Introduction
Alzheimer’s disease (AD) is a progressive
neurodegenerative disease accompanied by neuronal loss in the brain
and cognitive impairments. The exact pathogenesis is still unknown
although several theories have been revealed and accepted.
Cholinergic system dysfunction was one of the most important
pathogenesis of AD (1–3). Acetylcholinesterase inhibitor, a
target drug of the cholinergic system, has been used to treat mild
to moderate AD in clinical trials in recent years (4,5).
Scopolamine (SCOP), an anticholinergic agent, has been reported to
induce some features that are similar to AD, such as affective
disorder and memory impairment, and was widely used to induce the
cognitive impairment model for investigation of AD (6–8).
Studies have shown that it induced oxidative stress in SCOP-treated
animals (9). Oxidative stress,
another important pathogenesis of AD (10), was associated with neuronal loss
or apoptosis (11,12). Since the pathogenesis is
complicated, the target of therapy is diverse. Multi-targeted drugs
play an increasingly important role in the treatment of AD
(13). Chinese medicine recipes,
comprising multi-components, serve as a potential multi-targeted
drug. Certain compound recipes (14–16) exert beneficial effects on
cognitive ability in memory-impaired animal models and are crucial
in the clinical therapy of AD patients. In the traditional Chinese
medicine theory, deficiency of kidney was considered to be the root
cause of AD. Thus, the therapy of reinforcing kidney was widely
used in the clinic by using ‘kidney-reinforcing’ herbs or
prescription (17) which were
reported to possess anti-AD effects. Moreover, different herbs had
different effects on brain functions, such as antioxidant,
anti-apoptotic and anti-acetylcholinesterase activities (18,19). The Bushen-Yizhi formula (BSYZ) is
a traditional Chinese medicine compound recipe consisting of common
Cnidium fruit (CCF), tree peony bark (TPB), ginseng root
(GR), Radix Polygoni Multiflori Preparata (RPMP), barbary wolfberry
fruit (BWF) and Fructus Ligustri Lucidi (FLL). In traditional
Chinese medicine theory, BSYZ had an effect of ‘kidney-reinforcing’
and ‘brain nourishing’ based on the single effect of those six
herbs as well as the effect of compatibility of traditional Chinese
medicine, which was considered to play a more important role in
treatment of disease. Previous studies have shown that the
medicine-containing serum of BSYZ exerted effects of enhancing
choline acetyltransferase (ChAT) activity and neurotransmitter
release in a cell model of Aβ25–35-induced AD (20,21). However, additional evidence is
required to reveal the potential therapeutic effects of BSYZ in AD.
In this study, we investigated the effects of BSYZ extraction on
improving cognitive disorder in SCOP-induced senescence in mice by
Morris water maze test, a common method for assessing learning and
memory abilities of animals. Additionally, the effects of BSYZ on
oxidative stress-related apoptosis were investigated to illuminate
the underlying mechanisms.
Materials and methods
Materials
Ginsenoside Rb1, Ginsenoside Rg1, Osthole,
Imperatorin, Paeoniflorin, Paeonolum, Oleanic acid and
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside were purchased from
the National Institutes for Food and Drug Control (Beijing, China).
Acetonitrile [high performance liquid chromatography (HPLC) grade]
was bought from Honeywell International Inc. (Burdick &
Jackson, Muskegon, MI, USA). SCOP hydrobromide injection (Guangzhou
Baiyun mountain Mingxing Pharmaceutical Co., Ltd., Guangzhou,
China) was purchased from Guangzhou Pharmaceuticals Corporation
(Guangzhou, China). Acricept (Henan Joyline & Joysun
Pharmaceutical Stock Co., Ltd., Zhengzhou, China) was dissolved in
0.9% physiological saline. Kits used for determination of
superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione
(GSH) were purchased from the Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). Primary antibodies (Bcl-2, caspase-3
and β-actin) were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Anti-Bax antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Secondary antibodies
(horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse
IgG) were purchased from Cell Signaling Technology, Inc. Other
reagents were of AR grade.
Preparation of sample solution
BSYZ consisted of six medicinal plants (Table I). All the raw herbs were
purchased from the Guangxi Yifang Chinese Herbal Medicine
Department and identified by Professor Jiannan Chen,
pharmacognosist of the School of Chinese Materia Medica, Guangzhou
University of Chinese Medicine. All of these accorded with the
standard described in the 2010 edition of China Pharmacopoeia. The
contents of BSYZ or dried powder of single herb were weighed and
subjected to an ultrasonic extraction with 60 ml of 70% methanol
for 30 min. The extract solution was then filtered through a 0.45
μm filter membrane prior to analysis.
 | Table IConstituents of BSYZ. |
Table I
Constituents of BSYZ.
| Components | Ratio |
|---|
| She Chuang Zi
(Cnidium monnieri L. Cuss., fruit) | 3 |
| Ren Shen (Panax
ginseng C. A. Mey., rhizome) | 3 |
| Zhi He Shou Wu
(Preparata of Polygonum multiflorum Thuna., radix) | 2 |
| Mu Dan Pi
(Paeonia suffruticosa Andr., cortex) | 2 |
| Nv Zhen Zi
(Ligustrum lucidum Ait., fruit) | 2 |
| Gou Qi (Lycium
barbarum L., fruit) | 2 |
HPLC analysis
The HPLC equipment was Dionex Summit HPLC system,
equipped with a PDA-100 detector, a P680 pump, an ASI-100 automatic
sampler, and a STH585 thermostatic column compartment. The
chromatographic separation was carried out at 35°C with a flow rate
of 0.8 ml/min on a Gemini-C18 110A (150×2.00 mm, 5 μm). The mobile
phase was A (acetonitrile) and B (water-phosphoric acid, 100:0.1,
v/v), and 10 μl capacity per injection was used. The elution
program was optimized and conducted as follows: 0–23 min, linear
gradient 5–19% A; 23–33 min, linear gradient 19–22% A; 33–48 min,
linear gradient 22–32% A; 48–60 min, linear gradient 32–75% A;
60–61 min, linear gradient 75–80% A; 61–66 min, linear gradient 80%
A; 66–78 min, linear gradient 80–5% A; and 78–80 min, linear
gradient 5% A. Monitoring was performed at 203 nm with PDA
detector. Data analysis was performed by a similarity evaluation
system for chromatographic fingerprint of traditional Chinese
medicine (Version 2004A, The Pharmacopoeia Commission of PRC,
Beijing, China), which was recommended by the State Food and Drug
Administration (SFDA) of China. The software was usually used to
evaluate the similarities of different chromatograms and calculate
the correlative coefficient of different patterns.
Animals and drug administration
Male Kunming mice (8-month-old, weighing 50–60 g)
were purchased from the Experimental Animal Center of Sun Yat-Sen
University (Guangzhou, China). Mice were maintained on standard
laboratory conditions with food and water ad libitum for the
duration of the study. The animal experiments were approved by the
Animal Ethics Committee of Guangzhou University of Chinese
Medicine. Mice were randomly divided into six groups (n=9): the
vehicle control group (0.9% NaCl treatment), SCOP group (SCOP 2
mg/kg), Aricept group (SCOP + Aricept 3 mg/kg), low dose BSYZ group
(SCOP + BSYZ 1.46 g/kg), medium dose BSYZ group (SCOP + BSYZ 2.92
g/kg) and high dose BSYZ group (SCOP + BSYZ 5.84 g/kg). Mice were
orally administered saline, Aricept or BSYZ, once per day for two
weeks. In the vehicle control and SCOP groups, mice were treated
similarly with corresponding volumes of saline. The mice, with the
exception of the vehicle control group, were intraperitoneally
administered SCOP 30 min prior to the Morris water maze test.
Morris water maze test
The Morris water maze test was similar to the method
of Morris (22), with minor
modifications (23). The
equipment (Guangzhou Feidi Biology Technology Co., Ltd., Guangzhou,
China) consisted of a black circular pool (120 cm in diameter and
40 cm in height), filled to a depth of 30 cm with water (22–26°C)
and a non-toxic water-soluble black colored dye. The pool was
divided into four equal quadrants and a black escape platform (8 cm
in diameter, 1 cm below the water surface) was placed in the center
of one of the pool quadrants. The learning and memory ability of
mice was detected by the Morris water maze test in a dark room.
Mice were given a place navigation test for five consecutive days.
On each training day, there were four sequential training trials
for each mouse from four different entry positions equally spaced
around the perimeter of the pool. A trial began by placing the
animal in the water facing the wall of the pool at one starting
point and the escape latency was recorded at the end. If it failed
to find the platform within 60 sec, the mouse was guided to the
platform by the experimenter and allowed to stay there for 20 sec
and its escape latency was recorded as 60 sec. After four trials,
the mouse was dried and returned to its cage at the end. On the
sixth day, the probe test was performed in the absence of the
platform with a cut-off time of 60 sec. The number of crossing
through the original position of the platform and the time spent in
the target quadrant were measured.
Biochemical analysis (assay of SOD
activity, MDA and GSH level)
After the Morris water maze test, six mice from each
group were anesthetized and decapitated. Brains were removed
carefully and dissected into hippocampus and cortex on an ice-cold
plate. Tissues were rapidly stored at −80°C until use. Parts of
samples were used for biochemical analysis and western blot
analysis.
For the biochemical analysis, the hippocampus was
weighed and homogenized with ice-cold saline in a glass homogenizer
to make 10% (weight/volume) tissue homogenate. Homogenate was
centrifuged at 3,000 × g for 10 min at 4°C and the supernatant was
used to assay SOD activity, MDA and GSH contents by using the
commercial kits according to the manufacturer’s instructions. The
absorbance was read at 550, 532 and 420 nm, respectively, using
Universal Microplate Spectrophotometer (Bio-Rad, Hercules, CA,
USA). The levels of SOD activity, MDA and GSH contents were
expressed as U/mg protein, nmol/mg protein and μg/mg protein,
respectively.
Preparation of sections
Three mice from each group were anesthetized and
decapitated. Brains were removed carefully and quickly fixed in 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4)
for 24 h, dehydrated with a graded series of ethanol, embedded in
paraffin blocks and sliced at 4 μm thickness.
TUNEL staining
TUNEL staining was performed using the In Situ Cell
Death Detection kit (Roche Diagnostics GmbH, Mannheim, Germany),
according to the manufacturer’s instructions. Briefly, the sections
were heated at 60°C for 1 h, washed in xylene and rehydrated
through a graded series of ethanol and double-distilled water.
After treating sections with 0.1 M citrate buffer (pH 6.0) by
microwave oven for 1 min and cooling them to room temperature, the
sections were washed in PBS and incubated with 50 μl TUNEL reaction
mixture for 1 h at 37°C in the dark. Further incubation with 50 μl
converter-POD was performed at 37°C for 30 min. The sections were
then rinsed with PBS and stained with DAB substrate for 10 min at
room temperature. Images were captured and analyzed at a
magnification of ×200 by using a light microscope and LEICA QWin
plus (Leica Microsystems, Wetzlar, Germany). Average TUNEL-positive
cells of each animal were obtained from three adjacent
sections.
Immunofluorescent staining of Bcl-2 and
Bax proteins
Sections were dewaxed and rehydrated by conventional
methods. After quenching endogenous peroxidase with 3% hydrogen
peroxide for 10 min and blocking with normal goat serum for 10 min
at 37°C, sections were incubated with rabbit anti-Bax antibody
(1:200) and mouse anti-Bcl-2 antibody (1:200) (both from Santa Cruz
Biotechnology, Inc.) at 4°C overnight. After washing in PBS, the
sections were incubated with FITC-conjugated anti-rabbit IgG
(1:500) and Cy3 conjugated anti-mouse IgG (1:200) (both from
Beijing Cowin Biotech Co., Ltd., Beijing, China) for 1 h at room
temperature in the dark. Images were captured at a magnification of
×200 for analysis. The mean fluorescence intensity (MFI) was
measured, and expression levels of Bax and Bcl-2 were calculated as
change of the percentage in MFI compared to the vehicle control
mice.
Western blot analysis
For western blot analysis assay, the hippocampus was
homogenized and lysed in ice-cold RIPA buffer (containing 1:100
PMSF, 1:100 inhibitor proteases and phosphatases cocktail) for 15
min. The lysate was centrifuged at 12,000 × g for 15 min at 4°C and
the supernatant was removed to a new 1.5 ml centrifuge tube. The
protein concentrations were detected according to the
manufacturer’s instructions of the BCA protein assay kit (Nanjing
Biobox Biotech. Co., Ltd., Nanjing, China). Samples (40 μg of
protein) were subjected to SDS-PAGE analysis in 12% gel. The
separated protein was then transferred to PVDF membranes. The
membranes were blocked with 5% non-fat milk dissolved in
Tris-buffered saline-Tween-20 (TBST) for 1 h at room temperature
and subsequently incubated with rabbit anti-Bcl-2 (1:2,000, Cell
Signaling Technology, Inc.), rabbit anti-Bax (1:2,000, Santa Cruz
Biotechnology, Inc.), rabbit anti-caspase-3 (1:2,000) and mouse
anti-β-actin (1:5,000) (both from Cell Signaling Technology, Inc.)
overnight at 4°C. The membranes were subsequently washed three
times in TBST for 10 min each time and then incubated with
horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG
antibody (diluted at 1:5,000) for 1 h at room temperature. After
washing the membranes in TBST three times, immunopositive bands
were visualized using a super-enhanced chemiluminescense western
blot analysis-detection reagent (ECL; Applygen Technologies Inc,
Beijing, China). The optical density (OD) of bands on X-ray film
was determined. β-actin was used as internal control. Results were
expressed as the percentage of OD values by using the Image J2x
software system.
Statistical analysis
Data were shown as the mean ± SE and analyzed using
the Statistical Package for Social Science (SPSS) 17.0 software. In
the Morris water maze test, escape latency was analyzed using
repeated measures analysis of variance (ANOVA). When the Mauchly’s
test was significant, the differences between pairs of means were
assessed by the multivariate analysis together with the least
significant difference (LSD) post-hoc test. Other data obtained
from the Morris water maze, and biochemical, TUNEL and western blot
analyses were analyzed using one-way ANOVA. Values of P<0.05
were considered to be statistically significant.
Results
HPLC analysis of the main components in
BSYZ
The proposed HPLC analytical method was applied to
acquiring the fingerprint of different batches of BSYZ samples.
HPLC fingerprint of BSYZ is shown in Figs. 1 and 2. The relative retention time (RRA) and
relative peak area (RPA) of all common peaks, whose relative
standard deviation (RSD) values were ≤3.7%, were obtained with
reference to this substance. The results indicated the good
stability and reproducibility of the fingerprint analysis by HPLC.
The similarity indices of 10 batches of BSYZ samples were
calculated using a similarity evaluation system. The results
demonstrated that the samples showed good correlation and shared a
similar chromatographic pattern with the similarity indices at
>0.986. By comparing the retention times and UV spectra of the
reference standards, eight compounds (Paeoniflorin,
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside, Paeonolum,
Ginsenoside Rg1, Ginsenoside Rb1, Imperatorin, Osthole, and Oleanic
acid) in BSYZ were identified.
 | Figure 1High-performance liquid chromatography
(HPLC) pattern of Bushen-Yizhi formula (BSYZ) and single herbal
extracts. (A) Structures of the constituents identified from BSYZ,
(B) She Chuang Zi (Cnidium monnieri L. Cuss., fruit), (C)
Ren Shen (Panax ginseng C. A. Mey., rhizome), (D) Zhi He
Shou Wu (Preparata of Polygonum multiflorum Thuna., radix).
(E) Mu Dan Pi (Paeonia suffruticosa Andr., cortex), (F) Nv
Zhen Zi (Ligustrum lucidum Ait., fruit), (G) Gou Qi
(Lycium barbarum L., fruit). |
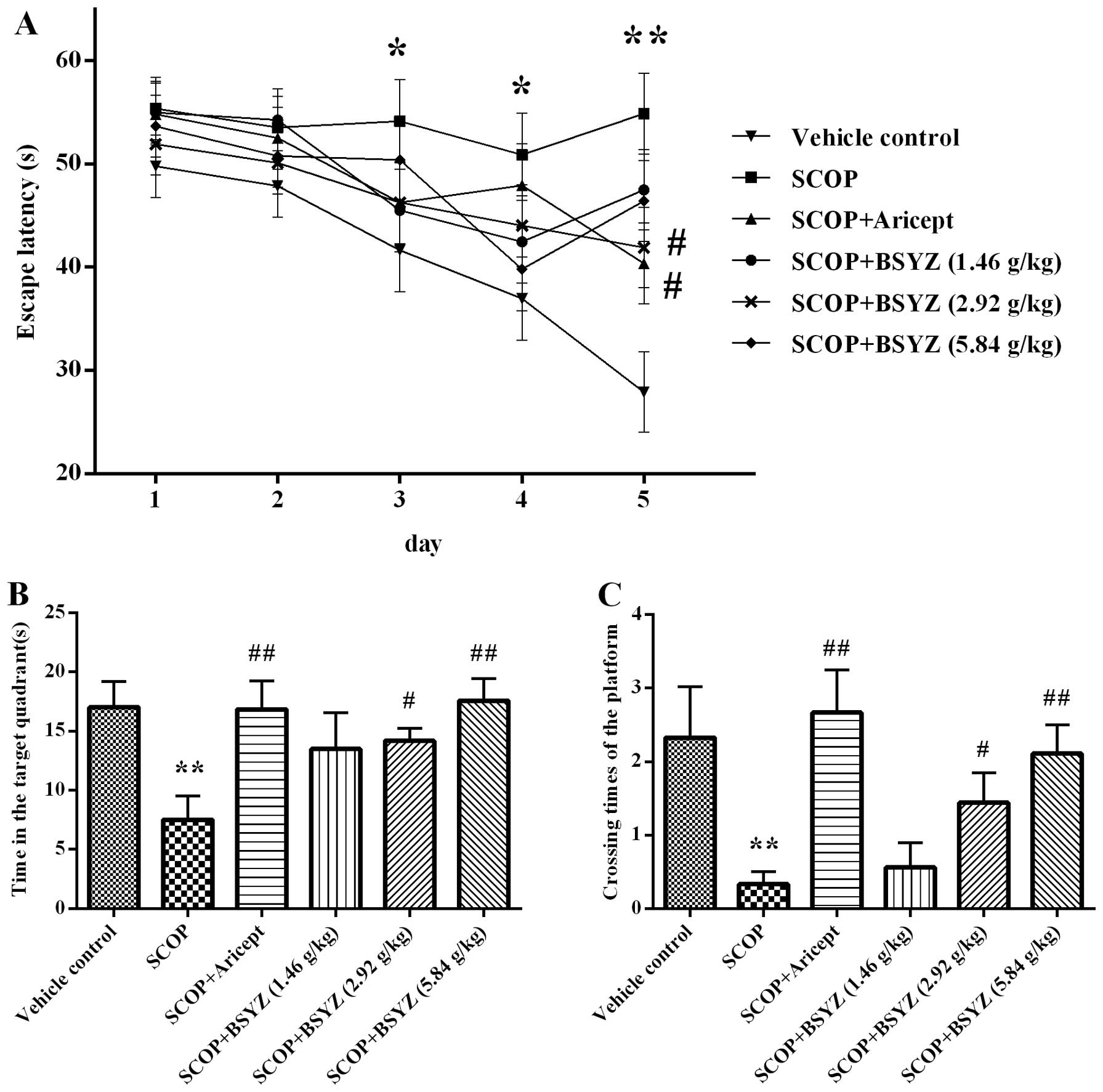
Morris water maze test
The spatial learning and memory ability of mice was
tested by the Morris water maze test. As shown in Fig. 3A, the escape latency declined
progressively during the five training days. The SCOP-treated mice
spent longer period of time in finding the platform than the
vehicle control mice from the third to fifth days (P<0.05,
P<0.05 and P<0.01, respectively). These results revealed that
the SCOP-treated mice had significant cognitive impairment.
Moreover, Aricept (3 mg/kg)- and BSYZ (2.92 g/kg)-treated mice
significantly shortened the escape latency compared with the
SCOP-treated mice on the fifth day (both P<0.05). In the spatial
probe test, the time spent in the target quadrant and the crossing
times of the platform location were showed as Fig. 3B and C. Compared with the vehicle
control group, SCOP-treated mice spent less time (P<0.01) in the
target quadrant and crossed to the platform fewer times
(P<0.01). In the Aricept (3 mg/kg)- and BSYZ (2.92 and 5.84
g/kg)-treated mice, the test revealed a significant increase both
in time spent in the quadrant of the platform placed and crossing
counts compared with the SCOP-treated mice (P<0.01, P<0.05
and P<0.01, respectively). Fig.
3D showed the swim tracks of mice in the fourth trial of the
second and fifth days in the place navigation test. Mice tended to
swim in circles around the wall of the pool on the second day. The
mice gradually changed this search strategy within five training
days. On the fifth day, SCOP-treated mice took a longer period of
time and complex swimming tracks were noted, while the vehicle
control mice swam in the direction of the platform. Aricept- and
BSYZ-treated mice performed similar tracks to the vehicle control
mice.
Effect of BSYZ on the SOD activity and
MDA, GSH levels
The antioxidant effects of BSYZ in SCOP-treated mice
are shown in Table II. SCOP
treatment induced SOD activity decrease of 36.75% in the
hippocampus. However, Aricept and BSYZ (2.92 g/kg) treatment
resulted in a significant elevation of enzyme activity in
SCOP-treated mice, by increases of 39.37 and 34.82%, respectively.
The MDA level in the hippocampus of SCOP-treated mice induced an
increase of 88% more than the vehicle control group (P<0.01).
This increase was reversed by treatment with Aricept and BSYZ (2.92
g/kg), with a percentage of 36.17 and 44.68%, respectively. The GSH
content significantly decreased in the hippocampus of SCOP-treated
mice compared with the vehicle control mice (P<0.01). Aricept
and BSYZ (2.92 and 5.84 g/kg) treatment induced increases of the
GSH level in the hippocampus of ~1.54-, 1.84-, and 1.48-fold,
respectively, compared with the SCOP-treated mice.
 | Table IIEffects of BSYZ on SOD activity, and
MDA and GSH content in the hippocampus of scopolamine-treated
mice. |
Table II
Effects of BSYZ on SOD activity, and
MDA and GSH content in the hippocampus of scopolamine-treated
mice.
| Group | SOD (U/mg
protein) | MDA (nmol/mg
protein) | GSH (μg/mg
protein) |
|---|
| Vehicle
control | 45.22±1.61 | 0.25±0.11 | 5.68±1.14 |
| SCOP | 28.60±4.42a | 0.47±0.14a | 2.81±0.52a |
| SCOP + Aricept (3
mg/kg day) | 39.89±7.02b | 0.30±0.14b | 4.34±0.96b |
| SCOP + BSYZ (1.46
g/kg day) | 34.68±6.24 | 0.38±0.08 | 3.40±0.45 |
| SCOP + BSYZ (2.92
g/kg day) | 38.56±8.00b | 0.26±0.04c | 5.18±0.40c |
| SCOP + BSYZ (5.84
g/kg day) | 30.50±3.02 | 0.35±0.07 | 4.17±0.76b |
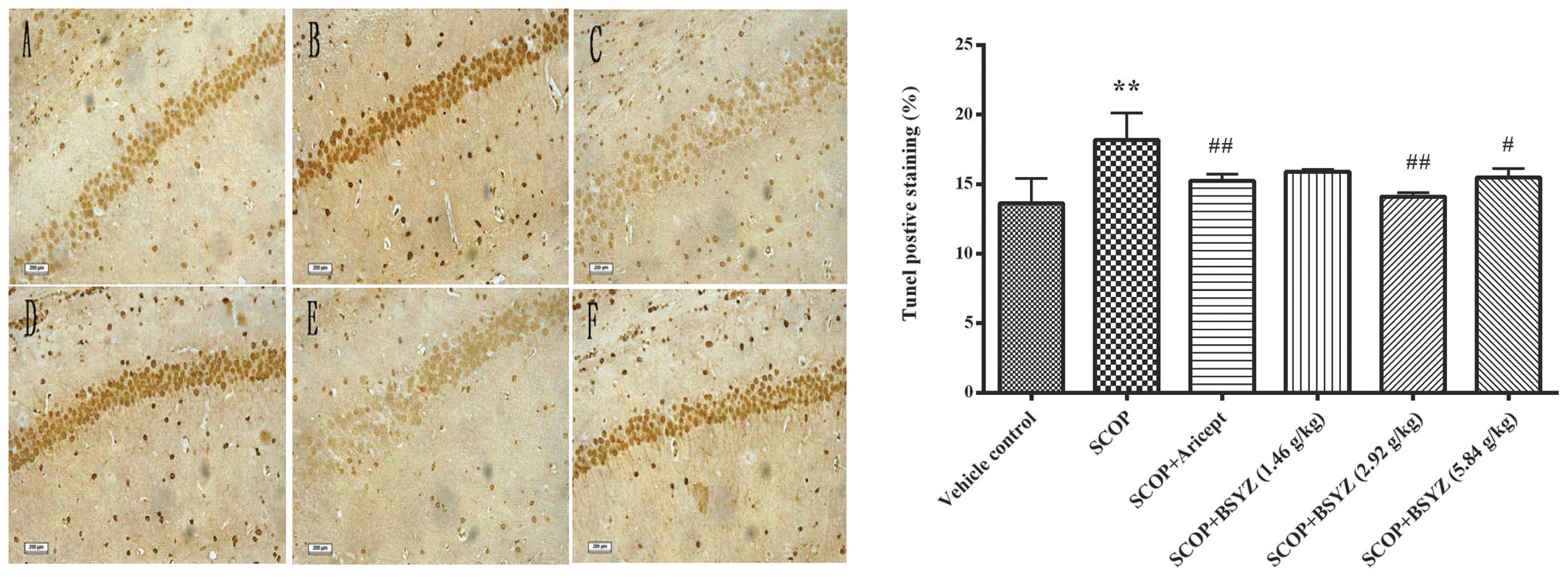
Effect of BSYZ on neuronal apoptosis in
the hippocampus
Representative images of the TUNEL staining in
hippocampus are shown in Fig. 4.
TUNEL-positive cells were stained a deep brown in the hippocampus.
Cell counting of the neuronal apoptosis in the hippocampus of
SCOP-treated mice (18.18±1.94%, P<0.01) was prominently more
than in the vehicle control mice (13.82±1.78%). Aricept and BSYZ
(2.92 and 5.84 g/kg) treatments markedly attenuated neuronal
apoptosis in SCOP-treated mice (15.24±0.47%, P<0.01;
14.11±0.26%, P<0.01; and 15.48±0.64%, P<0.05, respectively).
These results indicated that BSYZ attenuated SCOP-induced apoptotic
cell death in the hippocampus.
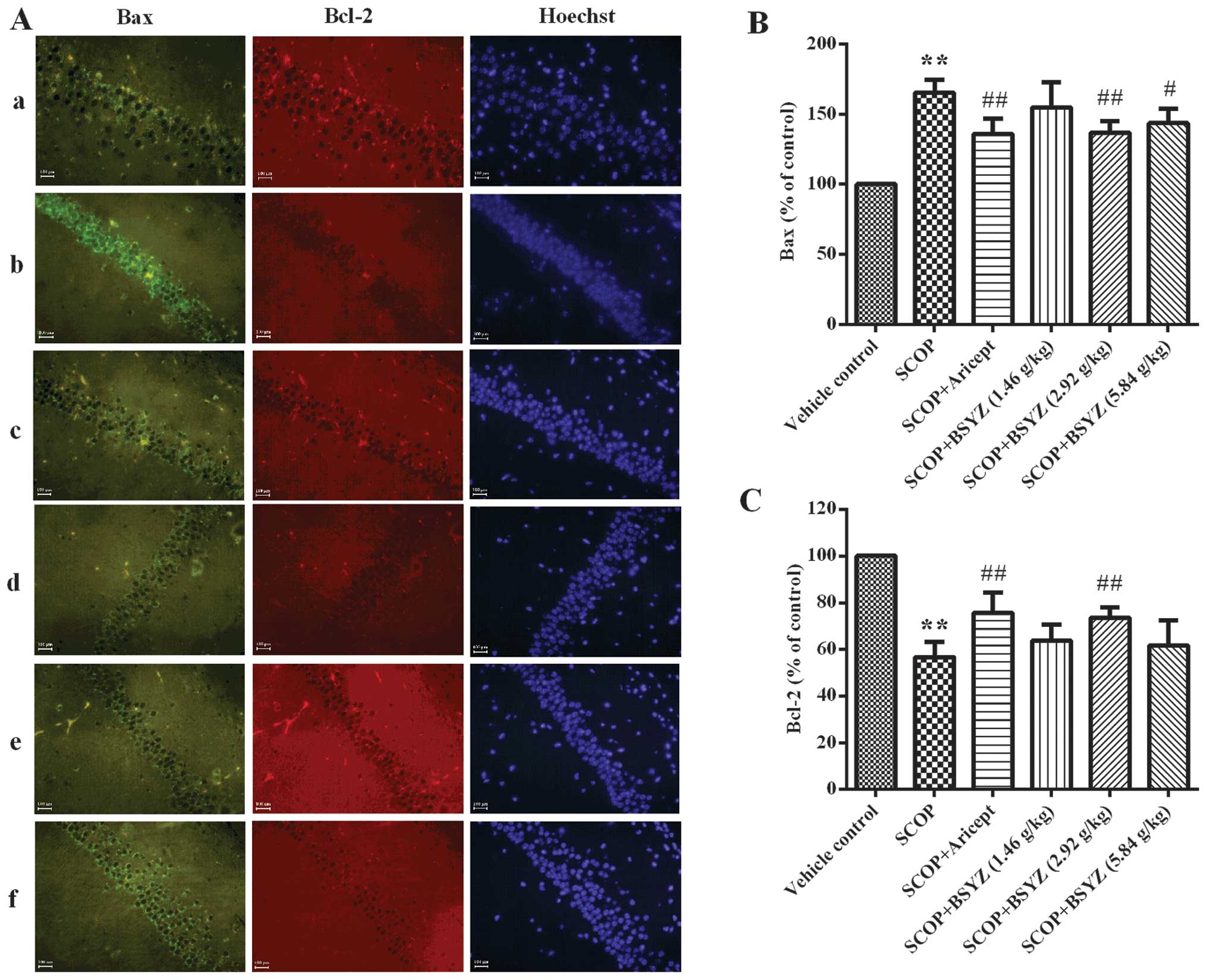
Effect of BSYZ on the expression of Bax
and Bcl-2 proteins by immunofluorescent staining
The expression of Bax and Bcl-2 in the hippocampus
of SCOP-treated mice was analyzed by immunofluorescent staining
(Fig. 5A). As shown in Fig. 5B and C, the MFI of Bax in the
hippocampus of SCOP-treated mice was higher than the vehicle
control group (165.40±9.20% of control, P<0.01), while MFI for
Bcl-2 was lower (56.73±6.57% of control, P<0.01). Following
treatment with BSYZ at a dose of 2.92 g/kg, MFI of Bax was markedly
reduced (136.72±8.26% of control, P<0.01), while Bcl-2 was
significantly elevated (73.59±4.49% of control, P<0.01).
Treatment with Aricept showed a similar effect with BSYZ.
Effect of BSYZ on the expression of Bax,
Bcl-2 and caspase-3 proteins by western blot analysis
The expression of Bax, Bcl-2 and caspase-3 proteins
in the hippocampus of SCOP-treated mice was analyzed by western
blot analysis. As shown in Fig.
6, the mean OD of Bcl-2 was lower in the SCOP-treated mice than
the vehicle control group (P<0.01), while the mean optical
densities of Bax and caspase-3 were higher than the vehicle control
group (P<0.01). Following treatment with BSYZ at dose of 2.92
g/kg, the mean OD of Bcl-2 was significantly elevated (P<0.01),
and the mean densities of Bax and caspase-3 were markedly reduced
(P<0.01). The same effect was observed in the Aricept treatment
group. In addition, BSYZ treatment at a dose of 1.46 g/kg showed a
downregulated effect on the expression of Bax and caspase-3
proteins (P<0.05).
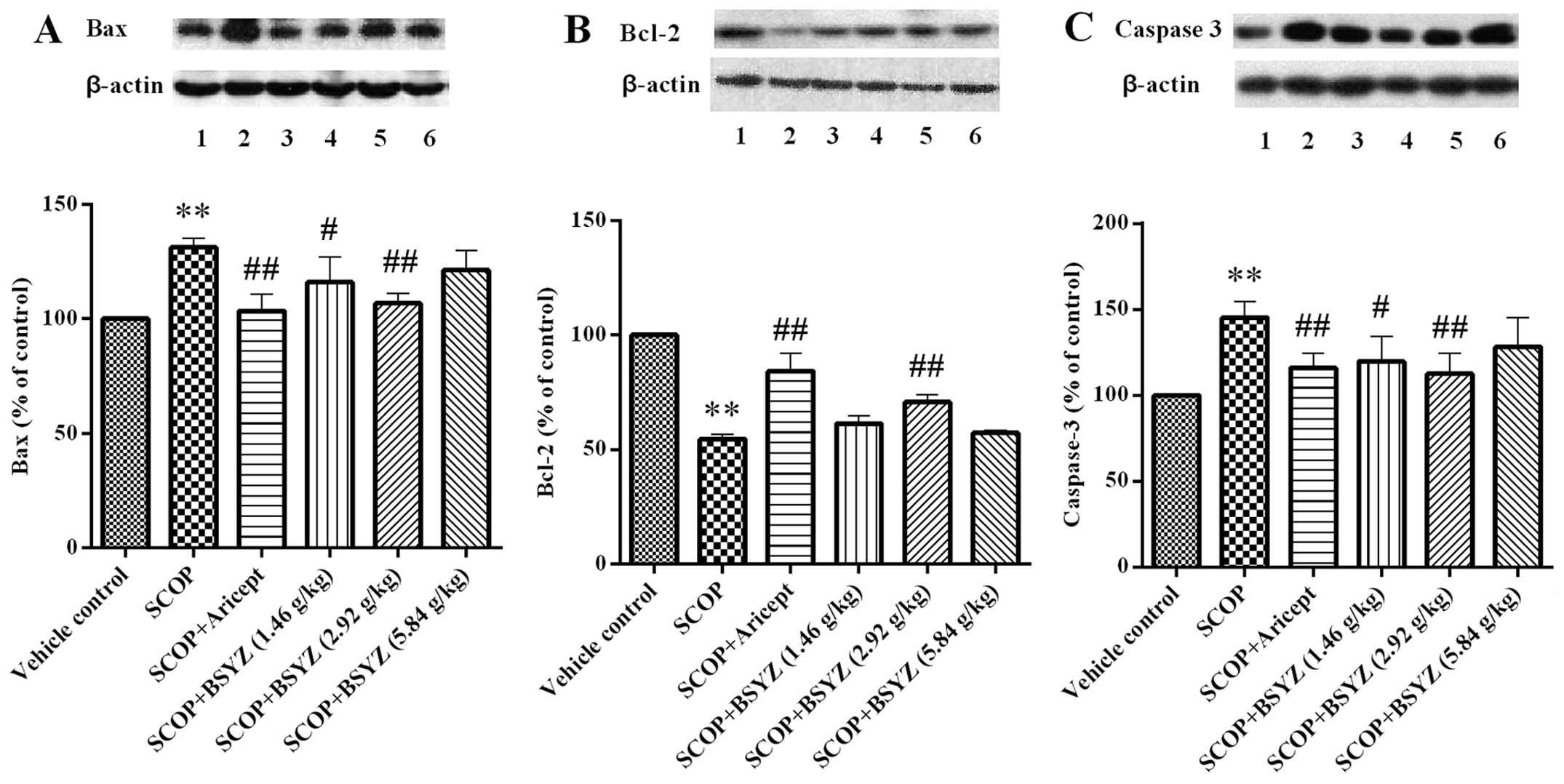 | Figure 6Effect of Bushen-Yizhi formula (BSYZ)
on the expression of Bax, Bcl-2 and caspase-3 proteins. A, B and C
show the expression of Bax, Bcl-2 and caspase-3 proteins,
respectively; lanes 1, Vehicle control; 2, SCOP; 3, SCOP + Aricept;
4, SCOP + BSYZ (1.46 g/kg); 5, SCOP + BSYZ (2.92 g/kg); 6, SCOP +
BSYZ (5.84 g/kg); Data are shown as the mean ± SE (n=3).
**P<0.01 versus the vehicle control mice.
#P<0.05 and ##P<0.01 versus the
SCOP-treated mice. |
Discussion
The gradual decline of learning and memory is a
typical symptom of AD. Findings of previous studies have shown that
a similar symptom was induced by SCOP (24), which is known as a cholinergic
receptor antagonist. SCOP-treated animals have been widely used to
estimate memory impairment and screening of potential
cognition-enhancing agents. Oxidative stress and apoptosis changes,
which are considered to be two important factors of pathogenesis of
AD, occur in this model (6,25,26). In this study, we evaluated the
effects of BSYZ on SCOP-induced memory impairments in mice by using
Morris water maze test, biochemical assessments of oxidative stress
indices and expression of apoptosis related proteins.
The Morris water maze test is widely used to
evaluate the spatial learning and memory ability of animals
(6,9,27).
In this study, it was shown that SCOP treatment prolonged the
escape latency, shortened the time spent in the target quadrant and
reduced the crossing times of the platform location compared with
the control group. This indicates that the animals had a cognitive
dysfunction. Moderate to high dose of BSYZ treatment showed a
reverse effect similar to Aricept (also known as Donepezil) which
is an acetylcholinesterase inhibitor and was approved to be used to
treat mild and moderate dementia patients by the US Food and Drug
Administration (FDA) in 1996. It has been widely used in studies of
AD (28–30) and has shown antioxidant and
anti-apoptotic activities (31).
Concerning oxidative stress, there was an imbalance
of antioxidant systems, such as a decrease of SOD activity and GSH
level, and an increase in the MDA level. In this study, the
assessments of oxidative stress indices showed that SCOP-treated
mice possessed a decreased SOD activity and GSH levels as compared
to the control mice, while MDA levels were elevated. However,
Aricept and BSYZ treatment increased SOD activity and the GSH level
while reducing the MDA level. These results indicate that BSYZ may
have potent antioxidant activity by exerting a protective effect
against oxidative damage induced by SCOP.
Apoptosis, another important pathogenesis of AD, has
been reported to be associated with the mechanism of central
cholinergic system dysfunction and oxidative stress. In the present
study, we found that SCOP markedly increased neuronal apoptosis in
the hippocampus by TUNEL staining. BSYZ and Aricept treatment
improved this brain damage.
Numerous genes are involved in the regulation of the
mitochondrial apoptotic pathway. The proto-oncogene Bcl-2 is an
inhibitor of apoptosis protein which exerts anti-apoptotic effects.
In our study, SCOP significantly reduced the expression of Bcl-2
protein in the hippocampus of mice, while BSYZ and Aricept both
induced an upregulation.
Bax, a pro-apoptotic protein, exerts an opposite
effect to Bcl-2. It was reported that a high expression of Bax
protein promoted cell death. The ratio of Bcl-2 to Bax determines
the susceptibility of cell apoptosis. Caspase-3, a key executioner
of apoptosis in programmed cell death, was able to induce neuronal
dysfunction (32). Studies have
shown that an increase of Bcl-2 and a decrease of Bax prevented the
release of cytochrome c in mitochondria, and therefore
inhibit the cascade of apoptosis. Our results demonstrate that BSYZ
arrested the upregulation of Bax in the hippocampus of SCOP-treated
mice, which enhanced the modulation of apoptosis. Moreover, the
results show that BSYZ reversed the elevation of caspase-3 activity
in the hippocampus of SCOP-treated mice by western blot
analysis.
Recently, multi-targeted therapy is employed in
various diseases, particularly those associated with different
pathogenesis. In traditional Chinese medicine, plant extracts from
different herbs in a formula may contain different ingredients,
which may play a different role in treating the same disease.
Furthermore, it was reported that different ingredients potentiated
each other’s effect (33). It was
identified by HPLC that BSYZ consisted of eight major components,
including Ginsenoside Rb1, Ginsenoside Rg1, Osthole, Imperatorin,
Paeoniflorin, Paeonolum, Oleanic acid and
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside, from its six herbs
respectively. The pharmacological effects of the components were
various, such as antioxidant, neuroprotective effect,
anti-apoptotic and anti-inflammatory (34–37). Moreover, it has been reported that
some of the components had beneficial effects in the improvement of
learning and memory ability (38,39).
Findings of our study suggest that BSYZ exerted a
neuroprotective effect in SCOP-treated mice. The mechanism involved
was presumably the regulation of oxidative stress and the
expression of mitochondrial-mediated apoptosis-related proteins.
However, it is essential to perform a further study to elucidate
the detailed mechanism.
In summary, SCOP induced learning and memory
impairment of mice. Moreover, BSYZ effectively improved cognitive
ability and restored the abnormal activity of SOD and levels of MDA
and GSH, reversed neural apoptosis, downregulated the expression of
Bax and caspase-3 and upregulated the expression of Bcl-2 in the
hippocampus. These data suggest that BSYZ exerted enhancing
cognitive function, which may result from the regulation of the
antioxidative defense system and mitochondrial-mediated apoptosis
mechanism. BSYZ is therefore a potential therapeutic agent for AD.
However, future investigations should be conducted to demonstrate
the effects of BSYZ on AD.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81273817), Doctoral Fund of
Education Ministry of China (no. 20114425110007 and
20124425120016), Guangdong Provincial Major Science and Technology
for Special Program of China (no. 2012A080202017), the Guangdong
Provincial Department of Science and Technology Foundation of China
(no. 2010A030100009), Guangdong Provincial Natural Science
Foundation of China (no. S2012040006514), the Scientific and
Technical Innovation Project of Guangdong Provincial Education
Department of China (no. 2012KJCX0032), the Characteristic Key
Discipline Construction Fund of Chinese Internal Medicine of
Guangzhou University of Chinese Medicine.
References
|
1
|
Pepeu G and Marconcini Pepeu I:
Dysfunction of the brain cholinergic system during aging and after
lesions of the nucleus basalis of Meynert. J Neural Transm Suppl.
44:189–194. 1994.PubMed/NCBI
|
|
2
|
Schliebs R, Rossner S and Bigl V:
Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic
system: a useful tool to produce cortical cholinergic dysfunction.
Prog Brain Res. 109:253–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niewiadomska G, Baksalerska-Pazera M and
Riedel G: The septo-hippocampal system, learning and recovery of
function. Prog Neuropsychopharmacol Biol Psychiatry. 33:791–805.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saykin AJ, Wishart HA, Rabin LA, Flashman
LA, McHugh TL, Mamourian AC and Santulli RB: Cholinergic
enhancement of frontal lobe activity in mild cognitive impairment.
Brain. 127:1574–1583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geldmacher DS: Donepezil (Aricept) for
treatment of Alzheimer’s disease and other dementing conditions.
Expert Rev Neurother. 4:5–16. 2004.
|
|
6
|
Gupta R and Gupta LK: Improvement in long
term and visuo-spatial memory following chronic pioglitazone in
mouse model of Alzheimer’s disease. Pharmacol Biochem Behav.
102:184–190. 2012.PubMed/NCBI
|
|
7
|
Richetti SK, Blank M, Capiotti KM, Piato
AL, Bogo MR, Vianna MR and Bonan CD: Quercetin and rutin prevent
scopolamine-induced memory impairment in zebrafish. Behav Brain
Res. 217:10–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knox LT, Jing Y, Fleete MS, Collie ND,
Zhang H and Liu P: Scopolamine impairs behavioural function and
arginine metabolism in the rat dentate gyrus. Neuropharmacology.
61:1452–1462. 2011. View Article : Google Scholar
|
|
9
|
Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC,
Jo TH, Park YI, Lee CK, Kim YB, Lee SY and Jang CG: Neuroprotective
effects of chlorogenic acid on scopolamine-induced amnesia via
anti-acetylcholinesterase and anti-oxidative activities in mice.
Eur J Pharmacol. 649:210–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Praticò D: Evidence of oxidative stress in
Alzheimer’s disease brain and antioxidant therapy: lights and
shadows. Ann NY Acad Sci. 1147:70–78. 2008.
|
|
11
|
Choi J, Conrad CC, Malakowsky CA, Talent
JM, Yuan CS and Gracy RW: Flavones from Scutellaria baicalensis
Georgi attenuate apoptosis and protein oxidation in neuronal cell
lines. Biochim Biophys Acta. 1571:201–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Liu Y, Lao M, Ma Z and Yi X:
Puerarin protects Alzheimer’s disease neuronal cybrids from
oxidant-stress induced apoptosis by inhibiting pro-death signaling
pathways. Exp Gerontol. 46:30–37. 2011.
|
|
13
|
Capurro V, Busquet P, Lopes JP, Bertorelli
R, Tarozzo G, Bolognesi ML, Piomelli D, Reggiani A and Cavalli A:
Pharmacological characterization of memoquin, a multi-target
compound for the treatment of Alzheimer’s disease. PLoS One.
8:e568702013.PubMed/NCBI
|
|
14
|
Li H, Li SL, Gong L, Wang JL, Li YZ and Wu
ZH: The effects of an herbal medicine Bu-Wang-San on learning and
memory of ovariectomized female rat. J Ethnopharmacology.
117:427–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang GR, Cheng XR, Zhou WX and Zhang YX:
Age-related expression of calcium/calmodulin-dependent protein
kinase II A in the hippocampus and cerebral cortex of senescence
accelerated mouse prone/8 mice is modulated by anti-Alzheimer’s
disease drugs. Neuroscience. 159:308–315. 2009.
|
|
16
|
Lan Z, Liu J, Chen L, Fu Q, Luo J, Qu R,
Kong L and Ma S: Danggui-Shaoyao-San ameliorates cognition deficits
and attenuates oxidative stress-related neuronal apoptosis in
d-galactose-induced senescent mice. J Ethnopharmacol. 141:386–395.
2012. View Article : Google Scholar
|
|
17
|
Li L, Wei HF, Zhang L, Chu J and Zhao L:
Modern biological basis of Chinese medical theory that ‘kidney
nourishes marrow and brain is sea of marrow’. Zhongguo Zhong Yao Za
Zhi. 31:1397–1400. 2006.(In Chinese).
|
|
18
|
Howes MJ and Houghton PJ: Plants used in
Chinese and Indian traditional medicine for improvement of memory
and cognitive function. Pharmacol Biochem Behav. 75:513–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin HQ, Ho MT, Lau LS, Wong KK, Shaw P and
Wan DC: Anti-acetylcholinesterase activities of traditional Chinese
medicine for treating Alzheimer’s disease. Chem Biol Interact.
175:352–354. 2008.PubMed/NCBI
|
|
20
|
Zhong ZG, Liu MC and Lai SL: Effect of
bushen yizhi formula on neurotransmitter release in rat models with
Alzheimer disease. Chin J Clin Rehabil. 44:167–170. 2005.
|
|
21
|
Chen YB, Lai SL, Hu JQ, Wang Q and Cheng
SY: Effects of drug serum in broken bushen yizhi formulas on cell
model of Alzheimer disease. Chin J Clin Rehabil. 32:250–253.
2005.
|
|
22
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar
|
|
23
|
Himeno E, Ohyagi Y, Ma L, Nakamura N,
Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T,
Tabira T, LaFerla FM and Kira J: Apomorphine treatment in Alzheimer
mice promoting amyloid-β degradation. Ann Neurol. 69:248–256.
2011.PubMed/NCBI
|
|
24
|
Molchan SE, Mellow AM, Hill JL,
Weingartner H, Martinez R, Vitiello B and Sunderland T: The effects
of thyrotropin-releasing hormone and scopolamine in Alzheimer’s
disease and normal volunteers. J Psychopharmacol. 6:489–500.
1992.PubMed/NCBI
|
|
25
|
Shi J, Liu Q, Wang Y and Luo G:
Coadministration of huperzine A and ligustrazine phosphate
effectively reverses scopolamine-induced amnesia in rats. Pharmacol
Biochem Behav. 96:449–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jahanshahi M, Nickmahzar EG and Babakordi
F: Effect of Gingko biloba extract on scopolamine-induced apoptosis
in the hippocampus of rats. Anat Sci Int. 88:217–222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim DH, Jeon SJ, Son KH, Jung JW, Lee S,
Yoon BH, Lee JJ, Cho YW, Cheong JH, Ko KH and Ryu JH: The
ameliorating effect of oroxylin A on scopolamine-induced memory
impairment in mice. Neurobiol Learn Mem. 87:536–546. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazareno S, Popham A and Birdsall NJ:
Towards a high-affinity allosteric enhancer at muscarinic M1
receptors. J Mol Neurosci. 19:123–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higgins GA, Enderlin M, Fimbel R, Haman M,
Grottick AJ, Soriano M, Richards JG, Kemp JA and Gill R: Donepezil
reverses a mnemonic deficit produced by scopolamine but not by
perforant path lesion or transient cerebral ischaemia. Eur J
Neurosci. 15:1827–1840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Birks J and Harvey RJ: Donepezil for
dementia due to Alzheimer’s disease (Review). Cochrane Database of
Systematic Reviews. 1:2006.
|
|
31
|
Takada Y, Yonezawa A, Kume T, Katsuki H,
Kaneko S, Sugimoto H and Akaike A: Nicotinic acetylcholine
receptor-mediated neuroprotection by donepezil against glutamate
neurotoxicity in rat cortical neurons. J Pharmacol Exp Ther.
306:772–777. 2003. View Article : Google Scholar
|
|
32
|
Qian YF, Wang H, Yao WB and Gao XD:
Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San,
inhibits apoptosis in hydrogen peroxide-induced PC12 cells by
preventing cytochrome c release and inactivating of caspase
cascade. Cell Biol Int. 32:304–311. 2008.
|
|
33
|
Yi LT, Xu Q, Li YC, Yang L and Kong LD:
Antidepressant-like synergism of extracts from magnolia bark and
ginger rhizome alone and in combination in mice. Prog
Neuropsychopharmacol Biol Psychiatry. 33:616–624. 2009. View Article : Google Scholar
|
|
34
|
Liu Q, Kou JP and Yu BY: Ginsenoside Rg1
protects against hydrogen peroxide-induced cell death in PC12 cells
via inhibiting NF-κB activation. Neurochem Int. 58:119–125.
2011.PubMed/NCBI
|
|
35
|
Qian YH, Han H, Hu XD and Shi LL:
Protective effect of ginsenoside Rb1 on beta-amyloid protein
1–42-induced neurotoxicity in cortical neurons. Neurol Res.
31:663–667. 2009.PubMed/NCBI
|
|
36
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun R, Wang K, Wu D, Li X and Ou Y:
Protective effect of paeoniflorin against glutamate-induced
neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia
Neuropathol. 50:270–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Wang J, Xing Y, Gong L, Li H, Wu
Z, Li Y, Wang J, Wang Y, Dong L and Li S: Effects of ginsenoside
Rg1 or 17β-estradiol on a cognitively impaired, ovariectomized rat
model of Alzheimer’s disease. Neuroscience. 220:191–200. 2012.
|
|
39
|
Zhou L, Hou Y, Yang Q, Du X, Li M, Yuan M
and Zhou Z: Tetrahydroxystilbene glucoside improves the learning
and memory of amyloid-β(1-42)-injected rats and may be
connected to synaptic changes in the hippocampus. Can J Physiol
Pharmacol. 90:1446–1455. 2012.
|