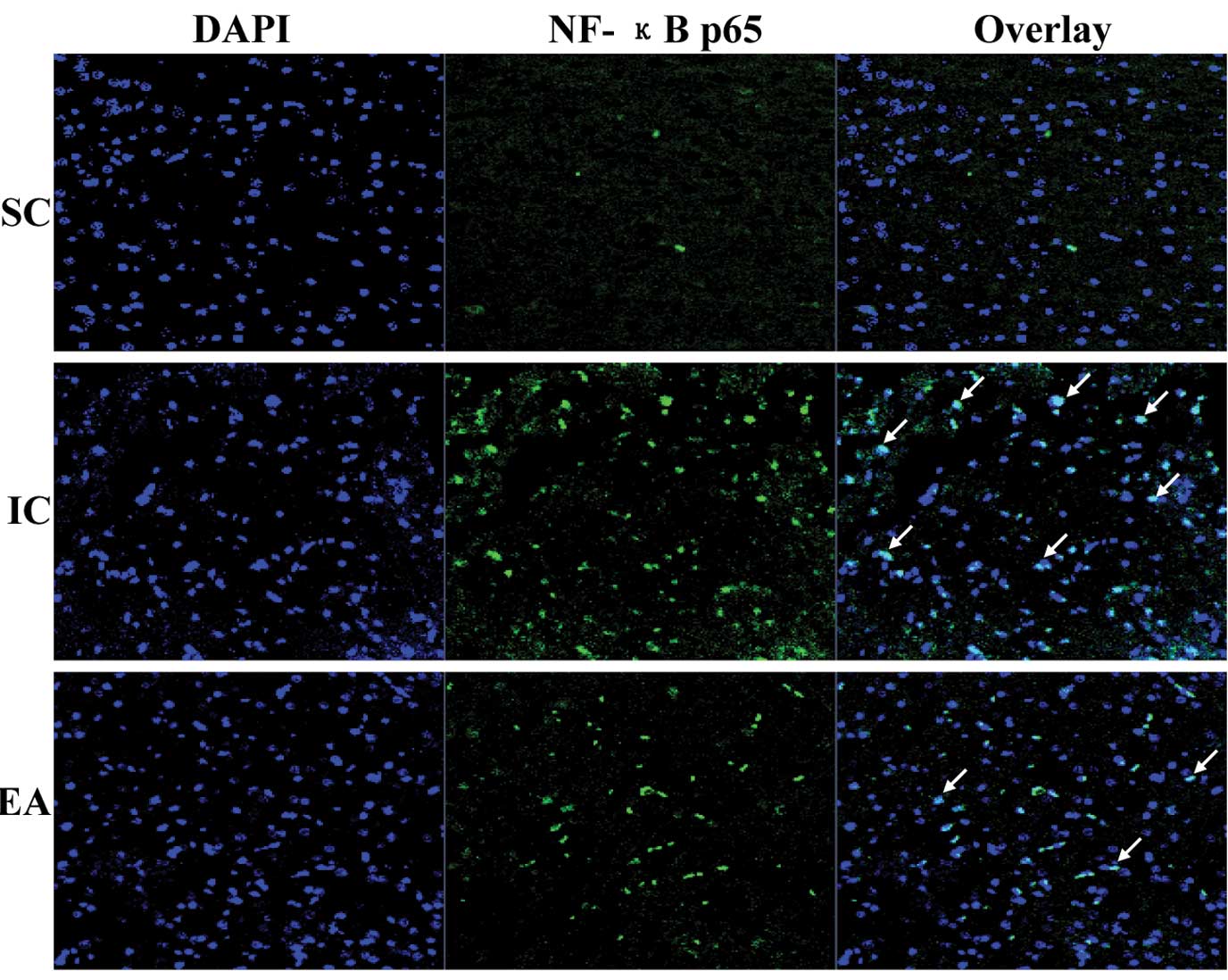
Electroacupuncture suppresses the
activation of the TLR4/NF-κB pathway in cerebral I/R injured
rats
TLR4 can activate a common signaling pathway by
inducing IκB phosphorylation and degradation and culminating the
translocation of NF-κB from cytoplasm into nucleus, which arouse
downstream-associated pro-inflammatory cytokines. To explore the
anti-inflammatory mechanism of electroacupuncture, we examined its
effect on the TLR4/NF-κB signaling pathway in ischemic cerebral
tissues. The expression of TLR4 and NF-κB p65 as well as the
phosphorylation level of IκB were significantly increased in the IC
group compared with those in the SC group, which, however, was
significantly neutralized by electroacupuncture (Fig. 3). To further verify these results,
we evaluated the effect of electroacupuncture on nuclear
translocation of NF-κB which is a critical step for NF-κB
activation. The NF-κB p65 subunit was visualized by
immunofluorescence staining and the cells were counterstained with
DAPI; NF-κB nuclear translocation was recognized by the
co-localization of p65 subunit with DAPI. Cerebral I/R injury
resulted in the nuclear translocation of NF-κB p65 subunit, which
was not observed in the sham operation group (Fig. 4). However, electroacupuncture
profoundly inhibited I/R-induced NF-κB nuclear translocation. This
indicates that the anti-inflammatory effect of electroacupuncture
at Zusanli and Quchi is mediated by suppression of TLR4/NF-κB
signaling in cerebral I/R injured rats.
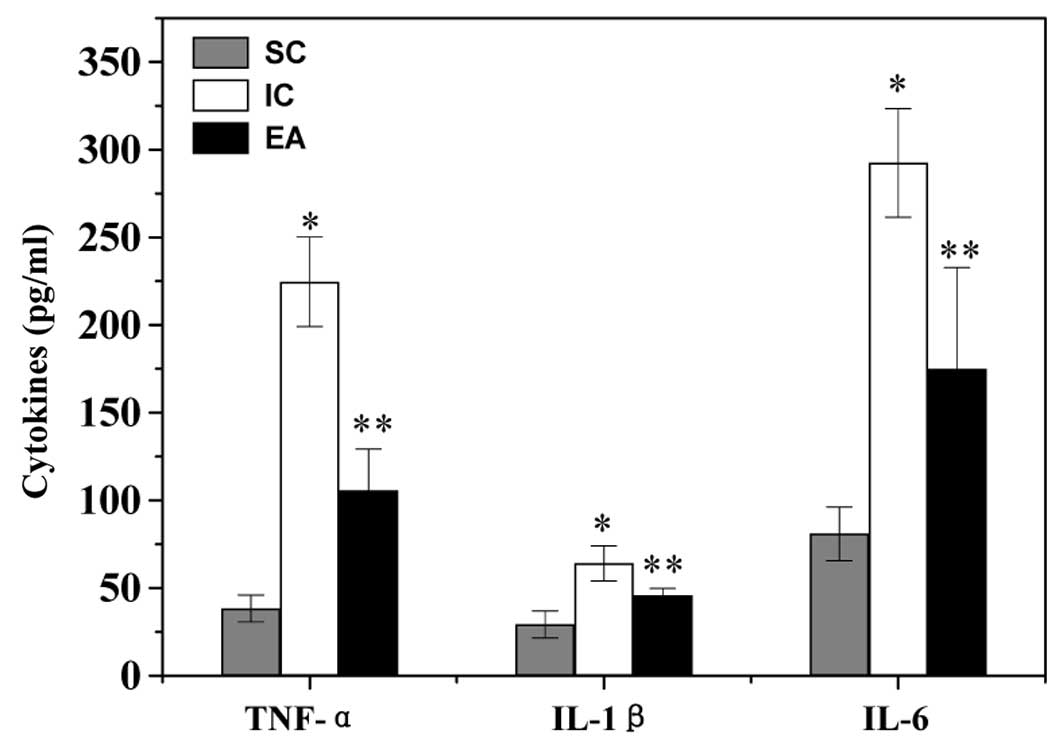
Electroacupuncture regulates the
secretion level of inflammatory cytokines in cerebral I/R injured
rats
To identify the downstream effectors in the
TLR4/NF-κB pathway, the secretion level of cytokines was examined
by ELISA. The serum level of pro-inflammatory TNF-α, IL-1β and IL-6
in the IC group was significantly increased, compared to that in
the SC group (P<0.05) (Fig.
5). However, electroacupuncture at Zusanli and Quchi profoundly
inhibited I/R-induced secretion of TNF-α, IL-1β and IL-6 (P<0.05
vs. the IC group).
Discussion
Acupuncture has served as a major complementary and
alternative therapy that supplements conventional medicine and has
been used in patients with acute ischemic stroke (5–9,22).
On the basis of a multitude of documents and materials, the Zusanli
(ST36) and Quchi (LI11) acupoints were commonly used in China to
clinically treat cerebral ischemia-reperfusion (I/R) injury
(11,12). In the present study, by using the
model of MCAO followed by reperfusion which is a classical model of
cerebral I/R, we demonstrated that electroacupuncture at Zusanli
and Quchi displayed neuroprotective effects as evidenced by
improving neurological deficits and reducing cerebral infarct
volume.
Brain damage caused by cerebral I/R is partly
attributed to secondary injury from inflammation for a short time
after the onset of cerebral ischemia (23). Therefore, developing
anti-inflammation therapies at the early stage of ischemia has been
an attractive strategy to combat cerebral lesion. Acupuncture has
been reported to exert anti-inflammatory effects in several
non-infectious disease models of the nervous system, such as spinal
cord injury and amyotrophic lateral sclerosis (24,25). To determine the anti-inflammatory
efficacy of acupuncture in cerebral I/R, we visualized the degree
of inflammation in cerebral I/R cord by H&E-staining. The
results confirmed that electroacupuncture at Zusanli and Quchi for
only 24 h already attenuated the histopathological changes
infiltration of neutrophils in the cerebral infarct areas.
Depending on the cerebral I/R injury, TLR4 can
activate a common signaling pathway by triggering IκB
phosphorylation and degradation and culminating the translocation
of NF-κB from cytoplasm into nucleus, which arouse
downstream-associated pro-inflammatory cytokines such as TNF-α,
IL-1β and IL-6 that in turn induce inflammation response (26–28). In the present study, we found that
the TLR4/NF-κB pathway was activated at 24 h after cerebral I/R
injury, which was consistent with previous studies (29–31). However, the activation of
TLR4/NF-κB signaling induced by cerebral ischemia was neutralized
by systemic electroacupuncture intervention through downregulating
important target genes of the TLR4/NF-κB pathway. As expected, we
also found that electroacupuncture decreased the serum levels of
pro-inflammatory cytokines (TNF-α, IL-1β and IL-6).
In conclusion, we report for the first time that
electroacupuncture at the Quchi (LI11) and Zusanli (ST36) acupoints
on the paralyzed limb reduces ischemic brain damage, improves
neurological deficits and exerts anti-inflammation function against
ischemic stroke via inhibition of the TLR4/NF-κB pathway. These
results suggest that electroacupuncture may be a potential
therapeutic modality for cerebral ischemia.
Acknowledgements
This study was sponsored by the Special Program for
Key Basic Research Project of the China Ministry of Science and
Technology (973 Program, no. 2010CB534900), and the National
Natural Science Foundation of China (no. 30901935).
Abbreviations:
|
TLR4
|
Toll-like receptor 4
|
|
NF-κB
|
nuclear factor-κB
|
|
I/R
|
ischemia/reperfusion
|
|
MCAO
|
middle cerebral artery occlusion
|
|
EA
|
electroacupuncture
|
|
TTC
|
2,3,5-triphenyltetrazolium
chloride
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Feigin VL: Stroke epidemiology in the
developing world. Lancet. 365:2160–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carolei A, Sacco S, De Santis F and Marini
C: Epidemiology of stroke. Clin Exp Hypertens. 24:479–483. 2002.
View Article : Google Scholar
|
|
3
|
Jorgensen HS, Nakayama H, Pedersen PM, et
al: Epidemiology of stroke-related disability. Clin Geriatr Med.
15:785–799. 1999.PubMed/NCBI
|
|
4
|
Kim SK and Bae H: Acupuncture and immune
modulation. Auton Neurosci. 157:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang GC, Fu WB, Xu NG, Liu JH, Zhu XP,
Liang ZH, Huang YF and Chen YF: Meta analysis of the curative
effect of acupuncture on post-stroke depression. J Tradit Chin Med.
32:6–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu HH, Chung C, Liu T, et al: A randomized
controlled trial on the treatment for acute partial ischemic stroke
with acupuncture. Neuroepidemiology. 12:106–113. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jansen G, Lundeberg T, Kjartansson J and
Samuelson U: Acupuncture and sensory neuropeptides increase
cutaneous blood flow in rats. Neurosci Lett. 97:305–309. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johansson K, Lindgren I, Widner H, Wiklund
I and Johansson B: Can sensory stimulation improve the functional
outcome in stroke patients? Neurology. 43:2189–2192. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magnusson M, Johansson K and Johansson BB:
Sensory stimulation promotes normalization of postural control
after stroke. Stroke. 25:1176–1180. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao J, Xue XH, Chen LD, Yang SL, Jiang M,
Gao YL and Wang XB: Electroacupuncture improves neurological
deficits and enhances proliferation and differentiation of
endogenous nerve stem cells in rats with focal cerebral ischemia.
Neurol Res. 32:198–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Gu HW, Ma WP, et al: Multicentral
randomized controlled study on effects of acupuncture at Zusanli
(ST 36) and Xuanzhong (GB 39) on cerebrovascular function in the
patient of ischemic stroke. Zhongguo Zhen Jiu. 26:851–853. 2006.(In
Chinese).
|
|
12
|
Fu WB, Guo Y, Chen XK, et al:
Comprehensive therapeutic protocol of electroacupuncture combined
with Chinese herbs and rehabilitation training for treatment of
cerebral infarction: a multi-center randomized controlled trial.
Zhongguo Zhen Jiu. 30:6–9. 2010.(In Chinese).
|
|
13
|
Iadecola C and Alexander M: Cerebral
ischemia and inflammation. Curr Opin Neurol. 14:89–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kriz J and Lalancette-Hébert M:
Inflammation, plasticity and real-time imaging after cerebral
ischemia. Acta Neuropathol. 117:497–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bowie A and O’Neill L: The interleukin-1
receptor/Toll-like receptor superfamily: signal generators for
pro-inflammatory interleukins and microbial products. J Leukoc
Biol. 67:508–514. 2000.PubMed/NCBI
|
|
16
|
Slack JL, Schooley K, Bonnert TP, et al:
Identification of two major sites in the type I interleukin-1
receptor cytoplasmic region responsible for coupling to
pro-inflammatory signaling pathways. J Biol Chem. 275:4670–4678.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson GB, Brunn GJ, Kodaira Y and Platt
JL: Receptor-mediated monitoring of tissue well-being via detection
of soluble heparan sulfate by Toll-like receptor 4. J Immunol.
168:5233–5239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehnardt S, Schott E, Trimbuch T, et al: A
vicious cycle involving release of heat shock protein 60 from
injured cells and activation of Toll-like receptor 4 mediates
neurodegeneration in the CNS. J Neurosci. 28:2320–2331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smiley ST, King JA and Hancock WW:
Fibrinogen stimulates macrophage chemokine secretion through
Toll-like receptor 4. J Immunol. 167:2887–2894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang H, Kwon YD and Yoon SS: Use of
acupuncture therapy as a supplement to conventional medical
treatments for acute ischemic stroke patients in an academic
medical centre in Korea. Complement Ther Med. 19:256–263. 2011.
View Article : Google Scholar
|
|
23
|
Ikeda K, Negishi H and Yamori Y:
Antioxidant nutrients and hypoxia/ischemia brain injury in rodents.
Toxicology. 189:55–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi DC, Lee JY, Moon YJ, et al:
Acupuncture-mediated inhibition of inflammation facilitates
significant functional recovery after spinal cord injury. Neurobiol
Dis. 39:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang JH, Yang EJ, Baek MG, et al:
Anti-inflammatory effects of electroacupuncture in the respiratory
system of a symptomatic amyotrophic lateral sclerosis animal model.
Neurodegener Dis. 8:504–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ridder DA and Schwaninger M: NF-kappaB
signaling in cerebral ischemia. Neuroscience. 158:995–1000. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barton GM and Medzhitov R: Toll-like
receptor signaling pathways. Science. 300:1524–1525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blanco AM, Pascual M, Valles SL and Guerri
C: Ethanol-induced iNOS and COX-2 expression in cultured astrocytes
via NF-kappa B. Neuroreport. 15:681–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Fang X, Tong Y, et al:
TLR4-mediated MyD88-dependent signaling pathway is activated by
cerebral ischemia-reperfusion in cortex in mice. Biomed
Pharmacother. 63:442–450. 2009. View Article : Google Scholar
|
|
30
|
Tu XK, Yang WZ, Shi SS, et al: Baicalin
inhibits TLR2/4 signaling pathway in rat brain following permanent
cerebral ischemia. Inflammation. 34:463–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan H, Li L, Zhang X, et al: Oxymatrine
downregulates TLR4, TLR2, MyD88, and NF-kappaB and protects rat
brains against focal ischemia. Mediators Inflamm.
2009:7047062009.PubMed/NCBI
|