Introduction
Advanced bone surgical therapies are currently based
on reconstruction surgery and tissue transplantation. However,
several therapeutic and methodological limitations, such as the
incomplete restoration of biological tissue functionality or the
progressive deterioration of implants have been observed (1). As an alternative clinical approach,
tissue engineering (2) has been
suggested and is currently used to restore tissue damage, promoting
the proliferation and differentiation of bone cells within
synthetic scaffolds. In particular, cell-induced bone regeneration
therapy is based on the transplantation of mesenchymal stem cells
(MSCs) combined with biomaterials favouring an efficient diffusion
of nutrients, a good gas exchange and 3D structure simulating in
vivo local environment. The low grafting rate of engineered
bone substitutes has been demonstrated to be greatly dependent on
the porosity grade and geometry of the scaffold (1). As previously demonstrated by Langer
and Vacanti (3), porous
biomaterials enhance the therapeutic efficacy of cellular
transplants, preserving cells from excretion and death. Synthetic
and natural materials, such as polymers, ceramics, metals or their
composites, have been largely investigated and their use has been
explored for bone repair applications using in vitro and
in vivo settings (2).
Polymers, including collagen, hydroxyapatite (HA), polylactic acid
(PLA), polyglycolic acid (PGA) and poly-ɛ-caprolactone (PCL) have
been used to obtain biodegradable and mechanically resistant
matrices using solution casting techniques (4), solvent casting particulate leaching
(5), gel casting (6), gas saturation (7) and phase separation (8,9).
The success of biomaterials to sustain a complete
bone repair is dependent on their ability to mimic the natural
extracellular matrix (ECM), thereby minimizing foreign body or
fibrotic responses. The mature bone matrix is composed of 65%
minerals predominantly including HA and 35% protein (10) as collagen I (Coll I) fibers and
proteoglycans (11). Bone ECM
components act as primary chemical effectors in cell signaling and
functionality. When HA (12,13) and/or Coll I (14,15) are combined with synthetic
scaffolds such as PCL, matrices become highly osteoconductive
(16) and acquire tensile and
bending force, but no compressive strength. PCL is a
semicrystalline, aliphatic polyester with a degradation grade rate
lower than collagen and strong mechanical strength (17). A composite matrix containing ECM
and PCL has been previously investigated by Phipps et al
(18) to obtain a useful scaffold
with defined mechanical properties and a high level of
biocompatibility. A tri-component electrospun scaffold composed of
PCL, Coll I and nanoparticulate HA was shown to blend the
advantageous mechanical resistance of PCL with the favourable
biochemical cues provided by native bone molecules, such as Coll I
and HA, guaranteeing the better adhesion, spreading and
proliferation of human MSCs (18).
In our previous study (9), experimental conditions were defined
to obtain porous PCL scaffolds using the phase separation technique
and alginate threads as porogen agents (PCL-AT). Although it was
demonstrated to sustain osteoblastic adhesion, growth and
differentiation, the mechanical properties of PCL-AT proved that it
was not sufficient for bone repair. Therefore, in this study, HA or
bone extracellular matrix powder (BP) was used to optimize the
mechanical properties of PCL-AT matrices, and the effects were
examined by a micro-CT analysis, while the improvement of the
osteogenic activity by rabbit bone marrow MSCs was evaluated by
gene expression analysis, MTS proliferation assay and an Alizarin
Red functionality test.
Materials and methods
Preparation of PCL scaffolds by the phase
separation technique
As previously reported (9), porous scaffolds were prepared using
PCL (molecular weight ~65 kDa; density, 1.145 g/cm3)
(Sigma Chemical Co., St. Louis, MO, USA) and Ca2+
alginate threads at 200/100 weight ratio (Ca2+
alginate/PCL). To increase mechanical resistance and osteogenic
properties, mineral components, such as HA or BP were added
(PCL-AT-HA, PCL-AT-BP). In parallel, PCL without porogen (PCL-WP)
was used as the control.
Hydroxyapatite
HA was prepared using calcium acetate aqueous
solution [Ca(Ac)2] (1 M) and ammonium phosphate aqueous
solution [(NH4) H2PO4] (1 M) as
follows: 5 Ca (Ac)2 + 3 (NH4) H2
PO4 + 7 (NH4) (OH) → Ca5
(PO4)3 (OH) + 6 H2O + 10
(NH4) (Ac). The final solution pH was corrected to 10
with 33% ammonium hydroxide solution. The mixture was stirred for
24 h and then heated at 70°C for 20 h under agitation. The
resulting precipitate was filtered, washed and dried overnight.
Bone extracellular matrix powder
BP was obtained using the femurs of Sprague Dawley
rats under animal care committee authorization. Following the
removal of residual covering tissues, the samples were frozen in
liquid nitrogen and pulverized using a mill. The resulting powder
was treated with 0.5 M HCl solution (25 mEq/g) for 2 h at 4°C and
then centrifuged at 4°C for 4 min at 4,000 rpm. To remove cellular
contaminants from BP, 4 repeated cycles of detergent-enzymatic
treatment were performed, as previously described by Meezan et
al (19), each one consisting
of the 3 following steps: i) distilled water for 72 h at 4°C; ii)
4% sodium deoxycholate solution for 4 h; and iii) 2,000 KU DNase I
in 1 M NaCl for 2 h.
Preparation of composite scaffolds
HA and BP were added to the PCL gel using 25/100
(PCL-AT-HA) and 13.3/100 (PCL-AT-BP) weight ratios, respectively.
The samples were kept at 30°C until complete solidification. The
residual solvent and Ca2+ alginate threads were removed
by washing with a sodium phosphate solution (0.1 M, pH 7.0) and
then with distilled water.
Scaffold characterization
Morphological analysis
The size and distribution of pores were examined in
the PCL matrices by scanning electron microscopy (SEM). The
specimens were lyophilized, frozen in liquid nitrogen, fractured,
coated with gold and observed using a Stereoscan-205 S scanning
electron microscope (Cambridge Instruments, Cambridge, MA,
USA).
Porosity measurement
The total porosity of the PCL scaffolds was
determined by micro-CT analysis and density measurement, as
previously described (9).
Parallel sections were manually prepared and then scanned using a
Skyscan 1172 HR Micro-CT scanner (Skyscan, Aartselaar, Belgium)
using the following settings: voltage, 48 kV; current, 167 μA;
exposition time, 363 msec; field of view (FOV), 1280×1024 pixels;
and an 8-μm isotropic voxel size. Moreover, all samples were
submitted to 360° rotation, a 0.4° rotation step and 1 frame
averaging. The reconstruction of raw data was performed using
N-Recon software (Skyscan) and a back projection algorithm was
applied to the subsequent axial images acquired in bitmap format.
Micro-CT images were analyzed using Ct-An software (Skyscan) and
focusing the selected volume of interest (VOI; 3×1.59 mm) in the
centre of each scaffold to prevent artifacts from cutting. All
samples were binarized with the same instrument settings. Sample
porosity was calculated as follows: Φ = 1 − BV/TV, where Φ is total
porosity, BV the bone volume, TV the total volume and BV/TV the
percentage bone volume. Trabecular thickness (Tb.Th) (or pore wall)
and trabecular separation (Tb.Sp) (or pore diameter) were computed
by direct measurements. A 3D reconstruction was performed using CT
Vol software (Skyscan) and OsiriX open-source software.
Biological properties of PCL
scaffolds
The PCL scaffolds were sterilized in 95% ethanol for
2 h, incubated in PBS containing 2% penicillin/streptomycin
solution and then washed 3 times in αMEM (Invitrogen, Grand Island,
NY, USA). The scaffolds were then placed in 24-well plates, seeded
with MSCs (3×104 cells/cm2) and cultured in
proliferative medium containing αMEM, 15% fetal bovine serum (FBS)
(Invitrogen), 2 mM glutamax (Invitrogen) and 1% antibiotic solution
(Sigma). At different time points, the samples were submitted to an
analysis of cell viability study morphological analysis by SEM. To
determine the effects of PCL matrices on osteogenic
differentiation, the samples were cultured in differentiation and
proliferative medium and subsequently analyzed by RT-PCR and
functionality tests.
Mesenchymal stem cells
MSCs were isolated in sterile conditions from femurs
and tibia of rabbits (rMSCs) acquired from private animal breeding.
Following the removal of bone tips, bone marrow was harvested using
Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented
with 0.2% penicillin-streptomycin (Invitrogen). The mononuclear
cell fraction was then isolated by centrifugation (2,000 rpm, 30
min) on Ficoll 1077 (Invitrogen) density gradient and subsequently
cultured in proliferative medium at 37°C, 95% humidity and 5%
CO2. The immunophenotypical profile of bone marrow MSCs
was verified on all primary cultures by detecting the expression of
CD105, CD44, CD29, CD90, CD34 and CD45 by flow cytometry (FACS).
The analysis was performed by indirect labeling using specific
anti-rabbit primary antibodies and FITC-conjugated secondary
antibodies (all from Santa Cruz Biotechnology, Inc., CA, USA).
Samples were loaded on a FACSCanto II cytometer (BD Biosciences,
San Jose, CA, USA) and the data were presented as a percentage of
positive cells relative to the labeling control.
SEM
SEM was performed to evaluate the morphology and
distribution of rabbit MSCs on the PCL scaffolds. At each time
point (24 h, 7 and 14 days), the samples were washed with PBS and
fixed with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH
7.4), for 24 h, at 4°C. The specimens were then rinsed 3 times with
sodium cacodylate buffer and dehydrated through a graded series of
ethanol and then air-dried. The scaffolds were coated with gold and
observed by SEM.
Analysis of cell viability
After seeding on PCL scaffolds, cell viability was
monitored at 24 h, 72 h, 7 and 14 days using the colorimetric
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. Metabolically active cells react with tetrazolium salt
in MTS reagent to produce a soluble formazan dye detectable at 490
nm. At each time point, all constructs were rinsed with PBS and
then incubated for 3 h with 20% MTS reagent in culture medium.
Thereafter, the aliquots were pipetted into 96-well plates and the
absorbance of each sample was read at 490 nm using ELx 808 Ultra
Microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The
data were expressed as the number of cells ×103. A
calibration curve (cell
number/cm2=3.8×105A490−1.1×104,
R2=0.98) was prepared.
Osteogenic differentiation of MSCs on
PCL matrices
At 48 h after seeding in proliferative medium, the
cells were stimulated from 7 to 21 days with αMEM, 10% FBS, 1%
antibiotic solution (APS), 0.1 μM dexamethasone, 10 nM
β-glycerophosphate and 0.05 mM ascorbate. Cultures of MSCs in
proliferative medium were used as the control. To verify the
maturation into osteoblastic-like cells, all samples were submitted
at different time points to the analysis of osteogenic markers by
RT-PCR (7 and 14 days), alkaline phosphatase (ALP) activity
analysis and the analysis of extracellular matrix calcium
deposition by Alizarin Red staining (24 h, 7 and 14 days) as
follows:
RT PCR. After 7 and 14 days, the constructs
were rinsed with PBS and then submitted to total RNA extraction
using TRIzol (Invitrogen). RNA was firstly quantified using a
NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA,
USA) at 260 and 280 nm, and then reverse transcribed into cDNA by
M-MLV Reverse Transcriptase (Sigma Aldrich), according to the
manufacturer’s instructions. For PCR amplification, ReadyMix™ Taq
PCR Reaction Mix with MgCl2 (Sigma) and specific oligo
primers (Invitrogen) designed on GenBank sequences (Table I) were used. PCR products were
then analyzed on 2% agarose gels and visualized using a Gel Doc
imaging system (Bio-Rad, Hercules, CA, USA) after GelRed staining.
The expression of β-actin was used as an internal control.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (3′→5′) |
|---|
| β-actin |
AGATCTGGCACCACACCTTCTACA |
ACTCGTCATACTCCTGCTTGCTGA |
| Runx2 |
AGTTTGTTCTCTGACCGCCTCAGT |
ATGGTCGCCAGACAGATTCATCCA |
| Osteocalcin
(OC) |
CATGAGAGCCCTCACA |
AGAGCGACACCCTAGAC |
| Osteopontin
(OPN) |
CCGACCAAGGAACAAT |
CTCTGAAGCACCAGGATA |
| Collagene type I
(Coll I) |
GGCAAACATGGAAACCG |
TCAAGGAAGGGCAAACG |
Alkaline phosphatase assay. ALP activity was
analyzed using p-nitrophenyl phosphate (p-NPP) as the substrate.
Following cell lysis and centrifugation at 12,000 × g for 10 min,
the supernatants were incubated with p-NPP for 30 min at 37°C. The
reaction was terminated by the addition of 3 N NaOH and the
absorbance was measured at 405 nm. All results were expressed as
the mean values ± SD of 3 separate experiments consisting of
triplicates.
Alizarin Red staining. To detect the calcium
deposition of MSCs on the PCL scaffolds, an Osteogenesis assay kit
(Millipore, Billerica, MA, USA) was used. The samples were fixed
with 10% formalin solution for 15 min, washed twice with distilled
water and then incubated with Alizarin Red S for 20 min at room
temperature. After washing with distilled water and incubation for
30 min with 10% acetic acid under agitation, the cellular
monolayers were then transferred to microcentrifuge tubes, heated
to 85°C for 10 min, placed on ice for 5 min and then centrifuged at
20,000 rpm for 15 min. After the addition of ammonium hydroxide,
the amount of extracted Alizarin Red dye was measured at 562 nm and
quantified using an Alizarin Red S standard curve. All results were
expressed as the mean concentration (μM) ± SD of 3 separate
experiments performed in triplicate on stimulated and unstimulated
samples with osteogenic factors.
Statistical analysis
Data are expressed as the means value ± SD of at
least 3 different samples. Significant differences were estimated
by one-way analysis of variance (ANOVA) followed by a
Student-Newman-Keuls post hoc test. Values of p<0.05 were
considered to indicate statistically significant differences.
Results
PCL scaffolds
The absence of alginate threads in PCL-WP (Fig. 1A) determined a random formation of
fine and not interconnected micropores. Homogeneously distributed
pores largely characterized by a diameter of ~10 μm were observed
by SEM in PCL-AT (Fig. 1B) and
PCL-AT-BP (Fig. 1D) or ranging
from 10 to 100 μm in PCL-AT-HA (Fig.
1C). Differently sized and distributed large pores due to air
bubbles were detected in all samples.
Micro-CT analysis
The analysis revealed a significative increase in
total porosity (Table II),
trabecular spacing (Tb.Sp) (Fig.
2B) and trabecular thickness (Tb.Th) (Fig. 3B). In comparison to PCL-WP
(Fig. 2A) characterized by a pore
diameter ranging from ~15 to 300 μm, PCL-AT (Fig. 2B) showed a porous structure with
cavities homogeneously distributed and sized (diameter, ~15 to
1,400 μm). In PCL-AT-HA (Fig. 2C)
and PCL-AT-BP (Fig. 2D), the
maximum size of the pores detected was ~700 μm and unimodal
(Fig. 2C) or bimodal (Fig. 2D) distribution was respectively
observed. In parallel, Tb.Th values ranging from ~15 to 600 μm were
observed with a similar distribution in PCL-AT (Fig. 3B) and PCL-AT-BP (Fig. 3D) in comparison to the control
characterized by Tb.Th values from ~15 to 150 μm (Fig. 3A). The highest Tb.Th value (~900)
was observed in PCL-AT-HA (Fig.
3C). 3D scaffold reconstruction confirmed the presence of
interconnected pores in all scaffolds relative to the control
(Fig. 4), suggesting a trabecular
bone-like structure. As shown in Fig.
5, the addition of HA did not significantly alter the
mechanical properties of PCL-AT. An increase in the displacement
value (3,900±2.17) and the shift/deformation due to the load
(3,273±4.73) observed on PCL-AT-BP suggested that the addition of
BP led to a reduction in the elastic module of the PCL-AT matrix,
preserving the porous structure.
 | Table IITotal porosity value of PCL matrices
detected by micro-CT analysis and density measurement. |
Table II
Total porosity value of PCL matrices
detected by micro-CT analysis and density measurement.
| Samples | Φ micro-CT (% value
± SD) | Φ density method (%
value ± SD) |
|---|
| PCL-WP | 21.59±7.06 | 70±3.75 |
| PCL-AT | 51.83±5.30 | 79.6±1.17 |
| PCL-AT-HA | 55.92±5.31 | 82.5±0.46 |
| PCL-AT-BP | 52.53±3.15 | 84±0.43 |
Biological properties of PCL
scaffolds
MSC cultures
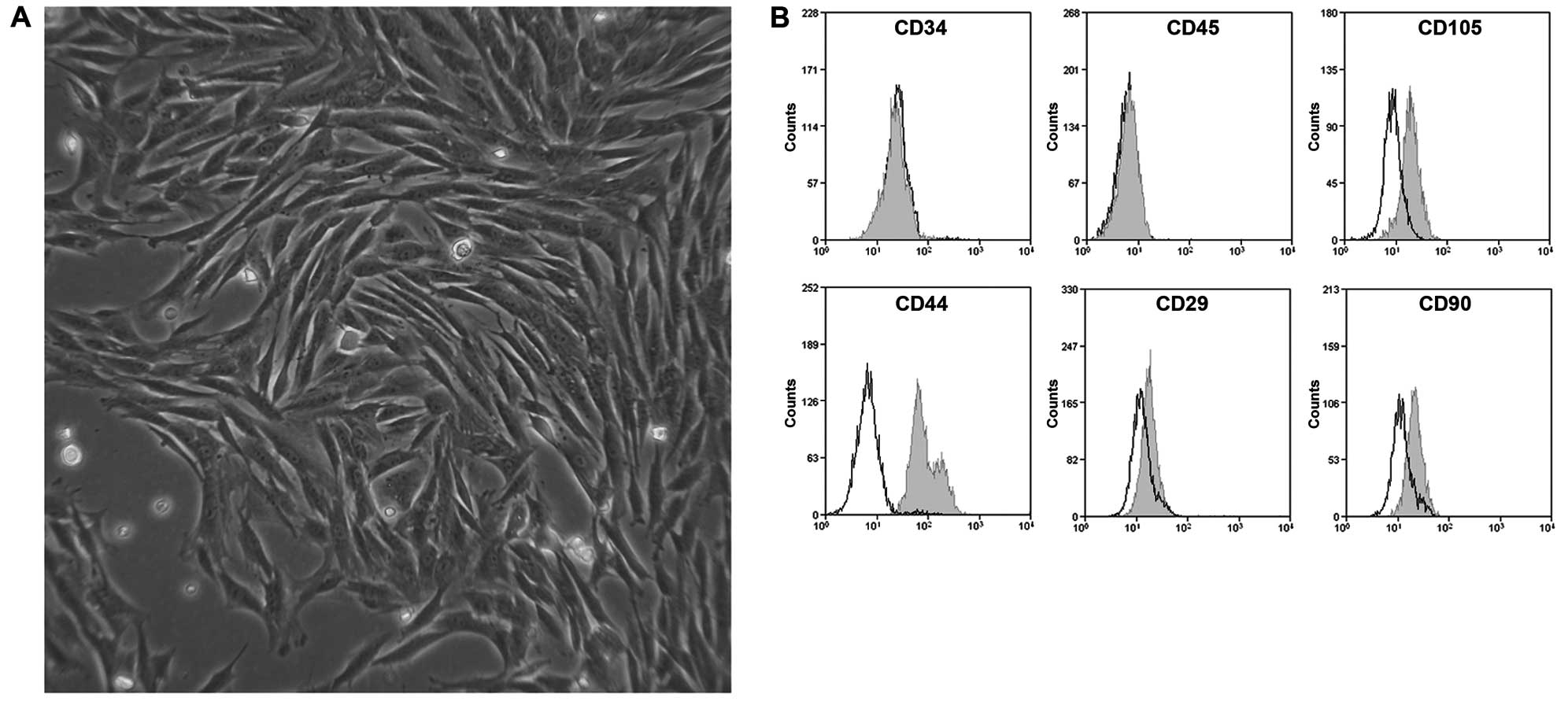
A total of 7 days after seeding, typical colony
forming units with fibroblastic cell morphology (Fig. 6A) were observed. FACS analysis
revealed that the rMSCs were positive for MSC markers, such as
CD105, CD44, CD29 and CD90 and negative for hematopoietic markers,
such as CD34 and CD45 (Fig.
6B).
MTS assay
From 24 h to 14 days, porous PCL matrices showed a
significant increase in cell viability relative to the control
(Fig. 8). Larger cavities were
shown to promote optimal conditions for cell growth at 72 h, as
detected in PCL-AT. After 1 week, all porous PCL scaffolds
presented similar cell proliferation rates (p<0.05) in
comparison to PCL-WP (Fig. 8).
Although cell viability markedly decreased in all samples at 14
days, possibly due to an impaired flux of nutrients and gas under
static growth conditions, the cell number detected on PCL-BP
significantly (p<0.05) diverged from that of the control,
suggesting that the porosity grade and BP prolonged the cell
viability on the PCL scaffolds over time.
SEM
At 24 h after cell seeding, the surface of all the
porous PCL scaffolds was shown to be largely populated by MSCs
(Fig. 7). From 7 to 14 days, the
cells were still organized in a monolayer. By contrast, the rare
cells observed on PCL-WP at the early (24 h), intermediate (7 days)
and late growth phase (14 days) suggested that the scaffold
porosity grade was not functional to guarantee cell viability,
adhesion and proliferation.
RT-PCR
The expression of osteopontin (OPN) and Coll I was
shown to be independent of PCL substrates (Fig. 9). Although its expression was
detected at 14 days in the samples under both proliferative and
osteogenic conditions, Runx2 was expressed as early as 7 days on
PCL-BP, possibly due to the combined stimulatory effects of the
composite matrix and differentiation medium. Independent of the
porosity grade of the scaffolds, the differentiation induction by
soluble factors was shown to strictly control the mRNA expression
of osteocalcin (OC) in rMSCs (Fig.
9A–D).
 | Figure 9(A) Expression of Runx2, osteopontin
(OPN), osteocalcin (OC) and collagen I (Coll I) mRNAs in MSCs
cultured for 7 (7d) and 14 days (14d) in (a–d) proliferative and
(A′–D′) osteogenic medium on (a, A′) PCL-WP, (b, B′) PCL-AT, (c,
C′) PCL-AT-HA and (d, D′) PCL-AT-BP scaffolds. In parallel, the
gene expression of the housekeeping, β-actin, was evaluated. All
RT-PCR products were electrophoresed on 2% agarose gels pre-stained
with GelRed. (B) Densitometry of agarose gel bands.
*p<0.05; **p<0.01. PCL,
poly-ɛ-caprolactone; WP, without porogen; AT, alginate threads;
AT-HA, alginate threads and hydroxyapatite; AT-BP, alginate threads
and bone powder. |
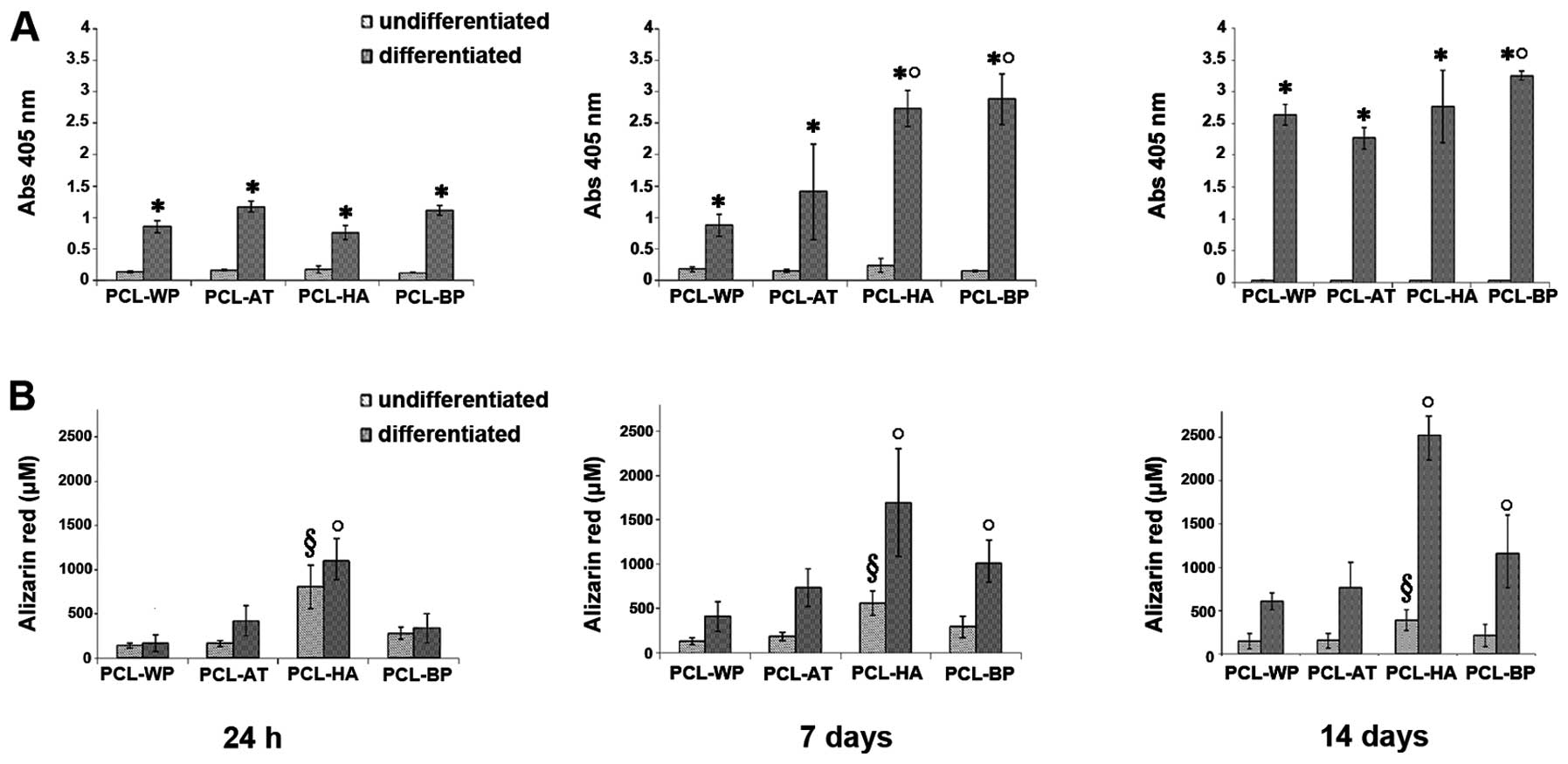
ALP assay and Alizarin Red
staining
From 24 h to 14 days, a low activity of the ALP
enzyme was detected in all porous PCL scaffolds and control samples
under proliferative conditions (Fig.
10A). When the MSCs were induced with osteogenic medium, the
ALP value significantly (p<0.05) increased. In particular, the
differentiated cells on PCL-HA (7 days) and PCL-BP (7, 14 days)
showed a significant (p<0.05) increase in ALP activity in
comparison to PCL-AT, suggesting that BP and HA enhanced the
osteogenic properties of the porous PCL scaffolds. As expected,
mineralized deposits (Fig. 10B)
were detected from 24 h to 14 days on PCL-HA under both
proliferative (p<0.05) and differentiation (p<0.05)
conditions. The BP extracts revealed to be effective (p<0.05) in
stimulatig ECM mineralization from 7 to 14 days only in samples
cultured in osteogenic medium.
Discussion
Following bone injury, the healing process develops
through the recruitment of immature cells that first originate from
osteoprogenitor cells (20) and
then differentiate into bone cells under local, biochemical and
biophysical stimuli. During bone implant incorporation, the
phenomenon of osteoinduction can be observed (20). It is dependent on scaffold
properties, such as porosity, which is defined as the percentage of
internal void space (21) and
pore size (22). Including
micropores (diameter size <10 μm) and macropores (diameter size
>50 μm), total porosity is a variable parameter among tissue
sites (~50–90% in trabecular bone and 3–12% in cortical tissue)
(23,24) and is crucial for the invasive
growth of cells, vascularization and the diffusion of nutrients and
gasses. Thus, for bone replacement, in order to achieve optimal
bone-tissue outcomes, the effects of pore size and morphology on
osteoinduction and scaffold mechanical properties have been
extensively investigated (25,26). As large pores favour direct
osteogenesis and small pores guarantee osteochondral ossification,
fabricating scaffolds with different grades of porosity and pore
size is appealing in terms of improving the bone regeneration
process.
Grandi et al (9) demonstrated that the biological
activities of PCL scaffolds obtained by the phase separation
technique were greatly enhanced when alginate threads were added as
the porogen (PCL-AT) and pore size was in the range of 15–1,400 nm.
As a bigger void volume implies a reduction in scaffold mechanical
strength, and the tensile strength of PCL-AT was not optimal for
in vivo bone applications, in the present study, mineral
components, such as HA or decellularized bone matrix powder (BP)
were used in order to prepare composite PCL-AT matrices with
increased mechanical strength. The obtained multi-scale porosity
and pore size in the range of ~15–800 nm in both PCL-AT-HA and
PCL-AT-BP was in agreement with structural properties previously
reported as functional to promote in vivo bone regeneration.
In particular, Hulbert et al (21) demonstrated that pore sizes in the
range of 100–150 and 150–200 μm resemble normal haversian systems
and stimulate in vivo substantial bone ingrowth. Macropores
with a diameter of 400–600 μm in porous HA implants have been shown
to promote the in vivo healing of rat femoral defects
(27), while pores ranging from
100 to 600 μm have been shown to sustain the in vitro
osteogenic differentiation of murine pluripotent C3H10T1/2 cells
(28).
As the in vivo bone environment is subjected
to mechanical compression, an elective scaffold for bone
regeneration must be designed to withstand this force, while
adequate porosity [Bignon et al (29)] is preserved. In the present study,
the porous PCL-AT scaffold demonstrated a significant increase in
structural resistance only when decellularized extracellular bone
matrix was added, suggesting that the reduction of total macropores
due to HA is not sufficient to modify the scaffold structural
strength. Despite being characterized by a high level of
osteoconductivity, HA is considered a bone graft extender or
carrier of growth factors rather than a ‘stand-alone’ bone graft
substitute. It is weak in tensile strength and toughness, and thus
when added to bone scaffolds, no significant change in mechanical
properties was detected while, as expected, a reduction in total
porosity, trabecular thickness and trabecular space was
observed.
As previously reported (9) and confirmed in this study, the
adhesion and proliferation of bone-forming cells in PCL-AT
scaffolds are enhanced by pores differentially organized into
‘blind’, ‘closed’ and ‘through’ pores (30). ‘Blind’ pores start from one
surface and terminate inside the material. They influence the
amount of fluids that can be stored within a matrix and contribute
to increasing the surface area of cell growth. ‘Closed’ pores are
void cavities not connected to the external surface and do not
contribute to the inflow of nutrients and gasses. ‘Through’ pores
are channels extending from one free scaffold surface to another
and are responsible for the flow of fluid through the material.
Although the role of porosity in bone substitution to promote the
migration and proliferation of osteoblasts and mesenchymal cells,
as well as vascularization (31)
has been demonstrated, the optimal porosity percentage and pore
size have been not yet been identified. In this study, we
demonstrated that the increased porosity of scaffolds due to AT was
associated with a major degree of cell adhesion and proliferation.
Moreover, the heterogeneity of scaffold pores in PCL-AT-HA and
PCL-AT-BP was shown to favour the growth and differentiation of
MSCs isolated from rabbit bone marrow.
The proliferation of osteoblasts and mesenchymal
cells has been largely demonstrated to be dependent on both
macropores, assuring a major interconnected network of void
cavities and an improved oxygen and nutrients inflow (31–33), as well as micropores, stimulating
high levels of bone-inducing protein adsorption, ion exchange and
bone-like apatite formation (34). Although the greater porosity in
PCL-AT guaranteed better conditions for the proliferation of MSCs
at the early phase, the presence of BP was shown to be crucial to
prolong the viability of cells at the late phase.
It is known that the microenviroment and soluble
factors interact to drive the proliferation and differentiation of
cells. When rMSCs were cultured for 14 days on porous PCL
scaffolds, the mRNA expression of Coll I and OPN was observed at
the early and late phase suggesting their expression is not
controlled by the microenviroment. The mRNA expression of Runx2 (at
7 days) and osteocalcin (at 14 days) was observed only following
stimulation with osteogenic factors, demonstrating that soluble
factors cooperate with the microenviroment to promote the
progression of the differentiation process. The transcription
factor, Runx2, is a key regulator of osteoblast differentiation
(35), controlling both
intramembranous and endochondral bone formation (36) and promoting chondrocyte maturation
(37). As the expression of Runx2
progressively increased during osteogenic differentiation, and the
activity of ALP and the levels of osteocalcin in the
culture-expanded MSCs significantly increased in response to Runx2,
it can be hypothesized that all porous PCL scaffolds sustain the
expression of certain osteogenic markers, but only in combination
with specific inducers (HA or BP) and that they are functional to
promote the activity of ALP and the mineralization of ECM,
suggesting a marked influence of HA and BP on osteogenic maturation
in bone substitutes.
In conclusion, the data presented in this study
indicate that PCL scaffolds prepared with AT as the porogen and HA
or BP acquire similar osteoinductive properties, but differ in
mechanical strength. Due to the more appropriate porosity grade,
structural resistance and biological properties, PCL-AT-BP was
shown to have greater potential for use in bone repair
applications.
Acknowledgements
We would like to thank San Luca Hospital, ULSS 18,
Trecenta (Rovigo), Italy and the University of Padua for providing
the laboratory facilities. We would also like to express our
gratitude to Thomas Bertalot for providing technical support.
References
|
1
|
Tabata Y: Biomaterial technology for
tissue engineering applications. J R Soc Interface. 6(Suppl 3):
311–324. 2009. View Article : Google Scholar
|
|
2
|
Porter JR, Ruckh TT and Popat KC: Bone
tissue engineering: a review in bone biomimetics and drug delivery
strategies. Biotechnol Prog. 25:1539–1560. 2009.PubMed/NCBI
|
|
3
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar
|
|
4
|
Schmitz JP and Hollinger JO: A preliminary
study of the osteogenic potential of a biodegradable
alloplastic-osteoinductive alloimplant. Clin Orthop Relat Res.
237:245–255. 1988.PubMed/NCBI
|
|
5
|
Mikos AG, Sarakinos G, Leite SM, Vacanti
JP and Langer R: Laminated threedimensional biodegradable foams for
use in tissue engineering. Biomaterials. 14:323–330. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal CM and Kennedy MB: The effects of
ultrasound irradiation on a biodegradable delivery system.
Transactions of the Society for Biomaterials. 19:2921993.
|
|
7
|
Mooney DJ, Baldwin DF, Suh NP, Vacanti JP
and Langer R: Novel approach to fabricate porous sponges of poly
(D,L-lactic-co-glycolic acid) without the use of organic solvents.
Biomaterials. 17:1417–1422. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schugens C, Maquet V, Grandfils C, Jerome
R and Teyssie P: Polylactide macroporous biodegradable implants for
cell transplantation. II Preparation of polylactide foams by
liquid-liquid phase separation. J Biomed Mat Res. 30:449–461. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grandi C, Di Liddo R, Paganin P, Lora S,
Dalzoppo D, Feltrin G, Giraudo C, et al: Porous
alginate/poly(ɛ-caprolactone) scaffolds: preparation,
characterization and in vitro biological activity. Int J Mol
Med. 27:455–467. 2011.
|
|
10
|
Costa-Pinto AR, Reis RL and Neves NM:
Scaffolds based bone tissue engineering: the role of chitosan.
Tissue Eng Part B Rev. 17:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karsenty G: The genetic transformation of
bone biology. Genes Dev. 13:3037–3051. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito Y, Hasuda H, Kamitakahara M, Ohtsuki
C, Tanihara M, Kang IK and Kwon OH: A composite of hydroxyapatite
with electrospun biodegradable nanofibers as a tissue engineering
material. J Biosci Bioeng. 100:43–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas V, Jagani S, Johnson K, Jose MV,
Dean DR, Vohra YK and Nyairo E: Electrospun bioactive nanocomposite
scaffolds of polycaprolactone and nanohydroxyapatite for bone
tissue engineering. J Nanosci Nanotechnol. 6:487–493. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teng SH, Lee EJ, Wang P and Kim HE:
Collagen/hydroxyapatite composite nanofibers by electrospinning.
Mater Lett. 62:3055–3058. 2008. View Article : Google Scholar
|
|
15
|
Ngiam M, Liao S, Patil AJ, Cheng Z, Chan
CK and Ramakrishna S: The fabrication of nano-hydroxyapatite on
PLGA and PLGA/collagen nanofibrous composite scaffolds and their
effects in osteoblastic behavior for bone tissue engineering. Bone.
45:4–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Venugopal J, Low S, Choon AT, Sampath
Kumar TS and Ramakrishna S: Mineralization of osteoblasts with
electrospun collagen/hydroxyapatite nanofibers. J Mater Sci Mater
Med. 19:2039–2046. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woodward SC, Brewer PS, Moatamed F,
Schindler A and Pitt CG: The intracellular degradation of poly
(epsilon-caprolactone). J Biomed Mater Res. 19:437–444. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phipps MC, Clem WC, Catledge SA, et al:
Mesenchymal stem cell responses to bone-mimetic electrospun
matrices composed of polycaprolactone, collagen I and
nanoparticulate hydroxyapatite. PLoS One. 6:e168132011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meezan E, Hjelle JT, Brendel K and Carlson
EC: A simple, versatile, nondisruptive method for the isolation of
morphologically and chemically pure basement membranes from several
tissues. Life Sci. 17:1721–1732. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Albrektsson T and Johansson C:
Osteoinduction, osteoconduction andosseointegration. Eur Spine J.
10(Suppl 2): 96–101. 2001. View Article : Google Scholar
|
|
21
|
Hulbert SF, Young FA, Mathews RS,
Klawitter JJ, Talbert CD and Stelling FH: Potential of ceramic
materials as permanently implantable skeletal prostheses. J Biomed
Mater Res. 4:433–456. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karageorgiou V and Kaplan D: Porosity of
3D biomaterial scaffolds and osteogenesis. Biomaterials.
26:5474–5491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaplan FS, Hayes WC, Keaveny TM, Boskey A,
Einhorn TA and Iannotti JP: Form and function of bone. Orthopaedic
Basic Science. Simon SR: American Academy of Orthopaedic Surgeons;
Rosemont, IL: pp. 127–185. 1994
|
|
24
|
Cooper DM, Matyas JR, Katzenberg MA and
Hallgrimsson B: Comparison of microcomputed tomographic and
microradiographic measurements of cortical bone porosity. Calcif
Tissue Int. 74:437–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauer TW and Muschler GF: Bone graft
materials. An overview of the basic science. Clin Orthop Relat Res.
10–27. 2000. View Article : Google Scholar
|
|
26
|
Keating JF and McQueen MM: Substitutes for
autologous bone graft in orthopaedic trauma. J Bone Joint Surg.
83:3–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Damien E, Hing K, Saeed S and Revell PA: A
preliminary study on the enhancement of the osteointegration of a
novel synthetic hydroxyapatite scaffold in vivo. J Biomed Mater Res
A. 66:241–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HD and Valentini RF: Retention and
activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J
Biomed Mater Res. 59:573–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bignon A, Chouteau J, Chevalier J,
Fantozzi G, Carret JP, Chavassieux P, Boivin G, Melin M and
Hartmann D: Effect of micro- and macroporosity of bone substitutes
on their mechanical properties and cellular response. J Mater Sci
Mater Med. 14:1089–1097. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahuja G and Pathak K: Porous carriers for
controlled/modulated drug delivery. Indian J Pharm Sci. 71:599–607.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuboki Y, Takita H, Kobayashi D, et al:
BMP-induced osteogenesis on the surface of hydroxyapatite with
geometrically feasible and nonfeasible structures: topology of
osteogenesis. J Biomed Mater Res. 39:190–199. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuruga E, Takita H, Itoh H, Wakisaka Y
and Kuboki Y: Pore size of porous hydroxyapatite as the
cell-substratum controls BMP-induced osteogenesis. J Biochem.
1:317–324. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gotz HE, Muller M, Emmel A, Holzwarth U,
Erben RG and Stangl R: Effect of surface finish on the
osseointegration of laser-treated titanium alloy implants.
Biomaterials. 25:4057–4064. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan H, Kurashina K, de Bruijn JD, Li Y,
de Groot K and Zhang X: A preliminary study on osteoinduction of
two kinds of calcium phosphate ceramics. Biomaterials.
20:1799–1806. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong SW, Ying DJ, Duan XJ, et al: Bone
regeneration using an acellular extracellular matrix and bone
marrow mesenchymal stem cells expressing Cbfa1. Biosci Biotechnol
Biochem. 73:2226–2233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komori T, Yagi H, Nomura S, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inada M, Yasui T, Nomura S, et al:
Maturational disturbance of chondrocytes in Cbfa1-deficient mice.
Dev Dyn. 214:279–290. 1999. View Article : Google Scholar : PubMed/NCBI
|