Introduction
Bone mass in adults is maintained locally by the
balance between osteoclastic bone resorption and osteoblastic bone
formation, each of which is subject to various factors aimed at
fulfilling bone function. This function, termed remodeling, is
influenced by a number of factors, including growth factors,
systemic hormones and the mechanical environment (1).
Mechanical loads affect the balance of bone
formation and resorption. Mechanical stimulation is recognized as a
major mediator of both osteogenic and angiogenic activities in
bone. An appropriate function of stress is beneficial to the normal
metabolism of bone tissue and the formation of new bone. Normal
bone tissue activity would normally result in osteoporosis and bone
resorption, if not for proper stimulation. Thus, stress stimulation
plays an important role in remodeling (2).
When mechanical loads stimulate bone tissue, the
interstitial fluid flow has an impact on the osteoblast, which is
in the porous bone tissue. By fluid shear stress stimulation, the
osteoblast transforms a mechanical signal to a biochemical signal,
and then regulates the formation and resorption of bone (3). Increased mechanical loads stimulate
bone formation and suppress resorption, whereas unloading has the
opposite effect (4). Wnts are
involved in this process.
Wnts are a large family of 19 secreted carbohydrate-
and lipid-modified polypeptides that mediate important biological
processes (5). The Wnt/β-catenin
pathway is one of the Wnt signaling pathways, and it is also a main
factor in the mediation of bone remodeling. The Wnt/β-catenin
signaling pathway facilitates the formation of new bone by inducing
the differentiation of pluripotent mesenchymal cells into
osteoblast progenitors, becoming osteoblasts, thus maintaining the
precursor status of these osteoprogenitors, inhibiting osteoblast
apoptosis, and promoting osteoblast proliferation and
differentiation (6,7).
As both mechanical stimulation and the Wnt pathway
are critical anabolic signaling factors affecting bone formation,
the demonstration of stress regulation of Wnt expression in
osteoblasts may provide new insight into the role of the functional
communication between stress and Wnt signaling pathways that affect
bone formation. In the present study, we assessed whether
mechanical stimulation by pulsating fluid flow (PFF) leads to
functional Wnt production.
Historically, two-dimensional (2D) cultures in
vitro have played a key role in the study of bone
mechanobiology; however, 2D experiments are performed on flat
surfaces, which do not reflect the environment of three-dimensional
(3D) architecture in vivo. Studies have proven that 2D
models do not always accurately represent what will occur under 3D
culture conditions (8–11).
Barron et al (12) demonstrated that a 3D grafting
structure and in vitro cultures under dynamic conditions
provide critical information to defining the fundamental
biochemical and biomechanical responses of bone cells. Therefore,
in a previous study, we designed and fabricated a 3D fluid flow
cell culture system, which can load shear stress on cells that are
cultured on scaffolds (13).
Therefore, the aim of this study was to evaluate
whether mechanical stimulation by means of PFF affects the function
of osteoblasts and Wnt production in osteoblasts.
Materials and methods
Cell culture
The osteoblast-like rat osteosarcoma cells,
ROS17/2.8, between passages 20 and 26 were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) - low-glucose medium with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin (all from
Gibco-Invitrogen, Carlsbad, CA, USA), and were set in a 37°C
incubator with 5% carbon dioxide and moisture.
Scaffolds
Partially deproteinized bone (PDPB; Yantai Zhenghai
Biology Technology Co., Ltd., China) was used as a scaffold for
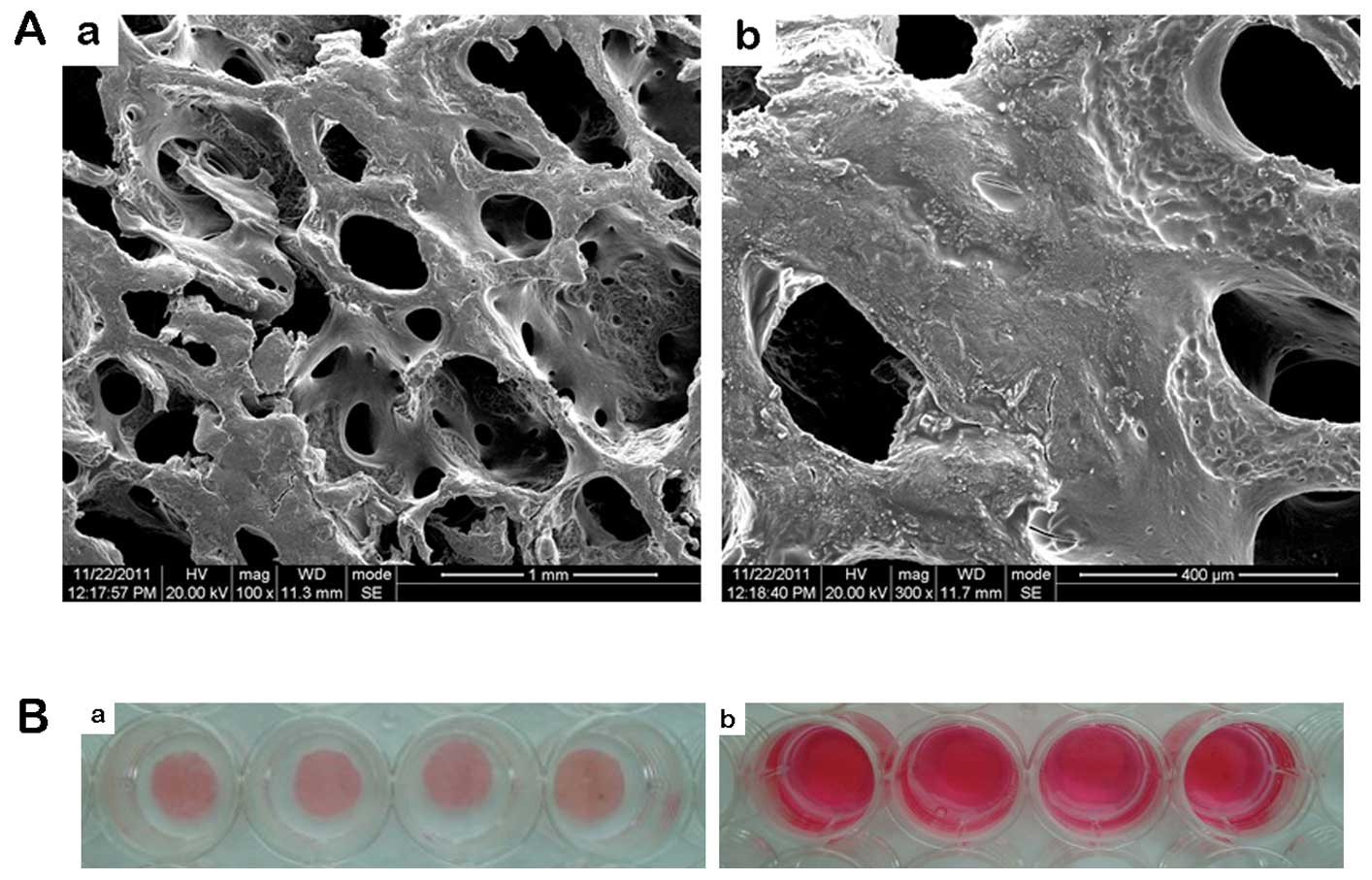
osteoblasts. The scaffolds were round, 10 mm in diameter and 2 mm
in thickness with an average porosity of 79±1.2% and a pore
diameter of 300–500 μm (Fig.
1A).
Cell seeding
The cells were harvested (trypsinized and
resuspended in culture medium) after reaching 80% confluency.
Subsequently, 2,000,000 cells in 100 μl medium were seeded onto
each 3D calcium phosphate scaffold in 24-well plates (2,000,000
cells/scaffold) (Fig. 1B-a).
After 2 h, 2 ml medium were added to each well and the cells were
allowed to attach for 24 h in a 37°C incubator (Fig. 1B-b).
Mechanical loading in 3D
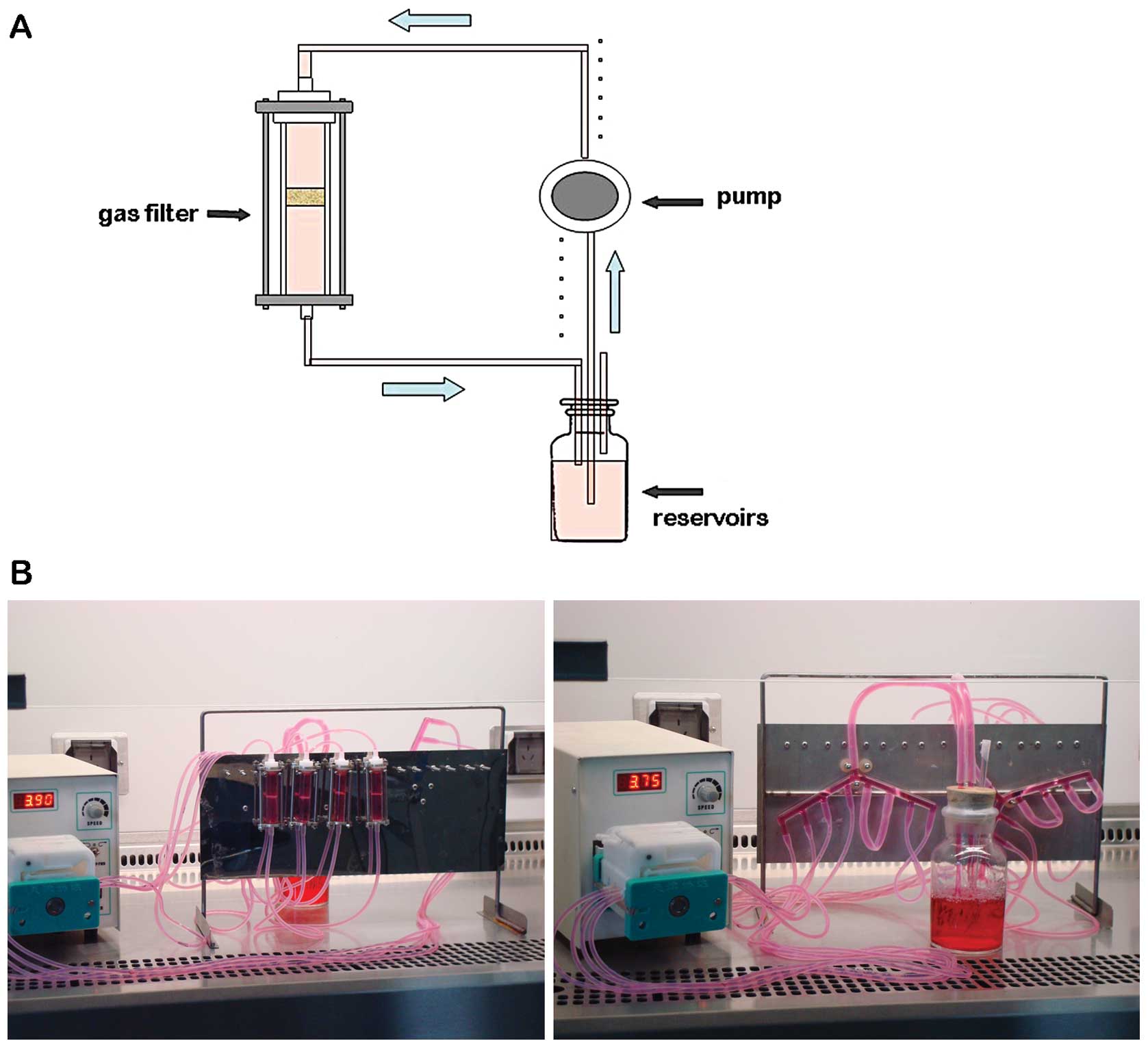
The 3D fluid flow cell culture system consists of 8
flow chambers, platinum-cured silicon tubing, reservoirs, a gas
filter and a pump (Tianjin Xieda Weiye Electron Co., Ltd., Tianjin,
China) (Fig. 2A). Scaffold/cell
constructs were then placed into each chamber, which was made of 2
ml glass syringes. The scaffolds were fixed by a cut radial plunger
(Fig. 2B).
Experimental design
The PFFs were generated by a roller pump, and the
value of 0.8 Pa used for shear stress was based on the outcome of
the theoretical model proposed in the study by Weinbaum et
al (20). There were 4
loading treatments. Teh scaffolds were thus divided into 4 groups:
group A remained incubated at 37°C with 5% CO2 for the
duration of the experiment. Groups B-D were placed in their
chambers and connected to the flow circuit. Loading occurred
immediately after the 3D fluid flow cell culture system was
connected and continued for 1 h and statically incubated for 23 h
for group B, 2 h and statically incubated for 22 h for group C, and
4 h and statically incubated for 20 h for group D. All samples were
removed for analysis at 24 h.
Scanning electron microscopy (SEM)
For SEM, the samples were rinsed twice with
calcium-free phosphate-buffered saline (PBS) and fixed in 2.5%
glutaraldehyde at 4°C overnight. The samples were then dehydrated
in a graded series of ethanol (30, 50, 70, 85, 90 and 100%), dried
with tetramethylsilane, sputter-coated with gold and examined using
an Inspect F scanning electron microscope (FEI Inspect F, The
Netherlands) at an acceleration voltage of 15 kV.
RNA isolation and qRT-PCR
The scaffolds was transferred into a cryotube and
immediately shock-frozen and pulverized in liquid nitrogen. Total
RNA was extracted using TRIzol reagent (Invitrogen) and frozen at
−70°C for subsequent RNA isolation.
cDNA synthesis was performed using 0.5–1 mg of total
RNA in a 20-μl reaction mixture consisting of 5 units of
PrimeScript™ RT reagent kit (Takara Bio, Inc., Shiga, Japan) at
37°C for 15 min, and terminated by heating at 85°C for 5 sec
followed by cooling at 4°C in a thermal cycler (i-Cycler; Bio-Rad,
Hercules, CA, USA). The cDNA was then used for real-time PCR for
the genes of interest and glyceraldehyde phosphate dehydrogenase
(GAPDH) was used as a housekeeping gene.
Real-time PCR was performed in 20 μl total volume
for each sample, containing 2 μl cDNA template, 10 μl
SYBR® Premix Ex Taq™ (Takara Bio, Inc.), 0.8 μl each of
forward and reverse primers, and 0.4 μl ROX Reference Dye with the
7300 real-time PCR system. The primers of alkaline phosphatase
(ALP), low density lipoprotein receptor-related protein 5 (LRP5),
catenin beta 1 [(CTNNB1), also known as β-catenin], Wnt3A,
adenomatous polyposis coli (APC) and GAPDH (synthesized by Takara
Biotechnology Co. Ltd., Dalian, China) are listed in Table I. Cycling conditions were as
follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec
and 60°C for 31 sec, then 95°C for 15 sec and 60°C for 31 sec, and
95°C for 15 sec.
 | Table IThe base of primers for quantitative
real-time RT-PCR. |
Table I
The base of primers for quantitative
real-time RT-PCR.
| Genes | Left primer | Right primer | Product size
(bp) |
|---|
| GAPDH |
TATGACTCTACCCACGGCAAGT |
ATACTCAGCACCAGCATCACC | 138 |
| ALP |
GACCCTGCCTTACCAACTCATT |
GTGGAGACGCCCATACCATCT | 166 |
| APC |
GAGTCAGCATCCAAAGGACTGA |
GACTGGCGTACTAATGCAGGTCT | 140 |
| LRP5 |
GAGTCAGCATCCAAAGGACTGA |
CCAGGCTCACAGAACTCATCA | 116 |
| Wnt3a |
ACGAGAGGATTGAGAGCGTCA |
GATAAGGGTCTTTGAGCGAGTCC | 118 |
| CTNNB1 |
GGGTCCTCTGTGAACTTGCTC |
CTTGTAGTCCTGTGGCTTGTCC | 167 |
Statistical analysis
All experiments were run 3 times with triplicate
samples. Significant differences were determined by one-way
analysis of variance (ANOVA) followed by a Newman-Keuls post hoc
test, and all data are expressed as the means ± standard deviation
(SD). In all the analyses, values of P<0.05 were considered to
indicate statistically significant differences. All the data were
analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL.
USA).
Results
SEM: PFF stimulates osteoblasts that are
larger, more spread out and thicker extracellularly, in a 3D fluid
flow cell culture system
SEM was used to visualize the scaffold/cell
constructs in order to assess cell morphology (×300, ×600 and
×1,200 magnification). Fig. 3A
shows microscopic images of the scaffold/cell constructs after 24 h
static culture, while Fig. 3B–D
shows microscopic images of group B (PFF for 1 h and statically
incubated for 23 h), group C (PFF for 2 h and statically incubated
for 22 h) and group D (PFF for 4 h and statically incubated for 20
h). In the PFF culture, the cells were larger, more spread out, and
appeared to be surrounded by thicker extracellular matrix
constructs than the cells cultured under static conditions.
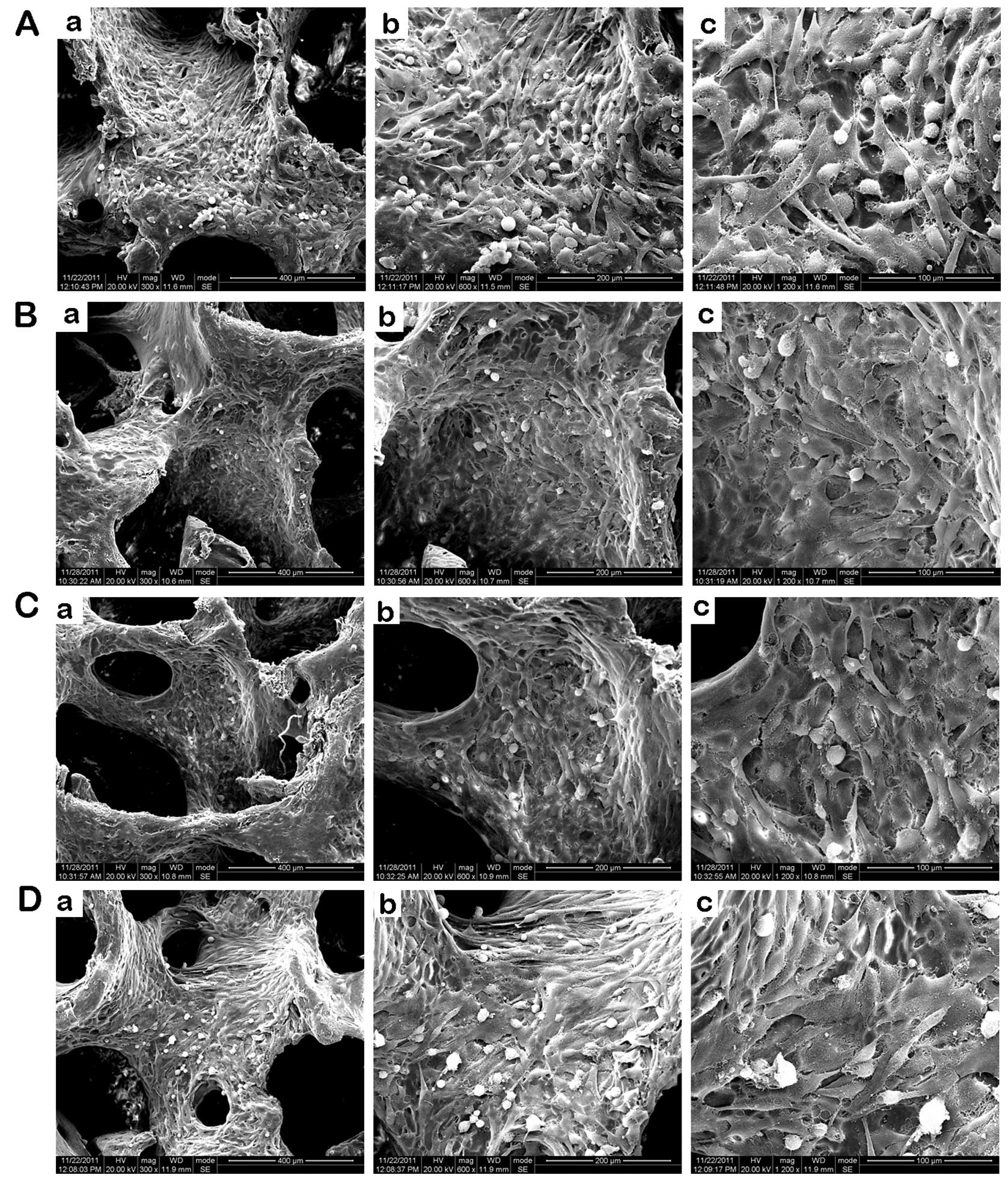 | Figure 3Scanning electron microscopy (SEM)
images of the surface of the seeded scaffold after 24 h. (A) Group
A: kept under static culture conditions (a, ×300; b, ×600; c, ×1200
magnification). (B) Group B: cells were subjected to pulsating
fluid flow (PFF) for 1 h and statically incubated for 23 h (a,
×300; b, ×600; c, ×1200 magnification). (C) Group C: cells
subjected to PFF for 2 h and statically incubated for 22 h (a,
×300; b, ×600; c, ×1200 magnification). (D) Group D: cells were
subjected to PFF for 4 h and statically incubated for 20 h (a,
×300; b, ×600; c, ×1200 magnification). |
Fig. 3A shows the
SEM view of the control group A, in which the osteoblasts covered
most of the scaffold surface, had a long fusiform or polygon shape
and were arranged in a disorderly manner. Group B-D scaffolds were
completely covered with osteoblasts and the cells were polygonal in
shape. In some osteoblasts, many tiny synaptic adhesions had
extended from the cells in the scaffolds, and the cells were
gathered together more closely and were integrated. It could be
seen the bigger and rougher cells that contained a large number of
processes (needle-like structures), indicating osteoblasts with
increased secretion. The cells were arrayed with obvious
directionality, according to the direction of PFF. In group C,
osteoblasts were covered with layers of some parts of the
scaffolds, and the number of cells had increased. In group D, the
elongated osteoblasts in the shear direction were more apparent,
the surface of cells became rougher, and some exfoliated cells were
observed occasionally. In addition, a higher cell density and more
extracellular matrix was observed as compared with the perfusion
groups and the static culture group.
PCR: PFF upregulates the expression of
osteogenic genes of osteoblasts and activates the Wnt/β-catenin
signaling pathway in the 3D fluid flow cell culture system
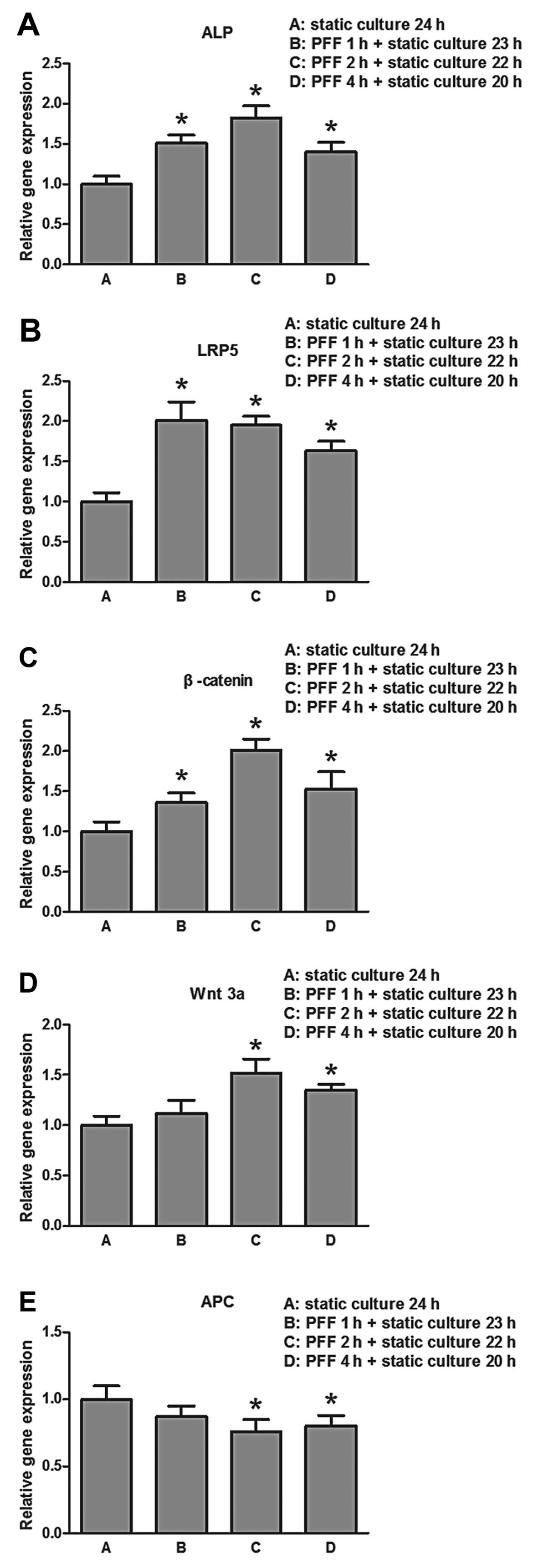
We assessed whether PFF affects the expression of
proteins related to osteogenesis, as well as genes involved in the
canonical Wnt signaling pathway. The results revealed that PFF
upregulated the expression of osteogenic genes in osteoblasts and
activated the Wnt/β-catenin signaling pathway. The mRNA expression
of ALP significantly increased in the groups in which the cells
were stimulated by PFF (P<0.05) (Fig. 4A). LRP5, β-catenin and Wnt3A, the
makers of the Wnt/β-catenin signaling pathway, demonstrated an
increased mRNA expression in the cells that were stimulated by PFF
(P<0.05) (Fig. 4B and C).
However, as the negative regulator of the key protein in the
Wnt/β-catenin signaling pathway, the mRNA expression of APC was
significantly decreased in all the PFF groups (P<0.05) (Fig. 4E).
Discussion
Bone is a dynamic tissue and is constantly under
conditions of growth and remodelling. It adapts to both
physiological and physical environments and undergoes continuous
remodeling throughout life. Osteoblasts secrete new bone, by
sensing and responding to mechanical stimulation via
stress-generated fluid flow inside the canalicular-lacunar networks
and trabecular spaces within bone tissue. Traditionally, 2D in
vitro culture [such as the parallel plate flow chamber (PPFC)]
(14) is a method normally used
in the study of fluid shear stress. A number of studies have shown
that the majority of adhered cells respond differently if cultured
in 2D and 3D substrates (15–19). In comparison to 2D monolayer
cultures, 3D culture system devices represent valuable tools for
the establishment of physiologically oriented in vitro
tissue models.
Previous studies have proven that the mechanical
loading of bone engenders the movement of extracellular fluid
through the bone lacunar-canalicular system. Such fluid flow may
stimulate bone cells via streaming potentials, wall shear stress,
or chemo-transport related effects. Biot’s porous media theory has
been used to relate whole bone stress to canalicular interstitial
fluid flow past osteocytic processes (20). It was predicted that loading
regimens engendering peak physiological strains will induce
fluid-induced shear stresses of 0.8–3 Pa. Subsequently, Bakker
et al (21) used PFF to
generate fluid shear stress of 0.2–1 Pa.
In this study, a higher cell density and greater
extracellular matrix were observed in the perfusion groups as
compared with the static culture group. In the fluid shear stress
group, the cells were larger in size and rougher, and contained a
large number of processes (needle-like structures). In some
osteoblasts, many tiny synaptic adhesions had extended from the
cells in the scaffolds, and the cells were gathered more closely
together and were integrated. The cells are arrayed with obvious
directionality, according to the direction of PFF. It is possible
that following stimulation with PFF, the cells deformed to adapt to
the direction of the PFF. These change were used to reduce stress
stimulation, maintain physiological function, promote the stress on
osteogenesis, and reduce osteoclast activity. With the extended
perfusion time, the cells proliferated more actively, cell
secretion increased, and the extracellular matrix presented an
increasing trend. All of these effects indicated that the
osteogenesis of osteoblasts was enhanced. This study reveals that a
fluid shear stress intensity of 0.8 Pa has an effect on
osteoblasts, conductive to the proliferation and differentiation of
osteoblasts within 4 h.
Recently, an important role for Wnt/β-catenin
signaling has been recognized in promoting bone anabolism. The Wnt
family includes more than 15 proteins that have been shown to play
an important role in organ development and the homeostasis of
several adult tissues, including bone (22). Considerable progress has been made
in terms of understanding the role of Wnt/β-catenin signaling in
osteogenesis and particularly in osteoblast biology; however,
little is known about the functions of this pathway in osteocytes
(23).
The β-catenin degradation complex consists of the
scaffold proteins, axin and APC, and the protein kinases, casein
kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3), thereby
relieving β-catenin (CTNNB1) from its constant phosphorylation by
CK1 and GSK3 and its subsequent degradation by the
ubiquitin-proteasome pathway (24,25). Hill et al (26) used β-catΔPrx/− mice to
study the effects of β-catenin in the limb and head mesenchyme.
Their findings revealed that β-catenin activity is required for the
early steps of osteoblast differentiation. On the other hand,
stabilization of the β-catenin function in the mesenchyme of
β-catΔex3Prx/+ animals suppressed chondrogenesis, rather
than stimulating osteoblastogenesis. Day et al (27) demonstrated the similar finding
that β-catenin signaling is required for osteoblasts to complete
differentiation and to synthesize properly formed bone.
LRP5 is the most well known member of the LRP
family, which constitutes a membrane receptor complex as Wnt
ligands. It is a Wnt co-receptor, and Wnt signaling through LRP5 is
required for mechanically induced bone formation. Sclerostin is
speculated to function as a secreted inhibitor of canonical
Wnt/β-catenin signaling by binding to LRP5 and LRP6 Wnt
co-receptors, preventing their association with the Wnt-Frizzled
(Fzd) receptor complex (28–30). Kato et al (31) reported that the germline deletion
of murine LRP5 reduced vertebral total bone volume by 40% at 8
weeks of age when peak bone mass occurred in LRP5+/+
mice, as determined by histomorphometry. They then disrupted exon 6
of the gene, and also noted that LRP5−/− mice had tibial
fractures by 2 months of age, resulting from low bone mass.
Furthermore, when the LRP5−/− mice were only 2 weeks
old, the total bone volume was found to be significantly reduced.
They also found that the loss of LRP5 did not alter osteoblast
apoptosis and differentiation, osteoclastogenesis, or bone
resorption. Thus, the deletion of LRP5, by reducing osteoblast
proliferation and activity, leads to decreased bone accrual in
early post-natal mice.
As shown in a previous study, osteoblast
differentiation was arrested at the early progenitor stages, and
only type I collagen and ALP were expressed (32). This suggests that β-catenin
signaling is required for osteoblasts to complete differentiation
and to synthesize properly formed bone (11). Thus, ALP is known to be an early
marker for the osteoblastic phenotype, being upregulated at the
onset of differentiation and subsequently decreasing as
differentiation progresses (33).
In this study, we observed the upregulation of the gene expression
of ALP in all PFF groups and the difference was not statistically
significant.
Wnt3a initiates canonical Wnt signaling by binding
to receptor complexes consisting of LRP5/6 and Frizzled on the cell
surface, which results in the nuclear translocation of β-catenin
and the activation of Lef1/Tcf transcription factors (34–36). Thus, we examined whether a
canonical Wnt pathway ligand (Wnt3A) that acts through the LRP5
receptor would have similar effects on PFF-induced gene expression.
We also observed that 1 h of PFF followed by 23 h of incubation
without PFF significantly upregulated gene expression.
A great deal of research has indicated that
mechanical stress and Wnt/β-catenin play important roles in bone
formation. The involvement of the Wnt/β-catenin pathway in the
mechanotransduction of signals from osteocytes has been revealed
(37,38). However, studies on mechanical
stress and Wnt signaling are limited. In this study, in a 3D cell
culture system, loading resulted in the promotion of osteoblast
proliferation and differentiation, as well as an increase in the
expression of Wnt pathway and Wnt/β-catenin target genes. However,
there are differences between in vivo and in vitro
environments; shear stress needs to be transformed inside the body
in future studies.
In conclusion, the results from our study suggest
that mechanical stimulation by PFF induces the differentiation,
proliferation and apoptosis of osteoblasts and the activation of
the Wnt/β-catenin signaling pathway in a 3D cell culture system.
The Wnt/Wnt/β-catenin signaling pathway is involved in the
progression of bone formation. These data provide a framework for
understanding the role of the Wnt/β-catenin signaling pathway in
the mechanical adaptation of bone.
Acknowledgements
We thank Xiaoyu Li, Yurong Liu, Xiangli Kong and
Chaoliang Zhang for their technical assistance. This study was
supported by the National Natural Science Foundation for the Youth
of China (NSFC) (grant 30801310).
References
|
1
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carter DR, Van der Meulen MC and Beaupre
GS: Mechanical factors in bone growth and development. Bone.
18(Suppl 1): 5S–10S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
You J, Yellowley CE, Donahue HJ, Zhang Y,
Chen Q and Jacobs CR: Substrate deformation levels associated with
routine physical activity are less stimulatory to bone cells
relative to loading-induced oscillatory fluid flow. J Biomech Eng.
122:387–393. 2000. View Article : Google Scholar
|
|
4
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: a review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodine PVN: Wnt signaling in bone
development. Bone and Development. Springer; London: pp. 137–152.
2010, View Article : Google Scholar
|
|
6
|
Zhang R, Oyajobi BO, Harris S, Chen D,
Tsao C, Deng HW and Zhao M: Wnt/β-catenin signaling activates bone
morphogenetic protein 2 expression in osteoblasts. Bone.
52:145–156. 2013.
|
|
7
|
Guo Y, Li PF, Shu XC, Deng H, Ma HL and
Sun L: Involvement of Wnt/β-catenin signaling in the osteogenesis
of bone marrow mesenchymal stem cells induced by drynaria total
flavonoids. Zhonghua Yi Xue Za Zhi. 92:2288–2291. 2012.(In
Chinece).
|
|
8
|
Fischbach C, Kong HJ, Hsiong SX,
Evangelista MB, Yuen W and Mooney DJ: Cancer cell angiogenic
capability is regulated by 3D culture and integrin engagement. Proc
Natl Acad Sci USA. 106:399–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byrne EM, Farrell E, McMahon LA, Haugh MG,
O’Brien FJ, Campbel VA, Prendergast PJ and O’Connell BC: Gene
expression by marrow stromal cells in a porous
collagen-glycosaminoglycan scaffold is affected by pore size and
mechanical stimulation. J Mater Sci Mater Med. 19:3455–3463. 2008.
View Article : Google Scholar
|
|
10
|
Helmke CD: Factors affecting bone cell
growth and differentiation under differing culture conditions.
Masters Thesis. Rice University, ETD. http://hdl.handle.net/1911/17342.
2000
|
|
11
|
Jarrahy R, Huang W, Rudkin GH, Lee JM,
Ishida K, Berry MD, Sukkarieh M, Wu BM, Yamaguchi DT and Miller TA:
Osteogenic differentiation is inhibited and angiogenic expression
is enhanced in MC3T3-E1 cells cultured on three-dimensional
scaffolds. Am J Physiol Cell Physiol. 289:C408–C414. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barron MJ, Tsai CJ and Donahue SW:
Mechanical stimulation mediates gene expression in MC3T3
osteoblastic cells differently in 2D and 3D environments. J Biomech
Eng. 132:0410052010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu YY, Ban Y, Geng N, Wang YY, Liu XG, Yu
T and Gong P: Evaluation of different culture techniques of
osteoblasts on 3D scaffolds. Cent Eur J Biol. 5:456–465. 2010.
View Article : Google Scholar
|
|
14
|
Fu Q, Liu YH, Xu YJ, Guo L and Wu CJ:
Design and use of flow chamber for fluid shear stress on cells in
vitro. Journal of Sun Yat-Sen University. S12009.(In Chinese).
|
|
15
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans ND, Minelli C, Gentleman E, LaPointe
V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ and Stevens MM:
Substrate stiffness affects early differentiation events in
embryonic stem cells. Eur Cell Mater. 18:1–14. 2009.PubMed/NCBI
|
|
17
|
Ghosh K and Ingber DE: Micromechanical
control of cell and tissue development: implications for tissue
engineering. Adv Drug Deliv Rev. 59:1306–1318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karamichos D, Skinner J, Brown R and
Mudera V: Matrix stiffness and serum concentration effects matrix
remodelling and ECM regulatory genes of human bone marrow stem
cells. J Tissue Eng Regen Med. 2:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orr AW, Helmke BP, Blackman BR and
Schwartz MA: Mechanisms of mechanotransduction. Dev Cell. 10:11–20.
2006. View Article : Google Scholar
|
|
20
|
Weinbaum S, Cowin SC and Zeng Y: A model
for the excitation of osteocytes by mechanical loading-induced bone
fluid shear stresses. J Biomech. 27:339–360. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E2 by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riancho JA, Olmos JM, Pineda B,
García-Ibarbia C, Pérez-Núñez MI, Nan DN, Velasco J, Cano A,
García-Pérez MA, Zarrabeitia MT and González-Macías J: Wnt
receptors, bone mass, and fractures: gene-wide association analysis
of LRP5 and LRP6 polymorphisms with replication. Eur J Endocrinol.
164:123–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonewald LF and Johnson ML: Osteocytes,
mechanosensing and Wnt signaling. Bone. 42:606–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
25
|
Kobayashi Y, Maeda K and Takahashi N:
Roles of Wnt signalingin bone formation and resorption. Jpn Dent
Sci Rev. 44:76–82. 2008. View Article : Google Scholar
|
|
26
|
Hill TP, Später D, Taketo MM, Birchmeier W
and Hartmann C: Canonical Wnt/beta-catenin signaling prevents
osteoblasts from differentiating into chondrocytes. Dev Cell.
8:727–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/β-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005.
|
|
28
|
Tüysüz B, Bursalı A, Alp Z, Suyugül N,
Laine CM and Mäkitie O: Osteoporosis-pseudoglioma syndrome: three
novel mutations in the LRP5 gene and response to bisphosphonate
treatment. Horm Res Paediatr. 77:115–120. 2012.PubMed/NCBI
|
|
29
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar
|
|
30
|
Johnson ML and Kamel MA: The Wnt signaling
pathway and bone metabolism. Curr Opin Rheumatol. 19:376–382. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato M1, Patel MS, Levasseur R, Lobov I,
Chang BH, Glass DA II, Hartmann C, Li L, Hwang TH, Brayton CF, Lang
RA, Karsenty G and Chan L: Cbfa1-independent decrease in osteoblast
proliferation, osteopenia, and persistent embryonic eye
vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell
Biol. 157:303–314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Owen TA, Aronow M, Shalhoub V, et al:
Progressive development of the rat osteoblast phenotype in vitro:
reciprocal relationships in expression of genes associated with
osteoblast proliferation and differentiation during formation of
the bone extracellular matrix. J Cell Physiol. 143:420–430. 1990.
View Article : Google Scholar
|
|
33
|
Rawadi G, Vayssière B, Dunn F, Baron R and
Roman-Roman S: BMP-2 controls alkaline phosphatase expression and
osteoblast mineralization by a Wnt autocrine loop. J Bone Miner
Res. 18:1842–1853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olivares-Navarrete R, Hyzy SL, Hutton DL,
Dunn GR, Appert C, Boyan BD and Schwartz Z: Role of non-canonical
Wnt signaling enhances osteoblast maturation on microstructured
titanium surfaces. Acta Biomater. 7:2740–2750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jullien N, Maudinet A, Leloutre B, Ringe
J, Häupl T and Marie PJ: Downregulation of ErbB3 by Wnt3a
contributes to wnt-induced osteoblast differentiation in
mesenchymal cells. J Cell Biochem. 113:2047–2056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Natsume H, Tokuda H, Matsushima-Nishiwaki
R, Kato K, Yamakawa K, Otsuka T and Kozawa O: Wnt3a upregulates
transforming growth factor-β-stimulated VEGF synthesis in
osteoblasts. Cell Biochem Funct. 29:371–377. 2011.
|
|
37
|
Sakai A: Space flight/bedrest
immobilization and bone. Osteocyte as a sensor of mechanical stress
and Wnt signal. Clin Calcium. 22:1829–1835. 2012.(In Japanese).
|
|
38
|
Sakai A: Mechanical stress and Wnt signal.
Clin Calcium. 23:839–845. 2013.(In Japanese).
|