Introduction
Puerarin
[7-hydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one
8-(β-D-glucopyranoside)] is a major isoflavone compound isolated
from Pueraria lobata (Willd.), and has a variety of
biological functions in cardiovascular diseases, gynecological
diseases, osteoporosis, cognitive capabilities and diabetic
nephropathy (1–3). A number of studies have demonstrated
that puerarin possesses numerous activities, including antioxidant
activity (4–7), anti-inflammatory (8–11)
and anti-apoptotic activities (12–16). Therefore, studies have
demonstrated that puerarin markedly reduces the levels of total
cholesterol (TC) in serum and liver (5,17).
Puerarin has also been shown to exert protective effects against
liver injury (1,7). However, the effects of puerarin on
liver injury induced by non-alcoholic fatty liver disease (NAFLD)
using oleic acid (OA)-treated hepatoma cells, namely its regulatory
effects on the levels of intracellular lipids and the potential
mechanisms involved have yet to be examined.
NAFLD is characterized by the hepatic manifestation
of metabolic syndrome and is the leading cause of chronic liver
disease in the absence of significant ethanol consumption. It is a
complex metabolic condition in which both lifestyle and genetic
factors play a pathogenic role and has been increasingly recognized
as a major cause of liver-related morbidity and mortality (18,19). Although the pathogenesis of
non-alcoholic steatohepatitis is not yet well understood, a
‘two-hit’ theory has been proposed. According to this theory,
hepatic steatosis is mainly caused by metabolic syndrome, the first
hit (20). Subsequently, hepatic
steatosis develops into non-alcoholic steatohepatitis due to the
effects of oxidative stress, reactive oxygen species, lipid
peroxidation and/or any cytokine, the second hit (20). In particular, NAFLD is
characterized by the accumulation of hepatic triglycerides (TG),
which result from an imbalance between the uptake, export,
synthesis and oxidation of fatty acids (1). It is considered that increased free
fatty acids (FFA) supplied to the liver play a major role in the
early stages of NAFLD (21).
Sterol regulatory element binding protein-1 (SREBP)-1c plays an
essential role in the regulation of lipogenesis involved in fatty
acid and TG synthesis, which result from the regulation of lipid
metabolism by SREBPs (22–24).
In addition, peroxisome proliferator-activated receptors (PPARs),
ligand-activated nuclear receptors, have been shown to mediate the
critical transcriptional regulation of genes associated with lipid
homeostasis and are abundantly expressed in the liver, diminishing
circulating TG and increasing high-density lipoprotein levels
(25).
The adenosine 5′-monophosphate (AMP)-activated
protein kinase (AMPK) plays a key role in energy homeostasis and
acts to simultaneously shut down ATP-consuming biosynthetic
processes and to facilitate ATP-producing catabolic processes
during periods of metabolic stress, leading to rapid changes in the
control of fatty acid metabolism (26). The AMPK stimulation of fatty acid
metabolism occurs as a result of AMPK phosphorylation (26). AMPK may play a key role in
regulating the activation of SREBP-1 and lipogenesis (27). The process of hepatic stellate
cell activation is accompanied by the depletion of intracellular
lipid droplets, the loss of lipid storage capacity and the
suppression of the expression of transcription factors, including
SREBP-1 and fatty acid synthase (FAS) (28,29).
In this study, we investigated the lipid-lowering
effects of puerarin using a human cellular model of steatosis
induced by OA. We determined the effects of puerarin on OA-treated
HepG2 cells and found that the activation of the AMPK pathway was
one of the underlying mechanisms responsible for its effects. These
effects also involved the regulation of the expression of genes
associated with lipid accumulation, the inhibition of hepatic
lipogenesis and the enhancement of hepatic antioxidant
activity.
Materials and methods
Materials
The puerarin used in this study was purchased from
Sigma-Aldrich (St. Louis, MO, USA) (Fig. 1). The Oil Red O stain and OA were
also purchased from Sigma-Aldrich. 3-(4,5-Dimethyl
thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) was purchased from Promega (Madison, WI, USA). Anti-β-actin
(sc-47778), PPARα (sc-130640), SREBP (sc-366) and FAS (sc-55580)
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Anti-pThr172-AMPK (#5759) and anti-AMPK (#2795)
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA).
Cell culture
HepG2 human hepatoma cells were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Cell
culture was carried out as previously described (30). Namely, cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 μg/ml penicillin, 100
μg/ml streptomycin and 2 mM L-glutamine (Thermo Scientific
HyClone, Logan, UT, USA). The cells were cultured at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Cytotoxicity assay
Cell viability was examined by MTS assay. Briefly,
HepG2 cells were seeded at a density of 1×104 cells/ml
in 96-well plates (Nunc A/S, Roskilde, Denmark). To determine the
non-toxic concentration for the cells, puerarin (10, 25, 50, 100
and 200 μM) was then added to each well. The plates were
then incubated for 24 h at 37°C under 5% CO2. MTS
solution (5 mg/ml) was added to each well and the cells were
cultured for a further 2 h, after which the optical density was
read at 490 nm using a plate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Cytotoxicity was then calculated using the
following formula: 1 - (mean absorbance value of treated cells/mean
absorbance value of untreated cells).
Oil Red O staining
Oil Red O staining was carried out as previously
described (30). Briefly, for the
examination of fat accumulation in the HepG2 cells, the cells were
treated for 24 h with puerarin. The cells were rinsed with cold
phosphate-buffered saline (PBS) and fixed in 10% paraformaldehyde
for 30 min. After the cells were washed with 60% isopropanol, they
were stained for at least 1 h in a freshly diluted Oil Red O
solution (6 parts Oil Red O stock solution and 4 parts
H2O; Oil Red O stock solution is 0.5% Oil Red O in
isopropanol). After the stain was removed and the cells were washed
with 60% isopropanol, images of each group of cells were acquired
(Nikon Imaging Korea, Seoul, Korea). The stained lipid droplets
were then extracted with isopropanol for quantification by
measuring the absorbance at 490 nm (BioTek Instruments, Inc.).
Measurement of lipid levels
The levels of TG and TC in the HepG2 cells were
quantified using a relevant kit (BioVision, Mountain View, CA, USA)
as per the manufacturer’s instructions.
Western blot analysis
Protein expression was assessed by western blot
analysis according to standard procedures. The HepG2 cells were
cultured in a 6-well plate (5×105 cells/ml) and
pre-treated with various concentrations of puerarin (25, 50 and 100
μM). After 1 h, the cells were treated with OA (0.5 mM) and
then incubated at 37°C. After 24 h of incubation, the cells were
washed twice in PBS. The cell pellets were resuspended in lysis
buffer on ice for 20 min, and the cell debris was removed by
centrifugation (13,000 rpm, 10 min, 4°C). The protein
concentrations were determined using the Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Hercules, CA, USA) according to the
manufacturer’s instructions. Equal amounts of protein (20
μg) were subjected to sodium dodecyl sulfate polyacrylamide
gel (SDS-PAGE) electrophoresis and then transferred onto a
polyvinylidene membrane (Millipore, Bedford, MA, USA). The membrane
was blocked with 5% non-fat milk in Tris-buffered saline with
Tween-20 buffer (150 mM NaCl, 20 mM Tris-HCl and 0.05% Tween-20, pH
7.4). After blocking, the membrane was incubated with primary
antibodies for 18 h. Antibodies against AMPK and phospho-AMPK were
purchased from Cell Signaling Technology, and FAS, SREBP-1c, PPARα
and β-actin antibodies were obtained from Santa Cruz Biotechnology,
Inc. The membrane was then washed with Tris-buffered saline with
Tween-20 and incubated with anti-mouse or anti-rabbit
immunoglobulin G horseradish peroxidase-conjugated secondary
antibodies. The proteins were then supplemented with ECL prime
Western blotting detection reagents and the ImageQuant LAS 4000
Mini Biomolecular Imager (both from GE Healthcare, Cleveland, OH,
USA) was used for evaluating the bands.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using an easy-BLUE total RNA
extraction kit according to the manufacturer’s instructions (iNtRON
Biotech, Seoul, Korea). Single-strand cDNA synthesis was performed
as previously described using 5 μg of RNA (30), oligo(dT)15 primers and reverse
transcriptase in a total volume of 50 μl. PCR reactions were
performed in a total volume of 20 μl comprising 2 μl
of cDNA product, 0.2 mM of each dNTP, 20 pmol of each primer and
0.8 units of Taq polymerase. The primer sequences for
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), SREBP-1, FAS and
PPARα are presented in Table I.
The PCR products increased as the concentration of the RNA
increased. Finally, the products were electrophoresed on a 2.0%
agarose gel and visualized by staining with ethidium bromide.
 | Table ISequences of oligonucleotide primers
designed for PCR. |
Table I
Sequences of oligonucleotide primers
designed for PCR.
| cDNA | Primer
sequences |
|---|
| hSREBP-1 | F:
5′-GTGGCGGCTGCATTGAGAGTGAG-3′ |
| R:
5′-AGGTACCCGAGGGCATCCGAGAAT-3′ |
| hFAS | F:
5′-CAAGAACTGCACGGAGGTGT-3′ |
| R:
5′-AGCTGCCAGAGTCGGAGAAC-3′ |
| hPPARα | F:
5′-CCTCTCAGGAAAGGCCAGTA-3′ |
| R:
5′-TCCACAGCAAATGATAGCAG-3′ |
| hGAPDH | F:
5′-TCCACCACCCTGTTGCTGTAAG-3′ |
| R:
5′-GTACCCGAGGGCATCCGAGAAT-3′ |
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance (ANOVA) followed by Dunnett’s t-test for
multiple comparisons. The data from the experiments are presented
as the means ± standard error of the mean (SEM). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of OA and puerarin in HepG2
cells
To evaluate the effects of OA and puerarin on the
viability of human HepG2 cells, an MTS assay was conducted. Due to
the fact that OA can induce cell apoptosis and reduce cell
viability when the concentrations of OA are over 1 mM, the effects
of OA on the intracellular lipid accumulation of HepG2 cells were
measured at a concentration range below 500 μM (data not
shown). Our data indicated that concentrations of 10, 25, 50 and
100 μM puerarin were not cytotoxic to the HepG2 cells.
However, the cytotoxicity of puerarin was evaluated to be at a high
concentration of 200 μM. Thus, we used OA (500 μM)
and pre-treatment with puerarin (25, 50 and 100 μM) to
investigate the effects on the viability of the HepG2 cells
(Fig. 2).
Effects of puerarin on intracellular
lipid accumulation in HepG2 cells
To verify the inhibition of OA-induced lipid
accumulation by puerarin, the HepG2 cells were treated with the
indicated concentrations of puerarin in the presence of OA for 24
h. The cells were then stained with Oil Red O and quantified by
measuring the absorbance at 490 nm. Pre-treatment with puerarin
weakened the intensity of Oil Red O staining induced by OA in a
dose-dependent manner (Fig. 3).
Thus, puerarin inhibits OA-induced lipid accumulation.
Effects of puerarin on TG and TC levels
in HepG2 cells
To further analyze the effects of puerarin on
OA-induced lipid accumulation, we measured the TG and TC levels in
the HepG2 cells. Pre-treatment with 25, 50 and 100 μM of
puerarin resulted in a 38, 40 and 82% decrease in TG levels,
respectively (Fig. 4). The TC
levels showed a 16, 19 and 34% decrease following pre-treatment
with 25, 50 and 100 μM puerarin, respectively in a
dose-dependent manner.
Effects of puerarin on the expression of
factors assiocated with hepatic lipid accumulation
To determine the mechanisms through which puerarin
reduces hepatic lipid accumulation, RT-PCR and western blot
analysis were performed to evaluate the expression of genes
associated with lipid metabolism. The expression levels of genes
involved in lipogenesis (SREBP-1 and FAS) significantly increased
in the OA-treated HepG2 cells both at the mRNA and protein level
(Fig. 5). Pre-treatment with
puerarin decreased the expression levels of SREBP-1 and FAS
(Fig. 5). These results suggest
that puerarin reduces hepatic lipid accumulation.
Effects of puerarin on the mRNA and
protein expression of PPARα
To evaluate the mechanisms through which puerarin
increases fatty acid β-oxidation, RT-PCR and western blot analysis
were performed. The expression levels of genes involved in fatty
acid β-oxidation (PPARα) significantly decreased in the OA-treated
HepG2 cells both at the mRNA and protein level. Pre-treatment with
puerarin increased the expression level of PPARα at the mRNA and
protein level (Fig. 6).
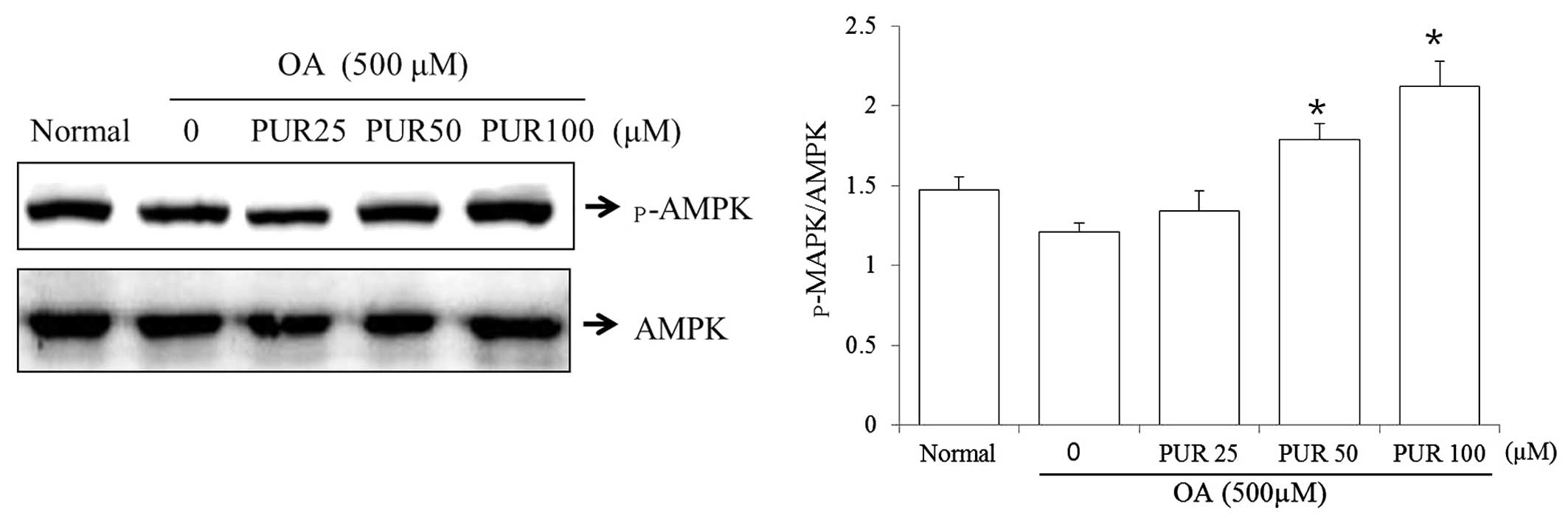
Effects of puerarin on AMPK activity in
HepG2 cells
AMPK is a key regulator of fatty acid oxidation and
lipogenesis in metabolic tissues. An alteration in AMPK activity in
HepG2 cells is strongly associated with intracellular lipid
metabolism (30). Thus, to
investigate the effects of puerarin on the phosphorylation of AMPK,
the HepG2 cells were treated with OA (500 μM), and incubated
with puerarin (25, 50 and 100 μM) for 24 h. Puerarin (50 and
100 μM) induced AMPK threonine 172 phosphorylation (Fig. 7).
Discussion
In the present study, we investigated the effects of
puerarin on hepatic lipid accumulation and metabolism, as well as
its possible mechanisms of action using HepG2 cells. Previous
studies have demonstrated that puerarin inhibits the oxidative
stress induced by acute alcoholism and regulates the signal
transduction of Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) in rats fed a high-fat diet
(7,10). However, the mechanisms of action
of puerarin and its effects on OA-treated human hepatoma cells are
not yet fully understood, although treatment with puerarin has been
shown to decrease nuclear factor (NF)-κB levels and prevent fatty
acid accumulation in the liver (23,31). In this study, we aimed to
investigate the effects of puerarin on lipid accumulation and
metabolism, and to elucidate the possible mechanisms involved.
Hepatic steatosis and hyperlipidemia are associated with the
expression of lipogenic enzymes, cholesterol biosynthesis, TG
biosynthesis and fatty acid β-oxidation in HepG2 cells. HepG2 cells
are derived from a human hepatoblastoma that is free of any known
hepatotropic virus and is thus very useful for the rapid screening
and assessment of the lipid metabolism potential of various agents.
OA, the richest source of fatty acid in olive oil, is a
monounsaturated fatty acid (MUFA, OA; 18:1n-9) (32). It has been demonstrated that diets
rich in monounsaturated fatty acids have anti-inflammatory effects
(33). Evidence indicates that
monounsaturated fatty acids, such as oleate may enhance the
recovery of antioxidant enzymes in the liver (34).
In this study, HepG2 cells were used to detect lipid
accumulation following exposure to OA. We investigated the effects
of puerarin on lipid homeostasis. We also examined cytotoxicity and
the viability of cells treated with various concentrations of
puerarin by MTS assay (Fig. 2).
We first analyzed the development of lipid accumulation in an in
vitro model of hepatic steatosis. Treatment with OA alone
induced a significant increase in lipid accumulation and treatment
with puerarin decreased the lipid accumulation (Fig. 3). In addition, the TG and TC
levels were reduced following treatment with 25, 50 and 100
μM of puerarin in a dose-dependent manner (Fig. 4). Lipid accumulation may be caused
by enhanced de novo lipogenesis, the lowering of lipid
catabolism and the activation of lipid uptake in the liver. FAS is
a key enzyme involved in de novo fatty acid and TG synthesis
in mammals. SREBP-1 is well known as a transcription factor
regulating the expression of these lipogenic enzymes in the liver
(23). Our results revealed that
the expression of levels of genes involved in lipogenesis (SREBP-1
and FAS) increased and those of genes involved in fatty acid
β-oxidation (PPARα) decreased in the OA-treated HepG2 cells at the
mRNA and protein level (Figs. 5
and 6). Puerarin decreased the
expression of genes and proteins involved in lipogenesis (SREBP-1
and FAS) (Fig. 5). As shown in
Fig. 6, puerarin increased the
mRNA and protein expression of PPARα. These results suggest that
puerarin reduces hepatic lipid accumulation in through 2
mechanisms: the downregulation of lipogenic proteins and the
upregulation of proteins involved in fatty acid β-oxidation.
AMPK is a central metabolic sensor and plays an
important role in regulating glucose, lipid and cholesterol
metabolism. Since AMPK is an important enzyme in maintaining
cellular energy homeostasis, a dysfunction in the AMPK signaling
pathway may result in metabolic disorders. It has been demonstrated
that the activation of AMPK effectively suppresses the expression
of SREBP-1 in the liver (27).
However, the association between AMPK and the proliferation of
hepatocellular carcinoma (HCC) cells is unknown. In this study,
treatment with puerarin increased AMPK phosphorylation (Fig. 7). It has bee demonstrated that
AMPK plays a key role in regulating carbohydrate and fat
metabolism, serving as a metabolic master switch response to
alterations in cellular energy charge (35). AMPK activation has been validated
as a therapeutic strategy for liver steatosis (36). In previous studies, it has been
shown that polyphenols derived from plants can suppress FAS and
activate AMPK expression, thus preventing the translocation of
SREBP-1 to the nucleus (37–39). In this study, we found puerarin
has the same ability to activate AMPK and then reduce SREBP-1
expression, finally leading to the inhibition of hepatic
lipogenesis. In accordance with these results, we confirmed that
puerarin decreased lipid synthesis and increased fatty-acid
oxidation by increasing p-AMPK and PPARα expression, further
inhibiting the protein expression of SREBP-1 and leading to the
reduction in the transcriptional activity of FAS. Thus, it can be
concluded that puerarin suppresses lipid accumulation in the liver
and may be used in the development of novel therapeutic strategies
to reduce the formation of a fatty liver.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (NRF-2013R1A1A2064673),
the Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education
(2013060380), the National Research Foundation of Korea (NRF) grant
funded by the Korea government (MSIP) (2008-0062484), and by the
Ministry Of Trade, Industry and Energy (MOTIE) and the Korea
Institute for Advancement of Technology (KIAT) through the Inter-ER
Cooperation Projects (R0002020).
References
|
1
|
Zhang S, Ji G and Liu J: Reversal of
chemical-induced liver fibrosis in Wistar rats by puerarin. J Nutr
Biochem. 17:485–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeung DK, Leung SW, Xu YC, Vanhoutte PM
and Man RY: Puerarin, an isoflavonoid derived from Radix puerariae,
potentiates endothelium-independent relaxation via the cyclic AMP
pathway in porcine coronary artery. Eur J Pharmacol. 552:105–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Xiao Y, Gong H, Shen D, Zhu F, Wu Q,
Chen H and Zhong H: Effect of puerarin on the expression of
extracellular matrix in rats with streptozotocin-induced diabetic
nephropathy. Natl Med J India. 22:9–12. 2009.PubMed/NCBI
|
|
4
|
Guerra MC, Speroni E, Broccoli M, Cangini
M, Pasini P, Minghett A, Crespi-Perellino N, Mirasoli M,
Cantelli-Forti G and Paolini M: Comparison between Chinese medical
herb Pueraria lobata crude extract and its main isoflavone puerarin
antioxidant properties and effects on rat liver CYP-catalysed drug
metabolism. Life Sci. 67:2997–3006. 2000. View Article : Google Scholar
|
|
5
|
Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ
and Xu HX: Puerarin decreases serum total cholesterol and enhances
thoracic aorta endothelial nitric oxide synthase expression in
diet-induced hypercholesterolemic rats. Life Sci. 79:324–330. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han RM, Tian YX, Becker EM, Andersen ML,
Zhang JP and Skibsted LH: Puerarin and conjugate bases as radical
scavengers and antioxidants: molecular mechanism and synergism with
beta-carotene. J Agric Food Chem. 55:2384–2391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao M, Du YQ, Yuan L and Wang NN:
Protective effect of puerarin on acute alcoholic liver injury. Am J
Chin Med. 38:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Hu W, Zhang Q, Wang Y and Sun L:
Puerarin inhibits C-reactive protein expression via suppression of
nuclear factor kappaB activation in lipopolysaccharide-induced
peripheral blood mononuclear cells of patients with stable angina
pectoris. Basic Clin Pharmacol Toxicol. 107:637–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KM, Jung DH, Jang DS, Kim YS, Kim JM,
Kim HN, Surh YJ and Kim JS: Puerarin suppresses AGEs-induced
inflammation in mouse mesangial cells: a possible pathway through
the induction of heme oxygenase-1 expression. Toxicol Appl
Pharmacol. 244:106–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng P, Ji G, Ma Z, Liu T, Xin L, Wu H,
Liang X and Liu J: Therapeutic effect of puerarin on non-alcoholic
rat fatty liver by improving leptin signal transduction through
JAK2/STAT3 pathways. Am J Chin Med. 37:69–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh AK, Jiang Y, Benlhabib E and Gupta
S: Herbal mixtures consisting of puerarin and either
polyenylphosphatidylcholine or curcumin provide comprehensive
protection against alcohol-related disorders in P rats receiving
free choice water and 15% ethanol in pure water. J Med Food.
10:526–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong FL, Sun XH, Gan L, Yang XL and Xu
HB: Puerarin protects rat pancreatic islets from damage by hydrogen
peroxide. Eur J Pharmacol. 529:1–7. 2006. View Article : Google Scholar
|
|
13
|
Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A,
Wang N, Fang N, Wang XC, Xiao XQ, Chen ZW and Li QL: Involvement of
ubiquitin proteasome system in protective mechanisms of puerarin to
MPP(+)-elicited apoptosis. Neurosci Res. 63:52–58. 2009. View Article : Google Scholar
|
|
14
|
Bo J, Ming BY, Gang LZ, Lei C and Jia AL:
Protection by puerarin against MPP+-induced
neurotoxicity in PC12 cells mediated by inhibiting mitochondrial
dysfunction and caspase-3-like activation. Neurosci Res.
53:183–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X and Zheng X: Potential involvement of
calcium and nitric oxide in protective effects of puerarin on
oxygen-glucose deprivation in cultured hippocampal neurons. J
Ethnopharmacol. 113:421–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mercer LD, Kelly BL, Horne MK and Beart
PM: Dietary polyphenols protect dopamine neurons from oxidative
insults and apoptosis: investigations in primary rat mesencephalic
cultures. Biochem Pharmacol. 69:339–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung MJ, Sung NJ, Park CS, Kweon DK,
Mantovani A, Moon TW, Lee SJ and Park KH: Antioxidative and
hypocholesterolemic activities of water-soluble puerarin glycosides
in HepG2 cells and in C57 BL/6J mice. Eur J Pharmacol. 578:159–170.
2008. View Article : Google Scholar
|
|
18
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
American Gastroenterology Association:
American Gastroenterological Association medical position
statement: nonalcoholic fatty liver disease. Gastroenterology.
123:1702–1704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koteish A and Diehl AM: Animal models of
steatosis. Semin Liver Dis. 21:89–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satia-Abouta J, Patterson RE, Schiller RN
and Kristal AR: Energy from fat is associated with obesity in U.S.
men: results from the Prostate Cancer Prevention Trial. Prev Med.
34:493–501. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimano H, Yahagi N, Amemiya-Kudo M, Hasty
AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K,
Gotoda T, Ishibashi S and Yamada N: Sterol regulatory
element-binding protein-1 as a key transcription factor for
nutritional induction of lipogenic enzyme genes. J Biol Chem.
274:35832–35839. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aggarwal BB: Targeting
inflammation-induced obesity and metabolic diseases by curcumin and
other nutraceuticals. Annu Rev Nutr. 30:173–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown MS and Goldstein JL: The SREBP
pathway:regulation of cholesterol metabolism by proteolysis of a
membrane-bound transcription factor. Cell. 89:331–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berger JP, Akiyama TE and Meinke PT:
PPARs: therapeutic targets for metabolic disease. Trends Pharmacol
Sci. 26:244–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N,
Hirshman MF, Goodyear LJ and Moller DE: Role of AMP-activated
protein kinase in mechanism of metformin action. J Clin Invest.
108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
You M, Matsumoto M, Pacold CM, Cho WK and
Crabb DW: The role of AMP-activated protein kinase in the action of
ethanol in the liver. Gastroenterology. 127:1798–1808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukamoto H, She H, Hazra S, Cheng J and
Miyahara T: Anti-adipogenic regulation underlies hepatic stellate
cell trans-differentiation. J Gastroenterol Hepatol. 21(Suppl 3):
S102–S105. 2006. View Article : Google Scholar
|
|
30
|
Kang OH, Kim SB, Seo YS, Joung DK, Mun SH,
Choi JG, Lee YM, Kang DG, Lee HS and Kwon DY: Curcumin decreases
oleic acid-induced lipid accumulation via AMPK phosphorylation in
hepatocarcinoma cells. Eur Rev Med Pharmacol Sci. 17:2578–2586.
2013.PubMed/NCBI
|
|
31
|
Shao W, Yu Z, Chiang Y, Yang Y, Chai T,
Foltz W, Lu H, Fantus IG and Jin T: Puerarin prevents high fat diet
induced insulin resistance and obesity via attenuating lipogenesis
in liver and inflammatory pathway in adipocytes. PLoS One.
7:e287842012. View Article : Google Scholar
|
|
32
|
Roche H, Zampelas A, Knapper JM, Webb D,
Brooks C, Jackson KG, Wright JW, Gould BJ, Kafatos A, Gibney MJ and
Williams CM: Effect of long-term olive oil dietary intervention on
postprandial triacylglycerol and factor VII metabolism. Am J Clin
Nutr. 68:552–560. 1998.PubMed/NCBI
|
|
33
|
Moreno JJ and Mitjavila MT: The degree of
unsaturation of dietary fatty acids and the development of
atherosclerosis (review). J Nutr Biochem. 14:182–189. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirimlioglu V, Kirimlioglu H, Yilmaz S,
Ozgor D, Coban S, Karadag N and Yologlu S: Effect of fish oil,
olive oil, and vitamin E on liver pathology, cell proliferation,
and antioxidant defense system in rats subjected to partial
hepatectomy. Transplant Proc. 38:564–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Winder WW and Hardie DG: AMP-activated
protein kinase, a metabolic master switch: possible roles in type 2
diabetes. Am J Physiol. 277:E1–E10. 1999.PubMed/NCBI
|
|
36
|
Brooks SC III, Brooks JS, Lee WH, Lee MG
and Kim SG: Therapeutic potential of dithiolethiones for hepatic
diseases. Pharmacol Ther. 124:31–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang JT, Park IJ, Shin JI, Lee YK, Lee
SK, Baik HW, Ha J and Park OJ: Genistein, EGCG, and capsaicin
inhibit adipocyte differentiation process via activating
AMP-activated protein kinase. Biochem Biophys Res Commun.
338:694–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Auger C, Teissedre PL, Gerain P, Lequeux
N, Bornet A, Serisier S, Besançon P, Caporiccio B, Cristol JP and
Rouanet JM: Dietary wine phenolics catechin, quercetin, and
resveratrol efficiently protect hypercholesterolemic hamsters
against aortic fatty streak accumulation. J Agric Food Chem.
53:2015–2021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weng MS, Ho CT, Ho YS and Lin JK:
Theanaphthoquinone inhibits fatty acid synthase expression in
EGF-stimulated human breast cancer cells via the regulation of
EGFR/ErbB-2 signaling. Toxicol Appl Pharmacol. 218:107–118. 2007.
View Article : Google Scholar
|