Introduction
Withaferin A is a steroidal lactone purified from
the Indian medicinal plant, Withania somnifera and has been
shown to exert anticancer effects in different types of cancer
cells, such as prostate cancer (1), myeloid leukemia (2), breast cancer (3) and renal cancer (4) cells. The mechanisms responsible for
the withaferin A-mediated anticancer effects are multiple. For
example, withaferin A has been shown to induce apoptosis through
the upregulation of intracellular reactive oxygen species (ROS)
generation (5,6), prostate apoptosis response-4 (Par-4)
induction (1), p38 MAP kinase
activation (7), Akt inactivation
(8) and the upregulation of
endoplasmic reticulum (ER) stress (4). It has been reported that withaferin
A induces apoptosis in head and neck carcinoma cells and inhibits
Akt activation (9). However, the
cellular and molecular mechanisms underlying withaferin A-induced
apoptosis in head and neck carcinoma cells are not yet fully
understood.
Cyclooxygenase (COX) converts arachidonic acid into
prostaglandin (PG)G2, which is then reduced to
PGH2, a is precursor of other prostanoids. COX is
divided into 2 isoforms. COX-1 is constitutively expressed in the
majority of tissues and has physiological functions, whereas COX-2
is induced by inflammatory cytokines (10), the mutation of oncogenes (11) and tumor promoters (12). COX-2 is overexpressed in multiple
types of cancer, such as pancreatic (13), colon (14), cervical (15), renal (16) and head and neck (17) cancer. The overexpression of COX-2
has been shown to be associated with the promotion of angiogenesis,
invasion and proliferation, and the inhibition of apoptosis.
Therefore, the downregulation of COX-2 expression and activity
enhances apoptosis (18,19). Furthermore, Limami et al
(20) reported that the
attenuation of COX-2 expression and COX-2 downregulation by siRNA
enhanced apoptosis in ursolic acid-treated colorectal cancer cells.
Therefore, the upregulation of COX-2 expression by anticancer drugs
may promote resistance to apoptosis; thus, the downregulation of
COX-2 expression and activity may enhance susceptibility to
apoptosis.
In the present study, we investigated whether the
withaferin A-induced COX-2 upregulation is involved in resistance
to apoptosis in the human head and neck carcinoma cells,
AMC-HN4.
Materials and methods
Cells and materials
The human head and neck cancer cells, AMC-HN4, were
obtained from the Asan Medical Center (Seoul, Korea). The cells
were cultured in Dulbecco’s modified Eagle’s medium that contained
10% fetal bovine serum, 20 mM HEPES buffer and 100 mg/ml
gentamicin. Withaferin A was purchased from Biomol Research
Laboratories, Inc. (Plymouth Meeting, PA, USA). z-VAD-fmk (a
pan-caspase inhibitor) and NS-398 (a COX-2 specific inhibitor) were
purchased from Calbiochem (San Diego, CA, USA).
Anti-poly(ADP-ribose) polymerase (PARP) antibodies (sc-25780) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Anti-actin antibody (A5441) was obtained from Sigma (St.
Louis, MO, USA). Anti-COX-2 (Cat#160106) antibody was purchased
from Cayman Chemical Co. (Ann Arbor, MI, USA). N-acetylcysteine
(NAC), dithiothreitol (DTT) and all the other chemicals were
obtained from Sigma.
Flow cytometric analysis and cell
morphology
Approximately 1×106 cells were suspended
in 100 μl PBS, and 200 μl 95% ethanol were added while vortexing.
The cells were incubated at 4°C for 1 h, washed with PBS and
resuspended in 250 μl 1,12% sodium citrate buffer (pH 8.4) together
with 12,5 μg RNase. Incubation was carried out at 37°C for 30 min.
Cellular DNA was then stained by applying 250 μl propidium iodide
(50 μg/ml) for 30 min at room temperature. The stained cells were
analyzed by fluorescence-activated cell sorting (FACS) on a FACScan
flow cytometer (E5464; Becton-Dickinson, Franklin Lakes, NJ, USA)
for relative DNA content based on red fluorescence. Cell morphology
was analyzed using a light microscope (Zeiss Axiovert 200M; Carl
Zeiss, Göttingen, Germany).
Western blot analysis
The cells were washed with cold PBS and lysed on ice
in modified RIPA buffer (50 mM Tris-HCl, pH 7,4, 1% NP-40, 0,25%
Na-deoxycholate, 150 mM NaCl, 1 mM Na3VO4,
and 1 mM NaF) containing protease inhibitors (100 μM
phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml
pepstatin and 2 mM EDTA). The lysates were centrifuged at 13,000 ×
g for 15 min at 4°C and the supernatant fractions were collected.
Proteins were separated by SDS-PAGE and transferred onto an
immobilon-P membrane (Amersham, Uppsala, Sweden). Specific proteins
were detected using enhanced chemiluminescence.
Prostaglandin E2
(PGE2) assay
PGE2 levels in the culture medium were
assayed using an enzyme immunoassay kit following the
manufacturer’s instructions (Cayman Chemical Co.). The assay is
based on the competition between peroxidase (or alkaline
phosphate)-conjugated tracer PGE2 and PGE2 in
the medium for a limited number of PGE2-specific Abs.
The amount of remaining tracer PGE2 was determined by
the addition of substrates for peroxidase (or alkaline
phosphatase). OD values were determined at 405 (or 450) nm, as
previously described (21).
Measurement of ROS production
The intracellular accumulation of ROS was determined
using the fluorescent probe, 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA). H2DCFDA is commonly used
to measure ROS generation (22).
The AMC-HN4 cells were pre-treated with 200 μM trolox and 500 μM
MnTBAP for 30 min, and the cells were then incubated with 3 μM
withaferin A for 30 min. The cells were stained with the
fluorescent dye, H2DCFDA, for an additional 10 min.
Subsequently, the cells were trypsinized and resuspended in PBS,
and fluorescence was measured at specific time intervals with a
flow cytometer (Becton-Dickinson) or was detected using a
fluorescence microscope (Zeiss, Goettingen, Germany).
Statistical analysis
The data were analyzed using one-way ANOVA and
post-hoc comparisons (Student-Newman-Keuls test) using the
Statistical Package for Social Sciences 22.0 software (SPSS Inc.,
Chicago, IL, USA).
Results
Withaferin A induces apoptosis and COX-2
expression in the human head and neck carcinoma cells, AMC-HN4
We have previously reported that withaferin A
induces apoptosis in human renal carcinoma Caki cells (23) and human leukemia U937 cells
(8). In this study, to determine
whether withaferin A induces apoptosis, AMC-HN4 cells were treated
with the indicated concentrations of withaferin A for 24 h. FACS
analysis for the measurement of DNA content and western blot
analysis for the detection of the cleavage of PARP, a substrate of
caspase-3, were performed. Withaferin A markedly increased the
sub-G1 population and the cleavage of PARP in a dose- and
time-dependent manner (Fig. 1).
COX-2-overexpressing cancer cells are resistant to anticancer
drug-mediated apoptosis (24).
Therefore, we examined whether withaferin A increases COX-2
expression in the AMC-HN4 cells. As shown in Fig. 1, withaferin A increased COX-2
expression in a dose- and time-dependent manner.
Subsequently, we investigated whether the withaferin
A-induced expression of COX-2 is a consequence of apoptosis.
Treatment with the pan-caspase inhibitor, z-VAD-fmk (z-VAD),
markedly inhibited the withaferin A-induced increase in the sub-G1
population and the cleavage of PARP (Fig. 2). However, the withaferin
A-induced increase in the expression COX-2 was not affected by
treatment with z-VAD. Therefore, these data indicate that the
withaferin A-mediated COX-2 expression is not a consequence of
apoptosis.
Withaferin A-induced COX-2 expression has
no effect on apoptosis
Previous studies have reported that the
downregulation of COX-2 expression and the inhibition of
PGE2 production enhances anticancer drug-mediated
apoptosis (19,20,22,23,25). Therefore, we examined whether the
inhibition of PGE2 production increases withaferin
A-mediated apoptosis. NS-398, a COX-2 inhibitor, markedly blocked
the withaferin A-mediated production of PGE2 (Fig. 3A). However, the withaferin
A-induced increase in the sub-G1 population and the cleavage of
PARP were not affected by NS-398 treatment (Fig. 3B). These data indicate that the
withaferin A-induced expression of COX-2 is not associated with
apoptosis in the head and neck carcinoma cells, AMC-HN4.
Withaferin A-mediated apoptosis is
independent of ROS signaling
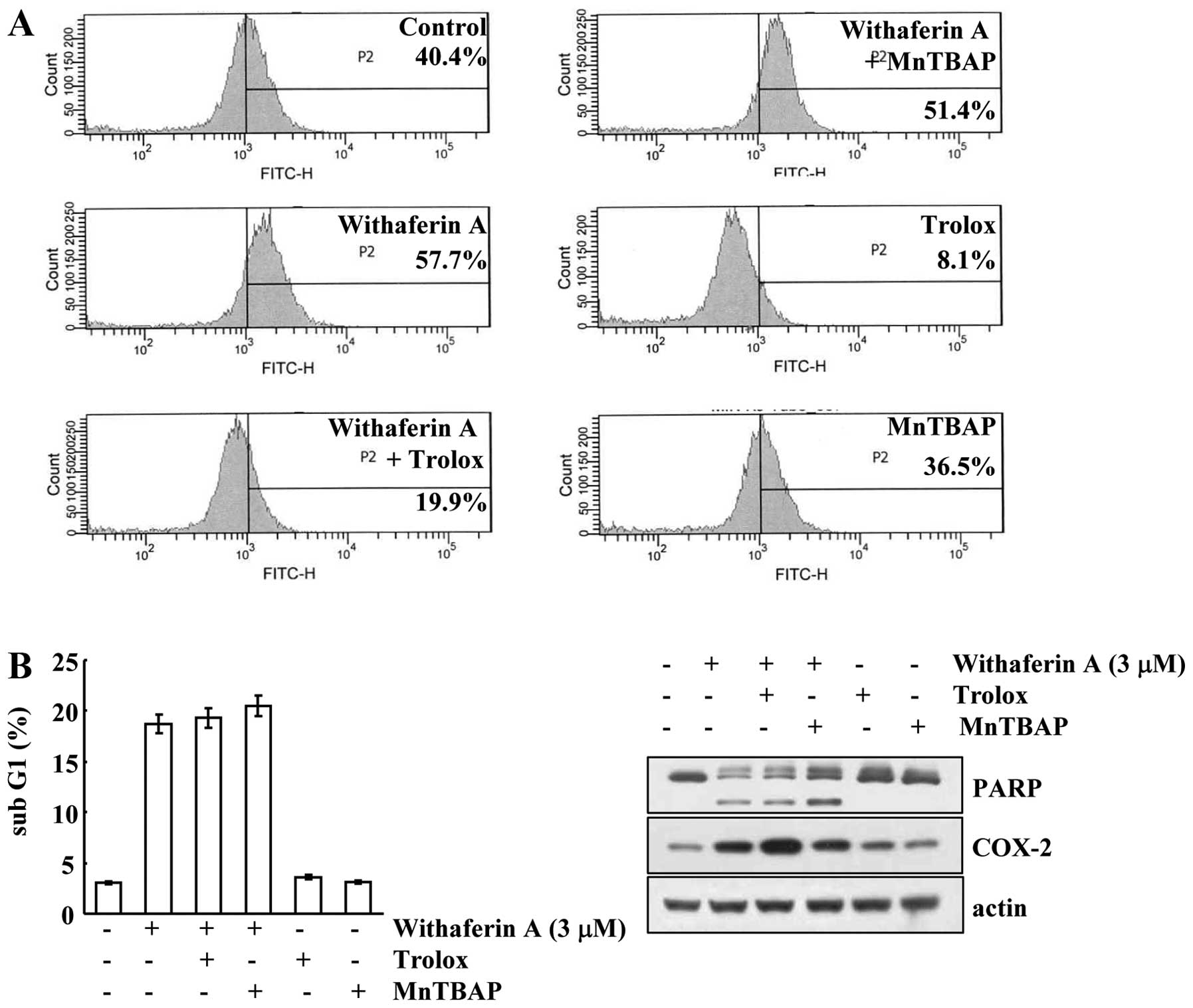
Withaferin A has been shown to increase ROS
production, and ROS is involved in apoptosis (5). Therefore, we investigated whether
withaferin A increases intracellular ROS levels in AMC-HN4 cells.
Withaferin A markedly increased intracellular ROS production
(Fig. 4A). Subsequently, we
wished to dertermine whether ROS are involved in withaferin
A-induced apoptosis. The ROS scavengers, trolox and MnTBAP,
inhibited withaferin A-mediated ROS production (Fig. 4A); however, the sub-G1 population
and the cleavage of PARP were not affected (Fig. 4B). Furthermore, the induction of
COX-2 expression by withaferin A was independent of ROS production
(Fig. 4B). These data indicate
that ROS is not associated with withaferin A-mediated
apoptosis.
Withaferin A-mediated apoptosis is
reversed by thiol donors
Previous studies have reported that excess amounts
of thiol donors block the effects of withaferin A (26,27). To determine whether thiol donors
inhibit withaferin A-induced apoptosis, AMC-HN4 cells were treated
with NAC and DTT. Both thiol donors markedly inhibited
morphological changes in the withaferin A-treated cells (Fig. 5A). Furthermore, NAC and DTT
blocked the increase in the sub-G1 population and the cleavage of
PARP (Fig. 5B). These data
suggest that the mechanism of thiol oxidation is important for
withaferin A-mediated apoptosis.
Discussion
In the present study, we demonstrated that
withaferin A induced apoptosis in the human head and neck carcinoma
cells, AMC-HN4. Withaferin A increased COX-2 expression and
PGE2 production, but PGE2 was not associated
with apoptosis. Furthermore, the upregulation of intracellular ROS
had no effect on apoptosis. Thiol donors only markedly inhibited
withaferin A-mediated apoptosis. Therefore, our results suggest
that thiol oxidation plays an important role in withaferin
A-mediated apoptosis.
COX-2 was overexpressed and the levels of PG, such
as PGE2, were increased in the cancer cells. Previous
studies have reported that COX-2 induces proliferation,
angiogenesis, migration and invasion, and inhibits apoptosis. A
selective COX-2 inhibitor (celecoxib) has been shown to induce
apoptosis in prostate carcinoma (25), colon carcinoma (28), cholangiocarcinoma (29), pancreatic carcinoma and melanoma
(30) cells. Furthermore, the
overexpression of COX-2 reduces apoptosis. Sun et al
(31) reported that COX-2
overexpression inhibits the release of cytochrome c and
caspase activation in colon carcinoma cells treated with COX-2
inhibitor and 5-fluorouracil. By contrast, the overexpression of
COX-2 in osteosarcoma cells has been shown to decrease cell
viability (32). Xu et al
(32) reported that apoptosis by
COX-2-overexpression is independent of PGE2, whereas the
inhibition of ROS production reduces apoptosis in osteosarcoma
cells. In head and neck carcinoma, celecoxib and sulindac have been
shown to reduce proliferation and induce apoptosis (33). Furthermore, the inhibition of
COX-2 enhances sensitivity to anticancer drugs, such as
doxorubicin, vincristine, cisplatin, bleomycin and 5-fluorouracil
(33). Therefore, we hypothesized
that the withaferin A-induced expression of COX-2 and
PGE2 production enhances resistance to apoptosis.
However, although NS-398 (a COX-2 inhibitor) markedly blocked
withaferin A-mediated PGE2 production, apoptosis was not
affected (Fig. 3B). Therefore,
the role of COX-2 in anticancer effects is dependent on cell type
and cell conditions.
Withaferin A exerts pro-apoptotic (4–8),
anti-proliferative (34),
anti-angiogenic (35), and
anti-invasive (36) effects
through multiple mechanisms. Among these, the upregulation of
intracellular ROS is important for withaferin A-mediated apoptosis
(37). We also detected
withaferin A-mediated ROS production in AMC-HN4 cells (Fig. 4A). ROS scavengers (trolox and
MnTBAP) reduced ROS production in the withaferin A-treated cells,
whereas the sub-G1 population and the cleavage of PARP were not
affected (Fig. 4B). Thiol
oxidation is important for the function of withaferin A. Withaferin
A inhibits IκB kinase-β activity, and DTT reverses the inhibitory
effects (27). Furthermore,
withaferin A-mediated apoptosis is reversed by DTT in
erythromyelogenous leukemia cells (26). In this study, the thiol donors,
DTT and NAC, markedly blocked withaferin A-mediated apoptosis
(Fig. 5B). Withaferin A has
α,β-unsaturated ketone moiety in the A ring, which reacts with
protein thiol nucleophiles (38).
Therefore, withaferin A may target cysteine residues of proteins,
such as kinases, phosphatases and chaperons. Therefore, further
studies are required to identify the target proteins of withaferin
A in head and neck carcinoma cells.
Taken together, our results suggest that the
withaferin A-mediated apoptosis is independent of COX-2 expression
and ROS production. Thiol oxidation is an important mechanisms of
withaferin A-induced apoptosis in head and neck carcinoma
cells.
References
|
1
|
Srinivasan S, Ranga RS, Burikhanov R, Han
SS and Chendil D: Par-4-dependent apoptosis by the dietary compound
withaferin A in prostate cancer cells. Cancer Res. 67:246–253.
2007. View Article : Google Scholar
|
|
2
|
Malik F, Kumar A, Bhushan S, et al:
Reactive oxygen species generation and mitochondrial dysfunction in
the apoptotic cell death of human myeloid leukemia HL-60 cells by a
dietary compound withaferin A with concomitant protection by
N-acetyl cysteine. Apoptosis. 12:2115–2133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stan SD, Hahm ER, Warin R and Singh SV:
Withaferin A causes FOXO3a- and Bim-dependent apoptosis and
inhibits growth of human breast cancer cells in vivo. Cancer Res.
68:7661–7669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi MJ, Park EJ, Min KJ, Park JW and Kwon
TK: Endoplasmic reticulum stress mediates withaferin A-induced
apoptosis in human renal carcinoma cells. Toxicol In Vitro.
25:692–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayola E, Gallerne C, Esposti DD, et al:
Withaferin A induces apoptosis in human melanoma cells through
generation of reactive oxygen species and down-regulation of Bcl-2.
Apoptosis. 16:1014–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hahm ER, Moura MB, Kelley EE, Van Houten
B, Shiva S and Singh SV: Withaferin A-induced apoptosis in human
breast cancer cells is mediated by reactive oxygen species. PLoS
One. 6:e233542011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandal C, Dutta A, Mallick A, Chandra S,
Misra L and Sangwan RS: Withaferin A induces apoptosis by
activating p38 Mitogen-activated protein kinase signaling cascade
in leukemic cells of lymphoid and myeloid origin through
mitochondrial death cascade. Apoptosis. 13:1450–1464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh JH, Lee TJ, Kim SH, et al: Induction of
apoptosis by withaferin A in human leukemia U937 cells through
down-regulation of Akt phosphorylation. Apoptosis. 13:1494–1504.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samadi AK, Tong X, Mukerji R, Zhang H,
Timmermann BN and Cohen MS: Withaferin A, a cytotoxic steroid from
Vassobia breviflora, induces apoptosis in human head and neck
squamous cell carcinoma. J Nat Prod. 73:1476–1481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanabe T and Tohnai N: Cyclooxygenase
isozymes and their gene structures and expression. Prostaglandins
Other Lipid Mediat. 68–69:95–114. 2002. View Article : Google Scholar
|
|
11
|
Tsuji S, Tsujii M, Kawano S and Hori M:
Cyclooxygenase-2 upregulation as a perigenetic change in
carcinogenesis. J Exp Clin Cancer Res. 20:117–129. 2001.PubMed/NCBI
|
|
12
|
Ledwith BJ, Pauley CJ, Wagner LK, Rokos
CL, Alberts DW and Manam S: Induction of cyclooxygenase-2
expression by peroxisome proliferators and non-tetradecanoylphorbol
12,13-myristate-type tumor promoters in immortalized mouse liver
cells. J Biol Chem. 272:3707–3714. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okami J, Yamamoto H, Fujiwara Y, et al:
Overexpression of cyclooxygenase-2 in carcinoma of the pancreas.
Clin Cancer Res. 5:2018–2024. 1999.PubMed/NCBI
|
|
14
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-regulation of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994.PubMed/NCBI
|
|
15
|
Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC
and Oh KS: High cyclooxygenase-2 expression in stage IB cervical
cancer with lymph node metastasis or parametrial invasion. Gynecol
Oncol. 76:320–325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hara S, Kondo Y, Matsuzawa I, et al:
Expression of cycloxygenase-2 in human bladder and renal cell
carcinoma. Adv Exp Med Biol. 507:123–126. 2002. View Article : Google Scholar
|
|
17
|
Celenk F, Bayramoglu I, Yilmaz A, Menevse
A and Bayazit Y: Expression of cyclooxygenase-2, 12-lipoxygenase,
and inducible nitric oxide synthase in head and neck squamous cell
carcinoma. J Craniofac Surg. 24:1114–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hara A, Yoshimi N, Niwa M, Ino N and Mori
H: Apoptosis induced by NS-398, a selective cyclooxygenase-2
inhibitor, in human colorectal cancer cell lines. Jpn J Cancer Res.
88:600–604. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawaoka H, Kawano S, Tsuji S, et al:
Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer
xenografts via induction of apoptosis in nude mice. Am J Physiol.
274:G1061–1067. 1998.PubMed/NCBI
|
|
20
|
Limami Y, Pinon A, Leger DY, et al: HT-29
colorectal cancer cells undergoing apoptosis overexpress COX-2 to
delay ursolic acid-induced cell death. Biochimie. 93:749–757. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang MS, Ji KA, Jeon SB, et al:
Interleukin-13 enhances cyclooxygenase-2 expression in activated
rat brain microglia: implications for death of activated microglia.
J Immunol. 177:1323–1329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
LeBel CP, Ischiropoulos H and Bondy SC:
Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of
reactive oxygen species formation and oxidative stress. Chem Res
Toxicol. 5:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Qu L and Tao H: Cyclo-oxygenase 2
up-regulates the effect of multidrug resistance. Cell Biol Int.
34:21–25. 2009.PubMed/NCBI
|
|
25
|
Hsu AL, Ching TT, Wang DS, Song X,
Rangnekar VM and Chen CS: The cyclooxygenase-2 inhibitor celecoxib
induces apoptosis by blocking Akt activation in human prostate
cancer cells independently of Bcl-2. J Biol Chem. 275:11397–11403.
2000. View Article : Google Scholar
|
|
26
|
Suttana W, Mankhetkorn S, Poompimon W, et
al: Differential chemosensitization of P-glycoprotein
overexpressing K562/Adr cells by withaferin A and Siamois
polyphenols. Mol Cancer. 9:992010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaileh M, Vanden Berghe W, Heyerick A, et
al: Withaferin A strongly elicits IkappaB kinase beta
hyperphosphorylation concomitant with potent inhibition of its
kinase activity. J Biol Chem. 282:4253–4264. 2007. View Article : Google Scholar
|
|
28
|
Arico S, Pattingre S, Bauvy C, et al:
Celecoxib induces apoptosis by inhibiting
3-phosphoinositide-dependent protein kinase-1 activity in the human
colon cancer HT-29 cell line. J Biol Chem. 277:27613–27621. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu GS, Zou SQ, Liu ZR, Tang ZH and Wang
JH: Celecoxib inhibits proliferation and induces apoptosis via
prostaglandin E2 pathway in human cholangiocarcinoma cell lines.
World J Gastroenterol. 9:1302–1306. 2003.PubMed/NCBI
|
|
30
|
Wu G, Yi J, Di F, Zou S and Li X:
Celecoxib inhibits proliferation and induces apoptosis via
cyclooxygenase-2 pathway in human pancreatic carcinoma cells. J
Huazhong Univ Sci Technolog Med Sci. 25:42–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Tang XM, Half E, Kuo MT and
Sinicrope FA: Cyclooxygenase-2 overexpression reduces apoptotic
susceptibility by inhibiting the cytochrome c-dependent apoptotic
pathway in human colon cancer cells. Cancer Res. 62:6323–6328.
2002.PubMed/NCBI
|
|
32
|
Xu Z, Choudhary S, Voznesensky O, et al:
Overexpression of COX-2 in human osteosarcoma cells decreases
proliferation and increases apoptosis. Cancer Res. 66:6657–6664.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashitani S, Urade M, Nishimura N, et al:
Apoptosis induction and enhancement of cytotoxicity of anticancer
drugs by celecoxib, a selective cyclooxygenase-2 inhibitor, in
human head and neck carcinoma cell lines. Int J Oncol. 23:665–672.
2003.PubMed/NCBI
|
|
34
|
Cai Y, Sheng ZY, Chen Y and Bai C: Effect
of Withaferin A on A549 cellular proliferation and apoptosis in
non-small cell lung cancer. Asian Pac J Cancer Prev. 15:1711–1714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohan R, Hammers HJ, Bargagna-Mohan P, et
al: Withaferin A is a potent inhibitor of angiogenesis.
Angiogenesis. 7:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thaiparambil JT, Bender L, Ganesh T, et
al: Withaferin A inhibits breast cancer invasion and metastasis at
sub-cytotoxic doses by inducing vimentin disassembly and serine 56
phosphorylation. Int J Cancer. 129:2744–2755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sen N, Banerjee B, Das BB, et al:
Apoptosis is induced in leishmanial cells by a novel protein kinase
inhibitor withaferin A and is facilitated by apoptotic
topoisomerase I-DNA complex. Cell Death Differ. 14:358–367. 2007.
View Article : Google Scholar
|
|
38
|
Fuska J, Fuskova A, Rosazza JP and
Nicholas AW: Novel cytotoxic and antitumor agents. IV Withaferin A:
relation of its structure to the in vitro cytotoxic effects on P388
cells. Neoplasma. 31:31–36. 1984.
|