Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
systemic disease of unknown etiology characterized by chronic
synovitis with subsequent articular bone and cartilage destruction
(1,2). Chronic inflammation with hyperplasia
of synovial lining cells, including synovial fibroblasts, are the
histological characteristics of RA. When activated, rheumatoid
arthritis synovial fibroblasts (RASFs) play a key role in the
pathogenesis of RA synovitis through proliferation and resultant
pannus formation. Inflammatory cytokines, matrix metalloproteinases
(MMPs) and cyclooxygenase (COX)-2 released from RASFs are involved
in the destruction of articular bone and cartilage (1,2).
On the basis that interleukin (IL)-1β induces the proliferation of
RASFs, resulting in the production of high levels of MMPs and
prostaglandin E2 (PGE2), it has become a major target of biological
therapy.
Flavonoids are natural polyphenols present in a wide
variety of fruits and vegetables (3) and have a number of biological
properties, such as antiviral (4), antitumor (5), antioxidant (6) and anti-inflammatory properties
(7). Kaempferol
(3,5,7,4′-tetrahydroxy flavone), which is found in tea, propolis
and grapefruit, is one of the most common dietary flavonoids
(8). It has been used as a
traditional therapeutic for a number of inflammatory disorders.
Previous studies have demonstrated that kaempferol reduces
lipopolysaccharide-induced COX-2 levels in RAW 264.7 cells
(9) and inhibits reactive oxygen
species production through the inhibition of inducible nitric oxide
synthase (iNOS) and tumor necrosis factor (TNF)-α protein
expression in aged gingival tissues (10). Kaempferol has also exhibited
anti-inflammatory effects through the inhibition of IL-4 (11), COX-2 and C-reactive protein (CRP)
expression and the downregulation of nuclear factor-κB (NF-κB) in
liver cells (12). Despite these
anti-inflammatory effects of kaempferol and the critical role of
RASFs in RA pathogenesis, to our knowledge, there are no studies to
date on the effects of kaempferol on inflammatory reactions,
including the production of MMPs, COX-2 and PGE2 by RASFs.
In the present study, we investigated the effects of
kaempferol on the production of pro-inflammatory mediators,
including MMPs, COX-2 and PGE2 produced by RASFs and the
proliferation of these cells, following stimulation with IL-1β.
Intracellular signaling factors were evaluated to elucidate the
mechanisms behind the effects of kaempferol. We demonstrate that
kaempferol inhibits the IL-1β-induced proliferation of RASFs and
inflammatory reactions by inhibiting the activation of
mitogen-activated protein kinases (MAPKs) and NF-κB pathways in
RASFs.
Materials and methods
Reagents and antibodies
Recombinant human IL-1β was purchased from R&D
Systems (Minneapolis, MN, USA) and kaempferol were obtained from
Sigma-Aldrich, Inc. (St. Louis, MO, USA) and dissolved in DMSO with
a concentration of 100 mM stock solution. Monoclonal antibodies
(mAbs) against COX-2, MMP-1, MMP-3 and TIMP were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). mAbs against
NF-κB (p65), IkBα, ERK, p-ERK, JNK, p-JNK, p38, p-p38 and β-actin
were purchased from Cell Signaling Technology (Beverly, MD, USA).
Fetal bovine serum (FBS) was obtained from Gibco BRL/Life
Technologies (Grand Island, NY, USA).
Isolation and culture of RASFs
Synovial tissues were obtained at the time of total
knee arthroplasty from patients who fulfilled the American College
of Rheumatology Criteria for RA (13), as previously described (14). Synovial tissue was digested for 2
h with 0.25% (w/v) collagenase and was then suspended in RPMI-1640
medium with 10% (v/v) FBS, 100 U/ml of penicillin and 100
μg/ml of streptomycin. The cells were incubated at 37°C in
5% CO2 for several days, after which the non-adherent
cells were removed. Synovial fibroblasts from passages 3–7 were
used for each experiment and were morphologically homogeneous and
had the appearance of RASFs with typical fibroblastoid
configuration under an inverse microscope. The purity of the cells
was determined by flow cytometry using phycoerythrin
(PE)-conjugated anti-Thy-1 (CD90) or anti-CD14 and fluorescein
isothiocyanate (FITC)-conjugated anti-CD3 mAb (BD Pharmingen, San
Diego, CA, USA). Informed consent was obtained from all patients,
and the study protocol was approved by the Chonbuk National
University Hospital Ethics Committee.
Cell viability analysis
Cell viability was determined by using the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Japan) according to
the manufacturer’s instructions. CCK-8 allows convenient assays
using Dojindo’s tetrazolium salt, 2-(2-
methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-
2H-tetrazolium, monosodium salt (WST-8), which produces a
water-soluble formazan dye upon bioreduction in the presence of an
electron carrier, 1-methoxy phenazinium methylsulfate (PMS)
(15). CCK-8 solution is added
directly to the cells; no pre-mixing of the components is required.
CCK-8 is a sensitive non-radioactive colorimetric assay for
determining the number of viable cells in cell proliferation and
cytotoxicity assays. WST-8 is bioreduced by cellular dehydrogenases
to an orange formazan product that is soluble in tissue culture
medium. The amount of formazan produced is directly proportional to
the number of living cells. RASFs [1×105 cells/well in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) FCS
in a 96-well U-bottom plate] were cultured in 200 μl medium/
well in the presence or absence of 1.0 ng/ml IL-1β and/or
kaempferol (100 μM) for 2 days according to the results of a
dose-dependent examination and a previous report (15), while the control RASFs were
incubated in DMEM with DMSO. CCK-8 (20 μl) was added to each
well of the plate and the cells were incubated for 2–3 h. The
absorbance was measured at 450 nm using a microplate reader to
determine the cell viability in each well.
Analysis of apoptosis
To evaluate the effects of kaempferol on the
apotosis of RASFs, RASFs were incubated in DMEM for 24 h with
kaempferol (100 μM), while the control RASFs were incubated
in DMEM with DMSO. The cells were then trypsinized and collected
for the detection of apoptosis with an Annexin V-FITC Apoptosis
Detection kit according to the manufacturer’s instructions.
Briefly, the cells were washed twice with cold PBS and resuspended
in 500 ml of binding buffer (10 mM HEPES-NaOH pH 7.4, 140 mM NaCl,
2.5 mM CaCl2) at a concentration of 0.5×105
cells/ml. After the addition of 5 μl of Annexin V-FITC
solution and propidium iodide (PI; 5 μl), the cells were
incubated for 15 min at room temperature. The cells were analyzed
using a flow cytometer (Beckman Coulter, Fullerton, CA, USA).
RNA isolation and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR( of COX,
MMPs and TIMP
Total RNA was extracted from the cultured RASFs
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s instructions. RNA (1 μg) was
reverse-transcribed using the Maxime RT Premix kit (iNtRON
Biotechnology, Seoul, Korea). cDNA was amplified using the
following primer sets: MMP-1 sense, 5′-GAA GGA GAT GAA GCA GCC CAG
ATG T-3′ and antisense, 5′-CAG TTG TGG CCA GAA AAC AGA AGT GAA
A-3′; MMP-3 sense, 5′-GAC ACC AGC ATG AAC CTT GTT-3′ and antisense,
5′-GGA ACC GAG TCA GGA CTA TG-3′; TIMP-1 sense, 5′-CCT TCT GCA ATT
CCG ACC TCG TC-3′ and antisense, 5′-CGG GCA GGA TTC AGG CTA TCT
GG-3′; COX-2 sense, 5′-TCC TTG CTG TTC CCA CCC ATG-3′ and
antisense, 5′-CAT CAT CAG ACC AGG CAC CAG-3′; GAPDH sense, 5′-AAA
TCA AGT GGG GCG ATG CT-3′ and antisense, 5′-AGC TTC CCG TTC AGC TCA
GG-3′. PCR products were electrophoresed using 1% agarose gels and
visualized by staining with ethidium bromide. Densitometric
analysis was performed on the relative intensity of each band using
the Multi Gauge software v3.0 (Fujifilm, Tokyo, Japan).
Immunoblotting
RASFs (1×106 cells) were seeded on 100-mm
culture dishes and harvested in phosphate-buffered saline (PBS).
After washing with PBS, cell pellets were lysed with the lysis
buffer [20 mM HEPES, pH 7.2, 1% Triton X-100, 150 mM NaCl, 0.1 mM
phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA and 1 μg/ml
aprotinin]. Following incubation for 30 min at 4°C, cellular debris
was removed by centrifugation at 100,000 × g for 30 min and the
supernatants were analyzed by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE). To determine membrane COX-2
expression in RASFs, cell membranes were prepared from isolated
RASFs as previously described (16). To determine NF-κB (p65)
expression, nuclear extracts were prepared using a previously
described method (14). To
determine cytoplasmic IkBα expression, cytoplasmic extracts were
prepared as previously described (14).
Protein concentration was determined using the
Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA,
USA). Samples (50 μg) were prepared with 4 vol of 0.5 M Tris
buffer (pH 6.8) containing 4% SDS, 20% glycerol and 0.05%
bromophenol blue at 95°C for 5 min. SDS-PAGE was performed on a 10%
slab gel. Proteins were transferred onto a nitrocellulose membrane.
The membrane was washed in blocking buffer (10 mM Tris-HCl pH 8.0,
150 mM NaCl, 5% fat-free milk) for 60 min at room temperature with
shaking and then washed with TBST (TBS, 0.01% Tween-20). Primary
antibodyies (10 μg/ml) against MMP-1, MMP-3, TIMP, COX-2,
ERK, p-ERK-1/2, p-38, p-p38 MAPK, JNK, p-JNK, NF-κB (p65), IkBα and
β-actin were then added followed by incubation at 4°C for 4 h. The
secondary HRP-conjugated antibody was goat anti-mouse IgG (Santa
Cruz Biotechnology, Inc.). Reactive proteins were detected by
enhanced chemiluminescence (ECL; Amersham Life Sciences, Arlington
Heights, IL, USA) using Fujifilm LAS-3000 (Fujifilm).
Assay of PGE2 production
RASFs (1×104 cells) were grown in 25
cm2 tissue-culture flasks for 48 h before treatment.
After washing with PBS (pH 7.4), cells were pre-treated with IL-1β
(1.0 ng/ml) or kaempferol (100 μM) at 37°C for 48 h in DMEM
containing 10% (v/v) FCS in an atmosphere of 5% CO2,
while the control RASFs were incubated in DMEM with DMSO. The
culture supernatant described above was collected at 2 days. The
level of PGE2 in the medium was determined by ELISA (R&D
Systems) in accordance with the instructions of the
manufacturer.
Statistical analysis
All data are expressed as the means ± SD of the
results of 3 experiments with different RASFs and all data were
analyzed using SPSS 12.0 software. Group mean values were compared
using the Student’s t-test or ANOVA where appropriate. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Kaempferol inhibits the IL-1β-induced
proliferation of RASFs
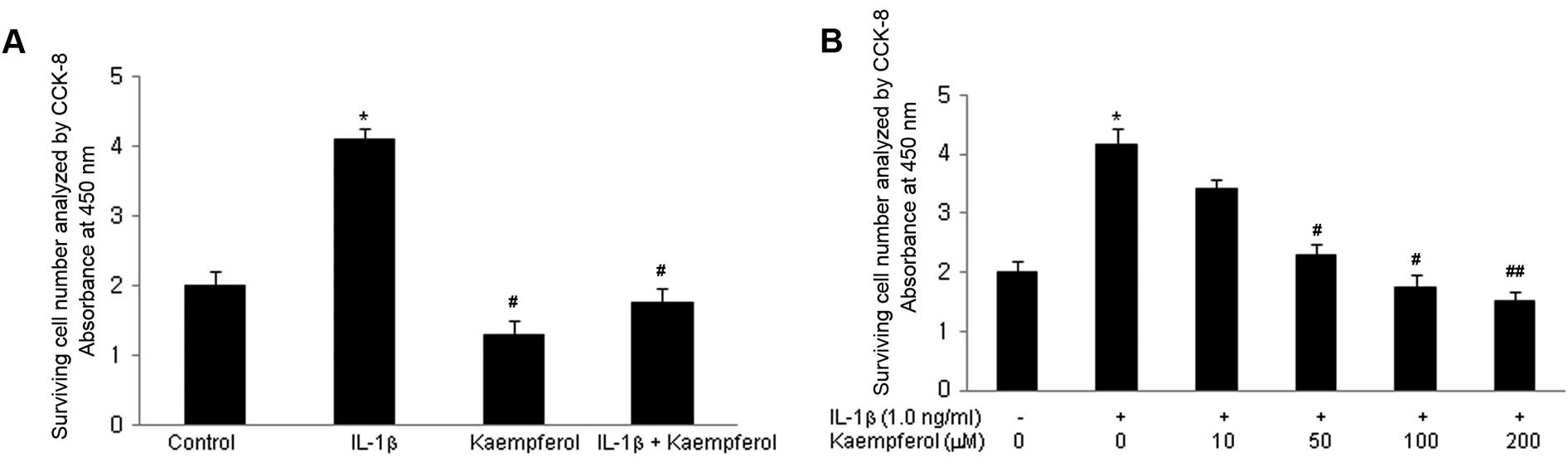
The effect of kaempferol on the growth ability of
RASFs was initially evaluated. Cell proliferation was measured
following treatment with IL-1β for 3 days; IL-1β is a well known
potent growth-promoting factor for RASFs (17). Cell proliferation was assayed as
described in Materials and methods. IL-1β increased the
proliferation of RASFs in a dose-dependent manner (from 0.1 to 10
ng/ml). To examine the effects of kaempferol on the IL-1β-induced
proliferation of RASFs, kaempferol (100 μM) was added to the
RASF cultures with/without IL-1β (1.0 ng/ml) for 2 days and the
CCK-8 assay was performed. IL-1β significantly increased the
proliferation of RASFs compared with the control cells cultured in
DMSO without IL-1β and kaempferol (P<0.05) (Fig. 1A). Kaempferol significantly
inhibited the proliferation of RASFs treated with or without IL-1β
(P<0.05). Various doses of kaempferol (10, 50, 100, 200
μM) were added to the RASF cultures with IL-1β (1.0 ng/ml)
for 2 days and CCK-8 assay was performed. The inhibitory effects of
kaempferol were significantly enhanced in a dose-dependant manner
(Fig. 1B).
Kaempferol induces the apoptosis of
RASFs
To elucidate the underlying mechanisms by which
kaempferol inhibits the IL-1β-induced proliferation of RASFs, the
effects of kaempferol on the apoptosis of RASFs were examined by
flow cytometry and staining with Annexin V and PI. The percentage
of Annexin V-positive cells was significantly increased in the
RASFs treated with kaempferol compared with the cells cultured in
DMEM with DMSO without kaempferol (P<0.05) (Fig. 2).
Effects of kaempferol on IL-1β-induced
MMP, TIMP-1 and COX mRNA expression in RASFs
RT-PCR was performed to determine the mRNA
expression of MMP-1, MMP-3 and TIMP-1 in the monocultured RASFs.
RASFs were stimulated with IL-1β (1.0 ng/ml) for 48 h in the
presence or absence of kaempferol (100 μM). IL-1β enhanced
the mRNA expression of MMP-1 and MMP-3 in RASFs (P<0.05), but
not that of TIMP-1. Kaempferol inhibited the effects of IL-1β on
the mRNA expression of MMP-1 and MMP-3 (P<0.05) (Fig. 3A). IL-1β also enhanced the mRNA
expression of COX-2 in RASFs (P<0.01), but not that of COX-1
(data not shown). Kaempferol inhibited the IL-1β-induced COX-2 mRNA
expression (P<0.05) (Fig. 3A).
Kaempferol also significantly decreased the mRNA expression of
MMP-1, MMP-3 and COX-2 compared with the control cells cultured in
DMSO without IL-1β and kaempferol (P<0.05).
Effects of kaempferol on IL-1β-induced
MMP, TIMP-1 and COX protein expression in RASFs
To determine the protein expression of MMPs, TIMP-1
and COX in the monocultured RASFs, we performed western blot
analysis. RASFs were stimulated with IL-1β (1.0 ng/ml) for 48 h in
the presence or absence of kaempferol (100 μM). IL-1β
enhanced the protein expression of MMP-1 and MMP-3 in RASFs
(P<0.05), but not that of TIMP-1; its effects on protein
expression were similar to those on mRNA expression. Kaempferol
inhibited the IL-1β-induced protein expression of MMP-1 and MMP-3
(P<0.05) (Fig. 3B). IL-1β also
enhanced the protein expression of COX-2 in RASFs (p < 0.01),
but not that of COX-1 (data not shown). Kaempferol inhibited the
IL-1β-induced protein expression of COX-2 (P<0.05) (Fig. 3B). Kaempferol also significantly
decreased the protein expression of MMP-1, MMP-3 and COX-2 compared
with the control cells cultured in DMSO without IL-1β and
kaempferol (P<0.05).
Kaempferol inhibits IL-1β-induced PGE2
production in RASFs
To confirm the effects of kaempferol on the
IL-1β-induced production of PGE2 by RASFs, we examined the
concentration of PGE2 in the culture supernatant. RASFs
(1×104 cells) were grown in 25 cm2
tissue-culture flasks for 48 h before and after treatment with
IL-1β (1.0 ng/ml) and/or kaempferol (100 μM). PGE2
production was increased following treatment with IL-1β (P<0.05)
compared with the control cells cultured with DMSO without IL-1β,
and it was significantly inhibited by treatment with kaempferol at
48 h (P<0.05) (Fig. 4); the
effects of kaempferol on PGE2 were similar to those on COX-2
expression. Kaempferol also significantly decreased the production
of PGE2 compared with the control cells cultured in DMSO without
IL-1β and kaempferol (P<0.05) (Fig. 4).
Effects of kaempferol on IL-1β-induced
signaling pathways in RASFs
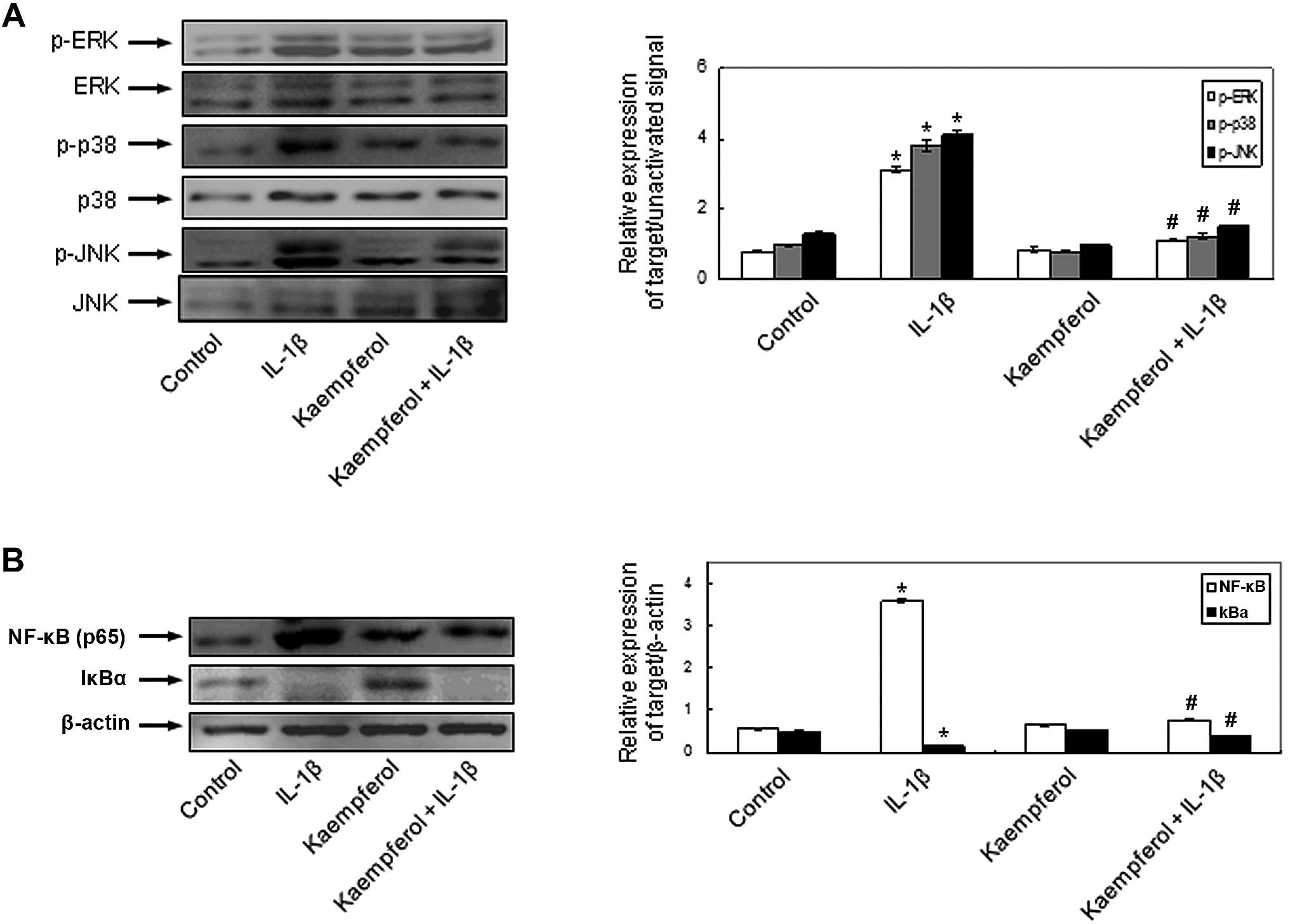
To demonstrate the involvement of the signal
transduction pathways and to elucidate the mechanisms behind the
effects of kaempferol on IL-1β-induced RASF proliferation and the
production of MMPs, COX-2 and PGE2, the activation of MAPKs and
NF-κB was evaluated in the RASFs. IL-1β phosphorylated
intracellular MAPKs, including ERK, p-38 and JNK and kaempferol
significantly inhibited the IL-1β-induced intracellular MAPK
activation (Fig. 5A). The
activation of NF-κB, p65 and the degradation of cytoplasmic IkBα
was observed in the RASFs treated with IL-1β. The effects of IL-1β
on NF-κB activation were abrogated by kaempferol (Fig. 5B). Kaempferol also inhibited the
activation of the above intracellular signaling pathways compared
with the control cells cultured in DMSO without IL-1β and
kaempferol. These results indicate that kaempferol inhibits the
IL-1β-induced proliferation of RASFs, the expression of COX-2 and
the production of PGE2 by inhibiting the activation of
intracellular MAPK and NF-κB pathways.
Discussion
In this study, we demonstrate that kaempferol
induces the apoptosis of RASFs and inhibits the IL-1β-induced
proliferation of RASFs. It inhibits the activation of the MAPK,
ERK1/2, p-38 and JNK and NF-κB signaling pathways, resulting in the
decreased expression of MMPs and COX-2, as well as in the decreased
production of PGE2 by RASFs. These findings suggest that kaempferol
may be used as a novel therapeutic agent for the management of RA
by decreasing synovial inflammation.
Kaempferol, a polyphenolic flavonoid extracted from
fenugreek seeds, has been shown to have strong antioxidant and
anti-inflammatory properties (18). Previous studies have demonstrated
that kaempferol reduces lipopolysaccharide-induced COX-2 levels in
RAW 264.7 cells (9) and inhibits
reactive oxygen species production through the inhibition of iNOS
and TNF-α protein expression in aged gingival tissues (10). Kaempferol has also exhibited
anti-inflammatory effects through the inhibition of IL-4 (11), COX-2 and CRP expression and the
downregulation of NF-κB in liver cells (12). Compared with other daily dietary
flavonols, kaempferol has been reported to be associated with a
decreased risk of various types of cancer (19–21). However, to our knowledge, there
are no reports to date on the effects of kaempferol on inflammatory
reactions, including the production of MMPs, COX-2 and PGE2 by
RASFs and the mechanisms involved, which play a crucial role in the
pathogenesis of synovitis and articular destruction in RA.
In RA, one of the most striking features is the
hyperplasia of synovial fibroblasts in the lining layer which is
considered to be the main mechanism responsible for the hyperplasic
growth of the RA synovium and eventually destroys articular bone
and cartilage (22,23). Given that the IL-1β-induced
proliferation of RASFs is closely involved in inflammatory
synovitis joint destruction, the response of RASFs to IL-1β plays a
crucial role in the physiopathology of RA (24). Our study demonstrated that
kaempferol significantly induced the apoptosis of RASFs and
inhibited the proliferation of unstimulated and IL-1β-stimulated of
RASFs in a dose- and time-dependent manner.
Pro-inflammatory cytokines, including IL-1β enhance
the expression of COX-2 and MMPs in human RASFs (25,26). MMPs are involved in the
destruction of the extracellular matrix in articular structures and
COX-2 converts free arachidonic acid into prostaglandins, including
a variety of bio-active compounds [prostacyclin (PGI2), thromboxane
A2 (TXA2), PGE2 and prostaglandin D2 (PGD2)]. PGE2, a pleiotropic
mediator of inflammation, is involved in several pathological
processes and plays a critical role in eliciting the signs and
symptoms of inflammation in the joints of RA patients when produced
in excess (27). We also found
that kaempferol inhibits the expression of MMPs and COX-2 and PGE2
synthesis in a dose-dependent manner in both unstimulated and
IL-1β-stimulated RASFs. This suggests that kaempferol may be a
potent therapeutic compound for RA. However, further studies are
required to investigate the overall effects of kaempferol on the
pathophysiology of synovitis in in vivo systems, such as
animal models of RA and collagen-induced arthritis (CIA) and to
elucidate the underlying mechanisms.
NF-κB and MAPKs participate in the pathogenic
mechanisms of inflammation and the destruction of joints in RA. It
is known that NF-κB, JNK, p-38 and ERK are expressed in cultured
RASFs and are readily activated by IL-1β and TNF-α (13,28,29). Numerous studies have demonstrated
that inhibitors of MAPKs or NF-κB decrease synovial inflammation,
bone destruction and cartilage damage in animal models of
arthritis, including adjuvant arthritis in rats and CIA in mice
(30,31). It has been described that
kaempferol suppresses IL-1β-induced inflammatory responses by
regulating signaling pathways, including NF-κB activation and MAPK
phosphorylation in human airway epithelial cells (32). In human synovial cells, the
addition of kaempferol has been shown to suppress the TNF-α-induced
increase in the mRNA expression of IL-8 and monocyte chemotactic
protein-1 (MCP-1) in a dose-dependent manner by inhibiting the
activation of NF-κB induced by TNF-α (33). In this study, to elucidate the
mechanisms behind the effects of kaempferol on IL-1β-induced RASF
proliferation, the expression of COX-2 and the production of PGE2,
as well as the activation of MAPKs and NF-κB were examined. Our
results revealed that kaempferol inhibited the IL-1β-induced
activation of NF-κB and the phosphorylation of ERK, p-38 and JNK.
However, further investigations are required to elucidate the
mechanisms by which kaempferol suppresses NF-κB activation, which
components of NF-κB are suppressed, and which intracellular
signaling factors are more specifically or directly involved in the
effects of kaempferol on the proliferation of RASFs and PGE2
production.
To our knowledge, this is the first study to report
that kaempferol induces the apoptosis of RASFs and inhibits the
IL-1β-induced proliferation of RASFs. It also suppresses the
expression of MMP-1, MMP-3 and COX-2 and the production of PGE2 by
RASFs by inhibiting the IL-β-induced activation of NF-κB and the
phosphorylation of the MAPK pathways, p-38, JNK and ERK. Based on
our findings, kaempferol has great potential as a novel therapeutic
agent and may prove useful in the treatment of inflammatory
diseases, including RA. However, further studies are required to
elucidate the exact mechanisms underlying the inhibitory effects of
kaempferol on synovial cell proliferation and inflammatory
reactions.
Acknowledgements
The present study was supported by a grant from the
Korea Healthcare technology R&D Project, Ministry for Health,
Welfare and Family Affairs, Korea (A084144).
References
|
1
|
Henderson B, Hardinham T, Blake S and
Lewthwaite J: Experimental arthritis models in the study of the
mechanisms of articular cartilage loss in rheumatoid arthritis.
Agents Actions Suppl. 39:15–26. 1993.PubMed/NCBI
|
|
2
|
Han MK, Kim JS, Park BH, et al:
NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial
fibroblasts by hypoxia and reoxygenation: potential role in
rheumatoid arthritis. J Leukoc Biol. 73:525–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosetti C, Rossi M, McLaughlin JK, et al:
Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol
Biomarkers Prev. 16:98–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Q, Zhao L, You Q, et al:
Anti-hepatitis B virus activity of wogonin in vitro and in vivo.
Antiviral Res. 74:16–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cárdenas M, Marder M, Blank VC and Roquin
LP: Antitumor activity of some natural flavonoids and synthetic
derivatives on various human and murine cancer cell lines. Bioorg
Med Chem. 14:2966–2971. 2006.PubMed/NCBI
|
|
6
|
Burda S and Oleszek W: Antioxidant and
antiradical activities of flavonoids. J Agric Food Chem.
49:2774–2779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
González-Gallego J, Sánchez-Campos S and
Tuñón MJ: Anti-inflammatory properties of dietary flavonoids. Nutr
Hosp. 22:287–293. 2007.
|
|
8
|
Olszewska M: Separation of quercetin,
sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC
determination of flavonoid aglycones in inflorescences, leaves and
fruits of three Sorbus species. J Pharm Biomed Anal.
48:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SK, Kim HJ, Choi SE, Park KH, Choi HK
and Lee MW: Anti-oxidative and inhibitory activities on nitric
oxide (NO) and prostaglandin E2 (COX-2) production of
flavonoids from seeds of Prunus tomentosa Thunberg. Arch
Pharm Res. 31:424–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HK, Park HR, Lee JS, Chung TS, Chung
HY and Chung J: Down-regulation of iNOS and TNF-alpha expression by
kaempferol via NF-kappaB inactivation in aged rat gingival tissues.
Biogerontology. 8:399–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes JR, Perez-G M, Rivas MD and
Zamorano J: Kaempferol inhibits IL-4-induced STAT6 activation by
specifically targeting JAK3. J Immunol. 179:3881–3887. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Mediavilla V, Crespo I, Collado PS,
Esteller A, Sánchez-Campos S, Tuñón MJ and González-Gallego J: The
anti-inflammatory flavones quercetin and kaempferol cause
inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and
reactive C-protein, and down-regulation of the nuclear factor
kappaB pathway in Chang Liver cells. Eur J Pharmacol. 557:221–229.
2007.
|
|
13
|
Arnett FC, Edworthy SM, Bloch DA, et al:
The American Rheumatism Association 1987 revised criteria for the
classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HY, Jeon HS, Song EK, et al: CD40
ligation of rheumatoid synovial fibroblasts regulates
RANKL-mediated osteoclastogenesis: evidence of NF-kappaB-dependent,
CD40-mediated bone destruction in rheumatoid arthritis. Arthritis
Rheum. 54:1747–1758. 2006. View Article : Google Scholar
|
|
15
|
Comalada M, Camuesco D, Sierra S,
Ballester I, Xaus J, Gálvez J and Zarzuelo A: In vivo quercitrin
anti-inflammatory effect involves release of quercetin, which
inhibits inflammation through down-regulation of the NF-kappaB
pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar
|
|
16
|
Hodgkin PD, Yamashita LC, Coffman RL and
Kehry MR: Separation of events mediating B cell proliferation and
Ig production by using T cell membranes and lymphokines. J mmunol.
145:2025–2034. 1990.PubMed/NCBI
|
|
17
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noh EM, Kim JS, Hur H, et al: Cordycepin
inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid
arthritis synovial fibroblasts. Rheumatology (Oxford). 48:45–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gates MA, Tworoger SS, Hecht JL, De Vivo
I, Rosner B and Hankinson SE: A prospective study of dietary
flavonoid intake and incidence of epithelial ovarian cancer. Int J
Cancer. 121:2225–2232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nöthlings U, Murphy SP, Wilkens LR,
Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk:
the multiethnic cohort study. Am J Epidemiol. 166:924–931.
2007.PubMed/NCBI
|
|
21
|
Garcia-Closas R, Gonzalez CA, Aqudo A and
Riboli E: Intake of specific carotenoids and flavonoids and the
risk of gastric cancer in Spain. Cancer Causes Control. 10:71–75.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komatsu N and Takayanagi H: Autoimmune
arthritis: the interface between the immune system and joints. Adv
Immunol. 115:45–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gitter BD, Labus JM, Lees SL and Scheetz
ME: Characteristics of human synovial fibroblast activation by IL-1
beta and TNF alpha. Immunology. 66:196–200. 1989.PubMed/NCBI
|
|
25
|
Crofford LJ, Wilder RL, Ristimaki AP, Sano
H, Remmers EF, Epps HR and Hla T: Cyclooxygenase-1 and -2
expression in rheumatoid synovial tissues. Effects of interleukin-1
beta, phorbol ester, and corticosteroids. J Clin Invest.
93:1095–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tolboom TC, Pieterman E, van der Laan WH,
et al: Invasive properties of fibroblast-like synoviocytes:
correlation with growth characteristics and expression of MMP-1,
MMP-3, and MMP-10. Ann Rheum Dis. 61:975–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martel-Pelletier J, Pelletier JP and Fahmi
H: New insights into prostaglandin biology. J Rheumatol. 31:14–16.
2004.PubMed/NCBI
|
|
28
|
Verma IM, Stevenson JK, Schwartz EM, Van
Antrerp D and Mitamoto S: Rel/NF-kappa B/I kappa B family: intimate
tales of association and dissociation. Genes Dev. 9:2723–2735.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han Z, Boyle DL, Chang L, et al: c-Jun
N-terminal kinase is required for metalloproteinase expression and
joint destruction in inflammatory arthritis. J Clin Invest.
108:73–81. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
McIntyre KW, Shuster DJ, Gillooly KM, et
al: A highly selective inhibitor of I kappa B kinase, BMS-345541,
blocks both joint inflammation and destruction in collagen-induced
arthritis in mice. Arthritis Rheum. 48:2652–2659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishikawa M, Myoui A, Tomita T, Takahi K,
Nampei A and Yoshikawa H: Prevention of the onset and progression
of collagen-induced arthritis in rats by the potent p38
mitogen-activated protein kinase inhibitor FR167653. Arthritis
Rheum. 48:2670–2681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon SH, Nam JI, Kim SH, Kim JH, Yoon JH
and Kim KS: Kaempferol and quercetin, essential ingredients in
Ginkgo biloba extract, inhibit interleukin-1beta-induced MUC5AC
gene expression in human airway epithelial cells. Phytother Res.
23:1708–1712. 2009. View
Article : Google Scholar
|
|
33
|
Kuhns DB, Priel DA and Gallin JI:
Induction of human monocyte interleukin (IL)-8 by fibrinogen
through the toll-like receptor pathway. Inflammation. 30:178–188.
2007. View Article : Google Scholar : PubMed/NCBI
|