Introduction
Age-related macular degeneration (AMD) is the
leading cause of irreversible visual impairment among the elderly
in western countries. AMD is primary characterized by the formation
of drusen, which are extracellular deposits between the retinal
pigment epithelium (RPE) and Bruch’s membrane (BrM), containing
glycolipids, proteins and cellular debris. The appearance of drusen
is strongly correlated with the development of AMD (1,2).
Amyloid-β (Aβ) peptide, a component of drusen, is
considered to contribute to neurodegenerative events and/or chronic
inflammation in the retina of patients with AMD. Aβ deposition
within the vesicles is particularly observed in the eyes of
patients with advanced AMD (3,4).
It has been postulated that the presence of Aβ triggers a local
inflammatory response that activates the immune system. Several
in vitro studies have demonstrated that inflammation
pathways and immune response are significantly upregulated in
Aβ-stimulated cells of the RPE (RPE cells) (5,6). A
single intravitreal injection of Aβ peptide has been shown to
induce inflammasome activation in the retinas of rats (7). A recent clinical trial reported that
RN6G, a humanized monoclonal antibody against Aβ, reduced the Aβ
deposits in the retina, thus preserving the retinal function of the
animals and maintaining normal RPE morphology (8).
Chronic inflammation is associated with aging and
plays an important role in AMD (9); however, the source of sterile
inflammation that fuels retinal degeneration in AMD remains
unknown. Age-related chronic inflammation may be derived from two
sources: i) an age-related decline in homeostatic immune function,
or ii) senescent cells. Cellular senescence may cause chronic
inflammation through the senescence-associated secretory phenotype
(SASP). SASP proteins include a wide range of chemokines and
cytokines [interleukin (IL)-6, IL-8, IL-1, granulocyte-macrophage
colony-stimulating factor, monocyte chemotactic protein (MCP) and
matrix metalloproteinases (MMPs)] that are known to stimulate
inflammation (10,11). Cellular senescence is defined as a
state of irreversible proliferative arrest caused by a wide range
of stimuli, including severe DNA damage, the expression of
oncogenes, oxidative stress and strong mitogenic signals (12–14). Aβ has been recently been shown to
be involved in the senescence of neuronal cells, astrocytes and
endothelial cells (15–18). Our previous in vitro study
demonstrated that Aβ peptide caused RPE cells to enter senescence
(19). However, the in
vivo effects of Aβ peptide on RPE cell senescence and
senescence-associated inflammation remain unclear.
The deposition of Aβ peptide, astrocyte senescence
and altered SASP expression have been observed in the brains of
patients with Alzheimer’s disease (AD) (17). This finding suggests the existence
of a correlation between Aβ peptide and senescence-associated
neuronal inflammation (17).
Previous in vivo studies have demonstrated that the
intravitreal or subretinal injection of Aβ peptide may cause
retinal degeneration and inflammation (7,20).
However, to the best of our knowledge, the effects of the
subretinal injection of Aβ peptide on RPE cell senescence have not
been investigated to date. Thus, in the present study, we aimed to
explore the role of cell senescence and inflammation in the retinal
degeneration induced by Aβ(1-42) peptide.
Materials and methods
Aβ(1-42) oligomerization
Aβ(1-42) peptide and its inactive reverse control
peptide Aβ(42-1) were prepared as described in our previous study
(19). Briefly, 0.5 mg of
lyophilized Aβ peptide (Sigma-Aldrich, Shanghai, China) was
dissolved in 140 μl hexafluoroisopropanol (HFIP) and incubated for
20 min, followed by the addition of 900 μl distilled H2O
and a 20-min incubation in the HFIP/water mixture. Subsequently,
the solvent was evaporated from the resulting supernatant under
constant stirring at room temperature for 5 days. Oligomerization
was verified by dot blot assay as previously described (19).
Animals and animal treatments
To elucidate the in vivo effects of Aβ
peptide on the retina, C57BL/6 mice received a single unilateral
subretinal injection of Aβ peptide (Fig. 1). Briefly, 5-month-old C57BL/6
mice were anesthetized by an intraperitoneal injection of 300 mg/kg
chloral hydrate (Sangon Biotech, Shanghai, China). The pupils were
dilated with 0.5% tropicamide and 0.5% phenylephrine (Santen
Pharmaceutical, Osaka, Japan); 0.5% proparacaine hydrochloride
(Alcon, Shanghai, China) was applied for topical anesthesia.
Following complete pupillary dilation, the anesthetized animals
were placed under an Nikon SMZ-1 stereoscopic microscope, and the
corneas were carefully punctured through the pars plana with a
30-gauge needle to produce a hole sufficiently large enought to
insert a 33-gauge blunt needle (Hamilton Co., Reno, NV, USA). The
blunt needle tip was inserted through the corneal puncture and
advanced into the anterior chamber. A slight resistance to the
movement of the needle indicated the penetration of the retina and
entrance into the subretinal matrix. The mice were divided into 3
groups as follows: i) mice injected with oligomeric Aβ(1-42)
[OAβ(1-42) (1 nmol/2 μl, n=24); ii) mice injected with OAβ(42-1) (1
nmol/2 μl, n=8), and iii) mice injected with 2 μl of
phosphate-buffered saline (PBS) (calcium- and magnesium-free,
n=8).
All animal experiments were performed according to
the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research. All surgical interventions and animal care procedures
were approved by the Ethics Committee of Tongji University,
Shanghai, China.
Electroretinography (ERG)
Flash ERG (FERG) was recorded using an ERG recording
system (APS-2000AER; Kanghua Ruiming Technology, Chongqing, China).
The animals were allowed to adapt to the dark overnight and
prepared for electroretinography and the scotopic electroretinogram
was recorded. The animals were anesthetized with an intraperitoneal
injection pf 300 mg/kg chloral hydrate (Sangon Biotech). Following
pupillary dilation and the topical anesthesia of the cornea, gold
loop electrodes were placed on the cornea with a drop of 2.5%
hydroxypropyl methylcellulose. Subsequently, the reference
electrode was placed into the mouth of the animal underneath the
tongue, and a ground electrode was subcutaneously inserted into the
midway of the tail. The ERG waveforms were then recorded in
response to a flash at 1.125 cd*s/m2. The
amplitude of the a-wave was measured from the baseline to the
trough of the a-wave. The amplitude of the b-wave was measured from
the trough of the a-wave to the peak of the b-wave. The amplitude
of the c-wave was measured from the baseline to the peak of the
c-wave.
Retinal tissue immunohistochemistry
On day 7 following surgery, the mice were terminally
anesthetized and subjected to whole-body perfusion with 4%
paraformaldehyde (PFA) in PBS and the whole eyes were then
enucleated and fixed in 4% PFA overnight. Only sections including
the optic nerve were used for histological and immunohistochemical
analyses. Sections (5-μm-thick) of paraffin-embedded specimens were
stained with hematoxylin and eosin (H&E). The slides were
dehydrated and placed on a coverslip.
For immunohistochemical analysis, antibody staining
was performed on sections of paraffin-embedded eyes. Antibodies to
RPE65 (ab67042) and p16INK4a (ab54210) were obtained
from Abcam (Hong Kong, China) and used at a dilution of 1:100. The
paraffin-embedded sections were heated to 60°C for 30 min, and then
deparaffinized and rehydrated in graded alcohol series. The
paraffin-embedded sections were incubated for 1 h in PBS containing
5% FCS to reduce non-specific binding, and then overnight at 4°C
with the p16INK4a and RPE65 primary antibodies. After
washing, the sections were incubated in a solution of 1:200 of goat
anti-mouse 594-conjugated secondary antibody or goat anti-rabbit
488-conjugated secondary antibody (Abcam). The sections were then
washed, stained for 5 min with DAPI, and washed again in PBS. The
sections were then mounted and examined under a fluorescence
microscope (Leica TCS SP5; Leica Microsystems, Wetzlar,
Germany).
Transmission electron microscopy
(TEM)
For ultrastructural analysis, the enucleated eyes
were dissected at the equator immediately and the posterior eye
cups were fixed in 2.5% glutaraldehyde in phosphate buffer at 4°C
overnight. The central 2×2 mm tissue temporal to the optic nerve
was post-fixed with 2% osmium tetroxide and alcohol dehydrated and
embedded in epoxy resin. The ultra-thin sections were stained with
lead citrate and uranyl acetate, and examined under an electron
microscope (CM120; Philips, Eindhoven, The Netherlands).
Fundus photography
For the funduscopic examination of the mice,
confocal scanning laser ophthalmoscopy (cSLO) was performed using a
device available for human fundus imaging (Spectralis HRA;
Heidelberg Engineering, Heidelberg, Germany). Fundus images were
captured using a 790-nm diode laser for infrared (IR) and 488 nm
for autofluorescence (AF).
Western blot analysis
RPE-choroid preparations for western blot analysis
and RT-PCR were dissected from the freshly harvested eyes and
preserved at −80°C until further processing. The RPE-choroid
complex was sonicated in radio-immunoprecipitation assay (RIPA)
buffer containing proteinase inhibitors for 15 min. Protein content
was determined by a bicinchoninic acid assay (Pierce, Rockford, IL,
USA). Aliquots (50 μg of total protein) of each sample from the
RPE-choroid were loaded per lane onto 10% SDS-PAGE gels for
electrophoresis and then transferred onto PVDF membranes. The
membranes were blocked and incubated with the primary antibody,
rabbit polyclonal antibodies against p16INK4a (1:500;
Abcam) and rabbit polyclonal antibodies against
glyceraldehyde-3-phosphate dehydrogenase [GAPDH (Cat. no. ab37168),
1:1,000] overnight. The membranes were then washed and incubated
with horseradish peroxidase-coupled secondary antibodies for 2 h.
The blots were washed and developed with chemiluminescence reagent.
The membranes were exposed to ImageQuant LAS 4000 imaging, and
densitometric analysis was performed using Photoshop CS4.0
software.
RT-PCR and real-time PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and was then reverse transcribed
into cDNA with RT Master Mix (Takara Biotechnology, Dalian, China).
The expression levels of IL-8 and IL-6 were measured using
quantitative PCR mix (Takara Biotechnology). The primers used for
PC were as follows: IL-6 sense, 5′-TTCCATCCAGTTGCCTTCTT-3′ and
antisense, 5′-CATTTCCACGATTTCCCAGA-3′; IL-8 sense, 5′-CCTT
GGTCTTCCTGCTTGA-3′ and antisense, 5′-ATCGTTG TTCCCATCCACAT-3′.
Real-time PCR was performed in a ABI7500 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA). GAPDH (sense,
5′-AGCAGTCCCGTACA CTGGCAAAC-3′ and antisense), 5′-TCTGTGGTGATGT
AAATGTCCTCT-3′ was used for normalization and the relative gene
expression was expressed as the relative ‘fold change’ calculated
using the ΔΔCt method.
Results
Aβ peptide leads to impairment of visual
function in animals
To elucidate the in vivo effects of Aβ
peptide on visual function, C57BL/6 mice received a subretinal
injection of Aβ peptide, and visual function was evaluated by ERG
on day 7 post-injection. Photoreceptors are the source of the
negative-going a-wave (21,22). Rod bipolar cells are the source of
the b-wave in the dark-adapted retina (23–25). RPE cells are the source of the
positive-going c-wave (26).
Representative waveforms of the maximal ERG scotopic response
evoked by a single flash at 1.125 cd*s/m2 are
illustrated in Fig. 2A. No
significant differences were observed between the Aβ(42-1)-injected
mice and the PBS control mice. However, a moderate but significant
decrease in a-, b- and c-wave amplitude was detected in the
Aβ(1-42)-injected mice (Fig. 2A and
B). These results indicated that the subretinal injection of
Aβ(1-42) induced an impairment of visual function in the
animals.
Aβ induces retinal degeneration and
ultrastructural alterations in the outer retina
To demonstrate the deleterious effects of Aβ(1-42)
on the retina, we compared its effects on retinal histology and RPE
structure with those of PBS and the inactive reverse peptide
Aβ(42-1). The H&E-stained sections of RPE/neural retina were
examined under a microscope (Fig.
3A). The mice injected with PBS or the reverse peptide showed a
normal appearance of retinal sections (Fig. 3A, left and middle panels). By
contrast, degenerative alterations, including extensive vacuolation
(V symbol), hyperpigmentation (arrow), hypopigmentation (asterisk)
and thickness of the BrM, were observed in the outer retina of the
Aβ(1-42)-injected mice (Fig. 3A,
right panel).
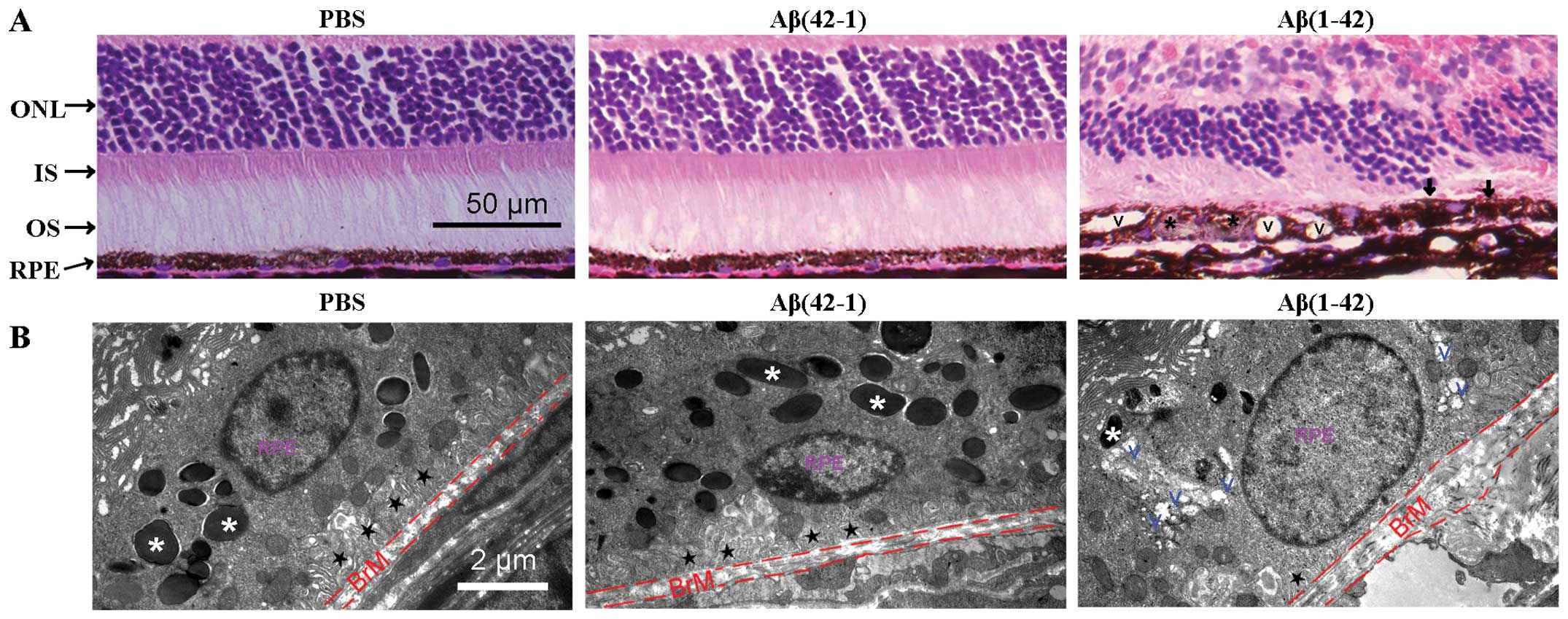 | Figure 3Histopathology of retinal
degeneration and deleterious alterations of the structure of the
retinal pigment epithelium (RPE) in amyloid-β (Aβ) peptide-injected
mice. (A) Hematoxylin and eosin (H&E)-stained paraffin sections
of the RP/neural retina. Signs of retinal degeneration, including
extensive vacuolation (V symbol), hyperpigmentation (arrow),
hypopigmentation (black asterisk) and thickness of Bruch’s membrane
(BrM), were observed in the Aβ(1-42)-injected mice. ONL, outer
nuclear layer; IS, inner segment; OS, outer segment. (B)
Transmission electron microscopy (TEM) of the interface between the
RPE and BrM in mice. RPE basal infoldings (black star) and BrM
thickness appear normal in the Aβ(42-1)-injected eye (middle panel)
compared with the phosphate-buffered saline (PBS)-injected control
mice (left panel). By contrast, in the Aβ(1-42)-injected mice, the
loss of pigmented granules, obvious thickening of the BrM, the loss
of normal basal infoldings and the formation of autophagic vacuoles
were observed. White asterisk, pigmented granules; black star,
normal basal infoldings; V, vacuoles. |
Subsequently, the RPE structures were examined by
TEM. The PBS- and Aβ(42-1)-injected mice showed normal structures
of the RPE, well-developed RPE infoldings and a consistent
thickness of the BrM (Fig. 3B,
left and middle panels). In the Aβ(1-42)-injected mice, the RPE
cells were highly vacuolated with membranous debris, the normal
basal infoldings were replaced by amorphous material deposits, the
number of pigment granules decreased and the BrM was thickened
(Fig. 3B, right panel). Taken
together, the results from histopathological analysis and TEM
demonstrated that the Aβ(1-42)-injected mice developed some
degenerative alterations on the neural retina layer, the RPE and
BrM, which are similar to some of the morphological characteristics
of patients with AMD (27).
RPE cell senescence is induced by the
subretinal injection of Aβ peptide
We then investigated whether the subretinal
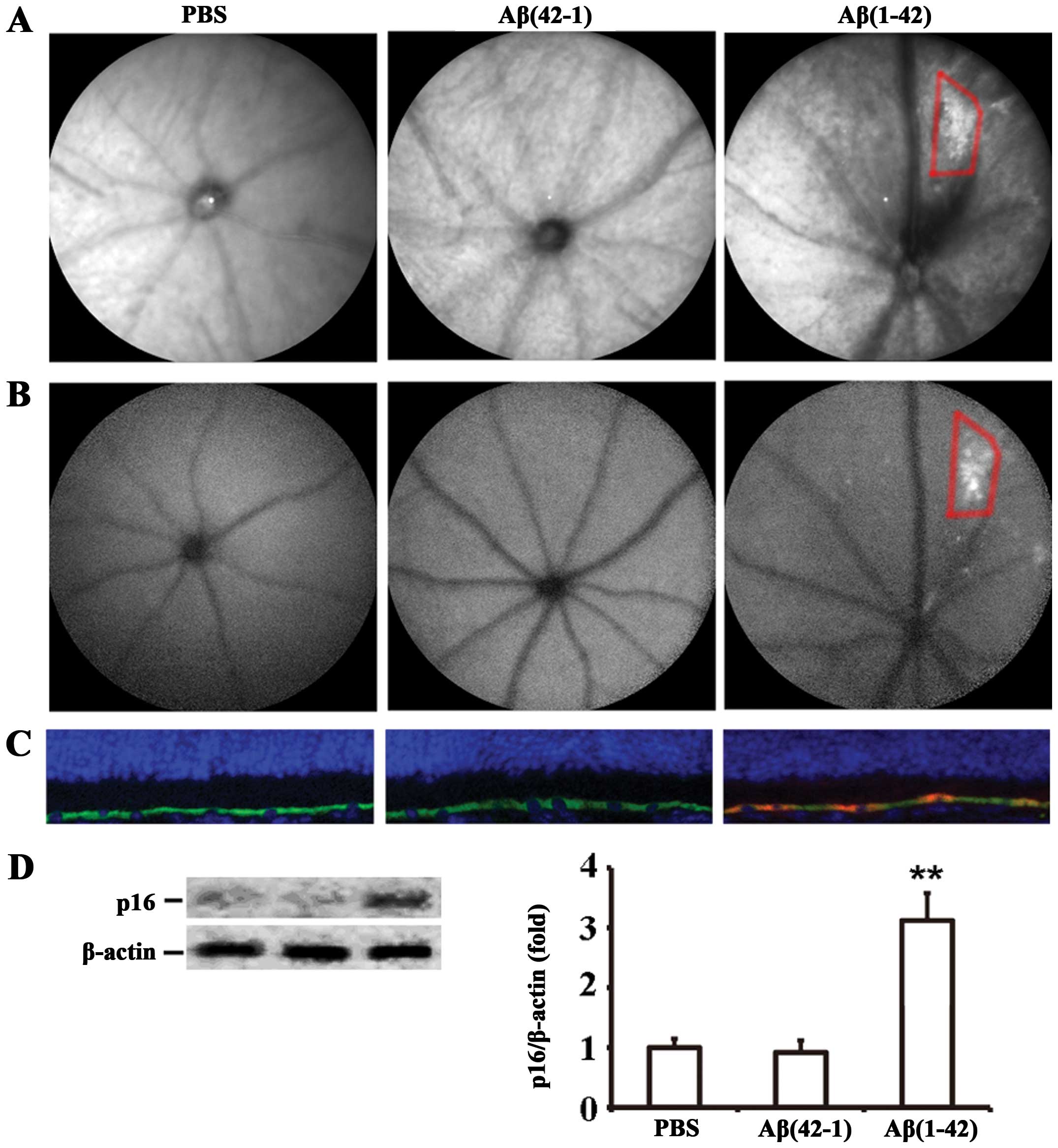
injection of Aβ(1-42) induces RPE cell senescence. cSLO, a clinical
imaging method, is used to examine the fundus autofluorescence
(FAF) pattern as a means of assessing the health of the RPE in AMD.
Therefore, funduscopic examination was used to assess RPE
alterations/senescence in vivo. IR-cSLO revealed that the
Aβ(1-42)-injected mice developed an increase in the number of
drusen-like lesions characterized by irregularly shaped bright
regions (Fig. 4A, right panel),
and the increased granular autofluorescent spots were observed by
AF-cSLO in almost the same areas (Fig. 4B, right panel). By contrast, no
significant distribution of fluorophores was observed in the ocular
fundus of the PBS- or Aβ(42-1)-injected mice (Fig. 4A and B). The analysis of cell
senescence on RPE/neural retinal sections by p16INK4a
staining confirmed that Aβ(1-42) induced RPE cell senescence in the
mice on day 7 post-injection (Fig.
4C, right panel). However, in the retinal sections of the PBS-
or Aβ(42-1)-injected mice, no p16INK4a-positive RPE
cells were detected (Fig. 4C,
left and middle panels). Subsequently, the expression level of
p16INK4a in RPE cells was determined by western blot
analysis; the injection of Aβ(1-42) significantly increased
p16INK4a expression by (3.12±0.46)-fold compared with
the injection of the reverse peptide or PBS (Fig. 4D). Taken together, these data
strongly suggest that Aβ(1-42) significantly induces RPE cell
senescence in the retinas of mice.
mRNA expression of IL-6 and IL-8 is
upregulated following the subretinal injection of Aβ(1-42)
peptide
Hallmarks of inflammation, including elevated IL-6
and IL-8 expression, are significantly associated with the severity
of AMD. Recent evidence indicates that cellular senescence is
accompanied by a marked increase in the secretion of 40–80 factors,
which has been termed the SASP (42). The key SASP factors, such as IL-6
and IL-8, are known to stimulate chronic inflammation in
age-related diseases (42). Thus,
to determine whether the subretinal injection of Aβ alters SASP
gene expression in RPE cells, the RPE-choroid layer was isolated
and IL-6 and IL-8 mRNA expression was examined by RT-PCR. Aβ(1-42)
injection significantly increased the mRNA expression of IL-6
(6.8±0.8-fold, P<0.05) and IL-8 (3.7±1.2-fold, P<0.05),
compared with PBS-injected group (Fig. 5), whereas the injection of
Aβ(42-1) did not induce any significant changes in the mRNA
expression of IL-6 and IL-8, compared with the PBS-injected group
(Fig. 5).
Discussion
The main results of the present study were as
follows: i) the subretinal injection of Aβ(1-42) impaired the
visual function of mice (Fig. 2);
ii) Aβ(1-42)-injected mice developed some degenerative alterations
on the retina and RPE cells (Fig.
3); iii) signs of RPE cell senescence, including increased FAF
and the expression of the senescence-associated marker,
p16INK4a, were observed in the Aβ(1-42)-injected mice
(Fig. 4); iv) the major SASP
factors, such as IL-6 and IL-8, were significantly upregulated in
the RPE-choroid of Aβ(1-42)-injected mice (Fig. 5). Taken together, our results
demonstrate a causal connection between Aβ peptide deposition and
the appearance of senescent RPE cells, and suggest that the
subretinal injection of Aβ induces senescence-associated chronic
inflammation. Our data also suggest that Aβ-injected mice represent
a useful animal model of AMD.
The electroretinogram is a non-invasive method to
evaluate the function of specific layers of the retina. The
negative-going a-wave and the positive-going b-wave originate from
photoreceptors and rod bipolar cells, respectively (21–25), whereas the sources of the
positive-going c-wave are the RPE and retinal glial cells (26). In our study, in Aβ(1-42)-injected
mice, there was a statistically significant decrease in the a-, b-
and c-wave amplitude compared to the control group (Fig. 2). Similar findings of decreased
ERG response have been reported in APP/PS1 transgenic mice, which
presented with accumulated Aβ deposition in the retina (28). Therefore, the decreased ERG
response or the impaired visual function may be secondary to the
retinal degenerative alterations induced by the subretinal
injection of Aβ(1-42).
Consistent with the decreased ERG response,
microscopic examination revealed severe degenerative alterations in
the retinas of the Aβ(1-42)-injected mice, including the loss of
inner and outer segments, extensive vacuolation, RPE
hypopigmentation and thickness of the BrM (Fig. 3A). These data are consistent with
those of other in vivo studies, which demonstrated that the
subretinal or intravitreal injection of Aβ peptide induced
progressive retinal degeneration and the disorganization of RPE
cells (7,20,29).
In Aβ(1-42)-injected mice, ultrastructural analysis
by TEM revealed that the RPE cells had multiple vacuoles in the
cytoplasm, and that basal infoldings were fewer or absent (Fig. 3B). The RPE appears to be a
specific target of Aβ. The subretinal injection of Aβ(1-42) induced
a loss of pigmentation and RPE hypertrophy (Fig. 2A). Intravitreal injections of Aβ
peptide have been shown to induce a comparable magnitude of gene
expression changes in the RPE-choroid compared with the neuroretina
(7), indicating that the RPE
plays a major role in response to Aβ peptide. The most obvious
ultrastructural sign of RPE injury was the loss of basolateral
infoldings, which are an established marker of epithelial cell
injury (30). Vacuole formation
was used as a second sign of RPE injury, as cytoplasmic vacuoles
have been identified in the RPE that overlie drusen deposits
(31). Of note, in a study on
colon cancer cells, Sox2-induced autophagy inhibited cell
proliferation and enhanced cellular senescence, suggesting that the
formation of autophagic vacuoles is involved in cellular senescence
(32). To the best of our
knowledge, our study is the first to demonstrate that the
subretinal injection of Aβ peptide induced two marked
ultrastructural alterations, including the loss of basal infoldings
and the formation of intracellular vacuoles, in RPE cells (Fig. 3B).
It has been suggested that Aβ(1-42) plays a central
role in the pathogenesis of AD as a mediator of oxidative stress
(33). A previous study
investigated the in vitro effects of Aβ(1-42) on RPE cells
and found that it induced an increase in reactive oxygen species
(ROS) production and caused mitochondrial dysfunction (29). Considering that persistent and
sublethal oxidative stress accelerates cellular senescence
(34), the effects of Aβ(1-42) on
RPE cell senescence were evaluated in the present study. First, the
increased granular autofluorescent spots were observed by cSLO in
the fundus of Aβ(1-42)-injected mice (Fig. 4A and B). During cell senescence,
autofluorescent lysosomal storage bodies known as lipofuscins or
age-pigments accumulate in many post-mitotic types of cells
(35,36). It is well known that the RPE
accumulates massive amounts of autofluorescent lysosomal storage
bodies (lipofuscins) during cell senescence (37–39). The formation of fundus
autofluroscence in human eyes is associated with RPE atrophy and
the progression to advanced AMD (40). Lipofuscins contained in the RPE
are the main source of FAF; it has been reported that FAF detected
at 488 nm excitation with a cSLO is largely attributable to RPE
lipofuscins (40,41). Therefore, increased fundus AF
detected by cSLO may be a signal of RPE cell senescence (Fig. 4A and B). Subsequently, the
increase in p16INK4a expression was proved by
immunostaining (Fig. 4C) and
western blot analysis (Fig. 4D),
indicating that the Aβ(1-42)-induced RPE senescence is regulated by
p16INK4a. This finding is consistent with our previous
in vitro study, indicating that Aβ peptide is involved in
the senescence of RPE cells (19). It is also consistent with the
results of other studies, suggesting that Aβ peptides induce
endothelial cells, astrocytes and neurons to enter senescence
(15,17,18).
We observed a significant overexpression of IL-6 and
IL-8 in the RPE-choroid of Aβ(1-42)-injected mouse eyes (Fig. 5). Senescent cells contribute to
aging and age-related disease by generating a low-grade
inflammatory state (42). Our
results suggested that cellular senescence may promote
inflammation, which is consistent with the findings of other
studies demonstrating an increased production of IL-6 and IL-8 by
senescent fibroblasts and epithelial cells (43,44). IL-6 and IL-8 may stimulate
angiogenesis, enforce macrophage function and induce innate immune
responses (45). Moreover, it has
been reported that IL-6 and IL-8 reinforce senescent growth arrest
(46,47). Kuilman et al (47) verified that the suppression of
IL-8, IL-6 or its cognate receptor, IL-6R, was sufficient to allow
these cells to re-enter the cell cycle and proliferate, supporting
the possibility that these cytokines function in vivo to
promote cellular senescence. In addition, the aqueous humor of
patients with AMD contains higher concentrations of IL-6 and IL-8
(48). We focused our attention
on these cytokines as they can act not only as pro-inflammatory
cytokines, but also as potent inducers of growth arrest (49). Overall, we conclude that
Aβ-induced senescent RPE cells may produce high levels of IL-6 and
IL-8, which are both required to amplify and sustain the
inflammatory network and senescence.
AMD is an age-related chronic inflammatory disease
(50,51). Our previous study (19) and the present study indicate that
Aβ-induced senescent RPE cells may constitute a link between
chronic inflammation and neuroretinal degeneration.
Senescence-causing inducers, such as DNA damage, protein
aggregation and increased ROS may activate the p53 and
p16INK4a pathways that initiate a senescence response
(52–56). Once initiated, senescence becomes
fully established and is irreversible. Subsequently, the senescent
cells affect the tissue microenvironment by secreting
pro-inflammatory cytokines, chemokines and proteases (12,57). It has been reported that the
clearance of p16INK4a-positive senescent cells prevents
age-related disorders and maximizes a healthy lifespan (58). Thus, the modulation of cellular
senescence, including the elimination of selected senescent cells,
the clearance of senescence inducers, such as Aβ peptide
deposition, the prevention of cellular senescence or affecting the
secretory phenotype may reduce age-related sterile chronic
inflammation in AMD.
Acknowledgements
We would like to thank the Biochemistry and
Molecular Biology Institute of Shanghai Tenth People’s Hospital and
are grateful for their technological support. This study was
supported by grants from the Outstanding Youths Program of Shanghai
Tongji University (No. 1501219084), the Science and Technology
Commission of Shanghai (No. 11JC1409900) and the National High
Technology Research and Development Program of China (863 program,
No. S2010GR0002).
References
|
1
|
Sarks SH: Drusen patterns predisposing to
geographic atrophy of the retinal pigment epithelium. Aust J
Ophthalmol. 10:91–97. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bressler NM, Silva JC, Bressler SB, Fine
SL and Green WR: Clinicopathologic correlation of drusen and
retinal pigment epithelial abnormalities in age-related macular
degeneration. 1994. Retina. 25:130–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dentchev T, Milam AH, Lee VM, Trojanowski
JQ and Dunaief JL: Amyloid-beta is found in drusen from some
age-related macular degeneration retinas, but not in drusen from
normal retinas. Mol Vis. 9:184–190. 2003.PubMed/NCBI
|
|
4
|
Johnson LV, Leitner WP, Rivest AJ, Staples
MK, Radeke MJ and Anderson DH: The Alzheimer’s A beta -peptide is
deposited at sites of complement activation in pathologic deposits
associated with aging and age-related macular degeneration. Proc
Natl Acad Sci USA. 99:11830–11835. 2002. View Article : Google Scholar
|
|
5
|
Kurji KH, Cui JZ, Lin T, et al: Microarray
analysis identifies changes in inflammatory gene expression in
response to amyloid-beta stimulation of cultured human retinal
pigment epithelial cells. Invest Ophthalmol Vis Sci. 51:1151–1163.
2010. View Article : Google Scholar
|
|
6
|
Cao L, Liu C, Wang F and Wang H: SIRT1
negatively regulates amyloid-beta-induced inflammation via the
NF-kappaB pathway. Braz J Med Biol Res. 46:659–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu RT, Gao J, Cao S, et al: Inflammatory
mediators induced by amyloid-beta in the retina and RPE in vivo:
implications for inflammasome activation in age-related macular
degeneration. Invest Ophthalmol Vis Sci. 54:2225–2237. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding JD, Johnson LV, Herrmann R, et al:
Anti-amyloid therapy protects against retinal pigmented epithelium
damage and vision loss in a model of age-related macular
degeneration. Proc Natl Acad Sci USA. 108:E279–E287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buschini E, Piras A, Nuzzi R and Vercelli
A: Age related macular degeneration and drusen: neuroinflammation
in the retina. Prog Neurobiol. 95:14–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coppé JP, Patil CK, Rodier F, et al:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar S, Millis AJ and Baglioni C:
Expression of interleukin 1-inducible genes and production of
interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA.
89:4683–4687. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campisi J: Senescent cells, tumor
suppression, and organismal aging: good citizens, bad neighbors.
Cell. 120:513–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben-Porath I and Weinberg RA: The signals
and pathways activating cellular senescence. Int J Biochem Cell
Biol. 37:961–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lombard DB, Chua KF, Mostoslavsky R,
Franco S, Gostissa M and Alt FW: DNA repair, genome stability, and
aging. Cell. 120:497–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He N, Jin WL, Lok KH, Wang Y, Yin M and
Wang ZJ: Amyloid-β(1–42) oligomer accelerates senescence in adult
hippocampal neural stem/progenitor cells via formylpeptide receptor
2. Cell Death Dis. 4:e9242013. View Article : Google Scholar
|
|
16
|
Golde TE and Miller VM:
Proteinopathy-induced neuronal senescence: a hypothesis for brain
failure in Alzheimer’s and other neurodegenerative diseases.
Alzheimers Res Ther. 1:52009. View
Article : Google Scholar
|
|
17
|
Bhat R, Crowe EP, Bitto A, et al:
Astrocyte senescence as a component of Alzheimer’s disease. PLoS
One. 7:e450692012. View Article : Google Scholar
|
|
18
|
Donnini S, Solito R, Cetti E, et al: Abeta
peptides accelerate the senescence of endothelial cells in vitro
and in vivo, impairing angiogenesis. FASEB J. 24:2385–2395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao L, Wang H, Wang F, Xu D, Liu F and Liu
C: Aβ-induced senescent retinal pigment epithelial cells create a
proinflammatory microenvironment in AMD. Invest Ophthalmol Vis Sci.
54:3738–3750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruban J, Maoui A, Chalour N, et al:
CCR2/CCL2-mediated inflammation protects photoreceptor cells from
amyloid-beta-induced apoptosis. Neurobiol Dis. 42:55–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hood DC and Birch DG: A quantitative
measure of the electrical activity of human rod photoreceptors
using electroretinography. Vis Neurosci. 5:379–387. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Penn RD and Hagins WA: Signal transmission
along retinal rods and the origin of the electroretinographic
a-wave. Nature. 223:201–204. 1969. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robson JG and Frishman LJ: Response
linearity and kinetics of the cat retina: the bipolar cell
component of the dark-adapted electroretinogram. Vis Neurosci.
12:837–850. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian N and Slaughter MM: Correlation of
dynamic responses in the ON bipolar neuron and the b-wave of the
electroretinogram. Vision Res. 35:1359–1364. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robson JG, Maeda H, Saszik SM and Frishman
LJ: In vivo studies of signaling in rod pathways of the mouse using
the electroretinogram. Vision Res. 44:3253–3268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanitzsch R and Lichtenberger T: Two
neuronal retinal components of the electroretinogram c-wave. Doc
Ophthalmol. 94:275–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Green WR and Enger C: Age-related macular
degeneration histopathologic studies. The 1992 Lorenz E Zimmerman
Lecture. Ophthalmology. 100:1519–1535. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perez SE, Lumayag S, Kovacs B, Mufson EJ
and Xu S: Beta-amyloid deposition and functional impairment in the
retina of the APPswe/PS1DeltaE9 transgenic mouse model of
Alzheimer’s disease. Invest Ophthalmol Vis Sci. 50:793–800. 2009.
View Article : Google Scholar
|
|
29
|
Bruban J, Glotin AL, Dinet V, et al:
Amyloid-beta(1–42) alters structure and function of retinal
pigmented epithelial cells. Aging Cell. 8:162–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drüeke T, Hennessen U, Nabarra B, et al:
Ultrastructural and functional abnormalities of intestinal and
renal epithelium in the SHR. Kidney Int. 37:1438–1448. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho YY, Kim DJ, Lee HS, et al: Autophagy
and cellular senescence mediated by Sox2 suppress malignancy of
cancer cells. PLoS One. 8:e571722013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Butterfield DA and Boyd-Kimball D: Amyloid
beta-peptide(1–42) contributes to the oxidative stress and
neurodegeneration found in Alzheimer disease brain. Brain Pathol.
14:426–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu AL, Fuchshofer R, Kook D, Kampik A,
Bloemendal H and Welge-Lüssen U: Subtoxic oxidative stress induces
senescence in retinal pigment epithelial cells via TGF-beta
release. Invest Ophthalmol Vis Sci. 50:926–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katz ML, Wendt KD and Sanders DN: RPE65
gene mutation prevents development of autofluorescence in retinal
pigment epithelial phagosomes. Mech Ageing Dev. 126:513–521. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rawes V, Kipling D, Kill IR and Faragher
RG: The kinetics of senescence in retinal pigmented epithelial
cells: a test for the telomere hypothesis of ageing? Biochemistry
(Mosc). 62:1291–1295. 1997.
|
|
37
|
Feeney-Burns L, Hilderbrand ES and
Eldridge S: Aging human RPE: morphometric analysis of macular,
equatorial, and peripheral cells. Invest Ophthalmol Vis Sci.
25:195–200. 1984.PubMed/NCBI
|
|
38
|
Katz ML and Robison WG Jr: Age-related
changes in the retinal pigment epithelium of pigmented rats. Exp
Eye Res. 38:137–151. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wing GL, Blanchard GC and Weiter JJ: The
topography and age relationship of lipofuscin concentration in the
retinal pigment epithelium. Invest Ophthalmol Vis Sci. 17:601–607.
1978.PubMed/NCBI
|
|
40
|
Holz FG, Bindewald-Wittich A, Fleckenstein
M, Dreyhaupt J, Scholl HP and Schmitz-Valckenberg S; FAM-Study
Group. Progression of geographic atrophy and impact of fundus
autofluorescence patterns in age-related macular degeneration. Am J
Ophthalmol. 143:463–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sparrow JR, Hicks D and Hamel CP: The
retinal pigment epithelium in health and disease. Curr Mol Med.
10:802–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Freund A, Orjalo AV, Desprez PY and
Campisi J: Inflammatory networks during cellular senescence: causes
and consequences. Trends Mol Med. 16:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsuji T, Aoshiba K and Nagai A: Alveolar
cell senescence exacerbates pulmonary inflammation in patients with
chronic obstructive pulmonary disease. Respiration. 80:59–70. 2010.
View Article : Google Scholar
|
|
44
|
Dagouassat M, Gagliolo JM, Chrusciel S, et
al: The cyclooxygenase-2-prostaglandin E2 pathway maintains
senescence of chronic obstructive pulmonary disease fibroblasts. Am
J Respir Crit Care Med. 187:703–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Acosta JC, O’Loghlen A, Banito A, et al:
Chemokine signaling via the CXCR2 receptor reinforces senescence.
Cell. 133:1006–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuilman T, Michaloglou C, Vredeveld LC, et
al: Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jonas JB, Tao Y, Neumaier M and Findeisen
P: Cytokine concentration in aqueous humour of eyes with exudative
age-related macular degeneration. Acta Ophthalmol. 90:e381–e388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hong DS, Angelo LS and Kurzrock R:
Interleukin-6 and its receptor in cancer: implications for
translational therapeutics. Cancer. 110:1911–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Parmeggiani F, Romano MR, Costagliola C,
et al: Mechanism of inflammation in age-related macular
degeneration. Mediators Inflamm. 2012:5467862012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Parmeggiani F, Sorrentino FS, Romano MR,
et al: Mechanism of inflammation in age-related macular
degeneration: an up-to-date on genetic landmarks. Mediators
Inflamm. 2013:4356072013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jeyapalan JC and Sedivy JM: Cellular
senescence and organismal aging. Mech Ageing Dev. 129:467–474.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ksiazek K, Mikula-Pietrasik J, Olijslagers
S, Jörres A, von Zglinicki T and Witowski J: Vulnerability to
oxidative stress and different patterns of senescence in human
peritoneal mesothelial cell strains. Am J Physiol Regul Integr Comp
Physiol. 296:R374–R382. 2009. View Article : Google Scholar
|
|
54
|
Weyemi U, Lagente-Chevallier O, Boufraqech
M, et al: ROS-generating NADPH oxidase NOX4 is a critical mediator
in oncogenic H-Ras-induced DNA damage and subsequent senescence.
Oncogene. 31:1117–1129. 2012. View Article : Google Scholar :
|
|
55
|
Sitte N, Merker K, Von Zglinicki T, Grune
T and Davies KJ: Protein oxidation and degradation during cellular
senescence of human BJ fibroblasts: part I - effects of
proliferative senescence. FASEB J. 14:2495–2502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar :
|
|
57
|
Campisi J and d’Adda di Fagagna F:
Cellular senescence: when bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Baker DJ, Wijshake T, Tchkonia T, et al:
Clearance of p16Ink4a-positive senescent cells delays
ageing-associated disorders. Nature. 479:232–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Timmers AM, Zhang H, Squitieri A and
Gonzalez-Pola C: Subretinal injections in rodent eyes: effects on
electrophysiology and histology of rat retina. Mol Vis. 7:131–137.
2001.PubMed/NCBI
|