Introduction
The physiological function of the intestinal barrier
regulates selective passage from the gut lumen, i.e. the transport
of ions or small molecules but not large molecules and
microorganisms (1). The
intestinal barrier is composed of both a cellular barrier and a
paracellular barrier. The paracellular barrier largely consists of
the tight junction (TJ) between epithelial cells (1). The TJ is composed of tight junction
proteins (TJPs) which is a group of proteins with various functions
and molecular structures. The TJPs regulate the function of the
intestinal barrier through their expression level and functional
status (2). A change in the
levels of TJPs is considered as a marker of impaired intestinal
barrier and increased mucosal permeability in many diseases
(3).
Previous studies have shown that TJPs are involved
in the regulation of intestinal permeability (4). Recently, changes in the cytoskeleton
dynamics have also been noted in the alteration of intestinal
permeability (5). It has been
shown that in the T84 colon cancer cell line, disrupted F-actin
results in increased paracellular permeability (6). The regulation of intestinal
permeability is a dynamic system in which the mobility of
intestinal epithelial cells is critically involved. It has been
noted that their interaction is the key to the regulation of
intestinal permeability (4).
However, little is known concerning the regulation of intestinal
paracellular permeability in regard to the interaction among TJPs
and cytoskeleton proteins.
Microtubes (MTs) are required for cellular mobility
and maintenance of cellular morphology (7). Dynamic cytoskeleton formation by
microfilaments and MTs determines cellular mobility and cell shape
(8). Kodama et al
(9) demonstrated that ACF7 is
critically required in MT-microfilament dynamics. ACF7 can
stabilize downstream cytoskeleton structure by either direct
binding to MTs or forming links between MTs and microfilaments.
In this study, we employed a conditional gene
targeting method to ablate the ACF7 gene in colonic mucosa in order
to create ACF7-conditional knockout (cKO) mice. We also employed
FITC-inulin, an effective tracer for paracellular permeability
assay (10). By utilizing a
Ussing chamber and FITC-inulin, we confirmed this mouse as a murine
model for decreased colonic paracellular permeability. Hematoxylin
and eosin (H&E) staining revealed histopatholigical changes in
the mucosal epithelial arrangement and interstitial proliferation.
Since TJPs are vital cytoskeletal proteins, we determined the
transcription and expression levels of ZO-1, occludin and
claudin-1, which are typical members of the TJPs. Our findings
revealed that the three TJPs were downregulated in this ACF7-cKO
model. Immunofluorescence for the three proteins also found weak
staining for ACF7 in the cKO mice.
Therefore, based on this significant evidence we
hypothesize that ACF7 regulates tight junction protein expression,
changes mucosal epithelial arrangement and consequently alters
colonic paracellular permeability.
Materials and methods
Generation of ACF7-cKO mice
Due to the lethal effect of ACF7 deficiency in
conventional knockout models (11), we crossed ACF7fl/fl
mice (12) and Vil-cre transgenic
mice to generate cKO mice. ACF7fl/fl mice were kindly
donated by Xiaoyang Wu of the Howard Hughes Medical Institute and
Laboratory of Mammalian Cell Biology and Development, Rockefeller
University, New York, NY, USA. B6.SJL-Tg(Vil-cre)997Gum/J mice of
C57BL/6 background were purchased from Jackson Laboratory (Bar
Harbor, ME, USA).
Vil-cre transgenic mice were identified by PCR using
DNA extracted from the peripheral blood of 4-week-old transgenic
mice using PCR primers: forward, 5′-GTGTGG GACAGAGAACAAACC-3′ and
reverse, 5′-ACATCTTCA GGTTCTGCG GG-3′. The PCR reaction generated a
1,100-bp product. Excision efficiency was assessed by PCR using the
following primers: upstream, 5′-AAAGAAACGGAAATA CTGGCC-3′ and
downstream, 5′-GCAGCTTAATTCTGCC AAATTC-3′; with 650- and 700-bp PCR
products, respectively. ACF7-cKO mice were generated by crossing F2
in a specific pathogen-free (SPF) environment. The Vil-cre and ACF7
genes were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China).
All mice were maintained in an SPF facility with a
regular light cycle (12 h light and 12 h dark) at a controlled
temperature (23±1°C) and relative humidity (50%). Food (SCXK
2008-0016; Shanghai Super B&K Laboratory Animal Corp., Ltd.,
Shanghai, China) and water were freely available to mice throughout
the experimental period. The Sixth People’s Hospital Animal Care
and Utilization Committee Affiliated to Shanghai Jiaotong
University approved all experiments relating to ethical
standards.
H&E staining
Intestinal samples were fixed in 10% formalin for 12
h, and then embedded in paraffin. Sections (3-μm) were stained with
H&E and evaluated by a pathologist. Microscopy of ×200 and ×400
fields was applied to capture typical images using a DSY5000X
microscope and Nikon D200 camera.
Measurement of colonic mucosal
paracellular permeability by Ussing chamber and FITC-inulin
Mice were sacrificed at 8–12 weeks of age by
cervical dislocation. The proximal segment of the colon was
dissected, of which the mucosa was stripped from the muscular layer
within 10 min. The mucosa was mounted in Lucite chambers exposing
the mucosal and submucosa surfaces to 10 ml of oxygenated Krebs
buffer. The buffer was maintained at 37°C by a heated water jacket
and circulated by CO2/O2 (13).
Non-absorbable tracer molecule FITC-inulin (1.0
mg/ml) (MW 5,000 kDa) (Sigma-Aldrich, USA) was added to the mucosal
side with a spread mucosa area of 0.3 cm2. Buffer
samples of 200 μl from the submucosa side were collected at 30, 60,
90 and 120 min, and samples were analyzed for fluorescence in black
walled 96-well plates (Greiner, Germany) using a Varioskan Flash
Scanner (Thermo Scientific, Finland) with excitation at 485 nm and
emission at 530 nm (14,15).
RNA extraction and qRT-PCR
ACF7-cKO and ACF7fl/fl mice were
sacrificed at 12 weeks of age by cervical dislocation. A segment of
the colon was dissected at 1.5 cm distal to the cecum. Each colon
sample was scissored to expose its interior on clean slides. The
mucosa was scraped from the muscular layer and preserved in
RNAlater (Invitrogen, USA) solution at −20°C for further
analysis.
We used qRT-PCR to estimate the expression of TJPs
(ZO-1, occludin and claudin-1). Briefly, 30 mg of the mucosa scraps
was homogenized for RNA extraction. RNA was extracted using a total
RNA extraction kit (SLNCO, China), followed by reverse
transcription using a qPCR-RT kit (Toyobo Co., Ltd., Japan). cDNA
was then evaluated by qRT-PCR using Real-Time PCR Master Mix
(Toyobo Co., Ltd.) in a FTC-3000 PCR Cycler (Funglyn Biotech, Inc.,
Canada) using the primers (Generay Biotech Co, Ltd., China) listed
in Table I. Denaturation,
annealing and extension temperatures were set at 95°C, 60°C and
68°C for 30 sec each, respectively, for 40 cycles according to
routine procedures.
 | Table IPrimers for qRT-PCR of GAPDH,
occludin, claudin-1 and ZO-1. |
Table I
Primers for qRT-PCR of GAPDH,
occludin, claudin-1 and ZO-1.
| Gene | Primers | Sequence (5′-3′) | Products (bp) |
|---|
| GAPDH | Ga-F |
AGGTTGTCTCCTGCGACTTCA | 143 |
| (internal
control) | Ga-R |
GAGGTCCACCACTCTGTTGCT | |
| Occludin | Oc-F |
GCAGCCTTCTGCTTCATCG | 168 |
| Oc-R |
CGTCGGGTTCACTCCCATTA | |
| Claudin-1 | Ca-F |
TGGGTTTCATCCTGGCTTCT | 163 |
| Ca-R |
TGTATCTGCCCGGTGCTTT | |
| ZO-1 | Zo-F |
TCACGATCTCCTGACCAACG | 271 |
| Zo-R |
GGCTGACGGGTAAATCCACA | |
Western blot analysis
Cytoplasmic proteins were extracted from mucosal
samples from ACF7-cKO and ACF7fl/fl mice using a
nucleic/plasma protein extraction kit (ViaGene, USA). After
quantitation, cytoplasmic protein was then separated by
SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Samples were
separated on 5% SDS-PAGE for ACF7 protein and on 10% SDS-PAGE for
ZO-1, occludin and claudin-1 for 1 h respectively (Bio-Rad, USA)
and transferred onto nitrocellulose membranes (Millipore, USA) at
4°C, 200 mA, overnight for ACF7 and 1 h for ZO-1, occludin and
claudin-1. The membranes were blocked for 1 h at room temperature
in 5% non-fat dried milk, and then incubated with the primary
antibody (1:500; Abcam, UK) with continuous gentle agitation
overnight at 4°C. The membranes were then incubated for 1 h with
HRP-conjugated secondary antibodies (1:2,000; Beyotime, China) at
room temperature, and finally developed by the ECL western blotting
system (Thermo Scientific-Pierce, USA).
Immunofluorescence
Colonic tissue sections were incubated with
anti-mouse occludin, anti-mouse claudin-1, anti-mouse ZO-1 (1:100;
Abcam), and FITC-labeled goat anti-mouse IgG (1:50; Sigma, USA) was
used as a secondary antibody. Typical images were captured using a
DSY5000X microscope and Nikon D200 camera at a ×400 magnification
(excitation at 485 nm).
Statistical analysis
Data are presented as means ± SEM. The differences
in mRNA levels of ACF7, ZO-1, occludin and claudin-1, between the
ACF7-cKO and ACF7fl/fl were compared using an unpaired
t-test. Differences in the FITC-inulin filtration rates using a
Ussing chamber were compared by monofactor ANOVA analysis
(GraphPad, Prism, USA). P-value below the level of α=0.05 was
considered to indicate a statistically significant result.
Results
Generation of conditional intestinal
ACF7-cKO mice
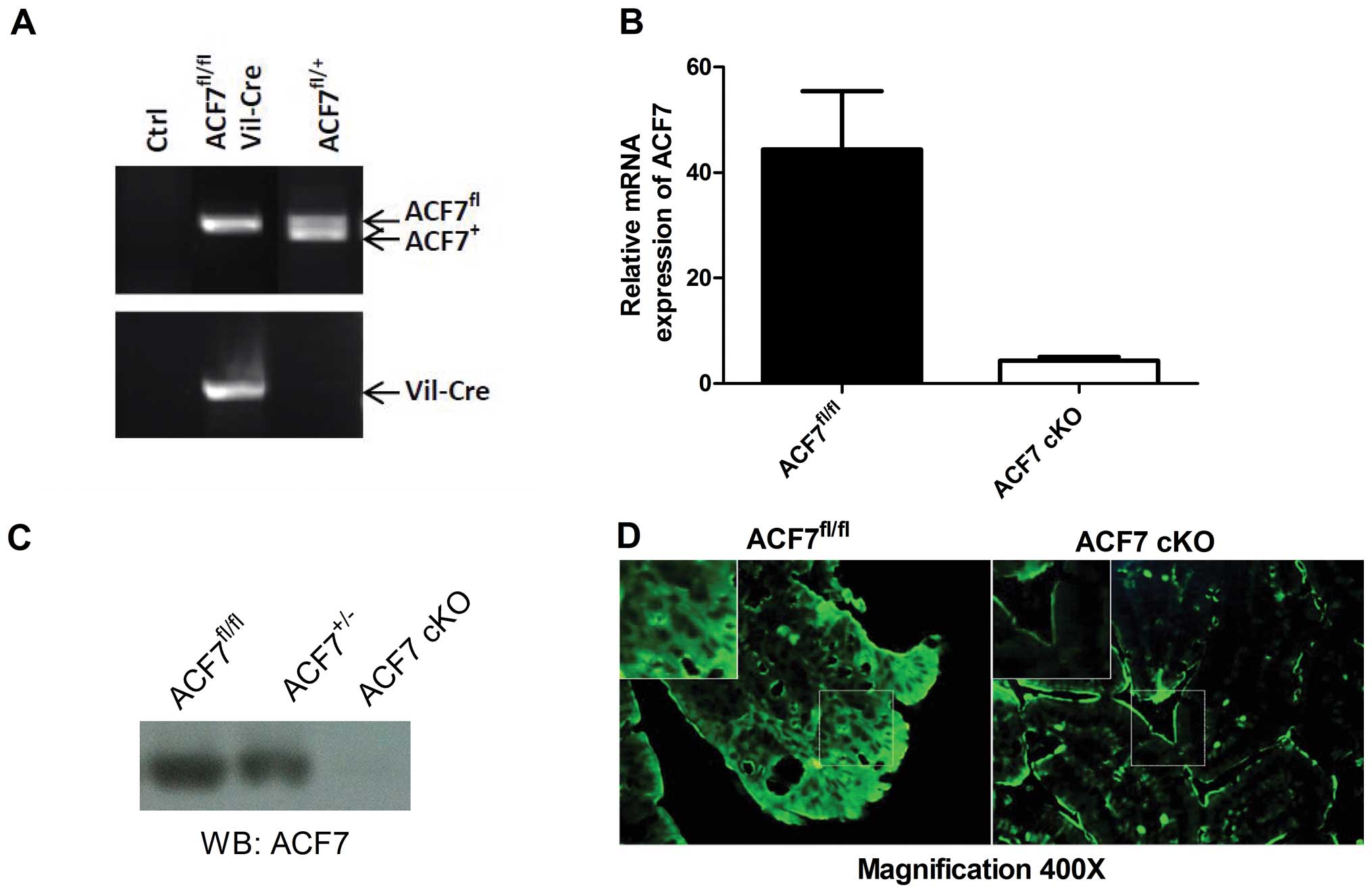
By genotyping, we successfully identified ACF7-cKO
mice (Fig. 1A). As expected, the
relative level of ACF7 mRNA declined drastically in the colonic
mucosa of the ACF7-cKO mice when compared to that in the control
ACF7fl/fl mice (0.097-fold; 4.309±0.73 vs. 44.36±11.08;
P<0.01, unpaired t-test; n=5/group) (Fig. 1B). Immunoblot analysis against the
ACF7 protein showed that ACF7 protein was decreased in the colonic
mucosa of the ACF7 mice and no ACF7 protein was evident in the
ACF7-cKO mice (Fig. 1C).
Fluorescent immunostaining also showed weak ACF7 expression in the
colonic mucosa in the ACF7-cKO mice compared to that in the control
ACF7fl/fl mice (Fig.
1D).
Rearrangement of mucosal epithelia and
interstitial proliferation as detected by H&E staining
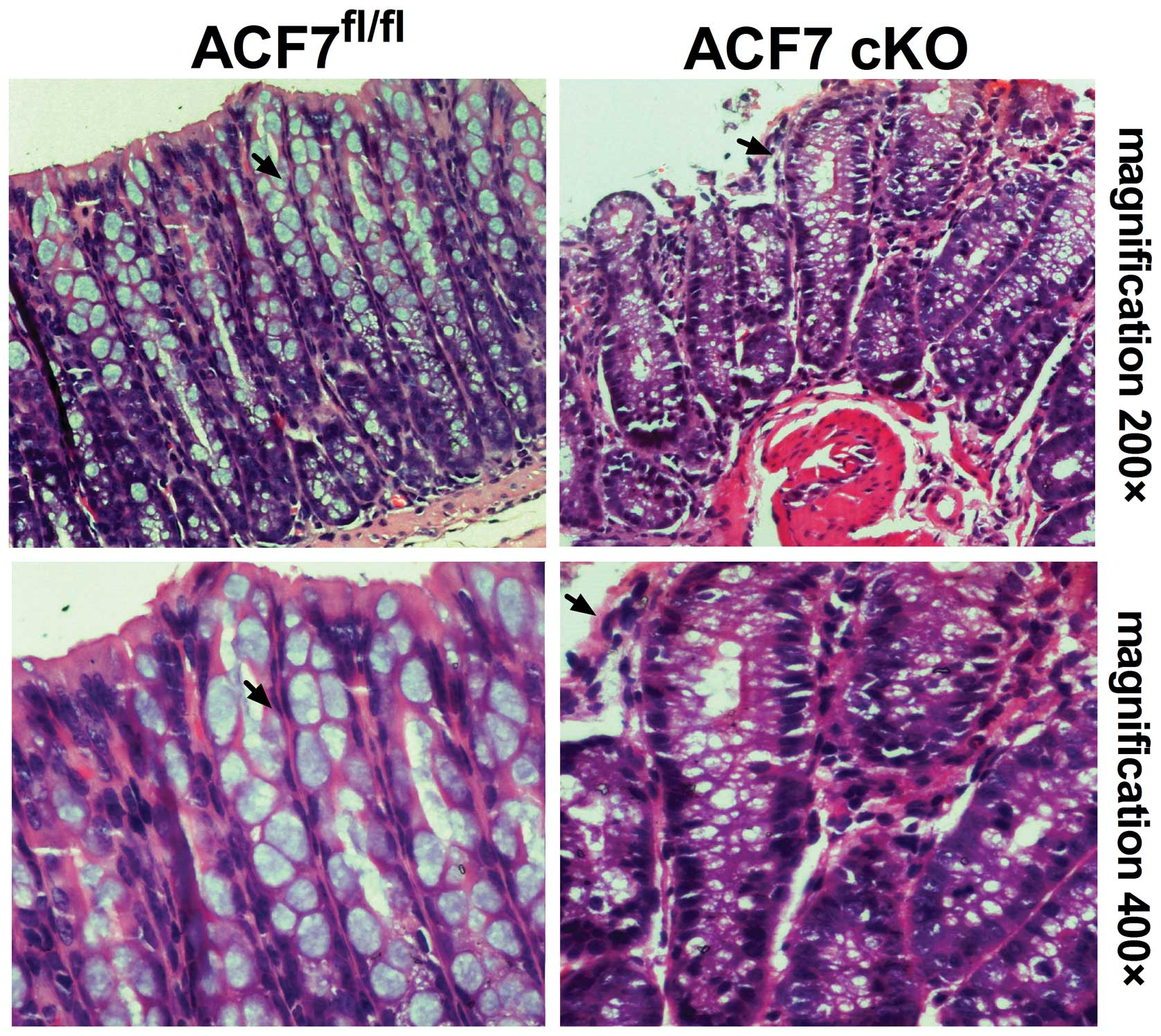
Through H&E staining, we demonstrated
interstitial proliferation in the colonic sections of the ACF7-cKO
mice. The results also revealed significant mucosal epithelial
rearrangement in the ACF7-cKO mice when compared to the control
(Fig 2).
Decreased colonic paracellular
permeability in ACF7-cKO mice
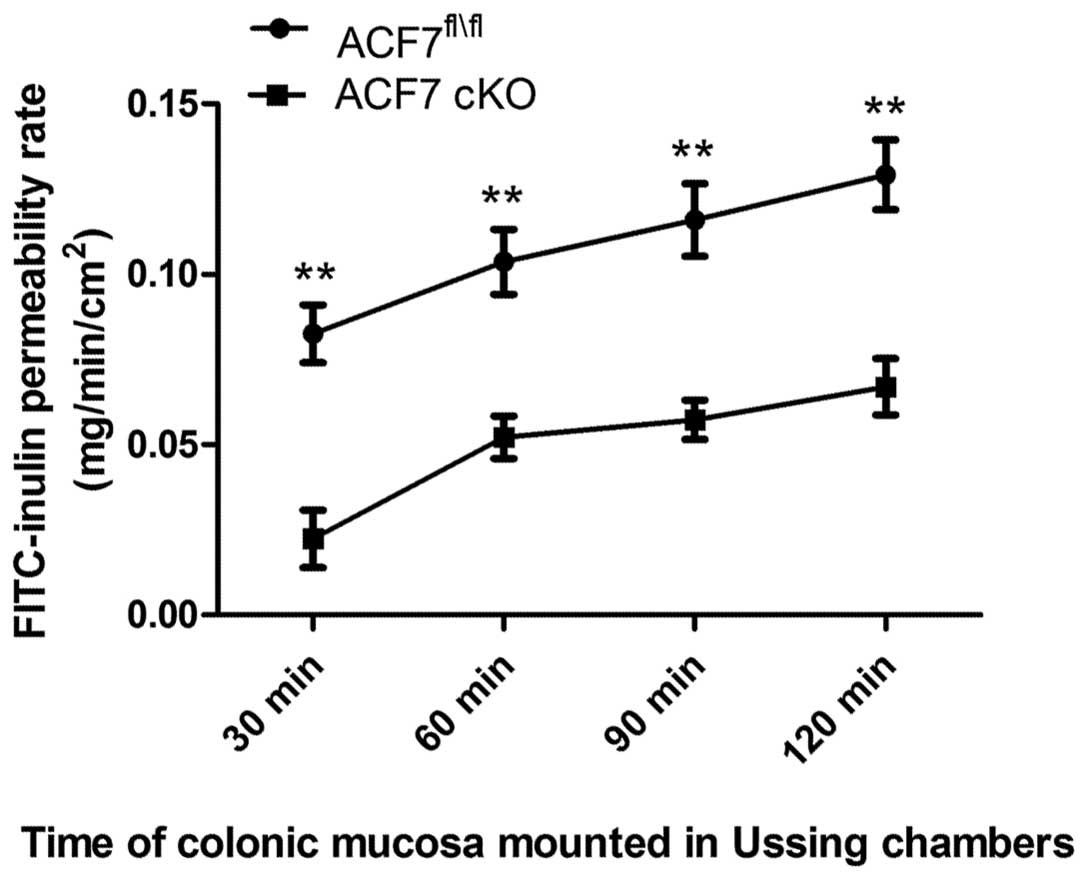
We found a significant decrease in the colonic
FITC-inulin permeability rate between the ACF7fl/fl and
ACF7-cKO mice at 30 min (0.083±0.0085 vs. 0.022±0.0086), 60 min
(0.104±0.0095 vs. 0.052±0.0066), 90 min (0.116±0.0106 vs.
0.057±0.0057) and 120 min (0.129±0.0106 vs. 0.067±0.0083) (one-way
ANOVA test; n=5/group) (Fig.
3).
Decreased occludin, claudin-1 and ZO-1
expression in ACF7-deficient colonic mucosa
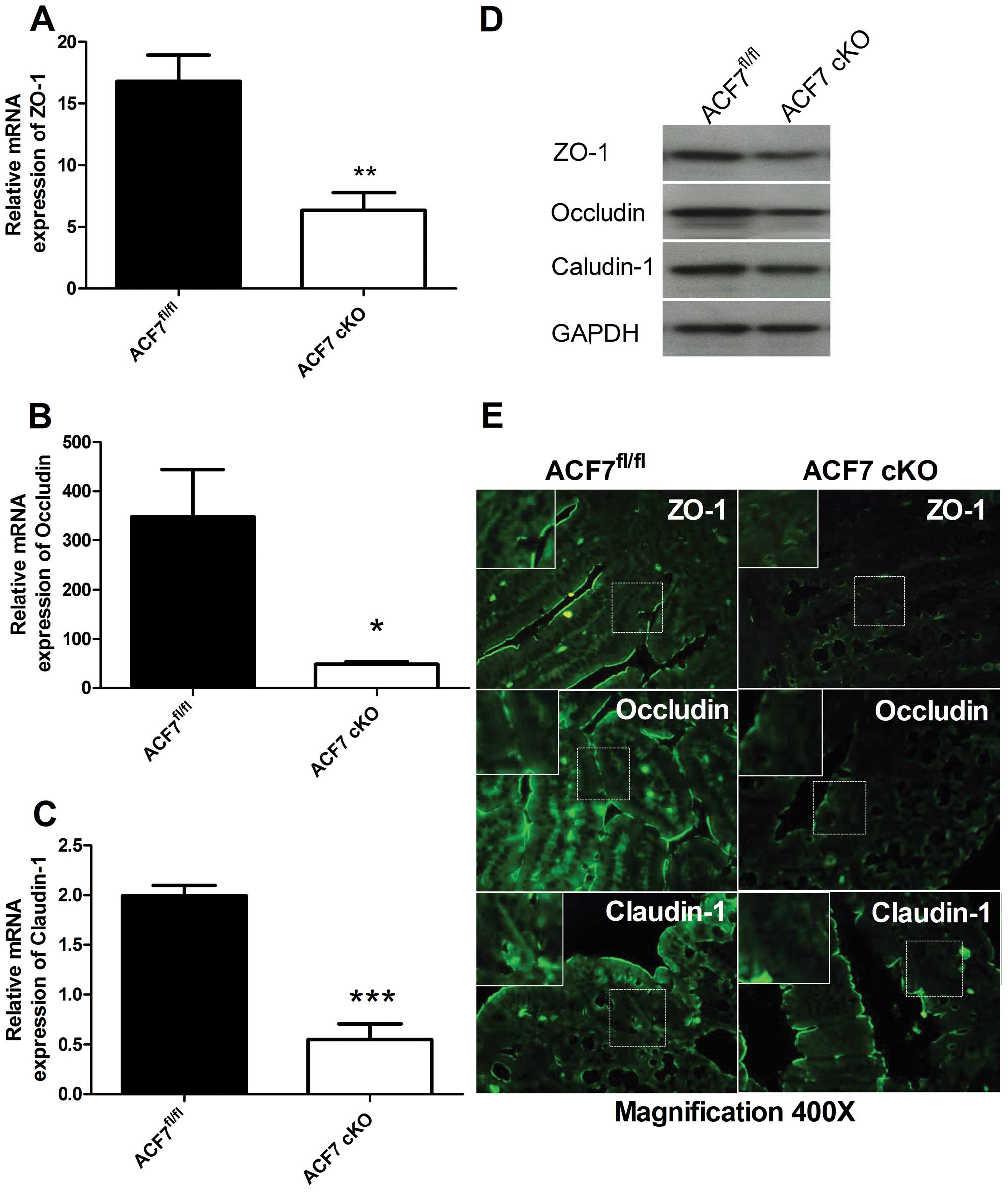
Compared to controls, the relative quantitative
value of mRNA expression of ZO-1, occludin and claudin-1 was
significantly lower in the ACF7-cKO mice by 2.65-fold (16.79±2.146
vs. 6.331±1.482, Fig. 4A),
7.22-fold (348.4±95.35 vs. 48.27±5.889, Fig. 4B) and 3.62-fold (1.990±0.104 vs.
0.550±0.157, Fig. 4C),
respectively (unpaired t-test; n=5/group). Western blot analyses of
the TJPs, ZO-1, occludin and claudin-1, revealed a decrease in
ZO-1, occludin and claudin-1 expression in the colonic tissues of
the ACF7-cKO mice vs. the ACF7fl/fl mice (n=5/group)
(Fig. 4D). Immunofluorescence
staining further demonstrated that ZO-1, occludin and claudin-1
were significantly downregulated in the colonic mucosa of ACF7-cKO
mice compared to levels in the ACF7fl/fl mice (Fig. 4E).
Discussion
ACF7 has recently been identified as a key
integrator in cellular skeleton dynamics and cell mobility
(16). Gene knockdown in an
animal, and observation of the entire animal phenotype are the most
effective methods of investigation of gene function (17–19). As gene knockout plays an important
role during development, traditional knockout sometimes leads to
the death of the embryo. Therefore, cKO utilizing the Cre-loxP
strategy may avoid this problem (20). Since the ACF7−/− mouse
displays fetal lethality (11),
we chose to establish a colonic mucosa ACF7-cKO mouse model.
Results of our Ussing chamber study indicated a significant
decrease in the colonic mucosal permeability in ACF7-cKO mice.
H&E staining of the colonic mucoca indicated a positive
association between the microscopy findings and cytoskeleton
dysregulation. By H&E staining, we observed that epithelial
arrangement was disturbed in the ACF7-deficient colonic mucosa, and
we hypothesized that cytoskeleton dysregulation results in an
alteration of colonic paracellular permeability. Ussing chamber and
FITC-inulin results of the colonic paracellular permeability were
also correlated with qRT-PCR, western blotting and
immunofluorescence results for ZO-1, occludin and claudin-1. The
significant decrease in these proteins indicated that TJPs were
correlated with ACF7 expression.
The interaction of epithelial TJPs with cytoskeleton
proteins has been recognized as essential. The tight junctions can
rapidly adapt, in response, to diverse external signals via
structural and functional linkage between TJPs and cytoskeleton
proteins (21). Tight junctions
are the most apical organelles of the apical junctional complex and
are primarily involved in the regulation of paracellular
permeability. Although previous researches have concerned with the
individual molecules of the tight junctions as well as their mutual
interactions. Only recently has it been noted to what extent TJPs
are important for the regulation of intestinal permeability and
which proteins are involved.
Occludin, claudin-1 are transmembrane proteins. They
are critically involved in the regulation of the TJ barrier
(22). Previous studies have
shown that the expression of occludin and claudin-1 is tightly
associated with intestinal paracellular permeability (23). Occludin expression levels
correlate with the number of tight junction strands in varied
colonic epithelia permeability. Occludin was found to be associated
with permeability for ions and small solutes in siRNA experiments
(24). In contrast, the role of
occludin in intestinal paracellular permeability has not been
recognized until one report revealed that occludin-deficient mice
exhibit normal epithelial and paracellular permeability (25). The family of claudin proteins
consists of a number of essential components associated with
deleterious barrier impairment in the event of their deficiency
(26). For example,
claudin-1-deficient mice died within 24 h of birth, and further
study found dramatic fluid and electrolyte loss through intestinal
mucoca and skin (27). It has
been shown in inflammatory bowel disease (IBD) that decreased ZO-1
expression is associated with intestinal paracellular permeability
(28). ZO-1 is a peripheral
membrane protein that binds to both the C terminal of occludin and
F-actin, thus stabilizing the cytoskeleton structure (29). ZO-1 connects to strand-forming
TJPs with cytoskeletal proteins (actin and microfilaments)
(30). One animal model with
upregulation of TJPs showed a decrease in intestinal paracellular
permeability under GvHD condition (31). Thus, there are different points of
view and some are paradoxical.
The previously mentioned studies reflect one
important fact. Since these models are established on a complex
physiological shock condition and lack long-term homeostasis, the
results are varied. Thus, we chose to establish ACF7-knockout mice
instead, generating a homogeneous and stable model with which to
provide strong evidence for a change in paracellular permeability.
Consequently, based on these findings, ACF7 alters the mucosal
epithelial arrangement, colonic paracellular permeability and
regulates tight junction protein expression. The ACF7-cKO mouse is
a suitable model with which to study intestinal permeability
regulatory mechanisms and related diseases.
Acknowledgements
The authors acknowledge Dr Xiaoyang Wu of the Howard
Hughes Medical Institute and Laboratory of Mammalian Cell Biology
and Development, Rockefeller University, New York, NY, USA for his
kind donation of ACF7fl/fl mice; Professor Dazheng Wu of
the Shanghai University of TCM for supplying the facilicities for
Ussing Chamber test and his assistance in the research; Sibo Zhu of
the CinoAsia Institute, Shanghai, China for his techinical
guidance. This study was supported by three grants from Medical
School of Shanghai Jiaotong University PhD Inovation Funding
(BXJ201236), LIJIESHOU Funding (LJS_201108) and NFSC Funding
(81070293).
References
|
1
|
Catalioto RM, Maggi CA and Giuliani S:
Intestinal epithelial barrier dysfunction in disease and possible
therapeutical interventions. Curr Med Chem. 18:398–426. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hossain Z and Hirata T: Molecular
mechanism of intestinal permeability: interaction at tight
junctions. Mol Biosyst. 4:1181–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gasbarrini G and Montalto M: Structure and
function of tight junctions. Role in intestinal barrier. Ital J
Gastroenterol Hepatol. 31:481–488. 1999.PubMed/NCBI
|
|
4
|
Ulluwishewa D, Anderson RC, McNabb WC,
Moughan PJ, Wells JM and Roy NC: Regulation of tight junction
permeability by intestinal bacteria and dietary components. J Nutr.
141:769–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen L, Su L and Turner JR: Mechanisms and
functional implications of intestinal barrier defects. Dig Dis.
27:443–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Madara JL, Stafford J, Barenberg D and
Carlson S: Functional coupling of tight junctions and
microfilaments in T84 monolayers. Am J Physiol. 254:G416–G423.
1988.PubMed/NCBI
|
|
7
|
Watanabe T, Noritake J and Kaibuchi K:
Regulation of microtubules in cell migration. Trends Cell Biol.
15:76–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsvetkov AS, Samsonov A, Akhmanova A,
Galjart N and Popov SV: Microtubule-binding proteins CLASP1 and
CLASP2 interact with actin filaments. Cell Motil Cytoskeleton.
64:519–530. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kodama A, Karakesisoglou I, Wong E, Vaezi
A and Fuchs E: ACF7: an essential integrator of microtubule
dynamics. Cell. 115:343–354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghandehari H, Smith PL, Ellens H, Yeh PY
and Kopecek J: Size-dependent permeability of hydrophilic probes
across rabbit colonic epithelium. J Pharmacol Exp Ther.
280:747–753. 1997.PubMed/NCBI
|
|
11
|
Chen HJ, Lin CM, Lin CS, Perez-Olle R,
Leung CL and Liem RK: The role of microtubule actin cross-linking
factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev.
20:1933–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Kodama A and Fuchs E: ACF7 regulates
cytoskeletal-focal adhesion dynamics and migration and has ATPase
activity. Cell. 135:137–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arrieta MC, Madsen K, Doyle J and Meddings
J: Reducing small intestinal permeability attenuates colitis in the
IL10 gene-deficient mouse. Gut. 58:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baker NT and Graham LL:
Campylobacter fetus translocation across Caco-2 cell
monolayers. Microb Pathog. 49:260–272. 2010. View Article : Google Scholar
|
|
15
|
Volpe DA: Application of method
suitability for drug permeability classification. AAPS J.
12:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Shen QT, Oristian DS, et al: Skin
stem cells orchestrate directional migration by regulating
microtubule-ACF7 connections through GSK3β. Cell. 144:341–352.
2011.PubMed/NCBI
|
|
17
|
Austin CP, Battey JF, Bradley A, et al:
The knockout mouse project. Nat Genet. 36:921–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown SD and Hancock JM: The mouse genome.
Genome Dyn. 2:33–45. 2006. View Article : Google Scholar
|
|
19
|
Dinnyes A and Szmolenszky A: Animal
cloning by nuclear transfer: state-of-the-art and future
perspectives. Acta Biochim Pol. 52:585–588. 2005.PubMed/NCBI
|
|
20
|
Kühn R and Schwenk F: Advances in gene
targeting methods. Curr Opin Immunol. 9:183–188. 1997.
|
|
21
|
Madara JL, Barenberg D and Carlson S:
Effects of cytochalasin D on occluding junctions of intestinal
absorptive cells: further evidence that the cytoskeleton may
influence paracellular permeability and junctional charge
selectivity. J Cell Biol. 102:2125–2136. 1986. View Article : Google Scholar
|
|
22
|
Tsukita S and Furuse M: Occludin and
claudins in tight-junction strands: leading or supporting players?
Trends Cell Biol. 9:268–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye D, Guo S, Al-Sadi R and Ma TY: MicroRNA
regulation of intestinal epithelial tight junction permeability.
Gastroenterology. 141:1323–1333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu AS, McCarthy KM, Francis SA, et al:
Knockdown of occludin expression leads to diverse phenotypic
alterations in epithelial cells. Am J Physiol Cell Physiol.
288:C1231–C1241. 2005. View Article : Google Scholar
|
|
25
|
Schulzke JD, Gitter AH, Mankertz J, et al:
Epithelial transport and barrier function in occludin-deficient
mice. Biochim Biophys Acta. 1669:34–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuse M and Moriwaki K: The role of
claudin-based tight junctions in morphogenesis. Ann NY Acad Sci.
1165:58–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furuse M, Hata M, Furuse K, et al:
Claudin-based tight junctions are crucial for the mammalian
epidermal barrier: a lesson from claudin-1-deficient mice. J Cell
Biol. 156:1099–1111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piche T, Barbara G, Aubert P, et al:
Impaired intestinal barrier integrity in the colon of patients with
irritable bowel syndrome: involvement of soluble mediators. Gut.
58:196–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keon BH, Schäfer S, Kuhn C, Grund C and
Franke WW: Symplekin, a novel type of tight junction plaque
protein. J Cell Biol. 134:1003–1018. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fanning AS and Anderson JM: Zonula
occludens-1 and -2 are cytosolic scaffolds that regulate the
assembly of cellular junctions. Ann NY Acad Sci. 1165:113–120.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noth R, Lange-Grumfeld J, Stüber E, et al:
Increased intestinal permeability and tight junction disruption by
altered expression and localization of occludin in a murine graft
versus host disease model. BMC Gastroenterol. 11:1092011.
View Article : Google Scholar : PubMed/NCBI
|