Introduction
The term ‘prehypertension’ was first introduced in
JNC-7 in 2003 to define individuals whose systolic blood pressure
(BP) levels are between 120 and 139 mmHg or diastolic BP between 80
and 89 mmHg (1,2). The aim of this definition by the
Joint National Committee on Prevention, Detection, Evaluation, and
Treatment of High Blood Pressure is to target the population with
hypertension who has a higher-than-normal BP and is at a
higher-than-normal risk of developing cardiovascular disease.
Results of previous studies have shown that individuals with
prehypertension had an increased risk of developing hypertension
compared with the normotensive population (3,4),
and had an increased age-related risk of cardiovascular and
cerebrovascular diseases including stroke and carotid
atherosclerotic plaque (5,6).
As with hypertension, one of the target organs for prehypertension
is brain. Findings of the Framingham Heart Study suggested that
prehypertension caused impairment to the structural integrity of
white matter and shrinkage of gray matter in young populations even
before these individuals developed hypertension, and the damage was
associated with premature aging but not stroke (7). Thus, it appears that BP-associated
brain damage may start at a relatively younger age than expected
and may continue over a long period of time if the abnormal BP is
not controlled or corrected in time.
There has been controversy regarding whether
antihypertensive drug treatment should be applied to
prehypertensive populations. Findings of the TROPHY study showed
that two years of treatment with candesartan, an angiotensin
receptor blocker, had a short-term effect on preventing the
progression of prehypertension to hypertension compared with the
placebo group (8). However, the
long-term benefit following drug withdrawal remains to be clarified
(8). Thus, although it is
important to lower BP during the prehypertension period to prevent
or decrease the occurrence of cardiovascular diseases at mid- or
late-life, how to efficiently achieve this long-term objective
remains to be determined. Lifelong drug treatment for
prehypertension is required, as in the case for hypertension,
however, a number of factors should be considered for any given
prehypertensive individual, such as the tolerance of drug,
concomitant diseases and drug treatment. Adoption of a healthier
lifestyle such as an increase in physical activity and diet
modification has been proven effective, however, it is difficult to
be realized, particularly in the young and middle-aged
prehypertensive populations (9).
The aforementioned potential organ damages caused by
prehypertension have therefore led to a search for an appropriate
drug for the long-term treatment of prehypertension.
In the clinic, angiotensin II (Ang II) receptor
antagonists and calcium channel blockers are two classes of drugs
that have been widely used to treat patients with hypertension. In
spontaneously hypertensive rats (SHR), prehypertensive treatment
with losartan maintained cardiac protection until the age of 48
weeks, but not with hydralazine, a general vasodilator (10). In a previous study it was
suggested that transient losartan treatment on SHR produced
improved cardioprotective and renoprotective effects compared with
amlodipine in the long-term (11). The abovementioned studies
demonstrated that different drugs have different impacts on
BP-associated organ damage. By contrast, long-term treatment with
losartan on salt-loaded stroke-prone SHR (SHRSP) reduced
cerebrovascular damage and provided full protection against
mortality (12). However, whether
transient administration of any of these drugs leads to long-term
protection against brain damage associated with prehypertension,
and whether there is any difference in the effectiveness of these
drugs in prehypertensive treatment against cerebrovascular
impairment remain to be determined.
In the present study, prehypertensive administration
of losartan and amlodipine was performed for 6 and 16 weeks,
respectively, on SHRSP. Continuous brain damage up to 40 weeks
among these groups was monitored by activity observations,
histological and biochemical examinations. Losartan is therefore a
preferred alternative to amlodipine for prehypertensive treatment
in lowering long-term BP as well as effectively limiting brain
damage associated with prehypertension in SHRSP. Therefore,
losartan is a good candidate drug that may be used in the clinic to
treat prehypertensive patients who have an increased risk of
developing adverse cerebrovascular events.
Materials and methods
Animals
SHRSP and Wistar Kyoto rats (WKY) were purchased
from SLAK Laboratory Animal. A total of 120 male, 4-week-old SHRSP
were randomly divided into 5 groups (n=24 rats per group): i)
SHRSP-Veh: SHRSP treated with saline, 2 ml/kg; ii) SHRSP-Los6:
SHRSP treated with 20 mg/kg/day losartan for 6 weeks; iii)
SHRSP-Los16: SHRSP treated with 20 mg/kg/day losartan for 16 weeks;
iv) SHRSP-Aml6: SHRSP treated with 10 mg/kg/day amlodipine for 6
weeks; v) SHRSP-Aml16: SHRSP treated with 10 mg/kg/day amlodipine
for 16 weeks. Twenty-four untreated WKY rats were used as the
control group. The rats were followed up until they were 40 weeks
of age. Systolic blood pressure (SBP) was measured using the
tail-cuff method at different ages (weeks). The clinical scores of
stroke of each group were evaluated at ages of 10, 20 and 40 weeks,
based on the symptomatological classification (13) with slight modifications as
follows: level 0, normal activity; level 1, slightly decreased
activity and/or slightly agitated; level 2, significantly decreased
activity and/or highly agitated; level 3, lethargic and
depression-like symptoms; level 4, paralyzed (either one or two
sides). Rats (4 animals per cage) were housed in a room with
controlled temperature (23±2°C) under a 12-h light/dark cycle. The
animals had access to standard food and tap water ad
libitum. Procedures were approved by the Animal Ethics
Committee of Fujian Medical University and performed in accordance
with institutional guidelines.
Reagents
The reagents used in the present study were:
Losartan (Hanzhou Merck Sharp & Dohme), amlodipine (Pfizer),
antibodies against AT1R, AT2R, gp91phox and SOD1
(Abcam), anti-β-actin antibody and HRP-conjugated secondary
antibody (ZSGB-Bio). 125I-labeled angiotensin II and
aldosterone (Ald) radioimmunoassay kits (Beijin North Institute of
Biological Technology), and in Situ Cell Death Detection Kit POD
(Roche) were also utilized.
Radioimmunoassay
Ang II and Ald levels in rat brain were measured
using radioimmunoassay kits according to the manufacturer’s
instructions.
Histology, apoptosis staining and
transmission electron microscopy (TEM)
For regular histology, rat brains were fixed in 10%
formaldehyde and sectioned at 10 μm. The sections were stained with
hematoxylin and eosin (H&E) according to the standard protocol.
TUNEL staining was performed according to the manufacturer’s
instructions. TEM was performed using standard procedures.
Western blot analysis
Western blots were performed as previously described
(11). Briefly, 100 μg of protein
lysates purified from brains of rats from each group was loaded
into SDS denatured gel, transferred to PVDF membrane, and probed
with designed antibodies. Protein bands were visualized by
electrochemiluminescence on high-performance chemiluminescence film
and quantified using image analysis software. The results were
expressed as a ratio of gp91phox, SOD, AT1R, and AT2R
over β-actin.
Statistical analysis
ANOVA and the unpaired Student’s t-test were applied
to determine statistical significance between groups when
applicable and shown in each figure legend. To evaluate the
significance of clinical scores of stroke, the Kruskal-Wallis H
test was used to analyze the significance of data distribution in
the six groups, followed by the Mann-Whitney U test for comparison
between two groups. Levels of statistical significance were defined
as P<0.05.
Results
Changes in BP in rats at different time
points
To determine whether and how losartan and amlodipine
would affect prehypertension-associated brain damage, we employed
SHRSP as a model system. Animals were divided into six groups (n=24
animals per group): WKY, vehicle (SHRSP-Veh), SHRSP-Los6 (6 weeks
of losartan treatment), SHRSP-Los16 (16 weeks of losartan
treatment), SHRSP-Aml6 (6 weeks of amlodipine treatment), and
SHRSP-Aml16 (16 weeks of amlodipine treatment). Animals were
treated at 4 weeks of age, when the BP of SHRSP was higher than
normal, but lower than the defined high BP, which resembles that of
prehypertension in humans (11).
We first measured the SBP of these animals from each group at
different ages (weeks) up to 40 weeks (Fig. 1). At 4 weeks age, the starting BP
of SHRSP of each group was higher than that of the control (WKY)
group, but lower than that of the high BP group after maturation,
suggesting that SHRSP at the age of 4 weeks serves as a good model
system for investigation of prehypertension. At 10 and 20 weeks, a
significant decrease in SBP in all the treatment groups was
observed compared with SHRSP-Veh (P<0.05). However, at 20 weeks,
the SBP of animals in the SHRSP-Los16 or SHRSP-Aml16 groups was
significantly lower than those of animals in the SHRSP-Los6 and
SHRSP-Aml6 (P<0.05, SHRSP-Los16 vs. SHRSP-Los6, and P<0.05,
SHRSP-Aml16 vs. SHRSP-Aml6) groups, suggesting that long-term
treatment of either of the two drugs exerted more effects compared
with a short-term treatment. At 40 weeks, only SHRSP-Los16 animals
maintained markedly lower SBP compared with the remaining four
SHRSP groups (P<0.05), while the animals of the three drug
treatment groups, SHRSP-Los6, SHRSP-Aml6, and SHRSP-Aml16, showed
no difference in SBP compared with SHRSP-Veh.
The results therefore showed that i) 6 weeks
(short-term) treatment of losartan or amlodipine in SHRSP was able
to decrease BP and maintain it at a low level for a short period of
time (until 20 weeks, i.e., 10 weeks after administration
withdrawal), ii) 16 weeks (long-term) treatment of losartan or
amlodipine in SHRSP caused a more significant decrease in BP at 20
weeks than the 6-week treatment groups, and iii) SHRSP-Los16
maintained lower BP more effectively than SHRSP-Aml16 for a longer
period of time (up to 40 weeks).
Clinical scores of stroke of the six
groups
SHRSP is a proven model that may develop stroke
after maturation. To determine whether prehypertension treatment
with losartan or amlodipine had any impact on the occurrence of
stroke, we evaluated the clinical scores of stroke from each group
at the age of 10, 20 and 40 weeks based on the symptomatological
classification (13) with slight
modification. At 10 and 20 weeks, no behavioral abnormalities were
observed in the animals of any of these groups (data not shown). At
age of 40 weeks, the number of animals that had died in the
SHRSP-Veh, SHRSP-Aml6 and SHRSP-Aml16 groups was 3, 2 and 1,
respectively. The clinical score of each group was evaluated and
presented in Table I. The
SHRSP-Los6 and SHRSP-Los16 groups showed a significant improvement
in the daily activity of animals compared with that of the
SHRSP-Veh group, although they were worse compared with the WKY
group. Animals in the SHRSP-Aml6 and SHRSP-Aml16 groups did not
show any significant difference in this evaluation compared with
the SHRSP-Veh group. Thus, prehypertension treatment with losartan
is more effective in decreasing stroke occurrence and in improving
quality of life than amlodipine, in the animal model.
 | Table ISummary of clinical scores of stroke
of WKY, SHRSP-Veh and four drug treatment groups. |
Table I
Summary of clinical scores of stroke
of WKY, SHRSP-Veh and four drug treatment groups.
| Evaluation
level | WKY n=8 | SHRSP-Veh n=5 | SHRSP-Los6 n=8 | SHRSP-Los16
n=8 | SHRSP-Aml6 n=6 | SHRSP-Aml16
n=7 |
|---|
| 0 | 8 | 0 | 3 | 6 | 0 | 0 |
| 1 | 0 | 0 | 5 | 2 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 1 | 2 |
| 3 | 0 | 2 | 0 | 0 | 3 | 4 |
| 4 | 0 | 3 | 0 | 0 | 2 | 1 |
| Mean ranking | 9 | 36.5a | 16.5a,b | 12a,b | 33.5a,c | 31.36a,c |
Changes in the brain structure of the
animals in the six groups
To gain a better understanding of the structural
basis underlying brain damage in SHRSP and improvement by
treatments, we first globally viewed the brains from each group at
the age of 40 weeks (Fig. 2A):
WKY rat showed normal brain structure without edema and bleeding,
and the midline was straight and not shifted. SHRSP-Veh rat brain
exhibited shifted midline, edema and significant bleeding spots
(Fig. 2A–b, arrows). SHRSP-Aml6
and -Aml16 brains showed pathological changes similar to those
observed in SHRSP-Veh group (Fig.
2A–c and -d). However, SHRSP-Los6 and SHRSP-Los16 showed
improved brain morphology similar to that of the WKY rats (Fig. 2A–e and -f). Thus, either
short-term (6 weeks) or long-term (16 weeks) treatment with
losartan protects SHRSP brain from prehypertension-associated
damage.
Histological analysis by H&E staining on the
sections of brains from these groups further corroborated the above
findings. SHRSP-Los16 showed relatively normal structure in brain
and showed no bleeding, similar to the WKY rats, while the rats
from all the remaining groups had internal bleeding in brains
(Fig. 2B). The ultrastructural
changes on the sections of brains prepared from SHRSP vehicle and
treatment groups were examined and compared with those of the WKY
rats at the age of 40 weeks. WKY rat brain showed relatively normal
structure of mitochondria and closely connected brain cells, while
the examination of the brain section of SHRSP-Veh showed swollen
mitochondria that contained vacuoles with disintegrated cristae,
and a loose connection between brain cells (Fig. 2C). Similar to the findings in
Fig. 2A and B, the SHRSP-Los16
group showed a much better improvement in the brain ultrastructure
than the SHRSP-Los6 group, while the amlodipine treatment groups
showed comparable pathological changes similar to those of the
SHRSP-Veh group (Fig. 2C). These
data collectively suggest that SHRSP-Los16 has the best protective
effects among the treatment groups in terms of maintaining normal
brain structure.
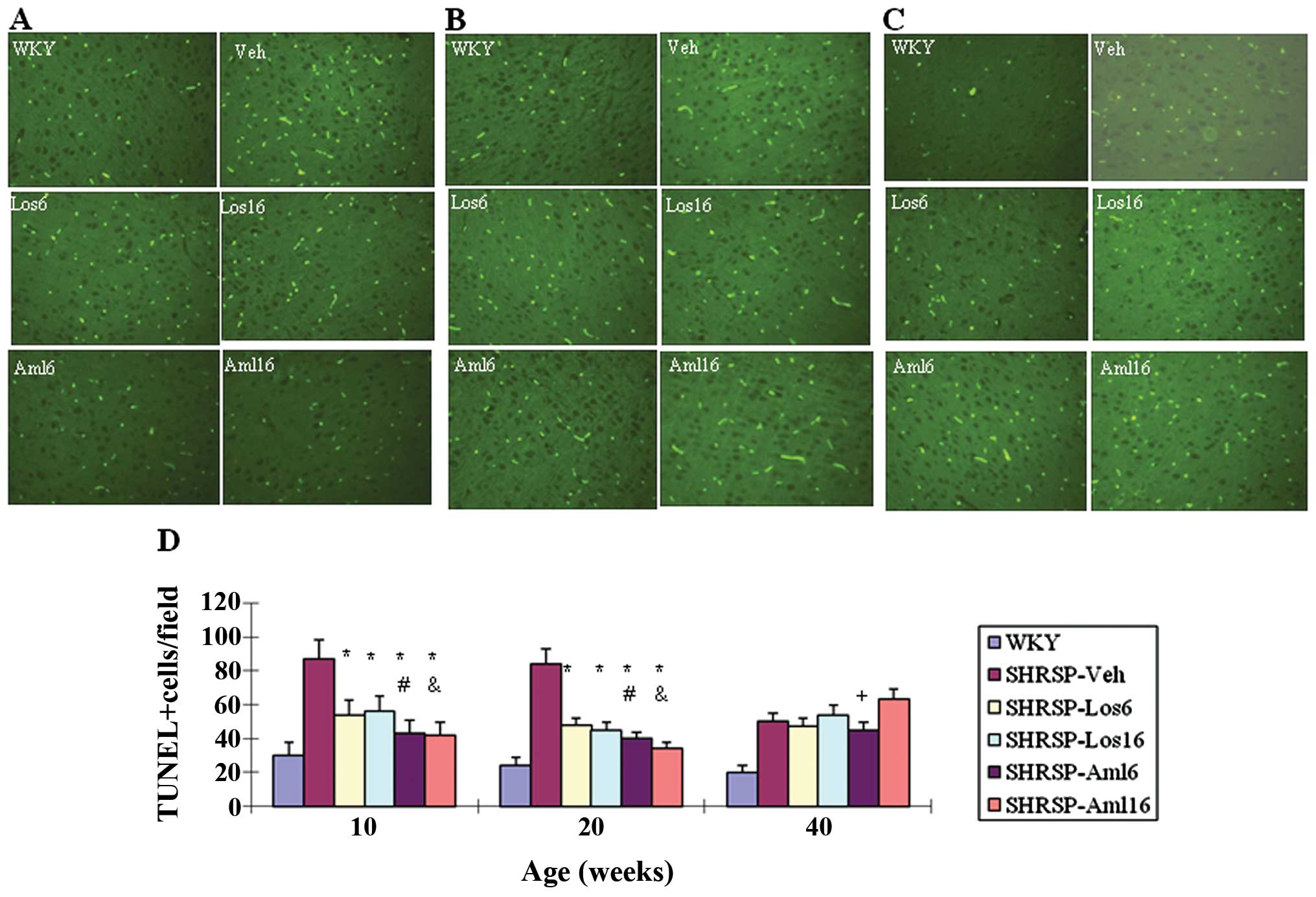
Apoptosis in the brain of the five
treatment groups and the control group
To determine the molecular basis underlying
pathological changes observed in SHRSP-Veh and other treatment
groups, we investigated apoptosis on brain sections prepared from
WKY, SHRSP-Veh and the four drug treatment groups at different ages
(10, 20 and 40 weeks). The number of apoptotic cells was
significantly reduced in each of the four treatment groups at 10
and 20 weeks compared with that in the SHRSP-Veh group (P<0.05)
(Fig. 3). At 10 and 20 weeks,
SHRSP-Aml6 and SHRSP-Aml16 were more effective in inhibiting
apoptosis compared with their respective counterparts, respectively
(P<0.05, SHRSP-Aml6 vs. SHRSP-Los6; P<0.05, SHRSP-Aml16 vs.
SHRSP-Los16). However, at 40 weeks, all the treatment groups and
SHRSP-Veh showed a comparable number of apoptotic cells.
Apoptosis was at least partly attributable to
increased oxidative stress (14),
and increased oxidative stress and free radicals were observed in
the brains of SHRSP (15,16).
Changes in the levels of gp91phox and
superoxide dismutase (SOD) in the cerebral cortex
To explore whether the treatment groups with
prehypertension had any impact on oxidative stress, we performed
western blots in brain of all the groups to evaluate the levels of
gp91phox, a heme binding subunit of the
superoxide-generating NADPH oxidase (17), and an important factor in
promoting oxidative stress-associated brain damage (18), and superoxide dismutase (SOD), an
anti-oxidative factor (19). At
10 weeks, the treatment groups showed a significant decrease in
gp91phox (Fig. 4A and
B, P<0.05), while the two amlodipine treatment groups were
more effective than their respective counterparts (P<0.05,
SHRSP-Aml6 vs. SHRSP-Los6, and P<0.05, SHRSP-Aml16 vs.
SHRSP-Los16). At 20 weeks, while all the treatment groups remained
effective in lowering gp91phox levels in the brains, two
long-term treatment groups were more effective compared with their
respective short-term treatments. Among these groups, SHRSP-Aml16
was the most effective at this time (P<0.05, SHRSP-Aml16 vs.
SHRSP-Los16). At 40 weeks, the levels of gp91phox in the
brains of the two short-term treatment groups returned to the
comparable levels similar to that of SHRSP-Veh, while the pattern
of the decreased levels of gp91phox in the two long-term
treatment groups was similar to the one shown at 20 weeks.
 | Figure 4Changes in the levels of
gp91phox and superoxide dismutase (SOD) in the cerebral
cortex of Wistar Kyoto rats (WKY), stroke-prone spontaneously
hypertensive rats (SHRSP)-Veh and the four drug treatment groups at
different ages (weeks). (A and B) Western blot was performed on
protein lysates extracted from brains of rats from each group at
different ages as indicated (A), and the statistical analysis is
shown in (B). β-actin served as the control, n=8/group. (C and D)
Western blot was performed as in (A), but the blot was reacted with
anti-SOD antibody (C), and the statistical analysis is shown in
(D), n=5/group. *P<0.05, compared with SHRSP-Veh;
#P<0.05, compared with SHRSP-Los6;
&P<0.05, compared with SHRSP-Los16;
+P<0.05, SHRSP-Aml6. The comparisons were made
between groups of the same age as indicated. Note that there is a
significant difference between WKY and any of the SHRSP groups. |
For SOD levels, the treatment groups showed a
significant increase compared with that of the SHRSP-Veh group at
10 and 20 weeks (Fig. 4C and D,
P<0.05), while SOD levels were higher in the two amlodipine
treatment groups compared with their respective counterparts
(P<0.05, SHRSP-Aml6 vs. SHRSP-Los6, and P<0.05, SHRSP-Aml16
vs. SHRSP-Los16). At 20 weeks, the long-term treatment groups had a
greater impact than their respective short-term treatment groups
(P<0.05, SHRSP-Los16 vs. SHRSP-Los6; P<0.05, SHRSP-Aml16 vs.
SHRSP-Aml6). It is noteworthy that the SOD levels in SHRSP-Aml16
were significantly higher than those in SHRSP-Los16 (P<0.05). At
40 weeks, the SOD levels in two short-term treatment groups
returned to levels similar to those of SHRSP-Veh, however, the two
long-term treatment groups maintained higher SOD levels than those
of SHRSP-Veh. Similar to 20 weeks, the SOD levels in SHRSP-Aml16
remained significantly higher than those in SHRSP-Los16 (P<0.05,
SHRSP-Aml16 vs. SHRSP-Aml6). Collectively, these findings suggested
that long-term (16 weeks) treatment with amlodipine is the most
effective in antagonizing BP-linked oxidative stress in the
treatment groups by at least partially increasing SOD and
repressing gp91phox.
Changes in the levels of angiotensin II
(Ang II) and aldosterone (Ald) in the cerebral cortex of the six
groups
The local activity of renin-angiotensin system (RAS)
was also involved in BP-associated target organ damage (20,21). To examine whether these
prehypertension treatments altered RAS activity in brain, we first
measured the levels of Ang II and Ald in the brains obtained from
WKY and SHRSP of each group. As shown in Fig. 5A, each of these treatments did not
lower Ang II levels in the SHRSP brains compared with SHRSP-Veh,
however, Ald levels were significantly downregulated in SHRSP-Los6
and SHRSP-Los16 at 10 and 20 weeks compared with SHRSP-Veh, and
remained low in SHRSP-Los16 up to 40 weeks (Fig. 5B). However, short- or long-term
treatment with amlodipine did not effectively affect the brain Ald
compared with SHRSP-Veh (Fig.
5B). Taken together, losartan is a preferred alternative
compared with amlodipine in decreasing brain Ald levels.
Additionally, long-term treatment with losartan is required to
maintain local Ald at low levels for the long-term.
Changes in the expression levels of AT1R
and AT2R in the cerebral cortex of the six groups
Hypertension has been associated with changes in the
AT2R/AT1R ratio (22,23). To determine whether the short- and
long-term administration of losartan and amlodipine for treatment
of prehypertension also caused changes in the AT2R/AT1R ratio in
brain, western blots were performed on protein lysates purified
from the brains of WKY, SHRSP-Veh, and the four treatment groups at
10, 20, and 40 weeks, respectively. As shown in Fig. 6A, at 10 weeks, all four treatment
groups showed a significant decrease in AT1R compared with
SHRSP-Veh (P<0.05), however, no significant difference among the
treatment groups was observed. At 20 weeks, the decrease in the
AT1R levels in all four treatment groups was maintained (P<0.05,
vs. SHRSP-Veh). However, a more significant decrease was only
observed in the two long-term treatment groups as compared to the
two short-term treatment groups (P<0.05, SHRSP-Los16 vs.
SHRSP-Los6; P<0.05, SHRSP-Aml16 vs. and SHRSP-Aml6), although
SHRSP-Los6 was more effective in reducing AT1R levels than
SHRSP-Aml6 (#P<0.05). At 40 weeks, persistent lower
AT1R levels were observed in all the treatment groups with the
exception of SHRSP-Aml6, with SHRSP-Los16 exhibiting the best
effects (P<0.05, SHRSP-Los16 vs. SHRSP-Los6; P<0.05,
SHRSP-Los16 vs. SHRSP-Aml16).
For AT2R, at 10 weeks, the losartan treatment groups
showed a significant increase compared with SRHSP-Veh (P<0.05),
unlike the amlopidine treatment groups. At 20 weeks, the two
losartan treatment groups continued to show a substantial increase
in AT2R, and SHRSP-Aml16 showed a slight but significant increase
in AT2R in brain compared with SHRSP-Veh (P<0.05). Compared with
SHRSP-Aml16, 16 weeks of treatment with losartan showed elevated
levels of AT2R in brain (P<0.05). During this period, a
time-dependent increase in AT2R by losartan treatments was also
observed (P<0.05, SHRSP-Los16 vs. SHRSP-Los6). For the same
length of time of treatment, losartan showed higher impacts on AT2R
than amlodipine (P<0.05). At 40 weeks, the pattern of the
difference in AT2R levels in these groups was identical to that
observed at 20 weeks, except that the levels of AT2R of SHRSP-Aml16
returned to the comparable level of that of the SHRSP-Veh group.
Taken together, these data demonstrated that long-term (16 weeks)
treatment with losartan has the most beneficial effects on
suppressing AT1R levels and elevating AT2R levels.
Discussion
In recent years much attention has been paid to
prehypertension as it can cause multiple target organ damage such
as hypertension. In a recent study, it was suggested that
prehypertension was linked to brain structural damage in humans
(7). To investigate whether
controlling prehypertension by short- or long-term drug
administration would benefit brain structure as well as prevent
stroke, and to explore which drug, losartan (AT1R blocker) or
amlodipine (calcium channel blocker), had more protective effects
on brain when used for prehypertension treatment, we utilized the
well-established SHRSP as a model system. As with SHR, SHRSP has a
relatively low BP at the age of 4 weeks. The BP progressively
increases between 4 and 10 weeks, and peaks between 10 and 12
weeks. Thus, BP of SHRSP at ages of 4–10 weeks is comparable to
clinically defined prehypertension. Those studies suggest that
losartan is a preferred candidate drug as comapred to amlodipine to
prevent brain from prehypertension-associated damage for long-term
treatment in the clinic.
A complete RAS is present in brain (24,25), which plays important roles in
maintaining normal function and the pathogenesis of brain (20,21). Short-term treatment with the AT1R
blocker candesartan on SHRSP during the prehypertension period
prevented hypertensive end organ damage, including cerebral edema,
after maturation, by at least partially inhibiting local RAS
(26,27). Consistent with those reports, the
present study has demonstrated that short- and long-term treatments
with losartan effectively reduced brain damage such as edema and
bleeding, and greatly improved clinical scores of stroke compared
with amlodipine treatment groups. Although short- and long-term
losartan treatments did not significantly affect Ang II levels in
the cerebral cortex, they substantially reduced Ald levels in the
brain, with the long-term treatment achieving reduced levels
compared with the short-term treatment after maturation.
Additionally, losartan treatments greatly decreased AT1R and
increased AT2R in the brains of SHRSP compared with amlodipine
treatments. AT1R and AT2R mediates the biological functions of Ang
II, and it is generally believed that AT2R functionally antagonized
AT1R (28). Moreover, a decrease
in AT1R and an increase in AT2R was reported to reduce hypertensive
end organ damage (29,30). In addition, the specific
activation of AT2R by its agonist protected high BP-associated
organ damage without exerting significant antihypertensive effects
(30). Obviously, the long-term
(16 weeks) losartan treatment had more sustained impacts on
AT1R/AT2R ratio than the short-term (6 weeks) treatment, and
exerted the most beneficial effects on brain structure as evidenced
by alleviated edema and bleeding compared with the other three
treatment groups. Given the above findings, it is highly likely
that one of the mechanisms underlying beneficial effects on brain
resulting from long-term losartan treatment is to suppress
AT1R-mediated local RAS activity and increase AT2R levels in
cerebral cortex.
Compared with the effective roles losartan played in
inhibiting local RAS activity, the short- and long-term treatments
with amlodipine were not as effective as losartan. Particularly,
the levels of AT2R in the brains of SHRSP following long-term (16
weeks) treatment with amlodipine increased at 20 weeks but returned
to the levels equivalent to that of SHRSP-Veh at 40 weeks. Thus,
amlodipine, unlike losartan, is not capable of persistently
repressing the local RAS activity, which is important for the
protection of BP-related end-stage brain damage. Consequently,
losartan is a better drug than amlodipine for treatment of
prehypertension in order to prevent mature stage cerebrovascular
damage.
In contrast to the impotent roles amlodipine played
in reducing local RAS activity, amlodipine decreased brain cell
apoptosis of SHRSP more effectively at the ages of 10 and 20 weeks
compared with losartan, although this effect was poorly sustained
at the age of 40 weeks. Increased apoptosis was observed in a
number of age-related diseases (31), including neurodegenerative
disorders (32). More recent
studies indicated that prehypertension was associated with
premature aging (7), although
whether this was attributable to enhanced apoptosis is not clear.
One-year-old hypertensive mice also exhibited elevated apoptosis in
brain (33). Thus, elevated
apoptosis is assumed to be a common phenomenon in BP-associated
brain pathophysiology. Mechanistically, oxidative stress was one of
the factors that promoted apoptosis (34). In the present study, we measured
the local levels of the factors gp91phox and SOD, which
are involved in regulating oxidative stress. At 10 and 20 weeks,
losartan and amlodipine significantly decreased gp91phox
and increased SOD in the brains of SHRSP while substantially
suppressing apoptosis, suggesting a potential connection of
decreased oxidative stress to decreased apoptosis by treatment.
Notably, at 40 weeks, the long-term treatments with losartan and
amlodipine persistently inhibited gp91phox and elevated
SOD, while they failed to repress apoptosis as was the case at 10
and 20 weeks. One possible explanation for this phenomenon is that
at early stages, increased apoptosis in the brain of SHRSP is
closely associated with increased oxidative stress, but at later
stages such as 40 weeks, increased oxidative stress contributes
little to hypertension-associated apoptosis in brain. The exact
mechanisms involved in hypertension-linked apoptosis in brain at
early and late stages should be investigated. Considering the
findings that i) amlodipine treatment inhibited apoptosis more
effectively than losartan but was less effective in preventing
brain damage, ii) at 40 weeks there was no significant difference
in apoptosis between SHRSP-Los16 and SHRSP-Veh, and iii) 16 weeks
losartan treatment most effectively prevented SHRSP from stroke and
improved brain function as evidenced by the greatly improved
clinical scores of stroke, we conclude that suppressing apoptosis
is not a significant mechanism by which losartan substantially
improved brain function.
In summary, we evaluated whether prehypertensive
treatment with different drugs (losartan and amlodipine) and
different lengths of treatment time (6 and 16 weeks) affected the
function and pathology of SHRSP brains up to 40 weeks, and the
associated mechanisms. Amlodipine is more effective in inhibiting
apoptosis and oxidative stress, however, losartan is more potent in
decreasing BP, repressing local RAS activity and increasing local
AT2R levels, leading to more protection from
hypertension-associated brain damage such as stroke. Findings of
this study provide evidence for the clinical selection of optimal
drugs for prehypertension treatment to protect BP-associated end
stage cerebrovascular diseases.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81070207).
References
|
1
|
Chobanian AV, Bakris GL, Black HR, et al:
The Seventh Report of the Joint National Committee on Prevention,
Detection, Evaluation, and Treatment of High Blood Pressure: the
JNC 7 report. JAMA. 289:2560–2572. 2003. View Article : Google Scholar
|
|
2
|
Chobanian AV, Bakris GL, Black HR, et al:
Seventh report of the Joint National Committee on Prevention,
Detection, Evaluation, and Treatment of High Blood Pressure.
Hypertension. 42:1206–1252. 2003. View Article : Google Scholar
|
|
3
|
Sun Z, Zheng L, Detrano R, et al: Risk of
progression to hypertension in a rural Chinese women population
with prehypertension and normal blood pressure. Am J Hypertens.
23:627–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasan RS, Larson MG, Leip EP, Kannel WB
and Levy D: Assessment of frequency of progression to hypertension
in non-hypertensive participants in the Framingham Heart Study: a
cohort study. Lancet. 358:1682–1686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greenlund KJ, Croft JB and Mensah GA:
Prevalence of heart disease and stroke risk factors in persons with
prehypertension in the United States, 1999–2000. Arch Intern Med.
164:2113–2118. 2004.PubMed/NCBI
|
|
6
|
Mainous AG III, Everett CJ, Liszka H, King
DE and Egan BM: Prehypertension and mortality in a nationally
representative cohort. Am J Cardiol. 94:1496–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maillard P, Seshadri S, Beiser A, et al:
Effects of systolic blood pressure on white-matter integrity in
young adults in the Framingham Heart Study: a cross-sectional
study. Lancet Neurol. 11:1039–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Julius S, Nesbitt SD, Egan BM, et al:
Feasibility of treating prehypertension with an
angiotensin-receptor blocker. N Engl J Med. 354:1685–1697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yambe M, Tomiyama H, Yamada J, et al:
Arterial stiffness and progression to hypertension in Japanese male
subjects with high normal blood pressure. J Hypertens. 25:87–93.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baumann M, Janssen BJ, Hermans JJ, et al:
Transient AT1 receptor-inhibition in prehypertensive spontaneously
hypertensive rats results in maintained cardiac protection until
advanced age. J Hypertens. 25:207–215. 2007. View Article : Google Scholar
|
|
11
|
Peng F, Lin J, Lin L and Tang H: Transient
prehypertensive treatment in spontaneously hypertensive rats: A
comparison of losartan and amlodipine regarding long-term blood
pressure, cardiac and renal protection. Int J Mol Med.
30:1376–1386. 2012.
|
|
12
|
Vacher E, Richer C and Giudicelli JF:
Effects of losartan on cerebral arteries in stroke-prone
spontaneously hypertensive rats. J Hypertens. 14:1341–1348. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamori Y, Horie R, Akiguchi I, Kihara M,
Nara Y and Lovenberg W: Symptomatological classification in the
development of stroke in stroke-prone spontaneously hypertensive
rats. Jpn Circ J. 46:274–283. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y and Song W: Regulation of RCAN1
translation and its role in oxidative stress-induced apoptosis.
FASEB J. 27:208–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishi T, Hirooka Y, Kimura Y, Ito K,
Shimokawa H and Takeshita A: Increased reactive oxygen species in
rostral ventrolateral medulla contribute to neural mechanisms of
hypertension in stroke-prone spontaneously hypertensive rats.
Circulation. 109:2357–2362. 2004. View Article : Google Scholar
|
|
16
|
Ito H, Torii M and Suzuki T: A comparative
study on lipid peroxidation in cerebral cortex of stroke-prone
spontaneously hypertensive and normotensive rats. Int J Biochem.
25:1801–1805. 1993.PubMed/NCBI
|
|
17
|
Yu L, Quinn MT, Cross AR and Dinauer MC:
Gp91(phox) is the heme binding subunit of the superoxide-generating
NADPH oxidase. Proc Natl Acad Sci USA. 95:7993–7998. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang J, Liu J, Zhou C, et al: Role of
NADPH oxidase in the brain injury of intracerebral hemorrhage. J
Neurochem. 94:1342–1350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii J and Taniguchi N: Down regulation
of superoxide dismutases and glutathione peroxidase by reactive
oxygen and nitrogen species. Free Radic Res. 31:301–308. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phillips MI and de Oliveira EM: Brain
renin angiotensin in disease. J Mol Med (Berl). 86:715–722. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wright JW and Harding JW: The brain
renin-angiotensin system: a diversity of functions and implications
for CNS diseases. Pflugers Arch. 465:133–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao L, Wang WZ, Wang W and Zucker IH:
Imbalance of angiotensin type 1 receptor and angiotensin II type 2
receptor in the rostral ventrolateral medulla: potential mechanism
for sympathetic overactivity in heart failure. Hypertension.
52:708–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda-Matsubara Y, Matsubara K, Ochi H,
Ito M, Iwai M and Horiuchi M: Expression of endothelial angiotensin
II receptor mRNA in pregnancy-induced hypertension. Am J Hypertens.
16:993–999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ganten D, Marquez-Julio A, Granger P, et
al: Renin in dog brain. Am J Physiol. 221:1733–1737.
1971.PubMed/NCBI
|
|
25
|
Fischer-Ferraro C, Nahmod VE, Goldstein DJ
and Finkielman S: Angiotensin and renin in rat and dog brain. J Exp
Med. 133:353–361. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamaguchi R, Takemori K, Inoue T, Masuno K
and Ito H: Short-term treatment of stroke-prone spontaneously
hypertensive rats with an AT1 receptor blocker protects against
hypertensive end-organ damage by prolonged inhibition of the
renin-angiotensin system. Clin Exp Pharmacol Physiol. 35:1151–1155.
2008. View Article : Google Scholar
|
|
27
|
Takemori K, Ishida H and Ito H: Continuous
inhibition of the renin-angiotensin system and protection from
hypertensive end-organ damage by brief treatment with angiotensin
II type 1 receptor blocker in stroke-prone spontaneously
hypertensive rats. Life Sci. 77:2233–2245. 2005. View Article : Google Scholar
|
|
28
|
Ichiki T: Regulation of angiotensin II
receptor expression. Curr Pharm Des. 19:3013–3021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mogi M and Horiuchi M: Effect of
angiotensin II type 2 receptor on stroke, cognitive impairment and
neurodegenerative diseases. Geriatr Gerontol Int. 13:13–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steckelings UM, Paulis L, Namsolleck P and
Unger T: AT2 receptor agonists: hypertension and beyond. Curr Opin
Nephrol Hypertens. 21:142–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muradian K and Schachtschabel DO: The role
of apoptosis in aging and age-related disease: update. Z Gerontol
Geriatr. 34:441–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar
|
|
33
|
Hamet P, Richard L, Dam TV, et al:
Apoptosis in target organs of hypertension. Hypertension.
26:642–648. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar
|