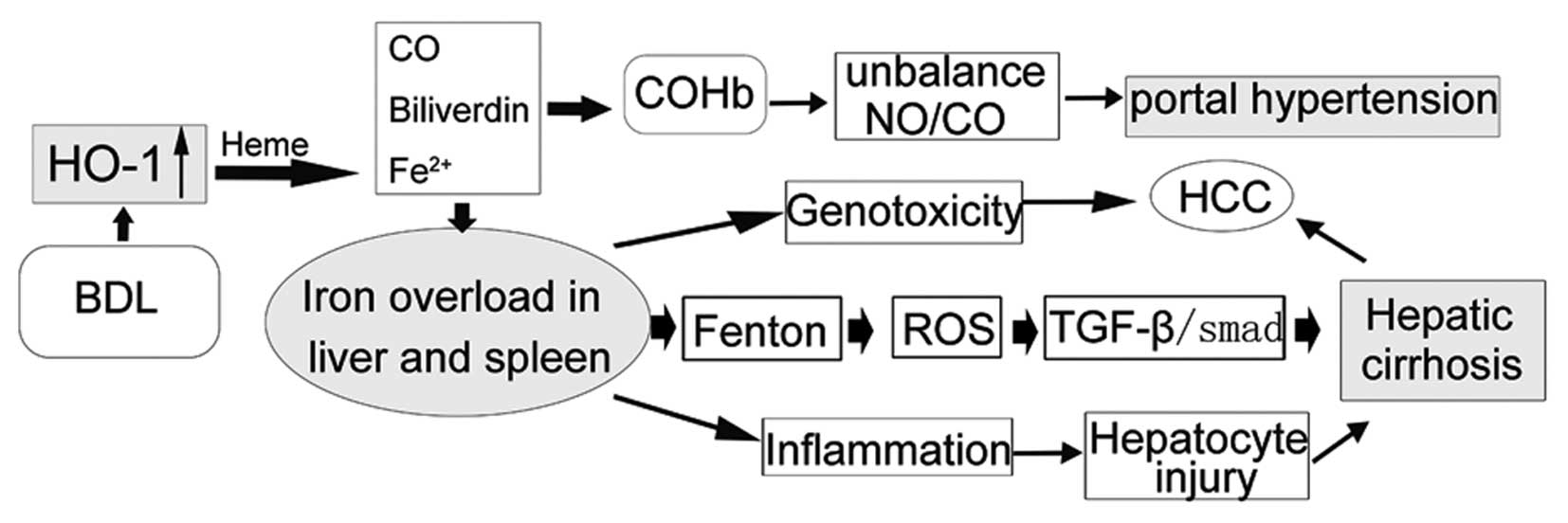
Introduction
The spleen is connected to the other abdominal
organs by the portal system. Portal hypertension and splenomegaly
are often found in end-stage hepatic cirrhosis. As a treatment
option, splenectomy is more widely accepted for patients without a
donor suitable for liver transplantation (1). It is reported that splenectomy has
beneficial effects on ameliorating reperfusion injury (2,3)
and that it also induces a significant reduction in portal system
pressure, improving hepatic function (4,5).
Splenectomy also improves hepatic microcirculation, decreases
spleen-derived endothelin-1, activates eNOS signaling, and inhibits
the Rho-kinase pathway in rats with secondary biliary cirrhosis
(6). Splenectomy also ameliorates
liver injury in rats by mediating heme oxygenase-1 (HO-1)
induction, thus yielding beneficial effects of massive hepatectomy
and ischemia/reperfusion (7,8).
The mechanism by which splenectomy influences cirrhotic liver
remains unclear.
Previous studies, including our own, show that the
HO/carbon monoxide (CO) pathway is involved in hepatic fibrosis and
contributes to the hyperdynamic circulatory syndrome (9,10).
HO-1 catalyses the oxidative degradation of heme to CO, free iron,
and biliverdin (11). CO, a
gaseous messenger similar to NO, leads to the production of cGMP,
which mediates vasodilation (12). Our previous studies indicated that
the HO/CO pathway may contribute to renal and pulmonary
vasodilation in cirrhotic rats induced by bile duct ligation (BDL)
(13,14). Whether splenectomy portal vein
pressure (PVP) decreases are associated with a change in the HO/CO
pathway is not clear.
Cirrhotic patients often have hypersplenism due to
splenomegaly. Therefore, leukopenia, thrombocytopenia, and anemia
are major concerns in cirrhotic patients (15). Damaged erythrocytes may lead to
iron overload as each unit of erythrocytes contains approximately
250 mg of iron, thus increasing the risk of iron accumulation
derived from increased heme degradation. Iron is an essential
nutrient for growth and survival, but excess liver iron in
particular induces oxidative stress and hepatic fibrogenesis
(16). Iron is primarily
accumulated in reticuloendothelial cells. The spleen also recycles
iron stored in bone marrow and is essential for the removal of
stored blood cells, and certain immune functions. Whether and how
splenectomy influences iron homeostasis remains unclear.
Given the above-mentioned issues, the current study
sought to evaluate how splenectomy impacts iron and CO levels, in
part by reducing HO-1 expression, which further decreased PVP and
reduced hepatic fibroproliferation.
Materials and methods
Animal care
The Animal Care and Use Committee of Dalian Medical
University (Liaoning, China) approved the experimental protocols in
accordance with guidelines established by the China Council on
Animal Care.
Bile duct ligation and treatment of
rats
The fifty healthy male SD rats, weighing 200–220 g,
were obtained from the Laboratory Animal Center of Dalian Medical
University and were randomly divided into six treatment groups:
sham (n=6), BDL (n=10), cobalt protoporphyrin (CoPP) (n=10), zinc
protoporphyrin (ZnPP) (n=8), iron-dextran (Fe) (n=8) and
splenectomy (n=8). They were housed in a specific pathogen free
(SPF) center at a room temperature of 24–26°C and relative humidity
of 60–65%. Water was given ad libitum.
The rats were fed and housed for three days prior to
any experimental protocols. Biliary cirrhosis was induced by BDL
(17,18). Five groups underwent BDL together
with a sham-operated animal group as a control. The Animal Care and
Use Committee of Dalian Medical University approved all surgical
procedures. Laparotomy was performed under anesthesia with ether.
The bile duct was isolated and double-ligated with a 3-0 silk
suture. The abdominal wall and skin were closed with a 4-0 silk
suture, and antibiotic Gentamicin (0.3 ml) was injected
intramuscularly. The sham group underwent laparotomy with the bile
duct isolated but not ligated. Two weeks after surgery, sham and
BDL rats received a saline intraperitoneal injection. For the
splenectomy group, the abdomen was opened through a midline
incision, and the spleen was immobilized to the centre of the
operative field after dissecting the surrounding ligaments. The
hilar vessels were ligated with a 3-0 silk suture. The spleen was
removed and the abdominal incision was closed. Other groups
received an intraperitoneal injection of CoPP, ZnPP, or
iron-dextran (5, 5 and 50 mg/kg body weight), three times a week,
respectively. Following establishment of the rat models, the number
of rats was reduced to six in each group due to death in some
groups.
ZnPP and CoPP (Sigma, St. Louis, MO, USA) were
dissolved in 0.2 mol/l of NaOH, adjusted to pH 7.4 and diluted in
0.85% NaCl to 1 mg/ml as previously described, and used to inhibit
and induce HO-1 expression. Fe (Sigma) was diluted in 0.85% NaCl to
20 mg/ml. Histostain™-Plus (SP9001) (Zhongshan Golden Bridge
Biotechnology, Beijing, China), hydroxyproline (HYP), malonaldehyde
(MDA) and glutathione (GSH) kits (Nanjing KeyGen Biotech Co. Ltd.,
China), transforming growth factor-β1 (TGF-β1) enzyme-linked
immunosorbent assay (ELISA) kit (HopeYear Medical Products Co.,
Ltd., China), and Takara RNA polymerase chain reaction (PCR) kits
(AMV) version 3.0 (Takara Bio, Inc., Dalian, China) were used in
this study.
Sample collection and examination
Two weeks after treatment, a catheter connected to a
Pressure Transducer (BL-420F biological experimental system;
Chengdu Technology and Market Co., Ltd., China), was placed in the
portal vein to measure PVP. Subsequently, 1 ml of arterial blood
was withdrawn to measure carboxyhemoglobin (COHb) using a RapidLab
1245 Blood Gas Analyzer (Siemens, New York, NY, USA). Levels of
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
total bilirubin (TBIL), and serum iron were detected using a
Hitachi 7600-110 Automatic Biochemical Analyzer (Hitachi Co.,
Tokyo, Japan). Levels of MDA and GSH were determined by a UV-2100
Spectrophotometer (Chemito Instruments Pvt., Ltd., Mumbai,
India).
Serum TGF-β1
TGF-β1 expression was determined by ELISA at 450 nm.
Serum samples were assayed using 96-well microtiter plates coated
with a polyclonal antibody against TGF-β1.
Liver iron
Liver iron was determined by atomic absorption
spectrometry with acetylene-air flame atomization. Analysis was
performed using a Varian atomic absorption spectrometer (Mulgrave,
Victoria, Australia) with a deuterium background correction.
Measurements were performed with the analytical 248.3 nm line at a
spectral interval of 0.2 nm. Iron concentration was determined by
the standard addition method. Samples were digested in an MDS 2000
microwave sample preparation system (CEM) in Teflon cartridges
using a mixture of nitric acid (5 ml) and hydrogen peroxide (2 ml)
(ultrapure grade; both from Merck KGa, Darmstadt, Germany) for 20
min at the 120 psi. Resulting products were analyzed directly in
the Teflon cartridges.
Histology and immunohistochemistry
Part of the liver and spleen lobe were excised,
fixed in 10% neutral formalin solution, and embedded in paraffin.
Hematoxylin and eosin (H&E) staining and Van Gieson’s (VG)
staining were performed according to standard procedures. Lesion
severity was graded according to the methods described previously
(19). Briefly, tissue sections
(4 μm) were treated with HCl (5%) to liberate ferric ions. Samples
were then treated with 5% potassium ferrocyanide to produce
insoluble ferric ferrocyanide. Slides were counterstained with
neutral red. For immunohistochemical examination, deparaffinized
sections were incubated with HO-1 antibodies (1:1,000 dilution;
Abcam, Cambridge, MA, USA), or anti-α-smooth muscle actin (α-SMA)
antibody (1:100 dilution; Boster Biological Technology Ltd., Wuhan,
China) and biotinylated secondary antibodies, followed by
avidin-biotin-peroxidase complex. Images were analyzed by
Image-Pro-Plus 6.0 software (Media Cybernetics, Rockville, MD, USA)
to calculate area and mean density of positive expression.
Statistical results from five visual fields were averaged from each
sample.
Hepatic hydroxyproline content
Liver tissue (100 mg) was prepared for HYP
determination according to a modification of the kit method
described. The HYP content of the liver served as an indirect
measure of tissue collagen content and was expressed as
microgram/gram of wet weight (μg/g).
Western blot analysis
The resected liver tissues were extracted with lysis
buffer (1% Triton X-100; 50 mmol/l Tris-HCl, pH 7.6; 150 mmol/l
NaCl; and 1% protease inhibitor cocktail). Western blot analysis
protocols were previously described (20). Western blot analyses were
performed with liver homogenates (30 μg protein) using anti-HO-1
antibody (1:2,000 dilution; Abcam), anti-β-actin antibody (1:500
dilution; Zhongshan Golden Bridge Biotechnology), and secondary
anti-rabbit and anti-mouse IgG (1:500 dilution; Biosynthesis
Biotechnology, Beijing, China). The intensity of each signal was
corrected by the values obtained from the immunodetection of
β-actin and the relative protein intensity expressed as fold of the
content in the control group.
RNA isolation and gene expression
analysis
Total RNA was extracted from livers following a
standard guanidinium phenol-chloroform extraction protocol. The
quantity of RNA was determined by measuring the optical density at
260 nm (A260 nm = 1 for 40 μg/ml RNA), and RNA purity was assessed
by determining the A260/A280 nm ratio (pure RNA: A260/A280 nm =
2.0) using a UV-1206 spectrophotometer (Shimadzu, Kyoto, Japan). An
aliquot of each mixture was used for reverse transcription (RT)-PCR
amplification using reagents purchased from Takara Bio, Inc. The
primer sequences for HO-1 were 5′-ATATCTATACGGCCCTGGAA-3′
(forward), 5′-GATGCTCGGGAAGGTGAA-3′ (reverse) and the product size
was 350 bp, while the primer sequences for β-actin were
5′-GAGGGAAATCGTGCGTGAC-3′ (forward), 5′-CTGGAAGGTGGACAGTGAG-3′
(reverse) and the product size was 445 bp. PCR products were
separated by 2.5% agarose gel electrophoresis. The product bands
were photographed and the density of each band was quantified. The
results are expressed as the ratio of the band density for the
target mRNA to that of β-actin mRNA.
Statistical analysis
All data are presented as the means ± standard
deviation. Statistical analysis was performed with SPSS software
version 16.0 (IBM, Chicago, IL, USA). Groups were compared using
one-way analysis of variance (ANOVA) with Dunnett’s multiple
comparison test (where applicable). Correlative comparison of two
non-hierarchical variances with normal distribution was evaluated
by Pearson’s test, whereas the Spearman test was used for data with
a non-normal distribution. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Splenectomy ameliorates liver
fibroproliferation and decreases PVP
The common bile duct dilation, ascites, and jaundice
were found in all BDL rats at four weeks post-operation, suggesting
that BDL was successfully established. Two weeks after the
operation, the spleens were much bigger and heavier in the BDL
group relative to the sham group (P<0.01), and the body weight
(g)/spleen weight (g) ratio was larger in the BDL group (P<0.01)
(Table I).
 | Table IBody weight and spleen weight in the
sham and BDL groups at 2 weeks. |
Table I
Body weight and spleen weight in the
sham and BDL groups at 2 weeks.
| 2 weeks |
|---|
|
|
|---|
| Body weight
(g) | Spleen weight
(g) | Body weight
(g)/Spleen weight (g) (%) |
|---|
| Sham | 257±6 | 0.96±0.07 | 0.37±0.03 |
| BDL |
219±12a |
1.49±0.19a |
0.68±0.04a |
The serum levels of AST, ALT and TBIL in the BDL
group were significantly higher than those in the sham group
(P<0.01). Of note, they were at much lower levels in the
splenectomy group than in the BDL group (P<0.01) (Table II). These data indicate that
splenectomy improves liver function.
 | Table IISerum biochemical index analysis in
different groups (means ± SD, n=6/group). |
Table II
Serum biochemical index analysis in
different groups (means ± SD, n=6/group).
| AST (U/L) | ALT (U/L) | TBIL (mg/dl) |
|---|
| Sham | 139.00±4.52 | 36.83±1.72 | 0.83±0.23 |
| BDL |
255.83±7.55a |
49.83±4.36a |
11.90±1.96a |
| CoPP |
406.67±14.81c |
72.00±3.52c |
13.75±2.23a |
| ZnPP |
216.00±7.54c |
37.17±2.48c |
5.53±2.23c |
| Fe |
309.83±13.61c |
43.83±4.96c |
13.82±1.94a |
| Splenectomy |
202.17±6.85cf |
37.67±7.06c |
5.10±0.48c |
Hepatic fibrosis was evaluated by H&E staining.
The sham group showed normal architecture (Fig. 1Aa), whereas the BDL group
exhibited inflammation, necrosis, and destruction of the lobular
architecture (Fig. 1Ab). Compared
with the BDL group, less fibrous hyperplasia and fibrosis
extensions with fibroblast proliferation were noted in the
splenectomy group (Fig. 1Af). The
histopathological scores for liver fibrosis were higher than for
those that received a splenectomy (Fig. 1Ag). HYP, a product of collagen
metabolism, is an amino acid characteristic of collagen. Compared
with the BDL group, HYP content was appreciably lower following
splenectomy (P<0.01) (Fig.
1Ah).
 | Figure 1Assessment of liver fibrosis by
different methods. (A) H&E staining and HYP content. (a) Normal
lobular architecture in the sham group; (b, c and e) fibrous
hyperplasia in the BDL, CoPP and Fe groups; (d and f) less fibrous
hyperplasia in the ZnPP and splenectomy groups; (g and h) hepatic
fibrosis was assessed using histopathological scores and HYP
content (original magnification, ×100). (B) Liver sections were
stained with α-SMA antibody. (a) There was only slight α-SMA
expression around central veins in the sham group. (b, c and e)
Elevated expression was observed in the interlobular region in the
BDL, CoPP and Fe groups. (d and f) There was less color in the ZnPP
and splenectomy groups. The density (mean) of α-SMA was measured by
IPP. (g) There was low α-SMA expressed in the splenectomy and ZnPP
groups compared with BDL (P<0.01). (h) Serum TGF-β1 was detected
by ELISA (original magnification, ×100). (C) H&E staining for
spleen structure and COHb levels was measured. (a) Macrophages were
distributed in the red pulp and marginal zone in normal spleen. (b
and c) There were fewer macrophages in the red pulp at two and four
weeks. (d) The levels of COHb were quantified (original
magnification, ×400). (D) VG staining for collagen I in spleen and
PVP measurement. (a) Little collagen I deposited in normal spleen,
(b and c) but more accumulated in the spleen at two and four weeks.
(d) PVP levels were measured (original magnification, ×400). The
data are represented as the means ± SD. **P<0.01,
*P<0.05 vs. sham; ##P<0.01,
#P<0.05 vs. BDL; $$P<0.01,
$P<0.05 vs. ZnPP. a, Sham; b, BDL; c, CoPP; d, ZnPP;
e, Fe; and f, splenectomy. |
Protein expression levels of α-SMA and TGF-β1 were
significantly higher in the BDL group than in the sham group
(P<0.01) (Fig. 1B). However,
they were much lower in splenectomy-treated rats than in those
receiving BDL (P<0.01) (Fig.
1Bg). PVP levels were significantly higher in BDL- than in
sham-treated rats (P<0.01). PVP levels were reduced following
splenectomy, relative to BDL treatment (P<0.01) (Fig. 1Dd). These results suggest that
splenectomy not only decreased PVP, but also improved liver
function and reduced TGF-β1 secretion, further decreasing hepatic
fibrosis.
Splenectomy reduces iron accumulation by
inhibiting HO-1 activity
The mRNA and protein expression levels of liver HO-1
increased significantly following BDL treatment compared to sham
controls (P<0.01); they were lower in the splenectomy and ZnPP
groups (P<0.01) than in the BDL group, but were elevated in the
CoPP and Fe groups (Fig. 2Ag and h,
Bg and h). Hepatic immunostaining showed that HO-1 was
expressed mainly near centrilobular veins located on Kupffer cells,
and partly in mesenchymal cells (Fig.
2Aa-f). Lower biochemical indicators, levels of α-SMA and
TGF-β1 were lower in ZnPP-treated rats than in those receiving BDL
treatment, yet higher in the CoPP and Fe groups. These results
suggest that inhibiting HO-1 expression may improve liver function
and decrease fibrosis. On the contrary, it aggravated liver injury
(Fig. 1A and B).
 | Figure 2HO-1 and iron levels in liver. (A)
Liver sections were stained with HO-1 antibody. Brown staining
indicated immunopositivity. (a) HO-1 expression was minimal around
central veins in the sham group. (b, c and e) HO-1 was observed
around central veins in the BDL, CoPP and Fe groups. (d and f)
There was reduced staining in the ZnPP and splenectomy groups. (g
and h) HO-1 mRNA increased in the BDL group relative to sham and
were enhanced in the CoPP and Fe groups relative to the BDL group,
and decreased in the ZnPP and DFX groups (original magnification,
×400). (B) Perls’ Prussian blue staining positive for iron was
observed as blue stain. (a) No iron accumulated in the sham group.
(b) A little iron accumulated mainly on Kupffer cells in the BDL
group. (c) Substantially more iron accumulation was found in
interlobular and macrophagocytes in the CoPP group. (d and f)
Slight iron accumulation was detected in the ZnPP and splenectomy
groups. (e) Substantial iron accumulation was present in the Fe
group. (g and h) HO-1 protein increased in the BDL group relative
to sham and were enhanced in the CoPP and Fe groups relative to the
BDL group, and decreased in the ZnPP and DFX groups. (C) Serum and
liver iron content. Iron content in both liver and serum increased
more in the BDL than in the sham group. (a and b) Iron content
decreased in the ZnPP and splenectomy groups compared with BDL, and
enhanced Fe was observed in the CoPP and Fe groups. The data are
represented as the means ± SD. **P<0.01,
*P<0.05 vs. sham; ##P<0.01,
#P<0.05 vs. BDL; $$P<0.01,
$P<0.05 vs. ZnPP. a, Sham; b, BDL; c, CoPP; d, ZnPP;
e, Fe; and f, splenectomy. |
Immunostaining of BDL spleen revealed multiple and
patchy patterns of HO-1 (brown positive expression). Patterns were
visible prominently in red pulp, while those in white pulp were
few, if any (Fig. 3A-b). By
contrast, the brown stain in the spleen collected from the CoPP and
Fe-treated groups, and from macrophages in the red pulp, was
markedly elevated. Cross-sections of red pulp appeared to be
enlarged compared with analogous cross-sections from the BDL group,
which decreased in splenectomy and ZnPP-treated rats (Fig. 3Aa-f). Changes in HO-1 protein
expression levels in the spleen were similar to those in the liver
(Fig. 3B).
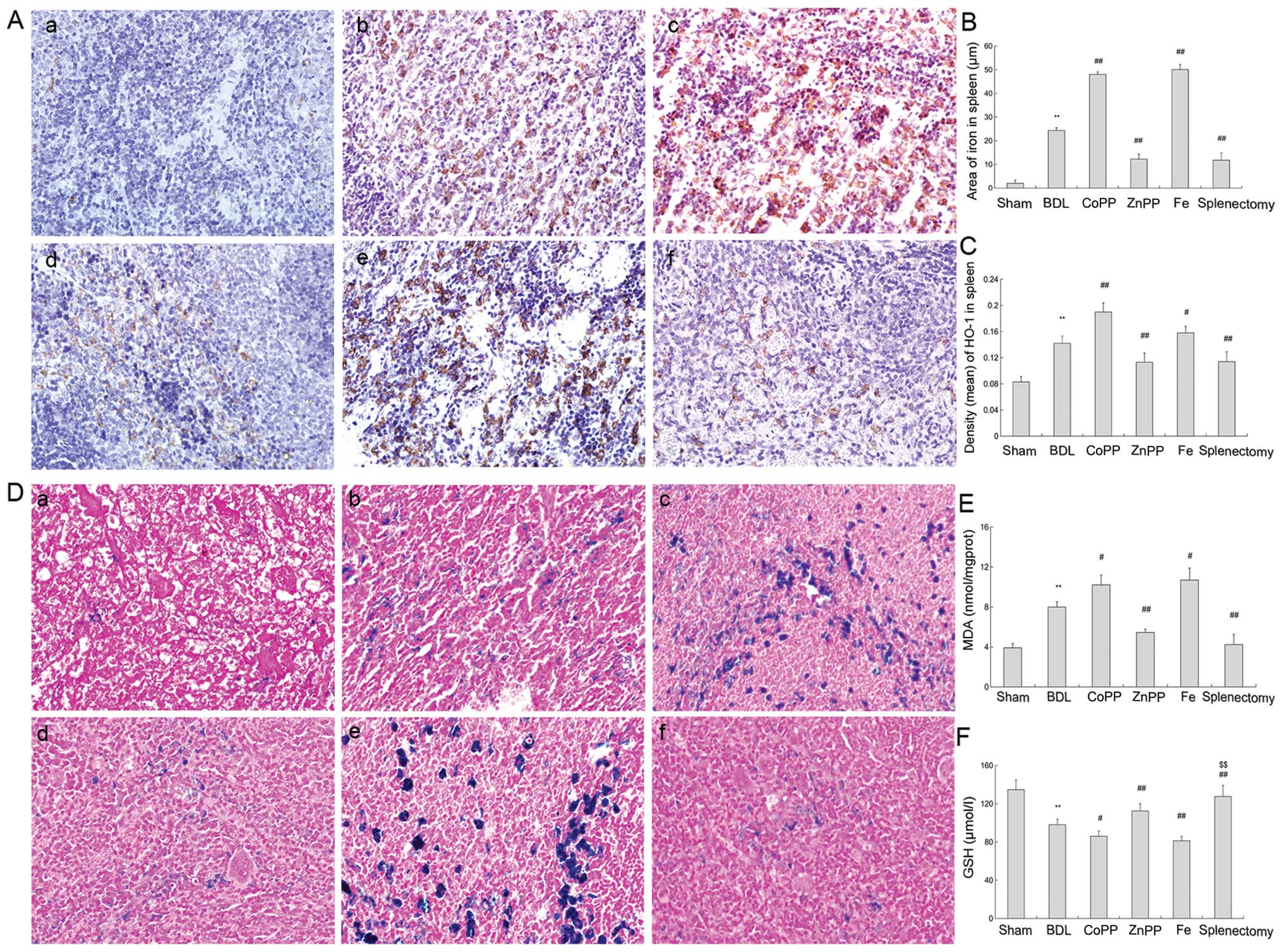 | Figure 3HO-1 and iron levels in the spleen.
(A) Spleen sections were stained with HO-1 antibody. (a) Slight
HO-1 expression was detected in the sham group. (b, c and e) There
was more HO-1 expression in red pulp in the BDL, CoPP and Fe
groups. (d and f) Compared with BDL rats, HO-1 expression decreased
in the ZnPP and splenectomy groups. (B) Density (mean) of HO-1 in
the spleen was measured by IPP, and was consistent with
immunohistochemistry results (P<0.01). (C) Area of iron in
spleen was detected by IPP. The iron levels were higher in BDL than
in sham rats. Iron levels were decreased in the ZnPP and
splenectomy groups compared with BDL, but enhanced in the CoPP and
Fe groups (P<0.01). (D) Perls’ Prussian blue staining in spleen.
(a) Little iron accumulated in the sham group. (b, c and e) Iron
accumulation was found in the red pulp in BDL, CoPP and Fe groups.
(d and f) Compared with the BDL group, less iron was found in the
ZnPP and splenectomy groups (original magnification, ×400). (E) MDA
levels in the liver were measured. MDA levels were markedly
enhanced in BDL rats relative to sham rats. HO-1 was increased in
the CoPP and Fe groups relative to the BDL group, but decreased in
the ZnPP and splenectomy groups (P<0.01). (F) GSH levels were
measured in the liver, and moved in opposition to MDA levels. The
data are represented as the means ± SD. **P<0.01,
*P<0.05 vs. sham; ##P<0.01,
#P<0.05 vs. BDL; $$P<0.01,
$P<0.05 vs. ZnPP. a, Sham; b, BDL; c, CoPP; d, ZnPP;
e, Fe; and f, spleen from the splenectomy group at two weeks. |
The iron serum levels in the BDL group were
significantly higher than in sham controls (P<0.01), and were
markedly lower following splenectomy and ZnPP treatments than after
BDL treatment (P<0.01) (Fig.
2Ca). Also, the change of liver iron content correlated with
the change of serum iron levels (Fig.
2Cb). We used Prussian blue stain to localize iron accumulation
in hepatic and splenic tissue. In the liver and spleen, iron
accumulated in Kupffer cells and macrophages in the BDL, CoPP and
Fe groups (Figs. 2B and 3D). However, iron staining was
essentially absent in the sham, splenectomy, and ZnPP treated
groups (Fig. 2Ba, d and f). The
areas of iron accumulation in the spleen are shown in Fig. 3C.
These results indicate that the spleen plays an
important role in HO-1 expression and iron accumulation, and that
splenectomy reduces liver HO-1 expression and reduces iron
accumulation. By contrast, enhanced HO-1 expression led to
increased accumulation of hepatic iron.
The HO/CO pathway is involved in
regulating PVP
Splenic structure was evaluated by H&E staining
(Fig. 1C). In normal spleen,
macrophages were distributed in red pulp and marginal zones, but
were less prevalent in BDL spleen (Fig. 1Ca-c). Collagen deposits were also
detected in the spleen. More collagen fibers were found around the
splenic corpuscle in the 4th week following BDL treatment than in
the 2nd week (Fig. 1Da-c). This
may be one factor regulating PVP formation in cirrhotic rats.
The COHb level in arterial blood was significantly
higher in the BDL group than in the sham group (P<0.01),
significantly lower following splenectomy and ZnPP treatment, and
higher in the CoPP and Fe-treated groups than in the BDL group
(P<0.01) (Fig. 1Dd). PVP was
significantly higher in BDL than in sham (P<0.01). Compared with
BDL, PVP decreased following splenectomy and ZnPP treatment
(P<0.01), and was enhanced in CoPP and Fe rats (P<0.01)
(Fig. 1Dd). Moreover, PVP
decreased following splenectomy relative to ZnPP treatment
(P<0.05). We hypothesized that splenectomy may decrease COHb
levels and regulate PVP through the HO/CO pathway.
Splenectomy reduces oxidative stress
partly through the HO/CO pathway
MDA levels increased significantly following BDL
compared to sham controls, and GSH levels were reduced (P<0.01)
(Fig. 3E and F). Splenectomy
resulted in a lower level of MDA and elevated GSH levels relative
to BDL (P<0.01) (Fig. 3E).
This suggests that oxidative stress was activated in cirrhotic
rats.
The level of MDA was slightly higher in the CoPP and
Fe-treated groups than in the BDL group (P<0.05), but lower in
ZnPP-treated rats. GSH levels were significantly enhanced by ZnPP
treatment relative to BDL treatment (P<0.01), but decreased
following CoPP and Fe treatment (Fig.
3F). Moreover, GSH levels were elevated following splenectomy
compared to ZnPP treatment (P<0.01). Markedly, inhibiting HO-1
expression reduced oxidative stress, and induced HO-1 expression
and iron accumulation, leading to liver injury.
Correlation analysis revealed that both SOD and MDA
were significantly correlated with HYP levels (R=−0.838, 0.871,
respectively, P<0.01). These data show that oxidative stress may
lead to extracellular matrix (ECM) deposition in the liver and
aggravate hepatic fibrosis. These data further indicate that
reducing oxidative stress may lighten hepatic fibrosis, partly
through the HO/CO pathway after splenectomy.
Discussion
Splenectomy is an effective operation for decreasing
PVP. In this study, we found that splenectomy also influences the
cirrhotic rat liver by altering HO-1 expression, resulting in iron
homeostasis. Two weeks after splenectomy, the levels of AST, ALT
and TBIL decreased, as did the serum concentrations of TGF-β1,
α-SMA and HYP in the liver. Recent studies suggest that splenectomy
may cause anti-inflammatory effects in the portal system in
addition to decreasing PVP and reversing hypersplenism, which
improves hepatic function (4,5).
Moreover, splenectomy may moderate fibrosis by decreasing TGF-β1
secretion. Spleen-derived TGF-β1 plays an inhibitory role in
healing hepatic cirrhosis by prohibiting regeneration of damaged
liver (21,22). We found it also improved liver
function and decreased liver fibrosis following BDL, although
splenectomy was initially performed to decrease PVP.
The majority of endogenous CO is catalyzed by
inducible expression of HO-1. The release of CO by vascular cells
may modulate blood flow and maintain the integrity of the vessel
wall (23). It has been suggested
that CO interacts with NO, which is a potent activator of soluble
guanylate cyclase and a vasodilator (24,25). Indeed, excessive CO production, a
consequence of HO-1 overexpression, could play a critical role in
modulating vascular tone under different pathological situations
(26). COHb levels can be used to
estimate HO activity in experimental animals (27). Notably, we observed that
upregulated COHb resulted from increased HO-1, which aggravated PVP
in BDL rats. Moreover, splenectomy and lower levels of COHb can
decrease PVP, with similar results found in the ZnPP treatment
group. We reasoned that CO may decrease PVP in early stages, and
excessive CO may be harmful by reducing PVP, leading to an
unbalanced NO/CO system in the last stage of hepatic fibrosis. It
therefore seems appropriate to reduce PVP by decreasing CO.
Our previous study indicated that HO-1 induction can
ameliorate immune liver fibrosis (28). Upregulation of HO-1 interferes
with chronic inflammation and prevents progression of liver
fibrosis in Mdr2 knockout mice (29). Our study found that inhibition of
HO-1 could modulate BDL-induced liver fibrosis, which was not in
accordance with previous results. We hypothesized that HO-1 plays
different roles in the progression of liver fibrosis. In early
stage liver fibrosis, inducing HO-1 may have a protective action,
but aggravates liver function and PVP during end stage cirrhosis
with portal hypertension. Moreover, fibrosis resulting from
different animal models may offer another explanation for these
results.
We investigated the relationship between HO-1
expression and iron accumulation by inhibiting or inducing HO-1
expression. Inhibiting HO-1 activity reduced iron accumulation in
the liver and spleen, and further attenuated hepatic fibrosis.
Splenectomy produced similar results, which may be due, partly, to
reduced HO-1 expression. Khan et al (30) reported that an increase in HO-1
expression is associated with iron accumulation, and HO-1 activity
contributes to increased levels of intracellular labile iron
(31). HO-1 gene expression is
upregulated by iron, suggesting that degradation of internalized
heme may be controlled by a positive feedback loop (32). In the present study, HO-1 was
upregulated following Fe treatment. However, anemia and iron
accumulation in the kidney and liver are found in HO-1-deficient
mice (33). Absent HO-1 and other
HO subtypes, such as HO-2 and HO-3, may play a major role in iron
homeostasis. We considered that HO-1 was necessary to form iron
homeostasis, and inhibiting it, but not knocking it out, may be
useful to reduce iron accumulation.
It has previously been shown that increased
deposition of iron in the liver often triggers oxidative stress and
inflammation and induces liver cell damage (34). Iron participates in the Fenton and
Haber-Weiss reaction, and excessive redox-active iron leads to
oxidative stress, with damage to membranes, proteins, and DNA
(35). Nontransferrin-bound iron
plays a key role in iron overload in severe cirrhosis (36). Our data show that iron
accumulation increases oxidative stress and aggravates liver injury
in CoPP and Fe-treated groups. This demonstrates that iron may play
a pivotal role in hepatic fibrosis.
In our study, iron levels in serum, liver, and
spleen all decreased in the splenectomy-treated animals, which
showed lower HO-1 than the BDL group. Similar results were found in
the ZnPP group. A previous study demonstrated that HO-1 activity is
found in the spleen (37). We
found enhanced HO-1 expression in the liver and spleen at two and
four weeks following BDL. Spleen is a source of HO-1 production and
splenectomy could therefore decrease HO-1 generation.
Previous studies have shown that spleen volume
negatively correlates with red blood cell and platelet counting
(38). Others have noted that
splenic hypertrophy partly explains the anemia process, and
splenomegaly is widely observed in human fascioliasis (39). These reports indicate that
splenomegaly is a key reason for RBC damage and iron production
from heme in cirrhotic patients. Splenectomy decreased red blood
cell damage and further reduced iron production from heme
degradation. This may be a benefit for cirrhotic patients with
splenomegaly.
Reactive oxygen species (ROS) may represent a
relevant profibrogenic stimulus for hepatic stellate cells (HSCs),
promote production of type I collagen, and act as an intracellular
signaling mediator of TGF-β (40,41). Splenectomy reduced oxidative
stress in BDL rats and further decreased collagen deposition. Of
note, however, similar results were found in the ZnPP group due to
inhibition of HO-1 expression. HO-1 protected hepatocytes from
ethanol-derived oxidative stress via the MAPK/Nrf2 pathway, in
primary human hepatocytes (42).
Iron accumulation increases liver injury through oxidative stress
in nutritional steatohepatitis (43). We thus reasoned that the
influences of pro-oxidant activities resulted from iron
accumulation and were more effective than the anti-oxidant effects
mediated by HO-1.
In conclusion, splenectomy decreases PVP. Moreover,
we found it improves liver function partly through the HO/CO
pathway during the hepatic cirrhotic process in rats. Inhibition of
HO-1 expression by splenectomy led to reduced iron production
(Fig. 4). However, we lacked a
large-scale clinical trial and in vitro study to better
clarify the exact role of the HO/CO pathway in cirrhotic patients
following splenectomy, as it is difficult to obtain samples from
patients who recover after surgery and who volunteer for a liver
biopsy. In addition, we have reason to believe that a reduction in
CO due to HO-1 inhibition may be a novel therapeutic option for
decreasing PVP. The HO/CO pathway may play a pivotal role in
patients with splenectomy to intervene in liver cirrhosis and
further reduce PVP.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China, no. 30970886, the Technology Project
of Dalian, no. 2008E13SF193 and the Doctoral Initial Funding of
Liaoning Province, no. 20121110.
References
|
1
|
Imura S, Shimada M, Utsunomiya T, et al:
Impact of splenectomy in patients with liver cirrhosis: Results
from 18 patients in a single center experience. Hepatol Res.
40:894–900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito K, Ozasa H, Noda Y and Horikawa S:
Splenic artery ligation ameliorates hepatic ischemia and
reperfusion injury in rats. Liver Int. 26:254–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ito K, Ozasa H and Horikawa S: Effects of
prior splenectomy on remnant liver after partial hepatectomy with
Pringle maneuver in rats. Liver Int. 25:438–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada M, Ijichi H, Yonemura Y, et al:
The impact of splenectomy or splenic artery ligation on the outcome
of a living donor adult liver transplantation using a left lobe
graft. Hepatogastroenterology. 51:625–629. 2004.PubMed/NCBI
|
|
5
|
Chen XP, Wu ZD, Huang ZY and Qiu FZ: Use
of hepatectomy and splenectomy to treat hepatocellular carcinoma
with cirrhotic hypersplenism. Br J Surg. 92:334–339. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uehara H, Akahoshi T, Kawanaka H, et al:
Endothelin-1 derived from spleen-activated Rho-kinase pathway in
rats with secondary biliary cirrhosis. Hepatol Res. 42:1039–1047.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arakawa Y, Shimada M, Uchiyama H, et al:
Beneficial effects of splenectomy on massive hepatectomy model in
rats. Hepatol Res. 39:391–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito K, Ozasa H, Yoneya R and Horikawa S:
Splenectomy ameliorates hepatic ischemia and reperfusion injury
mediated by heme oxygenase-1 induction in the rat. Liver.
22:467–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tarquini R, Masini E, La Villa G, et al:
Increased plasma carbon monoxide in patients with viral cirrhosis
and hyperdynamic circulation. Am J Gastroenterol. 104:891–897.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan ZJ, Yang D, Wang F, Sun YJ, Sun XY
and Zheng ML: Heme oxygenase-1 regulates the major route involved
in formation of immune hepatic fibrosis in rats. Chin Med J.
123:3304–3308. 2010.PubMed/NCBI
|
|
11
|
Sass G, Barikbin R and Tiegs G: The
multiple functions of heme oxygenase-1 in the liver. Z
Gastroenterol. 50:34–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naik JS, O’Donaughy TL and Walker BR:
Endogenous carbon monoxide is an endothelial-derived vasodilator
factor in the mesenteric circulation. Am J Physiol Heart Circ
Physiol. 284:H838–H845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo SB, Duan ZJ, Li Q and Sun XY: Effect
of heme oxygenase-1 on renal function in rats with liver cirrhosis.
World J Gastroenterol. 17:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo SB, Duan ZJ, Li Q and Sun XY: Effects
of heme oxygenase-1 on pulmonary function and structure in rats
with liver cirrhosis. Chin Med J. 124:918–922. 2011.PubMed/NCBI
|
|
15
|
Iwamoto T, Terai S, Mizunaga Y, et al:
Splenectomy enhances the anti-fibrotic effect of bone marrow cell
infusion and improves liver function in cirrhotic mice and
patients. J Gastroenterol. 47:300–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown KE, Dennery PA, Ridnour LA, et al:
Effect of iron overload and dietary fat on indices of oxidative
stress and hepatic fibrogenesis in rats. Liver Int. 23:232–242.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fallon MB, Abrams GA, McGrath JW, Hou Z
and Luo B: Common bile duct ligation in the rat: a model of
intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J
Physiol. 272:G779–G784. 1997.PubMed/NCBI
|
|
18
|
Luo B, Abrams GA and Fallon MB:
Endothelin-1 in the rat bile duct ligation model of hepatopulmonary
syndrome: correlation with pulmonary dysfunction. J Hepatol.
29:571–578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shackelford C, Long G, Wolf J, Okerberg C
and Herbert R: Qualitative and quantitative analysis of
nonneoplastic lesions in toxicology studies. Toxicol Pathol.
30:93–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakinuma S, Tanaka Y, Chinzei R, et al:
Human umbilical cord blood as a source of transplantable hepatic
progenitor cells. Stem Cells. 21:217–227. 2003.PubMed/NCBI
|
|
21
|
Ueda S, Yamanoi A, Hishikawa Y, Dhar DK,
Tachibana M and Nagasue N: Transforming growth factor-beta1
released from the spleen exerts a growth inhibitory effect on liver
regeneration in rats. Lab Invest. 83:1595–1603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akahoshi T, Hashizume M, Tanoue K, et al:
Role of the spleen in liver fibrosis in rats may be mediated by
transforming growth factor beta-1. J Gastroenterol Hepatol.
17:59–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giles TD, Sander GE, Nossaman BD and
Kadowitz PJ: Impaired vasodilation in the pathogenesis of
hypertension: focus on nitric oxide, endothelial-derived
hyperpolarizing factors, and prostaglandins. J Clin Hypertens.
14:198–205. 2012. View Article : Google Scholar
|
|
24
|
Johnson RA, Kozma F and Colombari E:
Carbon monoxide: from toxin to endogenous modulator of
cardiovascular functions. Braz J Med Biol Res. 32:1–14. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furchgott RF and Jothianandan D:
Endothelium-dependent and -independent vasodilation involving
cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and
light. Blood Vessels. 28:52–61. 1991.
|
|
26
|
Decaluwe K, Pauwels B, Verpoest S and Van
de Voorde J: Divergent mechanisms involved in CO and CORM-2 induced
vasorelaxation. Eur J Pharmacol. 674:370–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carter EP, Hartsfield CL, Miyazono M,
Jakkula M, Morris KG Jr and McMurtry IF: Regulation of heme
oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J
Physiol Lung Cell Mol Physiol. 283:L346–L353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Duan ZJ and Sun YJ: Influence of
heme oxygenase-1 expression on immune liver fibrosis induced by
cobalt protoporphyrin in rats. World J Gastroenterol. 15:3009–3014.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barikbin R, Neureiter D, Wirth J, et al:
Induction of heme oxygenase 1 prevents progression of liver
fibrosis in Mdr2 knockout mice. Hepatology. 55:553–562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan ZA, Barbin YP, Cukiernik M, Adams PC
and Chakrabarti S: Heme-oxygenase-mediated iron accumulation in the
liver. Can J Physiol Pharmacol. 82:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kartikasari AE, Wagener FA, Yachie A,
Wiegerinck ET, Kemna EH and Swinkels DW: Hepcidin suppression and
defective iron recycling account for dysregulation of iron
homeostasis in heme oxygenase-1 deficiency. J Cell Mol Med.
13:3091–3102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crichton RR, Wilmet S, Legssyer R and Ward
RJ: Molecular and cellular mechanisms of iron homeostasis and
toxicity in mammalian cells. J Inorg Biochem. 91:9–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Immenschuh S, Baumgart-Vogt E and Mueller
S: Heme oxygenase-1 and iron in liver inflammation: a complex
alliance. Curr Drug Targets. 11:1541–1550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramm GA and Ruddell RG: Iron homeostasis,
hepatocellular injury, and fibrogenesis in hemochromatosis: the
role of inflammation in a noninflammatory liver disease. Semin
Liver Dis. 30:271–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pietrangelo A: Metals, oxidative stress,
and hepatic fibrogenesis. Semin Liver Dis. 16:13–30. 1996.
View Article : Google Scholar
|
|
36
|
Deugnier Y and Turlin B: Pathology of
hepatic iron overload. Semin Liver Dis. 31:260–271. 2011.
View Article : Google Scholar
|
|
37
|
Chen YC, Gines P, Yang J, et al: Increased
vascular heme oxygenase-1 expression contributes to arterial
vasodilation in experimental cirrhosis in rats. Hepatology.
39:1075–1087. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi BM, Wang XY, Mu QL, Wu TH and Xu J:
Value of portal hemodynamics and hypersplenism in cirrhosis
staging. World J Gastroenterol. 11:708–711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Valero MA, Girones N, Garcia-Bodelon MA,
et al: Anaemia in advanced chronic fasciolosis. Acta Trop.
108:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rhyu DY, Park J, Sharma BR and Ha H: Role
of reactive oxygen species in transforming growth
factor-beta1-induced extracellular matrix accumulation in renal
tubular epithelial cells. Transplant Proc. 44:625–628. 2012.
View Article : Google Scholar
|
|
41
|
Urtasun R, Conde de la Rosa L and Nieto N:
Oxidative and nitrosative stress and fibrogenic response. Clin
Liver Dis. 12:769–790. viii2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao P, Nussler A, Liu L, et al: Quercetin
protects human hepatocytes from ethanol-derived oxidative stress by
inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol.
47:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okada K, Warabi E, Sugimoto H, et al: Nrf2
inhibits hepatic iron accumulation and counteracts oxidative
stress-induced liver injury in nutritional steatohepatitis. J
Gastroenterol. 47:924–935. 2012. View Article : Google Scholar : PubMed/NCBI
|