Introduction
There is increasing concern regarding the health
risks arising from exposure to low doses of ionizing radiation,
particularly within the medical diagnostic and therapeutic fields
(1,2). One of the challenging factors in the
study of the health effects of exposures to low doses of ionizing
radiation is the lack of epidemiological data, primarily due to
confounding factors related to inter-individual variability, life
style, and genetic background (3). Furthermore, in contrast to the
well-studied mechanisms and the thoroughly investigated health
effects of high doses, where cancer is a well-validated endpoint,
the mechanisms and the biological responses, induced by low doses
of ionizing radiation, are largely not agreed upon in the published
studies (4).
Previously, we demonstrated that low doses of
ionizing radiation induce immune responses in whole blood samples,
with the gene set enrichment analysis showing the involvement of
both innate and adaptive immunity (5). Monocytes are key players in the
immune response. These cells originate from the bone marrow and are
then released into the bloodstream following maturation, while in
tissues, they differentiate into macrophages and myeloid dendritic
cells (DCs) (6). Monocytes are
important in antigen presentation, release of immune-regulatory
cytokines, T-cell stimulation and differentiation, thus
orchestrating the immune system in response to ‘attacking signals’
(7). Toll-like receptors (TLRs)
are crucial in initiating an immune response in monocytes. TLRs are
involved in connecting the innate and adaptive immune responses
together and activating monocytes to secrete pro-inflammatory
cytokines (8,9). In humans, there are 10 characterized
TLRs (TLR 1–10). They interact with evolutionary conserved
pathogen-associated molecular patterns (PAMPs), such as
lipopolysaccharides (LPS), but also with endogenous stress signals,
known as danger-associated molecular patterns (DAMPs), including
fibrinogen, heat shock proteins, high mobility group B1 (HMGB1)
protein, as well as extracellular matrix components (10). Four adapter proteins play a key
role in the signaling of TLR pathways: MyD88, Tirap, Trif and Tram.
These pathways activate the signaling of the transcription factors
NF-κB, activator protein 1 (Ap-1) and interferon factors (IRF) 3 or
7. MyD88 pathway is the major signaling adapter, and is involved in
the promotion of NF-κB translocation into the nucleus and
activation of mitogen-activated protein kinases, leading to growth
factors and cytokine secretion (8,11).
In this study, we investigated the activation of
different TLRs in response to low and high doses of ionizing
radiation by measuring the expression of TLR2, TLR9 as well as
TLR4, a key monocyte TLR. The three receptors were chosen as they
share a common DAMP (HMGB1), and due to the mounting evidence of
their involvement in response to radiation stresses (12–14). Furthermore, the investigation
included downstream signaling pathways; in particular, MAPKs, a
group of serine/threonine protein kinases involved in a wide
variety of cell processes, such as cell differentiation,
proliferation, apoptosis, as well as in the secretion of
inflammatory cytokines. There are three major MAPK pathways: the
extracellular-signal-regulated-kinase (ERK), the Jun N-terminal
kinase (JNK) and the p38 pathways (15). We also evaluated the
phosphorylation levels of IκBα, a protein that sequesters NF-κB in
the cytoplasm and prevents its translocation to the nucleus
(16). The results showed an
immune-stimulatory response at low doses of ionizing radiation
through the involvement of TLR4 axis of signaling
(HMGB1-TLR4-MyD88-IRAK1). This observation was confirmed by the
activation of MAPK and NF-κB signaling. By contrast, high doses
predominantly exhibit a damaging response, through the induction of
classical ionizing radiation damage and stress genes
(CDKN1A, POLH, DDB2 and AEN) and
activation of the p53 protein. Furthermore, the immune-suppressive
response was demonstrated, mainly via the suppression of NF-κB and
MAPK signaling.
Materials and methods
Monocyte isolation, culture and
irradiation
After signing an informed consent form, 48 ml of
peripheral blood was obtained from 8 healthy donors in EDTA
vacutainer tubes. The whole blood samples were divided into three
different 50 ml Falcon tubes (16 ml/tube), and 160 μl of 100 mM
EDTA and 800 μl of Rosettesep™ Human Monocyte Enrichment Cocktail
(StemCell™ Technologies, Vancouver, BC, Canada) were added to each
tube, according to the manufacturer’s instructions. Blood was
diluted at 1:1 with phosphate-buffered saline (PBS) (Gibco-BRL,
Ghent, Belgium) supplemented with 2% heat-inactivated fetal bovine
serum (FBS) (Gibco-BRL) and 1% 100 mM EDTA (Invitrogen Life
Technologies, Carlsbad, CA, USA). The diluted whole blood samples
(32 ml) were transferred into SepMate™-50 tubes (StemCell™
Technologies) and layered over 15 ml of density gradient medium,
Ficoll-Paque™ PLUS (GE Healthcare, Uppsala, Sweden), according to
the manufacturer’s instructions. SepMate™-50 (StemCell™
Technologies) tubes were centrifuged at 1,200 × g for 10 min at
room temperature. The top layer, containing the monocytes, was
transferred into a new tube and washed three times with the washing
buffer. All the washing steps were performed at 350 × g for 8 min
with brakes off. The cells were counted using a Moxi Z Mini
Automated Cell Counter (Orflo Technologies, Hailey, ID, USA). Cells
(1.2×106) were then cultured in each of the 6-well
plates (Grenier Bio-One, Wemmel, Belgium) at a concentration of
5×105 cells/ml of RMPI-1640 medium (Gibco-BRL)
supplemented with 20% heat-inactivated FBS (Gibco-BRL) and 1%
penicillin-streptomycin (100 U/ml penicillin, 0.1 mg/ml
streptomycin) (Invitrogen Life Sciences, Ghent, Belgium). The
culture plates were exposed at room temperature to sham irradiation
(control) or to three different X-ray doses (0.05, 0.1 and 1 Gy),
at a rate of 30 mGy/min (250 kV, 1.6 mA, 1 mm Cu). Subsequently,
the cultured plates were incubated for 6 h at 37°C in a humidified
incubator with 5% CO2.
RNA isolation, cDNA synthesis and
quantitative RT-PCR (qRT-PCR)
Adherent cells were scraped using Mini Cell Scrapers
(Biotium, Hayward, CA, USA) and washed three times with PBS
(Gibco-BRL). All the washing steps were performed at 350 × g for 8
min, then suspended in 350 μl of lysis buffer (RLT buffer + 10
μl/ml of β-mercaptoethanol) provided in the RNeasy® Mini
Kit (Qiagen, Germantown, MD, USA). Lysates were stored at −80°C
until processed. RNA was isolated using the RNeasy® Mini
Kit (Qiagen) following the manufacturer’s instructions. RNA
quantity was measured using a NanoDrop 2000 Spectrophotometer
(Thermo Scientific, Aalst, Belgium). The quality was assessed with
Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA,
USA), and average RIN was 8.9±0.5. For qRT-PCR experiments, we
selected six different genes belonging to TLR signaling
(HMGB1, TLR4, TLR9, TLR2, IRAK1
and MyD88) and four genes belonging to damage response
(CDKN1A, POLH, DDB2 and AEN), as well
as the PF4 gene, shown to be involved in MAPK signaling.
RPLP0 was used as a housekeeping gene. Briefly, cDNA was
prepared from 200 ng of total RNA using SuperScript®
Vilo™ Master Mix (Invitrogen Life Technologies) following the
manufacturer’s instructions. RT-PCR was performed using
TaqMan® Gene Expression Assays (Applied Biosystems,
Grand Island, NY, USA). Each assay comprised two gene-specific
primers and TaqMan assay: FAM (6-carboxyfluorescein) labeled with
MGB (minor groove binder) probe. Each TaqMan assay was run in
duplicate for each diluted cDNA sample using TaqMan®
Fast Advanced Master Mix (Applied Biosystems). The reactions were
run on 7500 Fast Real-Time PCR system (Applied Biosystems)
following the manufacturer’s recommended PCR program: 95°C for 20
sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec.
Relative expression values were calculated by the Pfaffl method
(17).
Protein extraction, quantification and
ELISA assay
Adherent cells were scraped using Mini Cell Scrapers
(Biotium) and washed three times with PBS (Gibco-BRL). All the
washing steps were performed at 350 × g for 8 min, then lysed with
Lysis Mix provided in Abcam PhosphoTracer kits (Abcam®,
Cambridge, UK) following the manufacturer’s instructions. Proteins
were quantified using Quick Start™ Bradford Protein Assay (Bio-Rad,
Hercules, CA, USA) following the manufacturer’s instructions.
Proteins were stored at −80°C until assays were performed.
Quantitative measurements of the proteins of interest were
performed using a PhosphoTracer ELISA kit (ab119661) for the
measurement of phosphorylated IκBα (Ser32/36) (Abcam®),
PhosphoTracer ELISA kit (ab119674) for the measurement of
phosphorylated ERK (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182) and
JNK 1/2/3 (Thr202/Tyr185) (Abcam®) and PhosphoTracer
ELISA kit (ab119666) for the measurement of phosphorylated p53
(Ser15). The immuno-sandwich ELISA assay was performed according to
the manufacturer’s instructions. Fluorescence signals were measured
using the microplate reader Fluoroskan Ascent CF (Thermo
Labsystems, Franklin, MA, USA) with an excitation filter of 544 nm
and an emission filter of 590 nm. To obtain protein fold changes,
the fluorescence signals from irradiated samples were normalized to
their matching sham-irradiated controls.
Statistical analysis software and
graphical representation
For statistical analysis, normality was checked
using the D’Agostino and Pearson omnibus normality test.
Statistical significance was estimated using the Friedman test or
repeated measures ANOVA and Dunn’s or Tukey tests for multiple
comparison. P<0.05 was considered to indicate statistical
significance. Statistical analysis and graphical representation
were performed using the GraphPad Prism 5 program.
Results
Cultured monocytes
Monocytes were isolated and purified from human
whole blood samples using density gradient centrifugation and human
monocyte enrichment antibody cocktail. The average number of
purified cells was 375±83×103 cells/ml whole blood.
Results of the trypan blue staining showed that at least 95% of the
cells cultured for 6 h were viable.
Low doses of ionizing radiation induced
the TLR signaling axis
Monocytes were activated via the induction of TLR
signaling. Our results showed a significant upregulation of TLR4 at
0.05 Gy (Fig. 1A). Furthermore,
there was a significant upregulation of TLR9 at 0.1 Gy (Fig. 1B). TLR2 did not show any
significant changes in response to any of the doses (Fig. 1D). TLR2, 4 and 9 interact with the
endogenous molecule HMGB1, which is secreted by activated monocytes
or released due to increased damage. All the doses showed increased
levels of HMGB1 (Fig. 1C).
Adapter molecules are important in initiating the
signal transduction of the activated TLRs. One of the key adapter
molecules is MyD88. TLR 2, 4 and 9 signaling is known to be
mediated via MyD88. Although our results demonstrated an
upregulatory trend for all the doses, only the samples irradiated
with 0.05 Gy achieved statistical significance (Fig. 2A). IRAK1 interacts with MyD88 for
further promotion of TLR signaling, leading to the activation of
NF-κB and MAPKs. Our results showed an upregulation in the levels
of IRAK1 at 0.05 and 0.1 Gy, but not at 1 Gy (Fig. 2B).
Low doses of ionizing radiation induced
positive NF-κB signaling and MAPK activation
IRAK1 is known to interact with IκBα, which is a
protein that sequesters the NF-κB transcription factor in the
cytoplasm and prevents its translocation to the nucleus. Following
activation of NF-κB signaling, IκBα is phosphorylated and degraded.
Phosphorylation at Ser32/36 is essential for the release of NF-κB
and it is considered a good marker for NF-κB activation. Our
results showed upregulation in the phosphorylation of IκBα at 0.05
and 0.1 Gy and downregulation in the phosphorylation levels at 1 Gy
(Fig. 3A). In addition to that,
MyD88-IRAK1 signaling molecules promote the activation of MAPKs.
These are serine/threonine protein kinases that are activated
subsequent to phosphorylation. The three major MAPKs are p38, ERK
and JNK. Our results showed an upregulation of the phosphorylated
form, thus the activated form, of the three MAPKs in response to
0.05 Gy (Fig. 3B, C and D). By
contrast, higher doses (0.1 and 1 Gy) exhibited a downregulation of
the three MAPKs (Fig. 3B, C and
D). To investigate the responses of the MAPKs at different
doses, we measured the levels of PF4 gene. PF4 (platelet
factor 4) is known to induce the activation of MAPKs (p38, ERK and
JNK) in monocytes. The expression levels were in agreement with the
phosphorylation levels of the proteins; PF4 showed lower
induction at 0.1 and 1 Gy with significant statistical difference
between 0.05 Gy in comparison to 0.1 and 1 Gy (Fig. 4).
High doses of ionizing radiation induced
damage responses and p53 activation
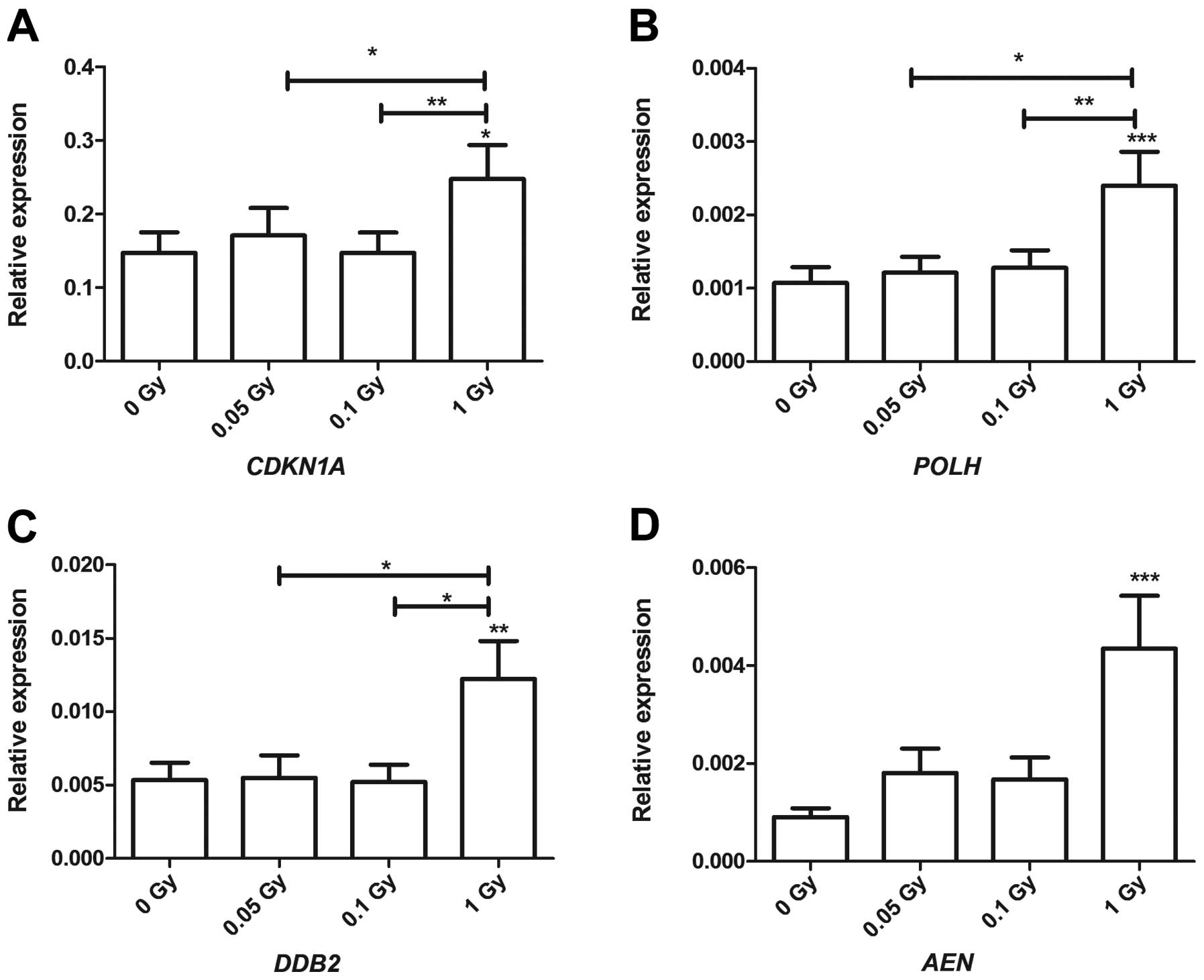
Samples irradiated with 1 Gy showed a significant
upregulation of the four genes involved in response to stress and
damage (Fig. 5). These included
cyclin-dependent kinase inhibitor 1A (CDKN1A), which plays a
role in cycle arrest in response to DNA damage. It showed a
statistical significance at 1 Gy compared to the sham-irradiated
samples and between the different irradiation doses, i.e., between
0.05 and 1 Gy, but also between 0.1 and 1 Gy (Fig. 5A). Additionally, polymerase
DNA-direct η (POLH), a gene that plays a role in DNA repair
in response to DNA damage was upregulated exclusively at 1 Gy. A
statistically significant difference between 1 Gy in comparison to
0.05 and 0.1 Gy (Fig. 5B) was
observed. Another DNA damage and repair gene, DNA damage binding
protein-2 (DDB2), showed a similar expression profile to
that of POLH. It showed statistical significance in response
to 1 Gy compared to the sham-irradiated samples with a statistical
difference between 1 Gy, compared to 0.05 and 0.1 Gy (Fig. 5C). The pro-apoptotic gene
apoptosis enhancing nuclease (AEN) showed an upregulatory
trend at low doses (0.05 and 0.1 Gy) without achieving statistical
significance; however, it was significantly upregulated in response
to 1 Gy (Fig. 5D). Furthermore,
the phosphorylated form of p53 showed a significant upregulation in
response to 1 Gy in comparison to the sham-irradiated samples, with
a statistical significance between the induced levels at 0.05 and 1
Gy (Fig. 6).
Discussion
Gaining a better understanding of the mechanisms
involved in cell and tissue responses to low doses of ionizing
radiation is of increased interest as medical diagnostic and
therapeutic applications involving X-rays are significant
contributory factors to the cumulative doses of the general
population (18). Previously,
whole genome expression studies showed that high doses of ionizing
radiation predominantly induced DNA damage and apoptosis with p53
signaling playing a key role, while low doses induced mainly
cytokine and chemokine signaling (5). To clarify the mechanisms behind this
immune response induction, we investigated the activation of
central immune pathways in the primary human monocytes following
exposure to low and high doses of ionizing radiation. Our results
showed immune-stimulatory and pro-survival responses at low doses
via the involvement of the TLRs, MAP kinases and positive
regulation NF-κB signaling pathways. On the other hand, a dose of 1
Gy showed less involvement of the pro-survival and pro-inflammatory
pathways, of NF-κB signaling and MAP kinase activation; however,
damage responses were induced.
TLR4 plays a crucial role in activating monocytes
and in initiating an innate immune response. TLR4 detects
lipopolysaccharides (LPS) and mediates its signal transduction via
CD14 receptors (10). Our results
have shown that low doses of ionizing radiation induced the
activation of TLR4, while there was no significant change in its
expression following exposure to 1 Gy (Fig. 1A). Previously, it has been
reported that doses in the range of 0.075–2 Gy increased the
expression of TLR4-MD2 in mouse macrophages (12). Additionally, TLR4 was shown to be
involved in the induction of radiation resistance (19), cell proliferation and the
promotion of radiation-induced lymphomas (20), as well as the promotion of
reactive oxygen and nitrogen species (ROS/RNS) causing the
aggravation of chronic inflammatory diseases (21). One of the novel activators of TLR4
is HMGB1 (22). The expression of
HMGB1 increased significantly at all doses (Fig. 1B). Principally, HMGB1 is a nuclear
DNA binding protein that plays a role in the stabilization of
chromosomes and transcription regulation. However, recently, the
role of HMGB1 extended to include inflammation and necrosis
(22). Activated monocytes and
macrophages secrete HMGB1 and promote immune-stimulatory signaling
(23). On the other hand, damaged
cells ‘passively’ release HMGB1 into the extracellular environment
(24), which could explain the
dose-dependent response demonstrated in our data (Figs. 1B and 7). Another TLR that is known to interact
with HMGB1 is TLR2 (25). Results
of the present study did not show any significant changes in the
expression of TLR2 at all doses (Figs. 1C and 7). It has been reported that the
interaction between HMGB1 and TLR2 is structurally and
mechanistically different to TLR4 as TLR2 interacts only with
nucleosome-bound HMGB1 proteins (26).
 | Figure 7Schematic representation of the
mechanisms involved in the induction of immune responses after
exposure to a low (0.05 Gy) and a high dose (1 Gy) of ionizing
radiation. The dashed arrows represent 0.05 Gy, while the complete
arrows represent 1 Gy. The direction of the arrows indicates the
regulation status. Low doses of ionizing radiation induce TLR4 but
not TLR2. TLR2, 4 and 9 are known to interact with HMGB1, leading
to the activation of TLR signal transduction. The adapter molecule
MyD88 plays a central role in the activation process, as it
interacts with different molecules, such as IRAK1, which is
involved in releasing NF-κB transcription factor, thus facilitating
its translocation to the nucleus via the phosphorylation of IκBα.
Downstream IRAK1, MAPKs (p38, ERK and JNK) are activated, thus
promoting pro-inflammatory signaling. PF4 is involved in the
activation of MAPKs in monocytes. NF-κB and MAPKs signaling were
activated at low but not high doses; activation of these pathways
stimulate inflammatory and survival responses. HMGB1, high mobility
group binding protein 1; TLR, toll-like receptor; MAPKs,
mitogen-activated protein kinases; PF4, platelet factor 4. |
Bacterial CpG DNA sequences and DAMPs, including
HMGB1, activate TLR9 (10,13).
Our results showed an upregulatory trend of TLR9 at all doses
(Figs. 1D and 7). Ermakov et al (27,28) showed that irradiation of
lymphocytes and endothelial cells with 0.1 Gy led to the secretion
of extracellular DNA fragments, which promotes the activation of
TLR9. Furthermore, they reported that these DNA fragments, along
with TLR9, played a role in bystander signaling between irradiated
and non-irradiated cells.
Downstream of the TLR signaling, the MyD88 adapter
molecule is known to be crucial for the secretion of
pro-inflammatory cytokines, such as IL-12 and IL-18 (29,30). MyD88 is involved in the downstream
signaling of TLR4 and TLR9. It showed statistically significant
overexpression in response to 0.05 Gy, while the two other doses
(0.1 and 1 Gy) were upregulatied without reaching statistical
significance (Figs. 2A and
7).
Following activation, MyD88 interacts with the IRAK1
molecule, which plays a role in releasing the NF-κB transcription
factor via the phosphorylation of the sequestering protein IκBα
(31). IRAK1 was overexpressed
significantly at 0.05 and 0.1 Gy but not at 1 Gy (Fig. 2B). Furthermore, the downstream
molecule IκBα was upregulated by 2- and 2.5-fold changes at 0.05
and 0.1 Gy, respectively (Figs.
3A). By contrast, the phosphorylation levels of IκBα exhibited
a dephosphorylation pattern in response to 1 Gy (Fig. 3A). The positive regulation of
NF-κB signaling at low doses indicates the promotion of
pro-survival and pro-inflammatory responses, whereas the
suppression of NF-κB signaling at high doses indicates a
pro-apoptotic response (16).
MAPKs such as p38, ERK 1/2 and JNK, are activated
downstream of the HMGB1-TLR4-MyD88-IRAK1 signaling axis (15). Our results showed increased fold
changes of phosphorylation, thus activation, of the three MAPKs at
0.05 Gy (Fig. 3B–D). However, and
in contrast to our expectations, the phosphorylation levels did not
show any increased fold changes at 0.1 Gy, which showed a similar
response to that of 1 Gy (Fig.
3B–D). Previously, we have demonstrated that PF4 gene
was upregulated in whole blood samples exposed to low doses of
ionizing radiation (5). PF4
encodes for the platelet factor-4 protein, a chemokine expressed in
platelets, T cells, monocytes, endothelial and muscle cells
(32). In monocytes, it induces
phagocytosis and activates MAPK family proteins (p38, ERK and JNK)
leading to ROS formation (33).
Our results showed a significant downregulation in PF4 at
0.1 and 1 Gy compared to 0.05 Gy (Figs. 4 and 7). This result is in agreement with the
activated MAPK levels measured at the protein levels. This suggests
a role of PF4 in the activation or suppression of MAPKs in
response to ionizing radiation.
Previously, we have reported that MAPK cascades play
a central role in whole blood samples irradiated with 0.05 Gy
(5). In addition to that, studies
on exposure to low doses have reported a proliferative response
through the activation of ERK 1/2, p38 and JNK (34,35). A study by Kim et al
(36) showed that in normal
diploid cells, there is a transient phosphorylation of ERK 1/2 and
p38 but not JNK 1/2 in response to 0.05 Gy of γ-irradiation, which
was accompanied with the activation of several downstream
transcription factors that promote growth signaling, including
Elk-1, p90RSK and ATF-2. Another study performed by Liang et
al (37) showed that MAPK
proteins c-Raf, ERK 1/2 and MEK 1/2 were activated in mesenchymal
stem cells in response to 0.075 Gy of X-rays, thus a proliferative
response was concluded.
In contrast to results obtained at the exposure dose
of 0.05 Gy, our results showed suppressed MAPKs levels after
exposure to 1 Gy (Fig. 3B–D).
Tsukimoto et al (38)
demonstrated that increased levels of MAPK phosphatase-1 (MKP-1)
promote dephosphorylation of p38 and ERK1/2 in the macrophage cell
line (RAW2674.7) irradiated with 0.5–1 Gy γ-irradiation. In
addition to that, studies performed by Lödermann et al
(39) demonstrated a decreased
induction of NF-κB and p38 in response to 0.5–0.7 Gy of
γ-irradiation.
However, our results demonstrated that the damage
responses were very prominent in response to 1 Gy. CDKN1A
(Fig. 5A) a cell cycle arrest
inducer gene; POLH (Fig.
5B) and DDB2 (Fig.
5C), DNA damage and repair genes; and AEN, (Fig. 5D) a pro-apoptotic gene, were shown
to be exclusively induced in response to 1 Gy. These genes were
extensively validated in response to ionizing radiation (40–44) and shown to be activated in
response to p53 signaling (45),
where as our results showed upregulation in the phosphorylated form
of p53 in response to 1 Gy (Fig.
6). Altogether, the 1 Gy results demonstrate immuno-suppressive
and damaging responses.
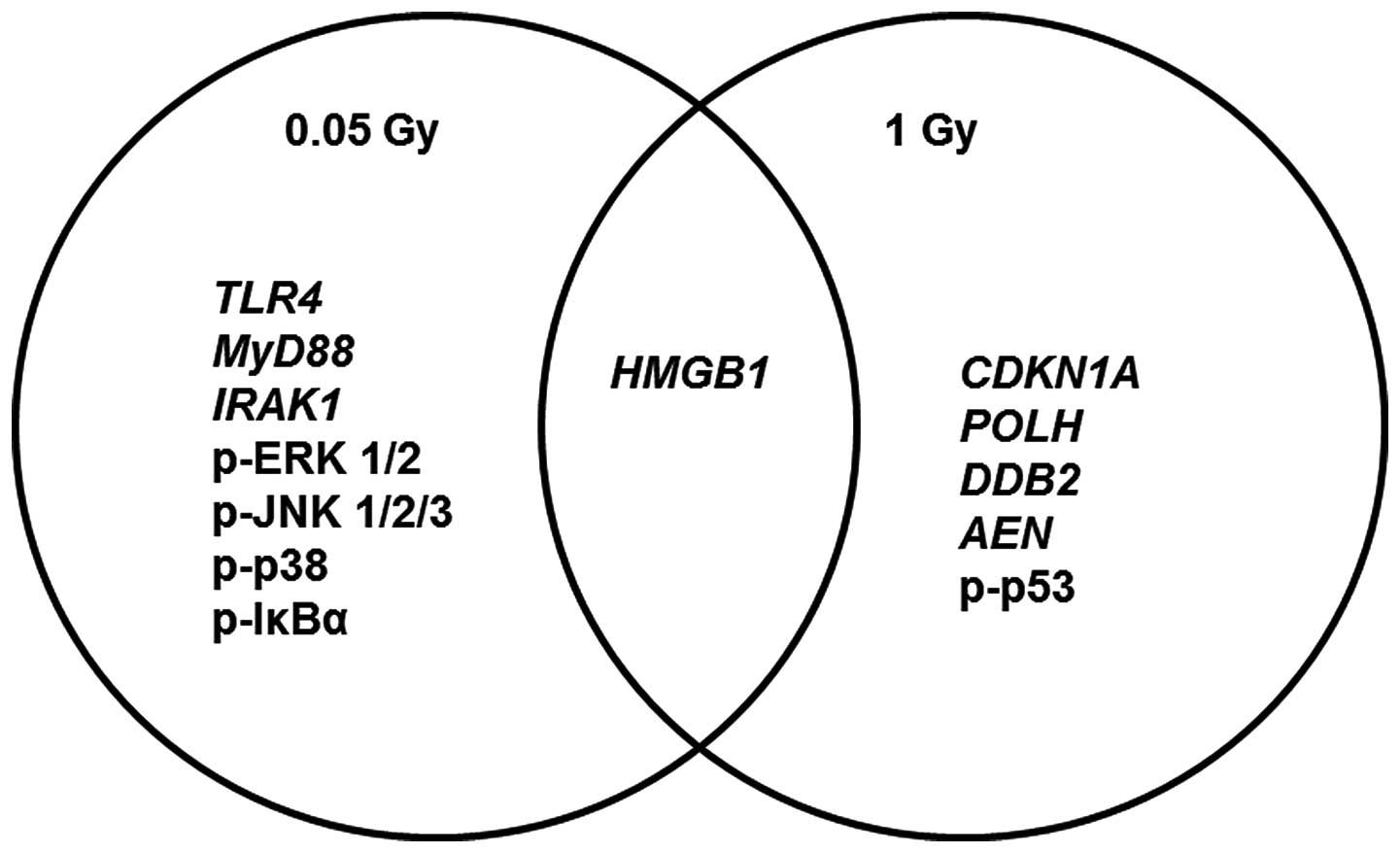
In conclusion, we have demonstrated that monocytes
exposed to low doses of ionizing induce immune-stimulatory and
pro-survival responses, while those exposed to high doses induced
immuno-suppressive and damaging responses. Low-dose responses were
transduced via the TLR4 signaling axis, i.e.,
HMGB1-TLR4-MyD88-IRAK1-MAPKs-NF-κB, which showed a particular
upregulation in response to 0.05 Gy. By contrast, the same axis did
not demonstrate similar induction in response to 1 Gy. Of note, 0.1
Gy showed a ‘borderline’ immune-stimulatory response between low
and high doses, i.e., TLRs and NF-κB signaling were upregulated but
not MAPKs. On other hand, damaged genes and p53 were exclusively
involved in response to 1 Gy but not at lower doses (Fig. 8). In view of these results, we
clearly address the necessity to consider the health risks that can
be induced in response to low doses of ionizing radiation, with the
TLR, NF-κB and MAPK signaling pathways being involved in tumor
proliferation, autoimmunity and chronic inflammation (15,16,22,46). Furthermore, there is accumulation
of evidence that has demonstrated an increased cancer risk due to
exposure to low doses from medical diagnostics. Thus, there is a
need for the re-evaluation of the radiation protection measures
within the medical field.
Acknowledgements
The authors are thankful to all the blood donors and
SCK-CEN medical staff who kindly accepted to participate in our
study. In addition, we appreciate Dr P. Willems [Federal Agency for
Nuclear Control (FANC), Belgium] for the useful scientific
discussions carried out through the preparation of the study. H.
El-Saghire is supported by a doctoral SCK-CEN/Ghent University
grant. This study was funded by the FANC CT-SCAN contract
(CO-90-09-2329-00) and by the FP7 EU EPI-CT contract (grant
agreement 269912).
References
|
1
|
Hall EJ and Brenner DJ: Cancer risks from
diagnostic radiology. Br J Radiol. 81:362–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pearce MS, Salotti JA, Little MP, et al:
Radiation exposure from CT scans in childhood and subsequent risk
of leukaemia and brain tumours: a retrospective cohort study.
Lancet. 380:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pernot E, Hall J, Baatout S, et al:
Ionizing radiation biomarkers for potential use in epidemiological
studies. Mutat Res. 751:258–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah DJ, Sachs RK and Wilson DJ:
Radiation-induced cancer: a modern view. Br J Radiol.
85:e1166–e1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Saghire H, Thierens H, Monsieurs P,
Michaux A, Vandevoorde C and Baatout S: Gene set enrichment
analysis highlights different gene expression profiles in whole
blood samples X-irradiated with low and high doses. Int J Radiat
Biol. 89:628–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hume DA: The mononuclear phagocyte system.
Curr Opin Immunol. 18:49–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer M, Goldstein M, Christmann M, Becker
H, Heylmann D and Kaina B: Human monocytes are severely impaired in
base and DNA double-strand break repair that renders them
vulnerable to oxidative stress. Proc Natl Acad Sci USA.
108:21105–21110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krutzik SR, Tan B, Li H, et al: TLR
activation triggers the rapid differentiation of monocytes into
macrophages and dendritic cells. Nat Med. 11:653–660. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piccinini AM and Midwood KS: DAMPening
inflammation by modulating TLR signalling. Mediators Inflamm.
2010:6723952010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shan YX, Jin SZ, Liu XD, Liu Y and Liu SZ:
Ionizing radiation stimulates secretion of pro-inflammatory
cytokines: dose-response relationship, mechanisms and implications.
Radiat Environ Biophys. 46:21–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivanov S, Dragoi AM, Wang X, et al: A
novel role for HMGB1 in TLR9-mediated inflammatory responses to
CpG-DNA. Blood. 110:1970–1981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menendez D, Shatz M, Azzam K, Garantziotis
S, Fessler MB and Resnick MA: The Toll-like receptor gene family is
integrated into human DNA damage and p53 networks. PLoS Genet.
7:e10013602011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012.
|
|
17
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
UNSCEAR. Report of the fifty seventh
session: Summary of low-dose radiation effects on health. 2010.
|
|
19
|
Liu C, Zhang C, Mitchel RE, et al: A
critical role of toll-like receptor 4 (TLR4) and its’ in vivo
ligands in basal radio-resistance. Cell Death Dis. 4:e6492013.
|
|
20
|
Liu C, Gao F, Li B, et al: TLR4 knockout
protects mice from radiation-induced thymic lymphoma by
downregulation of IL6 and miR-21. Leukemia. 25:1516–1519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lucas K and Maes M: Role of the Toll like
receptor (TLR) radical cycle in chronic inflammation: possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaue D and McBride WH: Links between
innate immunity and normal tissue radiobiology. Radiat Res.
173:406–417. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang FP, Li L, Li J, Wang JY, Wang LY and
Jiang W: High mobility group box-1 promotes the proliferation and
migration of hepatic stellate cells via TLR4-dependent signal
pathways of PI3K/Akt and JNK. PLoS One. 8:e643732013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersson U and Tracey KJ: HMGB1 is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng H, Deng Y, Xie Y, Liu H and Gong F:
Expression and significance of HMGB1, TLR4 and NF-κB p65 in human
epidermal tumors. BMC Cancer. 13:3112013.
|
|
26
|
Mittal D, Saccheri F, Venereau E, Pusterla
T, Bianchi ME and Rescigno M: TLR4-mediated skin carcinogenesis is
dependent on immune and radioresistant cells. EMBO J. 29:2242–2252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ermakov AV, Konkova MS, Kostyuk SV,
Egolina NA, Efremova LV and Veiko NN: Oxidative stress as a
significant factor for development of an adaptive response in
irradiated and nonirradiated human lymphocytes after inducing the
bystander effect by low-dose X-radiation. Mutat Res. 669:155–161.
2009. View Article : Google Scholar
|
|
28
|
Kostyuk SV, Ermakov AV, Alekseeva AY, et
al: Role of extracellular DNA oxidative modification in radiation
induced bystander effects in human endotheliocytes. Mutat Res.
729:52–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ichikawa S, Miyake M, Fujii R and Konishi
Y: MyD88 associated ROS generation is crucial for Lactobacillus
induced IL-12 production in macrophage. PLoS One. 7:e358802012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiedler K, Kokai E, Bresch S and Brunner
C: MyD88 is involved in myeloid as well as lymphoid hematopoiesis
independent of the presence of a pathogen. Am J Blood Res.
3:124–140. 2013.PubMed/NCBI
|
|
31
|
Windheim M, Stafford M, Peggie M and Cohen
P: Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination
of IL-1 receptor-associated kinase 1 to facilitate NEMO binding and
the activation of IkappaBalpha kinase. Mol Cell Biol. 28:1783–1791.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao Z, Visentin GP, Dayananda KM and
Neelamegham S: Immune complexes formed following the binding of
anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human
neutrophil activation and cell adhesion. Blood. 112:1091–1100.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasper B, Brandt E, Brandau S and Petersen
F: Platelet factor 4 (CXC chemokine ligand 4) differentially
regulates respiratory burst, survival, and cytokine expression of
human monocytes by using distinct signaling pathways. J Immunol.
179:2584–2591. 2007. View Article : Google Scholar
|
|
34
|
Narang H and Krishna M: Mitogen-activated
protein kinases: specificity of response to dose of ionizing
radiation in liver. J Radiat Res. 45:213–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rizvi A, Pecaut MJ, Slater JM, Subramaniam
S and Gridley DS: Low-dose γ-rays modify CD4(+) T cell signalling
response to simulated solar particle event protons in a mouse
model. Int J Radiat Biol. 87:24–35. 2011.
|
|
36
|
Kim CS, Kim JM, Nam SY, et al: Low-dose of
ionizing radiation enhances cell proliferation via transient ERK1/2
and p38 activation in normal human lung fibroblasts. J Radiat Res.
48:407–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang X, So YH, Cui J, et al: The low-dose
ionizing radiation stimulates cell proliferation via activation of
the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J
Radiat Res. 52:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsukimoto M, Homma T, Mutou Y and Kojima
S: 0.5 Gy gamma radiation suppresses production of TNF-alpha
through up-regulation of MKP-1 in mouse macrophage RAW264.7 cells.
Radiat Res. 171:219–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lödermann B, Wunderlich R, Frey S, et al:
Low dose ionising radiation leads to a NF-κB dependent decreased
secretion of active IL-1β by activated macrophages with a
discontinuous dose-dependency. Int J Radiat Biol. 88:727–734.
2012.
|
|
40
|
Turtoi A, Brown I, Oskamp D and
Schneeweiss FH: Early gene expression in human lymphocytes after
gamma-irradiation-a genetic pattern with potential for
biodosimetry. Int J Radiat Biol. 84:375–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brengues M, Paap B, Bittner M, et al:
Biodosimetry on small blood volume using gene expression assay.
Health Physics. 98:179–185. 2010.PubMed/NCBI
|
|
42
|
Kabacik S, Mackay A, Tamber N, et al: Gene
expression following ionising radiation: identification of
biomarkers for dose estimation and prediction of individual
response. Int J Radiat Biol. 87:115–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mayer C, Popanda O, Greve B, et al: A
radiation-induced gene expression signature as a tool to predict
acute radiotherapy-induced adverse side effects. Cancer Lett.
302:20–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Riecke A, Rufa CG, Cordes M, Hartmann J,
Meineke V and Abend M: Gene expression comparisons performed for
biodosimetry purposes on in vitro peripheral blood cellular subsets
and irradiated individuals. Radiat Res. 178:234–243. 2012.
View Article : Google Scholar
|
|
45
|
Rashi-Elkeles S, Elkon R, Shavit S, et al:
Transcriptional modulation induced by ionizing radiation: p53
remains a central player. Mol Oncol. 5:336–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|