Introduction
The growing prevalence of obesity constitutes a
major health problem worldwide (1). Obesity, particularly abdominal
obesity, has a strong relationship with insulin resistance and is a
major risk factor for type 2 diabetes and cardiovascular disease
(2,3). The imbalance between energy intake
and expenditure contributes to the development of obesity (1,4);
the cellular mechanisms for which include the expansion of white
adipose tissue via the hypertrophy of preexisting adipocytes and
hyperplasia resulting from the adipogenesis of preadipocytes
(4,5). When animals are maintained on a
high-fat diet, adipo cyte cell size initially increases, followed
by an increase in fat cell number upon prolonged over-nutrition
(6). In adults, ∼10% of fat cells
are renewed from preadipocytes annually (7). One study in adults demonstrated that
short-term overfeeding increases the adipocyte cell numbers
(8). Thus, adipogenesis probably
has a role in the pathology of obesity in human adults. However,
there are significant differences in lipid and glucose metabolism
between adipocyte hypertrophy and hyperplasia (9–11).
Recent studies have shown that adipocyte hypertrophy is negatively
correlated with dyslipidemia and insulin resistance, independent of
body composition (9,11). Notably, hyperplasia, which is
characterized by an increased number of small subcutaneous
adipocytes, may have a positive effect on lipid metabolism and
insulin sensitivity through preadipocyte differentiation (9,10).
Therefore, improving the characteristics of adipocytes may have
therapeutic potential for treating obesity and insulin
resistance.
Glucagon-like peptide 1 (GLP-1), which is secreted
from intestinal L-cells following nutrient ingestion, exerts
multiple biological effects through the GLP-1 receptor (GLP-1R)
such as enhancing glucose-dependent insulin secretion, reducing
glucagon levels, inhibiting the gastric emptying rate and
increasing pancreatic β-cell proliferation (12,13). Moreover, GLP-1 also increases
insulin sensitivity in liver, muscle and adipocyte models (14–16). Furthermore, recent studies have
revealed that GLP-1 improves insulin sensitivity in adipocytes by
upregulating the expression of insulin receptor and Glut-4
(16), reducing macrophage
infiltration and inhibiting inflammatory adipocytes (17). Boc5 (a GLP-1R agonist) also
significantly reduced fat mass and adipocyte hypertrophy in an
animal model of obesity (18).
However, the effect of GLP-1 on adipogenesis is less clear.
In this study, using 3T3-L1 preadipocytes, we
examined the effect of GLP-1 on preadipocyte differentiation and
investigated the mechanisms that could be involved in this
effect.
Materials and methods
Cell culture and treatment
3T3-L1 preadipocytes (CL-173™; ATCC) were cultured
in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlbad,
CA, USA) containing 10% fetal bovine serum (FBS) and 10 mg/ml
penicillin/streptomycin in an atmosphere of 10% CO2 at
37°C. Two days after the 3T3-L1 preadipocytes reached confluence,
differentiation was induced by culture with 0.5 mmol/l
3-isobutyl-1-methylxanthine (IBMX), 1 μM dexamethasone
(DEX), 5 μg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA)
and 10% FBS in DMEM for 48 h. The cell culture medium was then
replaced with DMEM containing 5 μg/ml insulin and 10% FBS
for an additional 48 h. The cells were fed DMEM containing 10% FBS
every other day for the next 5–8 days, and at day 8, >90% of the
cells demonstrated an adipocyte phenotype. Recombinant human
glucagon-like peptide (7–36) GLP-1 (Huayi BIO-Lab Co., Shanghai,
China) was added to the culture medium at different concentrations
during the adipogenic period of 8 days.
MTT cell viability assay
The 3T3-L1 preadipocytes were seeded into 96-well
culture plates at a density of 6×105 cells/well. GLP-1
was added to the medium at different concentrations and was then
incubated with the cells for 48 h. Subsequently, the medium was
replaced with 100 μl FBS-free DMEM and the cells were
incubated with 5 mg/ml MTT solution for 4 h at 37°C. Next, the
medium was removed, and the cells were solubilized in DMSO (Huayi
BIO-Lab Co.). The absorbance at 490 nm was measured using a
spectrophotometer (Varioskan Flash; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Quantitative real-time PCR
Total RNA was extracted, and the concentrations were
measured using a spectrophotometer (Bio-Rad, Hercules, CA, USA). A
total of 1 μg mRNA was reverse-transcribed into cDNA and was
amplified using the SYBR-Green I reagent (Takara, Tokyo, Japan) in
a fluorescence thermocycler (LightCycler; Roche Diagnostics,
Mannheim, Germany). Mouse β-actin was used as an internal control.
The sequences of the primers used in the study were as follows:
peroxisome proliferator activated receptor-γ (PPAR-γ), 5′-GTG AAG
CCC ATC GAG GAC A-3′ (forward) and 5′-TGG AGC ACC TTG GCG AAC A-3′
(reverse); CCAAT/enhancer-binding protein α (C/EBPα), 5′-GCG GGA
ACG CAA CAA CAT C-3′ (forward) and 5′-GTC ACT GGT CAA CTC CAG
CAC-3′ (reverse); lipoprotein lipase (LPL), 5′-TGT AAC AAT CTG GGC
TAT GAG ATC AAC-3′ (forward) and 5′-TGC TTG CCA TCC TCA GTC CC-3′
(reverse); free fatty acid binding protein 4 (aP2), 5′-AGG CTC ATA
GCA CCC TCC TGT G-3′ (forward) and 5′-CAG GTT CCC ACA AAG GCA TCA
C-3′ (reverse); and β-actin, 5′-GTG ACG TTG ACA TCC GTA AAG A-3′
(forward) and 5′-GCC GGA CTC ATC GTA CTC C-3′ (reverse). Target
gene mRNA levels were normalized to those of β-actin using the
2−ΔΔCT method.
Western blot analysis
Total protein was extracted and then quantified
using a BCA protein quantification kit (Beyotime, Shanghai, China).
A total of 30 μg protein from each sample was separated by
SDS-PAGE (10–15%) and transferred to a polyvinylidene difluoride
membrane (0.22-μm pore size; Millipore, Billerica, MA, USA).
The membranes were blocked in 5% non-fat milk in TBS-T for 2 h at
room temperature. After incubation with the primary antibodies at
4°C overnight, the membranes were washed extensively with TBS-T
prior to incubation with the secondary anti-rabbit/mouse
horseradish peroxidase-conjugated antibody (Beijing Zhongshan Gold
Bridge Biotechnology Co., Beijing, China) for 2 h at room
temperature. After the membranes were washed again with TBS-T, the
bands were visualized with enhanced chemiluminescence reagents
(Millipore). Primary antibodies against mouse PPAR-γ, aP2, β-actin,
phospho-Akt, Akt, phospho-P38, P38, phospho-ERK1/2 and ERK1/2 (Cell
Signaling Technology, Danvers, MA, USA) were used.
Oil Red O staining and lipid content
quantification
The cellular lipid content was assessed by Oil Red O
staining (Sigma-Aldrich). After 8 days of differentiation, cells
were washed, fixed in 4% formalin for 1 h, stained with an Oil Red
O working solution and then incubated for an additional 1 h at room
temperature. After washing 3 times with PBS, the cells were
photographed with a light microscope (Olympus, Osaka, Japan). The
size and number of lipid droplet were analyzed using Image Pro Plus
5.02. At least 10 different microscopic fields were used per well
to determine adipocyte size and number. Next, 125 μl
isopropyl alcohol was added to each well, and the cells were
maintained at room temperature for 5 min to stain the lipids with
Oil Red O. Then, 100 μl of the eluate from each well was
transferred to a 96-well plate, and the absorbance values at a
540-nm wavelength were measured using a spectrophotometer (Thermo
Fisher Scientific, Inc.).
Statistical analysis
The relative band densities were quantified using
Image Pro Plus 5.02. The data are presented as means ± SD and were
analyzed using the Student’s t-test and one-way ANOVA with SPSS
13.0 software. P-values <0.05 were considered to represent
statistically significant differences between groups.
Results
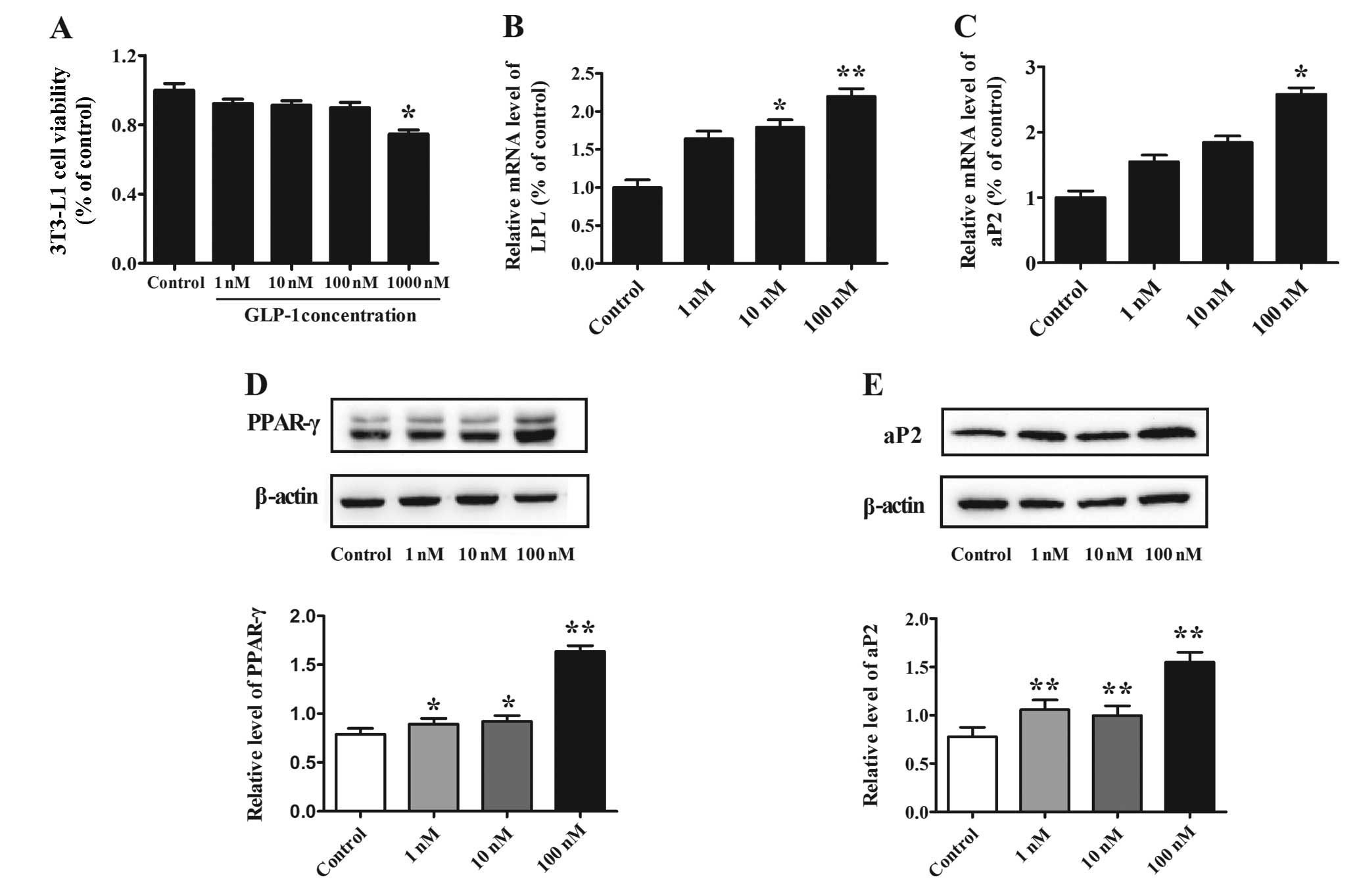
Effect of different concentrations of
GLP-1 on 3T3-L1 preadipocyte viability
3T3-L1 preadipocytes were treated with different
concentrations of GLP-1 (1, 10, 100 or 1,000 nM) for 48 h, and the
cell viability was assessed by MTT assay. For GLP-1 concentrations
between 1 and 100 nM, no effect on 3T3-L1 cell viability was
observed. However, cell viability was decreased by 25% in cells
treated with 1,000 nM in comparison to the non-treated control
(P<0.05) (Fig. 1A). Therefore,
the concentrations of 1, 10 and 100 nM were deemed suitable for use
in the cell study.
Effect of GLP-1 on adipocyte-specific
markers during preadipocyte differentiation
To determine the effect of GLP-1 on 3T3-L1
preadipocyte differentiation, increasing concentrations of GLP-1
(1, 10 and 100 nM) were added to the cells for 8 days. Western blot
analysis shown that the protein level of the transcription factor
PPAR-γ increased in a dose-dependent manner (P<0.05) (Fig. 1D) and that the maximal effect was
reached at a concentration of 100 nM GLP-1 (P<0.01). We next
examined the expression of the adipocyte-specific markers LPL and
aP2 and found that these markers were also increased in a
dose-dependent manner; the mRNA levels of LPL increased by
1.79-fold (P<0.05) and 2.20-fold (P<0.01) when cells were
treated with 10 and 100 nM GLP-1, respectively, as compared to
levels in the control (Fig. 1B).
In addition, the mRNA level of aP2 increased by 2.58-fold
(P<0.05) at 100 nM GLP-1 when compared to the level in the
control (Fig. 1C). Furthermore,
evaluation of the aP2 protein level demonstrated similar results
when cells were treated with 100 nM GLP-1, which elicited the
maximal effect (P<0.01) (Fig.
1E).
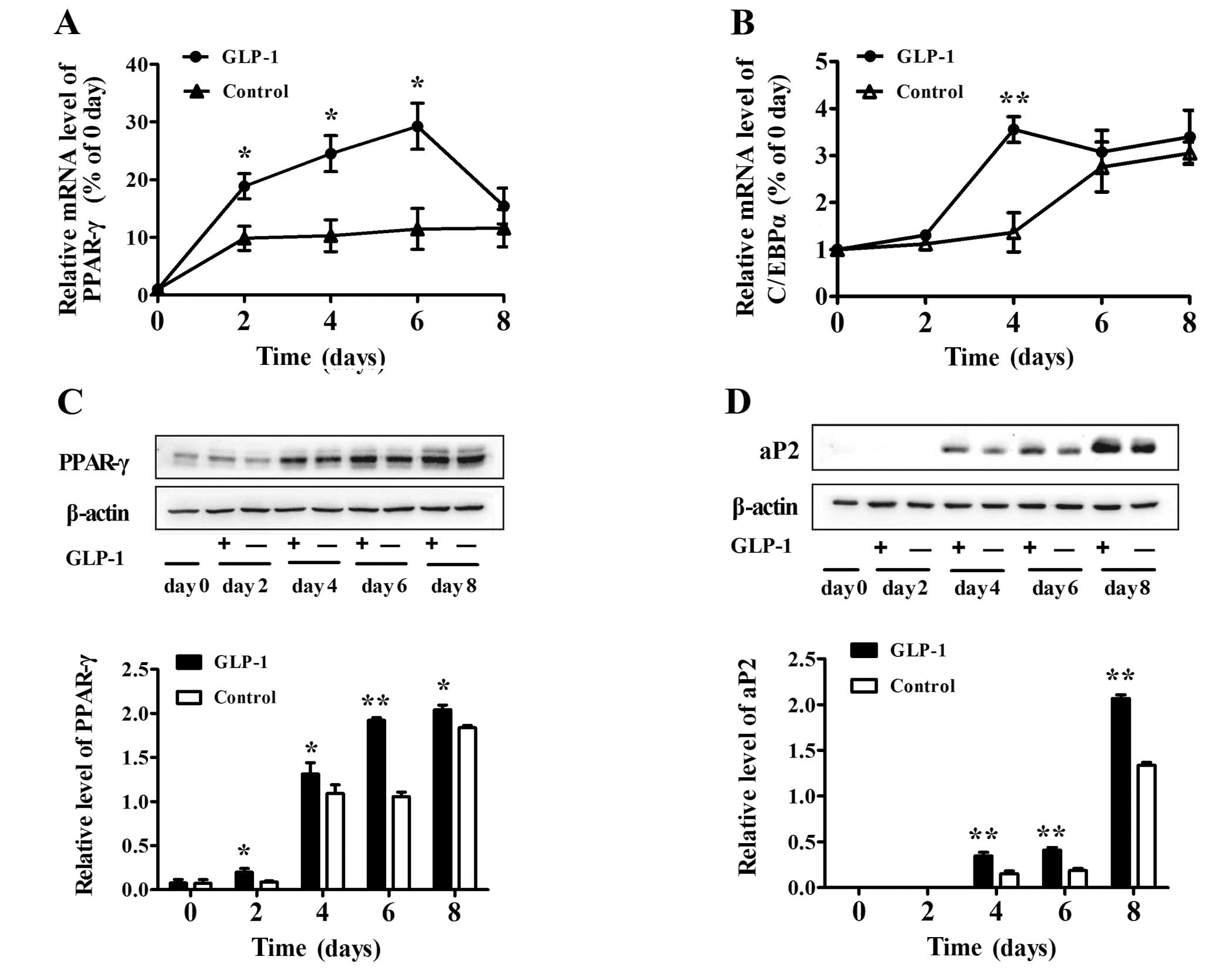
To further examine the effect of GLP-1 at varying
times during differentiation, we added 100 nM GLP-1 to the medium
and harvested the cells at days 0, 2, 4, 6 and 8. We observed that
GLP-1 enhanced the mRNA levels of the transcription factors PPAR-γ
and C/EBPα. In particular, the mRNA level of PPAR-γ increased by
∼3- to 4-fold from day 2 to 6 (both P<0.05) (Fig. 2A). The mRNA level of C/EBPα in the
100 nM GLP-1 group was also enhanced markedly at day 4 (P<0.01)
(Fig. 2B). In addition, GLP-1
enhanced the protein level of PPAR-γ in a time-dependent manner
(both P<0.05) (Fig. 2C).
Furthermore, we found that the amount of aP2 protein began to
increase at day 4 of differentiation and then increased gradually
at day 6 and 8 (Fig. 2D), and
GLP-1 significantly increased the expression of aP2 when compared
to that in the control (both P<0.01).
Effect of GLP-1 on Akt, P38 and ERK1/2
signaling pathways during adipogenesis
Previous studies have shown that the Akt, P38 and
ERK1/2 signaling pathways may be involved in adipogenesis. To
identify whether GLP-1 affects these signaling pathways during
adipogenesis, we evaluated the expression of phosphorylated-Akt
(pAkt), phosphorylated-P38 (pP38) and phosphorylated-ERK1/2
(pERK1/2) at the early phase of differentiation. 3T3-L1
preadipocytes were incubated in differentiation medium containing
100 nM GLP-1, and the cells were harvested at 0, 15, 30 min, 1, 2,
6, 12 and 24 h of culture. Western blotting showed that the level
of pAkt increased rapidly at 15 min and that the pAkt level reached
its maximum at 2 h, which lasted for 24 h. In contrast, the
addition of 100 nM GLP-1 significantly increased the expression of
pAkt during the first 24 h of culture as compared to the control
(P<0.01) (Fig. 3A). In
addition, pP38 and pERK1/2 were also activated during the early
phase of differentiation, while GLP-1 had no obvious effect on
these expression levels (Fig.
3B).
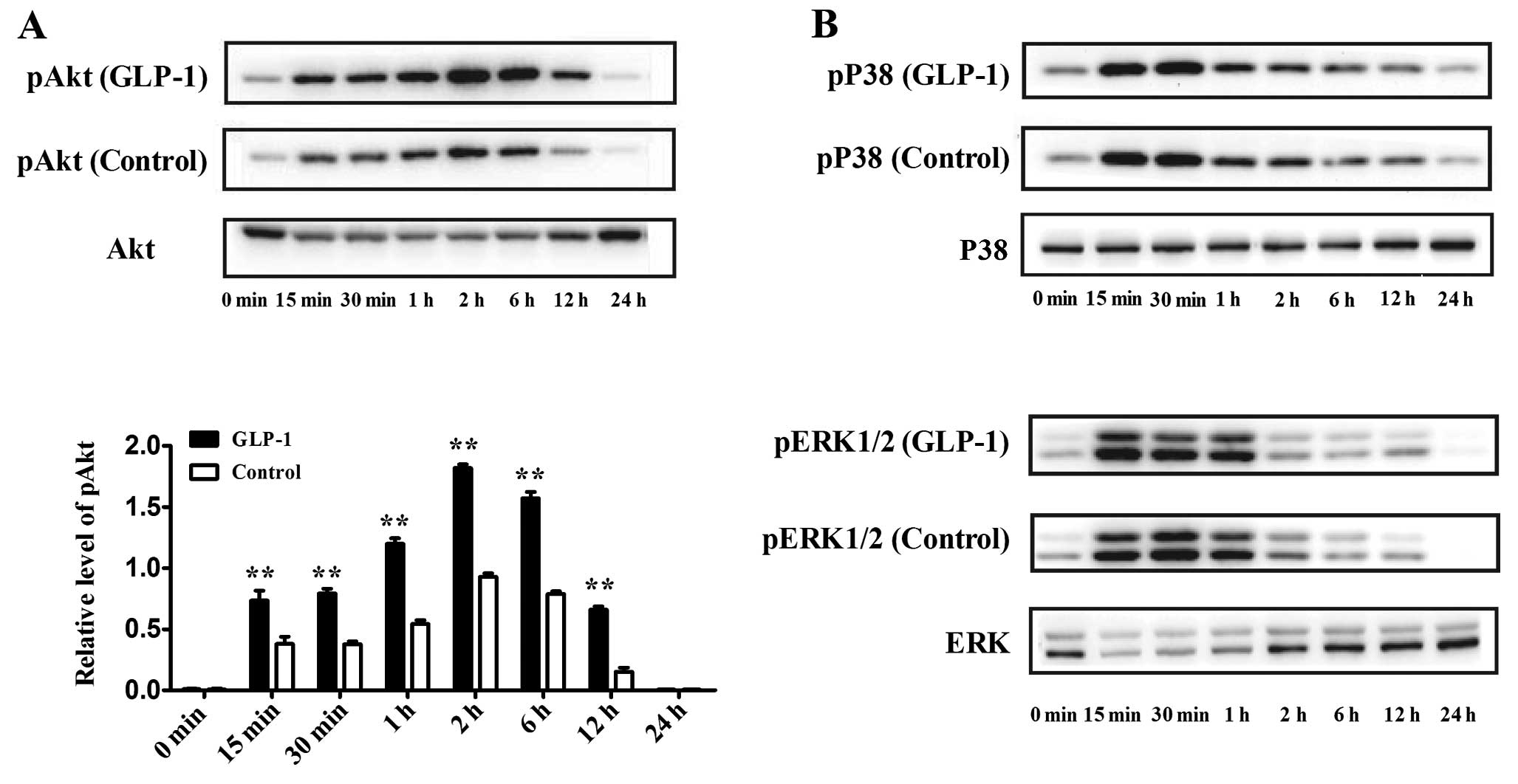 | Figure 3Effect of GLP-1 on the Akt, P38 and
ERK1/2 signaling pathways during the first 24 h of differentiation.
GLP-1 (100 nM) was added to the differentiation medium, and the
cells were harvested at 0, 15, 30 min, 1, 2, 6, 12 and 24 h of
culture. Western blot analyses were used to assess the protein
levels of (A) pAkt and Akt, (B) pP38, P38, pERK1/2 and ERK1/2. The
data shown represent the means ± SD of 3 independent experiments.
**P<0.01 vs. control. |
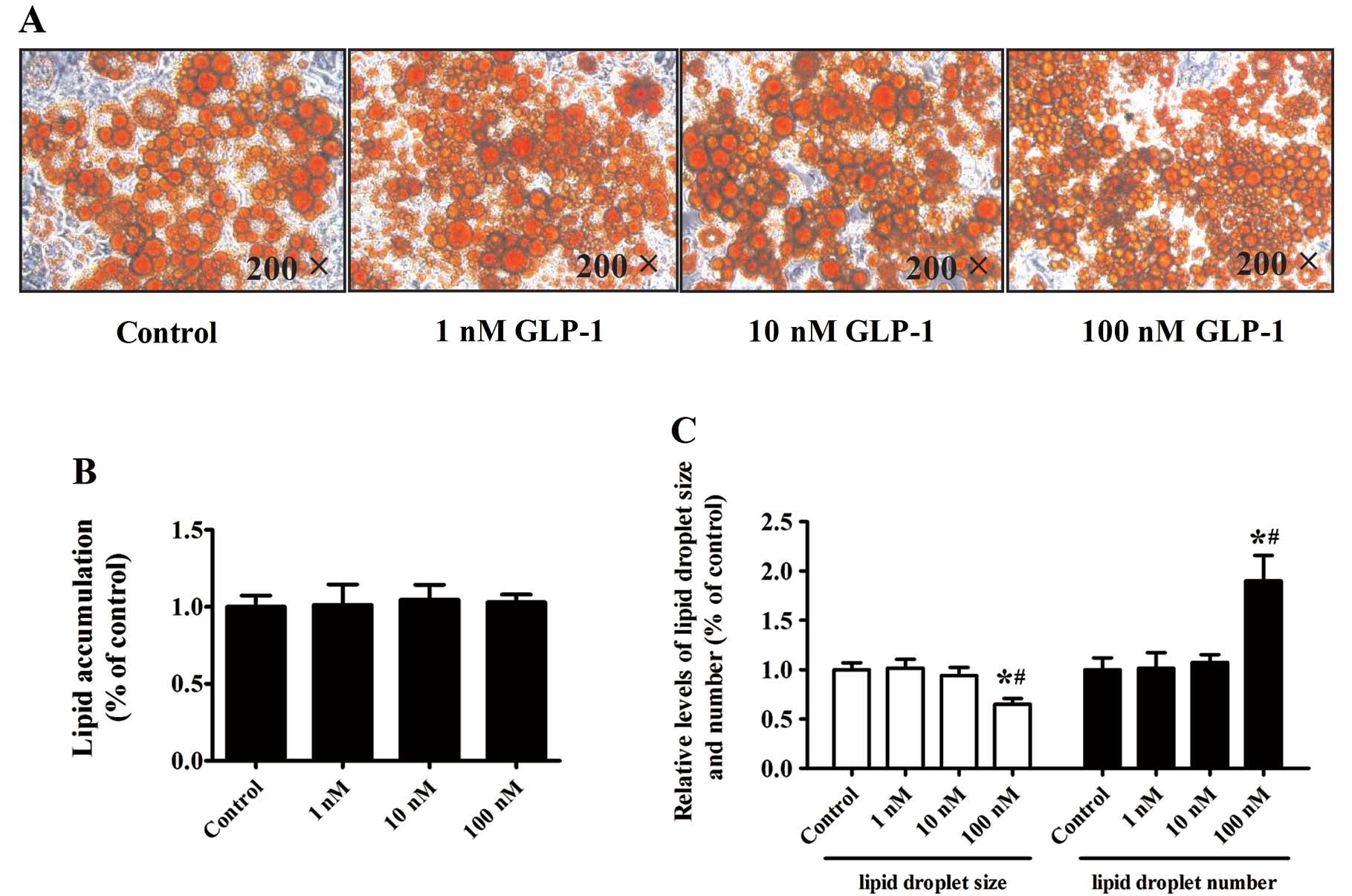
Effect of GLP-1 on lipid
accumulation
We next performed Oil Red O staining to examine the
effect of GLP-1 on lipid droplet accumulation. Differentiation of
the 3T3-L1 preadipocytes was induced for 8 days with increasing
doses of GLP-1 (1, 10 or 100 nM). Surprisingly, isopropyl alcohol
elutes of Oil Red O revealed that increasing concentrations of
GLP-1 had no significant effects on lipid accumulation in
comparison to the control cells (Fig.
4A and B) (P>0.05). The lipid droplet size decreased
markedly while the lipid droplet number increased in cultures
treated with 100 nM GLP-1, as compared to the control and other
concentrations of GLP-1 (P<0.05) (Fig. 4C).
Discussion
Obesity is a major characteristic of metabolic
syndrome; it is closely correlated with dyslipidemia, hyperglycemia
and hypertension and is associated with an increased propensity for
the development of cardiovascular disease (19). GLP-1 is an incretin hormone and
has multiple biological effects against obesity and other metabolic
diseases. Recent research has revealed that GLP-1 markedly improves
insulin resistance in adipose tissue by reducing fat mass and
adipocyte hypertrophy (18). In
the present study, we demonstrated that GLP-1 regulates
preadipocyte differentiation.
Preadipocyte differentiation is a complex process
that is regulated by many transcription factors, particularly
PPAR-γ and CCAAT/enhancer-binding proteins such as C/EBPα, C/EBPβ
and C/EBPδ. These transcription factors are the primary drivers of
adipocyte gene induction during terminal differentiation (20). The expression of C/EBPα and PPAR-γ
cross-regulate each other through a positive feedback loop and
induce the expression of downstream target genes, such as aP2 and
LPL, which leads to the appearance of lipid droplets. These markers
appear sequentially during differentiation, following a
time-dependent expression pattern (20,21). In the present study, we found that
GLP-1 significantly promoted the expression of adipocyte-specific
markers aP2 and LPL in a dose- and time-dependent manner. We also
showed that GLP-1 enhanced the expression of the upstream
transcription factors PPAR-γ and C/EBPα. Therefore, these results
demonstrate that GLP-1 promotes adipogenesis by upregulation of
adipocyte-specific markers aP2 and LPL in 3T3-L1 preadipocyte.
Moreover, the transcription factors PPAR-γ and C/EBPα are likely
involved in this process.
We next investigated the potential signaling pathway
involved in GLP-1-induced preadipocyte differentiation. The
activation and phosphorylation of the Akt, P38 and ERK1/2 signaling
pathways play a key role in the earliest phases of differentiation
(22,23). Akt signaling is indispensable for
the regulation of preadipocyte and adipocyte number. It is also
important for the regulation of insulin-stimulated metabolic
pathways in human adipocytes (24). Furthermore, it has been reported
that phosphorylation of Akt reduces the expression of forkhead
transcriptional factor 1 (Foxo1), which is an antagonist of PPAR-γ,
thus enhancing the expression of PPAR-γ indirectly and promoting
adipogenesis (25,26). In addition, Akt phosphorylation
activates C/EBPα during preadipocyte differentiation (21). Our findings revealed that for
cells cultured in induction medium, Akt phosphorylation was rapidly
induced after 15 min and reached a peak level at 2 h, which was
maintained for 24 h after the initial stimulation with induction
medium. As compared to the non-treated control, treatment with 100
nM GLP-1 significantly enhanced the level of pAkt. According to
these findings, our results suggest that Akt phosphorylation may be
involved in GLP-1-induced 3T3-L1 preadipocyte differentiation.
Although the P38 and ERK1/2 signaling pathways are also important
mediators of adipogenesis, GLP-1 treatment did not have a marked
effect on these mediators in this study.
Previously, one study found that GLP-1 promoted the
proliferation and cytoprotection of human bone marrow-derived
mesenchymal stem cells (hMSCs) but prevented cellular
differentiation into adipocytes (27). However, a recent study suggests
that GLP-1 promotes the proliferation and differentiation of
preadipocytes by GLP-1R (28).
The results of the present study support the latter view. We used
3T3-L1 preadipocytes as experimental subjects in this study because
these cells are precursor of fat cells and are more specialized.
Furthermore, we tested adipocyte-specific markers, including LPL
and aP2, in comparison to previous studies to further analyze the
effects of GLP-1 on preadipocyte differentiation. Notably, we found
that the lipid droplet size decreased significantly while the lipid
droplet number increased in cultures treated with 100 nM GLP-1, as
compared to the control and other concentrations. And the total
lipid accumulation did not enhance markedly.
It has also been reported that an increase in the
number of small subcutaneous adipocytes promotes lipid metabolism
and insulin sensitivity (9,29–31). The PPAR-γ agonist TZD has been
shown to stimulate preadipocyte differentiation, to increase the
number of small subcutaneous adipose cells, to improve insulin
sensitivity and to prevent excess lipid accumulation through the
upregulation of the expression of Glu-4 and adiponectin (29,30). Ascofuranone, which has an
anti-hyperlipidemia effect, can also increase the levels of PPAR-γ
and adiponectin in 3T3-L1 preadipocyte cells (31). Previous studies have revealed that
GLP-1 increases insulin sensitivity by upregulating Glut-4, thus
reducing macrophage infiltration, inhibiting inflammatory pathways
and reducing fat mass in adipocytes (29,30). Our results suggest an alternative
mechanism whereby GLP-1 increases insulin sensitivity.
In conclusion, our data demonstrated that GLP-1
promotes 3T3-L1 preadipocyte differentiation by promoting the
expression of the adipocyte-specific markers LPL and aP2 and the
transcription factors PPAR-γ and C/EBPα. Moreover, activation of
the Akt signaling pathway may be involved in this process. GLP-1
also increased the numbers of small adipocytes, which may increase
insulin sensitivity and consequently have a positive effect against
obesity.
Acknowledgements
The present study was supported by the
National Natural Science Foundation of China (no. 81100617), the
Medical and Health Science and Technology Development Projects of
Shandong Province (2011HD005), the National Science and Technology
Support Plan (2009BAI80B04), the Natural Science Foundation of
Shandong Province (ZR2012HM014), the Chinese Diabetes Society Key
Projects (07020470055), the International Science and Technology
Projects of Shandong Province (2010GHZ20201), the Science and
Technology Star Project of Jinan Sincere and Technology Bureau
(20100318), the Innovation Foundation of Shandong University
(2009TS054) and the Business Plan of Jinan Students Studying Abroad
(20110407).
References
|
1
|
Hossain P, Kawar B and El Nahas M: Obesity
and diabetes in the developing world - a growing challenge. N Engl
J Med. 356:213–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi H, Akunuru S, Bierman JC, et al:
Diet-induced obese mice are leptin insufficient after weight
reduction. Obesity. 17:1702–1709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seale P and Lazar MA: Brown fat in humans:
turning up the heat on obesity. Diabetes. 58:1482–1484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahlman I and Arner P: Obesity and
polymorphisms in genes regulating human adipose tissue. Int J Obes.
31:1629–1641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun K, Kusminski CM and Scherer PE:
Adipose tissue remodeling and obesity. J Clin Invest.
121:2094–2101. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cristancho AG and Lazar MA: Forming
functional fat: a growing understanding of adipocyte
differentiation. Nat Rev Mol Cell Biol. 12:722–734. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spalding KL, Arner E, Westermark PO, et
al: Dynamics of fat cell turnover in humans. Nature. 453:783–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tchoukalova YD, Votruba SB, Tchkonia T, et
al: Regional differences in cellular mechanisms of adipose tissue
gain with overfeeding. Proc Natl Acad Sci USA. 107:18226–18231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffstedt J, Arner E, Wahrenberg H, et al:
Regional impact of adipose tissue morphology on the metabolic
profile in morbid obesity. Diabetologia. 53:2496–2503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koenen TB, Tack CJ, Kroese JM, et al:
Pioglitazone treatment enlarges subcutaneous adipocytes in
insulin-resistant patients. J Clin Endocrinol Metab. 94:4453–4457.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veilleux A, Caron-Jobin M, Noel S, et al:
Visceral adipocyte hypertrophy is associated with dyslipidemia
independent of body composition and fat distribution in women.
Diabetes. 60:1504–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meier JJ: GLP-1 receptor agonists for
individualized treatment of type 2 diabetes mellitus. Nat Rev
Endocrinol. 8:728–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kielgast U, Holst JJ and Madsbad S:
Antidiabetic actions of endogenous and exogenous GLP-1 in type 1
diabetic patients with and without residual β-cell function.
Diabetes. 60:1599–1607. 2011.PubMed/NCBI
|
|
14
|
Ben-Shlomo S, Zvibel I, Shnell M, et al:
Glucagon-like peptide-1 reduces hepatic lipogenesis via activation
of AMP-activated protein kinase. J Hepatol. 54:1214–1223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chai W, Dong Z, Wang N, et al:
Glucagon-like peptide 1 recruits microvasculature and increases
glucose use in muscle via a nitric oxide-dependent mechanism.
Diabetes. 61:888–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao H, Wang X, Zhang Z, et al: GLP-1
amplifies insulin signaling by up-regulation of IRβ, IRS-1 and
Glut4 in 3T3-L1 adipocytes. Endocrine. 32:90–95. 2007.PubMed/NCBI
|
|
17
|
Lee YS, Park MS, Choung JS, et al:
Glucagon-like peptide-1 inhibits adipose tissue macrophage
infiltration and inflammation in an obese mouse model of diabetes.
Diabetologia. 55:2456–2468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He M, Su H, Gao W, et al: Reversal of
obesity and insulin resistance by a non-peptidic glucagon-like
peptide-1 receptor agonist in diet-induced obese mice. PLoS One.
5:e142052010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bass J and Takahashi JS: Circadian
integration of metabolism and energetics. Science. 330:1349–1354.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cristancho AG and Lazar MA: Forming
functional fat: a growing understanding of adipocyte
differentiation. Nat Rev Mol Cell Biol. 12:722–734. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim GS, Park HJ, Woo JH, et al: Citrus
aurantium flavonoids inhibit adipogenesis through the Akt signaling
pathway in 3T3-L1 cells. BMC Complement Altern Med. 12:312012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi SS, Cha BY, Iida K, et al: Honokiol
enhances adipocyte differentiation by potentiating insulin
signaling in 3T3-L1 preadipocytes. J Nat Med. 65:424–430. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Ikeda K, Xu JW, et al: Genistein
suppresses adipogenesis of 3T3-L1 cells via multiple signal
pathways. Phytother Res. 23:713–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer-Posovszky P, Tews D, Horenburg S,
et al: Differential function of Akt1 and Akt2 in human adipocytes.
Mol Cell Endocrinol. 358:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polvani S, Tarocchi M and Galli A: PPARγ
and oxidative stress: Con(β) catenating NRF2 and FOXO. PPAR Res.
2012:6410872012.
|
|
26
|
Dowell P, Otto TC, Adi S and Lane MD:
Convergence of peroxisome proliferator-activated receptor γ and
foxo1 signaling pathways. J Biol Chem. 278:45485–45491. 2003.
|
|
27
|
Sanz C, Vázquez P, Blázquez C, Barrio PA,
Alvarez Mdel M and Blázquez E: Signaling and biological effects of
glucagon-like peptide 1 on the differentiation of mesenchymal stem
cells from human bone marrow. Am J Physiol Endocrinol Metab.
298:E634–E643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Challa TD, Beaton N, Arnold M, et al:
Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol
Chem. 287:6421–6430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang JG, Park CY, Ihm SH, et al:
Mechanisms of adipose tissue redistribution with rosiglitazone
treatment in various adipose depots. Metabolism. 59:46–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dubuisson O, Dhurandhar EJ, Krishnapuram
R, et al: PPARgamma-independent increase in glucose uptake and
adiponectin abundance in fat cells. Endocrinology. 152:3648–3660.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang YC and Cho HJ: Ascofuranone
stimulates expression of adiponectin and peroxisome proliferator
activated receptor through the modulation of mitogen activated
protein kinase family members in 3T3-L1, murine pre-adipocyte cell
line. Biochem Biophys Res Commun. 422:423–428. 2012. View Article : Google Scholar
|