Introduction
Postoperative pain is a common clinical problem
encountered in patients undergoing thoracotomy, inguinal hernia
repair, living donor nephrectomy, amputation, gynecological
surgery, and gastro-intestinal surgery (1). Postoperative pain can be resolved
after an initial phase or transit into chronic postoperative pain
that seriously affects quality of life and productivity of
patients, including a number of negative effects on mood, daily
activities, sleep, cognitive function, and social life (2,3).
Thus, there is a need for therapeutic approaches to prevent chronic
postoperative pain.
Mounting evidence has demonstrated that adenosine
triphosphate-sensitive potassium (KATP) channels regulate
nociception. For example, Zoga et al (4) reported that KATP channel subunits
SUR1, SUR2 and Kir6.2, but not Kir6.1, were expressed in rat dorsal
root ganglion (DRG) neurons, peripheral nerve fibers, glial
satellite and Schwann cells. KATP channels were downregulated in
DRG neurons and Schwann cells following painful axotomy, suggesting
that loss of KATP currents in the DRG neurons may contribute to
neuropathic pain (4). Wu et
al (5) found that the KATP
channel subunits SUR1, SUR2 and Kir6.1 but not Kir6.2 were normally
expressed in the spinal cord and significantly downregulated after
nerve injury. Furthermore, nerve injury-induced downregulation of
the KATP channels in the spinal cord may interrupt the astroglial
gap junctional function and contribute to neuropathic pain. The
KATP channels opener cromakalim may reduce neuropathic pain,
probably partly by regulating the astroglial gap junctions
(5). Xia et al
demonstrated that the expression level of KATP channel subunit
Kir6.2 in the spinal cord was reduced in bone cancer pain.
Activation of KATP channels by the KATP channels opener pinacidil
(Pina) at the spinal level reduced pain hypersensitivity associated
with bone cancer pain (6). The
abovementioned studies suggested that the expression pattern of
KATP channel subunits in the spinal cord remains controversial and
the role of KATP channels in regulating spinal nociceptive
transmission remains to be elucidated.
In this study, we aimed to investigate alterations
of the protein expression for KATP channel subunits in the spinal
cord after skin/muscle incision and retraction (SMIR), a new model
that accurately reflects the clinical scenario of postoperative
pain (7). In addition, we
assessed the association between KATP channels and the chemokine
monocyte chemoattractant protein-1 (MCP-1) as recent findings
showed that MCP-1 is also activated in the spinal cord and
contributes to the development of inflammatory and neuropathic pain
hypersensitivity (8,9).
Materials and methods
Animals and grouping
Adult male Sprague-Dawley rats (200–250 g) were
purchased from the Experimental Animal Center of Nantong University
and kept in the animal housing facility with controlled room
temperature (23±1°C) and unlimited access to food and water. The
rats were allowed to habituate to the housing facility for 3 days
before the experiments were initiated. Surgical and experimental
procedures were approved by the Animal Use and Care Committee for
Research and Education of Nantong University. Animal treatments
were performed according to the Guidelines of the International
Association for the Study of Pain (10).
Rats were randomly and evenly divided into 6 groups
(n=5): i) normal group, ii) sham-operated group, iii) SMIR model
group, iv) SMIR + PBS group, v) SMIR + KATP channels opener Pina
group and vi) SMIR + Pina + KATP channel blocker glibenclamide
(Gli) group.
SMIR surgery was performed on rats as previously
described (7). Briefly, the
animals were anesthetized with intraperitoneal injection of
pentobarbital sodium (50 mg/kg) and placed in the supine position.
After the medial thigh on the right leg was shaved and sterilized,
a 1.5–2 cm skin incision, ~4 mm medial to the saphenous vein, was
made to expose the muscle of the thigh. A 7–10 mm incision, ~4 mm
medial to the saphenous nerve, was made in the superficial
(gracilis) muscle layer of the thigh. The superficial muscle was
further isolated by spreading blunt scissors within the muscle
incision site to allow the insertion of a micro-dissecting
retractor. The retractor was inserted into the incision site, and
the superficial muscle of the thigh was retracted by 2 cm. In the
period of retraction, the saphenous nerve was displaced and
potentially stretched around the retractor, but not compressed
against a hard surface such as bone. The animals were covered with
a heavy absorbent bench underpad to prevent surgical site
dehydration. After 1 h, the muscle and skin of the surgical site
was closed with 4.0 Vicryl® sutures. Sham-operated rats
underwent the same procedure with the exception of the skin/muscle
retraction.
Phosphate-buffered saline (PBS), Pina (4, 20 or 40
μg; Research Biochemicals International, Natick, MA, USA),
or Pina (20 μg) + Gli (20 or 50 μg; Sigma, St. Louis,
MO, USA) were intrathecally injected at 7 days after SMIR surgery.
For intrathecal injection, the animals were anesthetized with
isoflurane. The spinal cord puncture was made with a 30-gauge
needle between the L4 and L5 level to deliver the reagents (40
μl) to the cerebral spinal fluid. Immediately after the
needle entry into the subarachnoid space, a brisk tail flick was
observed (11).
Cell culture and treatment
Primary astrocytes were prepared from cerebral
cortexes of neonatal rats (postnatal day 1, P1). The cerebral
hemispheres were isolated and transferred to ice-cold Hank’s buffer
(Invitrogen, Carlsbad, CA, USA), and the meninges were carefully
removed. Tissues were then minced into 1-mm sections, triturated,
filtered through a 100-μm nylon screen, and collected by
centrifugation at 3,000 × g for 5 min. The cell pellets were
re-suspended in a medium containing 10% fetal bovine serum in
low-glucose DMEM. After filtration through a 10-μm screen,
the cells were plated in 6-well plates at a density of
2.5×105 cells/cm2 and cultured for 10–12
days. The medium was replaced twice a week. When the cells grew to
95% confluence (10–12 days), 15 mM dibutyryl cyclic adenosine
monophosphate (cAMP) (Sigma-Aldrich) was added to induce
differentiation. The cells were used 3 days later.
When the cells were ready, they were randomly
divided into four groups: i) normal control; ii) LPS treatment
group: the astrocytes were stimulated with LPS (1 μg/ml) for
3 h in a 37°C incubator; iii) Pina group: the astrocytes were
pre-incubated with Pina (200 mM) for 30 min; iv) Pina + LPS group:
the astrocytes were pre-incubated with Pina (200 mM) for 30 min,
followed by incubation with LPS for 3 h; v) Pina + Gli + LPS group:
the cells were pre-incubated with Pina (200 mM) + Gli (50 or 500
mM) for 30 min and subsequently exposed to LPS for 3 h; vi) Gli
group: the astrocytes were pre-incubated with Gli (200 mM) for 30
min. In addition, to examine whether the expression of p-JNK was
dependent on exposure time, the cells were treated with 1
μg/ml LPS for 15, 30 and 60 min, respectively, following
treatment with d-cAMP for 72 h. After the above treatments, the
cells were collected for RT-PCR and western blotting.
Measurement of mechanical allodynia
Mechanical allodynia was assessed using Up-Down
paradigm (12) with von Frey
filaments (IITC Life Science Inc., Victory Blvd, Woodland Hills,
CA, USA) ranging from 1.4 to 26 g. Animals were placed on an
elevated wire mesh floor and confined underneath individual
overturned Perspex boxes (26×20×14 cm). Tests were conducted in the
morning between 8:30 and 11:30 a.m. A series of von Frey hair
stimuli was delivered in an ascending order of forces to the
mid-plantar area of the hindpaw encircled by tori/footpads. The
responses were recorded in grams of paw withdrawal averaged over
3–5 applications referred to as mechanical withdrawal threshold
(MWT). Behavioral tests were performed prior to, and 1, 3, 5, 7,
10, 21, 28 and 32 days following SMIR surgery.
Reverse-transcriptase polymerase chain
reaction (RT-PCR)
At 3 and 7 days after SMIR surgery, the animals were
terminally anesthetized with pentobarbital (40 mg/kg,
intraperitoneal) and transcardially perfused with PBS at room
temperature. The L3-L5 spinal cord segments from each rat were
dissected. Total RNA was extracted from the spinal cord or cultured
astrocytes using TRIzol reagent (Invitrogen, Carlsbad, CA, USA).
Total RNA (1 μg) was reverse-transcribed to cDNAs using
PrimeScript reverse transcriptase (Takara, Kyoto, Japan). The
MCP-1, Kir6.1, Kir6.2, SUR1, and
SUR2 genes were amplified from the cDNA using the Rotor-Gene
3000 real-time DNA analysis system (Corbett Research, Sydney,
Australia). The detailed primer sequences for each gene are
provided in Table I.
Amplification conditions and incubation conditions were as follows:
30 sec at 50°C, then thermo-cycling for 40 cycles of 5 sec at 95°C,
45 sec at 60°C, then 20 sec at 72°C. The threshold cycle number and
reaction efficiencies of each condition were identified with
Rotor-Gene Analysis Software 6.1 (Corbett Research). The mean
relative mRNA levels were determined with at least three separate
reactions per condition. Melt curves were performed on completion
of the cycles to ensure that non-specific products were absent.
Quantification was performed by normalizing cycle threshold (Ct)
values with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
Ct and analyzed with the 2−ΔΔCt method.
 | Table IThe detailed primer sequences for the
six genes. |
Table I
The detailed primer sequences for the
six genes.
| Gene | Primer
sequences |
|---|
| GAPDH | F: 5′-TCC TAC CCC
CAA TGT ATC CG-3′ |
| R: 5′-CCT TTA GTG
GGC CCT CGG-3′ |
| MCP-1 | F: 5′-TGC TGC TAC
TCA TTC ACT GGC-3′ |
| R: 5′-CCT TAT TGG
GGT CAG CAC AG-3′ |
| Kir6.1 | F: 5′-AAA GGC ATC
ACG GAG AAG AGT-3′ |
| R: 5′-TGG AGA AGA
GAA ACG CAG AAG-3′ |
| Kir6.2 | F: 5′-AGC ATC CAC
TCC TTT TCG TCT-3′ |
| R: 5′-GCT TGC TGA
AGA TGA GGG TTT-3′ |
| SUR1 | F: 5′-TTT TGG ATG
ACC CTT TCT CG-3′ |
| R: 5′-AGT GTC CCC
TCC CTC TGA AT-3′ |
| SUR2 | F: 5′-TTT GCC TCT
CTG TCT CTC TTC C-3′ |
| R: 5′-CTG CTT CCT
GTT TAT CGG TTT T-3′ |
Western blotting
Tissue samples of the spinal cord at each time point
after SMIR were prepared in the same manner as for PCR. Spinal cord
tissues or astrocytes were homogenized in a lysis buffer containing
protease and phosphatase inhibitors (Sigma). Protein concentrations
were determined by the BCA Protein Assay (Pierce, Rockford, IL,
USA). Protein (30 μg) was loaded for each lane, separated by
SDS-polyacrylamide gel electrophoresis (SDS-PAGE-10%), and
transferred to polyvinylidene difluoride membranes. After the
transfer, the membranes were blocked with 5% milk in PBS with 0.1%
Tween-20 for 2 h at room temperature, and incubated overnight at
4°C with polyclonal antibody against Kir6.1 (rabbit, 1:200, Sigma),
Kir6.2 (goat, 1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA), SUR1 (rabbit, 1:200, Santa Cruz Biotechnology Inc.), SUR2
(goat, 1:200, Santa Cruz Biotechnology Inc.), and p-JNK (rabbit,
1:2,000, Santa Cruz Biotechnology Inc.). For the loading control,
the blots were also probed with GAPDH antibody (mouse, 1:20,000,
Millipore, Billerica, MA, USA). The membranes were washed three
times with TBST buffer and incubated with the secondary antibody
(1:2,000) for 2 h followed by three washings. Blots were visualized
in ECL solution and exposed on hyperfilms (Bio-Rad, Hercules, CA,
USA) for 2–5 min. Specific bands were then evaluated by apparent
molecular size. The intensity of the selected bands was analyzed
using Image J software (NIH, Bethesda, MD, USA).
Immunohistochemistry
On day 0 and day 7 after SMIR, the rats were
anesthetized with pentobarbital and perfused transcardially with
PBS followed by 4% paraformaldehyde in PBS (250 ml; pH 7.0). After
the perfusion, the L3-L5 spinal cord from each rat was extracted
and post-fixed in the same fixative at 4°C overnight and then
placed in 20% and subsequently in 30% sucrose solution at 4°C
overnight, respectively. After cryoprotection with 30% sucrose, the
spinal cord was cut at 30-μm on a freezing microtome. The
sections were first blocked with 5% goat serum for 2 h at room
temperature and then incubated with the following primary
antibodies: Kir6.1 (rabbit, 1:80, Sigma), Kir6.2 (goat, 1:80, Santa
Cruz Biotechnology Inc.), SUR1 (rabbit, 1:50, Santa Cruz
Biotechnology Inc.), SUR2 (goat, 1:100, Santa Cruz Biotechnology
Inc.), glial fibrillary acidic protein (GFAP) antibody (mouse,
1:5,000, Millipore), OX-42 antibody (mouse, 1:5,000, Serotec,
Kidlington, Oxford, UK), and NeuN antibody (mouse, 1:5,000,
Millipore) at 4°C overnight. The sections were then incubated for 2
h at room temperature with Cy3- or FITC-conjugated secondary
antibodies (1:400, Jackson ImmunoResearch, West Grove, PA, USA).
For double immunofluorescence, the sections were incubated with a
mixture of mouse and rabbit (or goat) primary antibodies followed
by a mixture of FITC- and CY3-conjugated secondary antibodies. The
stained sections were examined with a Leica fluorescence
microscope, and images were captured with a CCD Spot camera. The
sections with double staining were imaged with an FV10i confocal
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were presented as mean ± SEM and analyzed by
the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). For behavioral
studies, the data were analyzed with two-way analysis of variance
followed by a Bonferroni’s test for post-hoc analysis. Differences
in gene and protein expression between groups were compared using
one-way ANOVA followed by Newman-Keuls post-hoc test or using the
Student’s t-test if only two groups were applied. P<0.05 was
considered statistically significant.
Results
SMIR surgery induces persistent
significant mechanical hypersensitivity
In this study, we established the postoperative pain
model by using a retractor to properly open the skin and
superficial muscle for 1 h. As SMIR did not induce significant heat
hyperalgesia or cold allodynia (13), mechanical hypersensitivity became
the focus. No significant differences in basal MWT between the
normal, SMIR and sham-operated groups were identified. However, the
MWT of SMIR-operated rats was decreased at postoperative day 1 and
this decrease was maintained for >21 days. During these periods,
SMIR-induced mechanical hypersensitivity in the ipsilateral paw was
significant at postoperative day 3 (P<0.01) and most prominent
at postoperative day 10 compared with the baseline (Fig. 1). However, the sham-operated and
normal groups did not show any marked changes at any of the time
points, and no significant difference was observed between the sham
and normal groups (P>0.05). These results indicated that SMIR
induced persistent significant mechanical hypersensitivity.
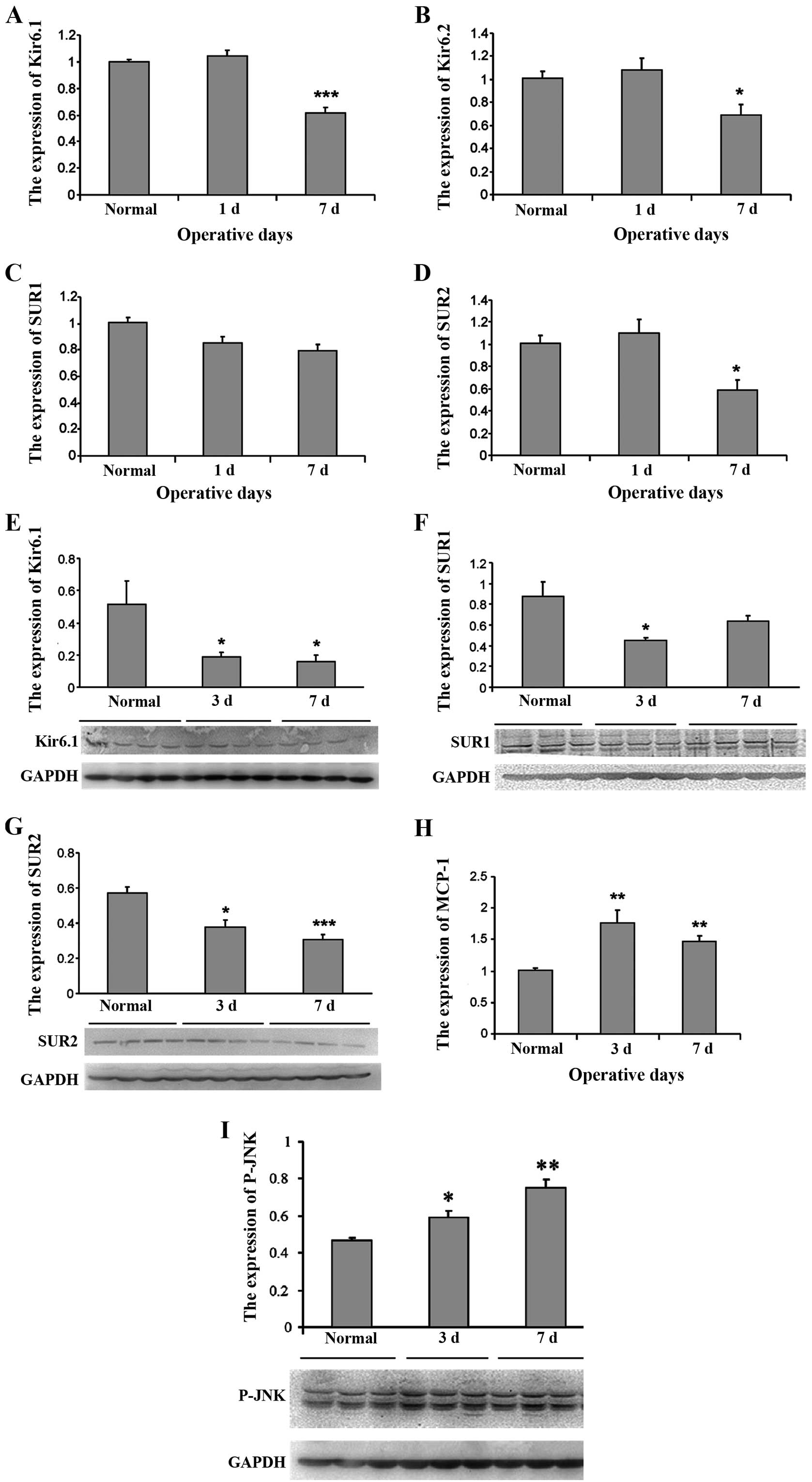
Decrease in KATP channel subunits and
increase in MCP-1 expression after SMIR in the spinal cord
To determine whether KATP plays a role during the
development of postoperative pain, we first performed a time-course
analysis of the subunits of KATP expression in the spinal cord
after the SMIR operations. The results showed that compared with
normal rats, SMIR caused a significant decrease in the expression
of the KATP channel subunits Kir6.1 (Fig. 2A), Kir6.2 (Fig. 2B) and SUR2 (Fig. 2D) on postoperative day 7
(P<0.05, Fig. 2). However,
from days 3 to 7 after SMIR, no significant variation of SUR1 mRNA
expression was observed compared with the normal rats (P>0.05,
Fig. 2C). The ressults from
western blot analysis revealed that the expression of the KATP
channel subunits Kir6.1 (Fig. 2E)
and SUR2 (Fig. 2E) in the spinal
cord was significantly decreased at 3 and 7 days after SMIR
(P<0.05). SUR1 expression was reduced at post-operative day 3,
but returned to the level of the control at post-operative day 7
(Fig. 2F), indicating it may not
be important for pain hypersensitivity. The protein expression for
Kir6.2 subunit was extremely low and not detectable in the spinal
cord. By contrast, the PCR results showed that MCP-1 expression in
the spinal cord was significantly increased at 3 and 7 days
(P<0.01) after SMIR (Fig.
2H).
Intrathecal administration of Pina
inhibits mechanical allodynia after SMIR and downregulates MCP-1
expression
SMIR produced long-lasting mechanical allodynia
associated with downregulation of the KATP channel subunits SUR2
and Kir6.1. We determined whether a KATP channel opener was capable
of reducing the allodynia. Therefore, we examined the analgesic
effect of KATP channel opener Pina by intracisternal injection at 7
days after SMIR. As shown in Fig.
3A, intracisternal injection of Pina markedly increased MWT
initiation within 1 h, peaked at 2 h, with the pain returning
within 3 h. In addition, the anti-nociceptive effect of 20
μg Pina was the optimum. Therefore, we utilized the 20
μg Pina to determine whether the anti-nociceptive effects of
the KATP activators could be relieved by the KATP blocker Gli (20
or 50 μg). As expected, injection of Gli together with the
Pina reversed the anti-mechanical nociceptive effects of Pina after
1, 1.5 and 2 h (Fig. 3A).
To determine the possible role of MCP-1 in the KATP
opener-induced inhibition of neuropathic pain, we then tested the
MCP-1 expression after Pina (20 μg) treatment at 7 days
after SMIR. The results indicated Pina significantly reduced
SMIR-induced MCP-1 mRNA in the spinal cord (Fig. 3B). These results suggested that
opening the KATP channel downregulated MCP-1 expression to
alleviate postoperative pain.
KATP channel opener Pina prevents
SMIR-induced JNK activation in spinal cord
Previously it was shown that c-jun-N-terminal kinase
(JNK) is an important intracellular kinase and plays a crucial role
in the pathogenesis of chronic pain by upregulating MCP-1
expression in spinal cord astrocytes (14). To investigate whether the effect
of Pina on SMIR-induced MCP-1 expression was mediated through the
JNK pathway, we first examined the activation of JNK by western
blotting with phospho-specific antibody at 3 and 7 days after SMIR.
The result showed that SMIR induced a persistent p-JNK increase in
the spinal cord peaking at day 7 (Fig. 2I). However, intrathecal
administration of the KATP channel opener Pina blocked the
SMIR-induced upregulation of p-JNK protein, reaching the level of
the normal group (Fig. 3C). These
results suggested that the JNK signaling pathway was involved in
KATP channel in postoperative pain transmission.
Cellular co-localization of Kir6.1 and
SUR2 in the spinal cord
Expression of Kir6.2 was not detected from the
spinal cord and SMIR did not cause any change in SUR1 expression at
postoperative days 1 and 7. Thus, Kir6.1 and SUR2 are involved in
post-operative pain. To define the cell distribution of Kir6.1 and
SUR2, we performed double staining of Kir6.1 and SUR2 with
different cell markers. As shown in Fig. 4, Kir6.1 was primarily co-localized
with the astrocytic marker GFAP, but not with neuronal marker NeuN
or microglial marker Iba-1, suggesting Kir6.1 is primarily induced
in astrocytes in the spinal cord. SUR2 was co-localized with NeuN,
while occasionally it was identified in a small amount with
GFAP.
KATP channel opener inhibits
LPS-triggered MCP-1 elevation in rat primary astrocytes
As Kir6.1, SUR2 and MCP-1 are expressed in
astrocytes, we determined the interaction of KATP and MCP-1 in
vitro, and prepared primary astrocytes from cerebral cortexes
of neonatal rats. Since astrocytes were known to be activated by
inflammatory mediators, we simulated astrocyte activation in
vitro with LPS, a critical trigger, for inflammatory responses
(15). As shown in Fig. 5C, after incubation with LPS (1
μg/ml) for 3 h, astrocytic MCP-1 expression was markedly
increased (P<0.01) compared with the control group. By contrast,
Kir6.1 and SUR2 expression was significantly decreased when
astrocytes were exposed to LPS for 3 and 6 h, respectively
(Fig. 5A and 5B).
To examine the involvement of KATP in MCP-1
expression, we pre-incubated astrocytes with Pina or Pina + Gli for
30 min followed by incubation with LPS for 3 h. As shown in
Fig. 5C, Pina significantly
alleviated MCP-1 upregulation in LPS co-treated astrocytes
(P<0.01) and Gli significantly reversed the inhibition of Pina
to MCP-1 expression (P<0.01). Pina or Glib alone had no effect
on MCP-1 expression (Fig. 6A).
These findings demonstrated that the protective role of Pina in
astrocytes was actually due to the opening of the KATP channel.
KATP agonist reduces the LPS-induced
protein level of p-JNK in primary cultured astrocytes
Mounting evidence suggests that JNK is important in
mediating the effects of LPS on MCP-1 production (16). Thus, we investigated whether the
KATP channel opener affected LPS-induced JNK phosphorylation in
astrocytes. After LPS exposure, there was a rapid activation
(phosphorylation) of JNK. The p-JNK induction was initiated at 15
min, reached a peak at 30 min, and decreased at 60 min, indicating
that the JNK signaling pathway was activated in response to the LPS
treatment in astrocytes (Fig.
6B). We detected whether the KATP channel opener regulated
LPS-induced JNK phosphorylation at the 30-min time point.
Pretreatment of cells with Pina (200 M) decreased LPS-induced
increase in phospho-JNK >40 and >50% (Fig. 6C).
Discussion
Ion channels play a vital role in pain signal
initiation and conduction (5).
The transient receptor potential channel family and voltage gated
sodium channels are among the most intensively studied ion channels
in pain signaling (17,18). Increasing attention has been paid
to the role of potassium (K+) channels in pain (19,20). K+ channels play an
essential role in setting the resting membrane potential and in
controlling the excitability of neurons. Thus, K+
channels are potentially attractive peripheral targets for the
treatment of pain. One K+ channel that is known to
regulate excitability in a variety of central and peripheral
neurons is the M channel. Another family of K+ channels
indicated in pain responses is the ATP-sensitive potassium channel
(KATP) family (21). The KATP
channels play critical roles in regulating membrane excitability
and neurotransmitter release, and providing neuroprotection
(22). The structure of the KATP
channel was initially determined in pancreatic b-cells to be an
octameric complex of two types of subunit: pore-forming Kir6.2 and
the sulfonylurea receptor SUR1, which belong to the ATP-binding
cassette superfamily (23).
Additional Kir and SUR subunits were subsequently identified to
form complexes with distinct pharmacological properties: the
cardiac and skeletal muscle type composed of Kir6.2 and SUR2A, an
isoform of SUR1 (24), and the
vascular smooth muscle type composed of Kir6.1 and SUR2B, a splice
variant of SUR2A. Previous findings have shown that the KATP
channel subunits SUR1, SUR2, and Kir6.2, but not Kir6.1, are
expressed in rat DRG neurons and in the satellite glial cells.
After painful axotomy, KATP channels were downregulated in DRG
neurons and Schwann cells (4),
and loss of the KATP current may contribute to neuropathic pain
through increased membrane excitability and amplified
neurotransmitter release (25).
Nevertheless in the spinal cord, the protein expression of Kir6.2
was extremely low and not detectable, which is different from that
in DRG. RT-PCR results revealed mRNA encoding all KATP channel
subunits. However, immunostaining or western blotting did not
detect any Kir6.2 at the protein level. This may be attributed to
the fact that immunohistochemistry and western blotting were not
sufficiently sensitive to detect Kir6.2 protein in the spinal cord,
or that Kir6.2 mRNA is not translated into protein in these cells
(26). However, the same
antibodies did not fail to detect Kir6.2 in DRG, indicating
competence of our antibodies, and, therefore the lack of
translation of Kir6.1 mRNA to protein in the spinal cord. This
finding suggested SUR1 or SUR2 subunits always co-assemble with
Kir6.1 or Kir6.2 subunits into functional KATP channel (27). Additionally, the different
expression of KATP channels in DRG and the spinal cord may suggest
different roles of these channels in DRG and the spinal cord.
However, this needs to be further investigated. Our findings from
these experiments emphasize the presence of KATP channels in the
spinal cord are of the Kir6.1/SUR1 and Kir6.1/SUR2 subtype complex.
Spinal administration of a KATP channel opener Pina can prevent or
reduce hyperalgesia and allodynia, but did not affect the
mechanical sensitivity in vehicle animals. The KATP blocker Gli
co-applied with Pina can reverse the effect of analgesic action by
Pina, suggesting the Kir6.1/SUR1 and Kir6.1/SUR2 subtype in the
spinal cord may be involved in the postoperative pain mechanism and
act as an early sensor of stress conditions. On the other hand, the
KATP blocker Gli did not affect any of the nocifensive behavior in
the absence of KATP activators. This result suggests that KATP does
not necessarily play a role in the nociception induced by these
stimuli, merely that the existence of these channels provides a
means of reducing hyperexcitability when KATP activity is enhanced
(28).
Our results show that Kir6.1 was co-localized with
astrocytes only and SUR1 and SUR2 were co-localized primarily with
neurons, in a small amount with astrocyte. It is now widely
recognized that activation of spinal glial cells, including
microglia and astrocytes, are involved in central sensitization and
mechanical hypersensitivity in acute and persistent pain states
(29–31). Astrocytes are recognized as
important contributors in pathological pain creation and
maintenance (32,33). In normal conditions, astrocytes
are relatively resting or quiescent. However, after injury or under
disease conditions, they can be converted to reactive states and
participate in the pathogenesis of neurological disorders (34,35). Astrocytes are also capable of
monitoring changes in synaptic activity and of spatially
integrating this information for signaling microvascular units, in
particular via dynamic Ca2+ signaling between astrocytes
and neurons or the endothelium via gap junctions and purinergic
transmission. Notably, astrocytes make very close contacts with
synapses and astrocyte reaction is more persistent following nerve
injury, arthritis, and tumor growth than microglial reaction,
exhibiting a better correlation with chronic pain behaviors
(32). A close contact with
neurons and synapses makes it possible for astrocytes to support
and nourish neurons, and regulate the external chemical environment
of neurons during synaptic transmission. Accumulating evidence
indicates that activated astrocytes can release gliotransmitters
such as ATP, glutamate, growth factors, pro-inflammatory cytokines,
and chemokines in the spinal cord to enhance and prolong persistent
pain states (8,9). MCP-1, also known as monocyte
chemotactic protein-1 (MCP-1), is a small 14-kDa protein that
signals through the G-protein-coupled receptor CCR2. MCP-1, similar
to other chemokines, was initially identified as an
immunomodulatory factor that regulates activation and migration of
peripheral immune cells. It has been shown to have a
neuromodulatory role in spinal nociceptive processing. Spinal nerve
ligation induced persistent neuropathic pain and MCP-1 upregulation
in the spinal cord (14). MCP-1
contributes to the maintenance of mechanical hypersensitivity after
plantar incision and establishes a role for neural glial signaling
in postoperative pain (36).
Consistent with the abovementioned studies, our results show that
the expression of mRNA for MCP-1 levels was significantly increased
at 3 and 7 days compared with the normal group in rat after
SMIR.
KATP channels and chemokine MCP-1 are expressed in
astrocytes and altered in the spinal cord by SMIR, and both of them
contribute to postoperative pain. We examined whether there was a
connection between them. Intrathecal administration of a KATP
channel opener Pina was applied and the results showed that Pina
significantly downregulated the expression of MCP-1 mRNA. In order
to further verify these results, we prepared primary astrocytes
from cerebral cortexes of neonatal rats. We detected MCP-1
production in astrocyte lysates and MCP-1 release in the culture
medium. In the non-stimulated conditions, astrocytes expressed low
levels of MCP-1. Exposure to LPS-induced rapid and time-dependent
increased MCP-1 expression, whereas the mRNA level of Kir6.1 and
SUR2 were significantly downregulated in astrocytes. The KATP
channel opener inhibited LPS-triggered MCP-1 elevation in rat
primary astrocytes. These results show KATP is involved in MCP-1
release in the astrocytes in vitro and in vivo.
However, how KATP regulates the MCP remains to be determined.
Previous findings suggest that activation of the
JNK/MCP-1 pathway in astrocytes is important in promoting
neuropathic pain (14).
Pre-incubation with Gli can prevent the activation of SAPK/JNK
(37). Moreover, diazoxide
suppressed rotenone-induced mitochondrial membrane potential loss
and p38/c-Jun N-terminal kinase activation in microglia, which may,
in turn, regulate the production of pro-inflammatory factors
(38). Those findings demonstrate
a possible link between the KATP channel and the downstream
mechanisms of MCP-1 expression in the spinal cord through
p-JNK-dependent manner. As expected, we proved that the KATP
channel opener inhibited the protein level of p-JNK elevation in
rat primary astrocytes, as well as in spinal cord induced by
SMIR.
In conclusion, this study has demonstrated that KATP
channel opener treatment is an effective approach to relieving
postoperative pain. This effect may be mediated by the activation
of the JNK/MCP-1 pathway in astrocytes in the spinal cord. As KATP
channels may have diverse functional roles in astrocytes and
neurons in the spinal cord, further studies are required to explore
the potential of KATP channels as targets of therapy against
postoperative pain and neurodegeneration.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (NSFC 31171062 and 31371121), Technical
Innovation and Demonstration Planning Project of Nantong
(HS2013061) and the Scientific Research Foundation of Xinglin
School of Nantong University (2012K133).
References
|
1
|
Deumens R, Steyaert A, Forget P, et al:
Prevention of chronic postoperative pain: cellular, molecular, and
clinical insights for mechanism-based treatment approaches.
Progress Neurobiol. 104:1–37. 2013. View Article : Google Scholar
|
|
2
|
Kehlet H, Jensen TS and Woolf CJ:
Persistent postsurgical pain: risk factors and prevention. Lancet.
367:1618–1625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caffo O, Amichetti M, Ferro A, Lucenti A,
Valduga F and Galligioni E: Pain and quality of life after surgery
for breast cancer. Breast Cancer Res Treat. 80:39–48. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zoga V, Kawano T, Liang MY, et al: KATP
channel subunits in rat dorsal root ganglia: alterations by painful
axotomy. Mol Pain. 6:62010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XF, Liu WT, Liu YP, Huang ZJ, Zhang YK
and Song XJ: Reopening of ATP-sensitive potassium channels reduces
neuropathic pain and regulates astroglial gap junctions in the rat
spinal cord. Pain. 152:2605–2615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia H, Zhang D, Yang S, et al: Role of
ATP-sensitive potassium channels in modulating nociception in rat
model of bone cancer pain. Brain Res. 1554:29–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flatters SJ: Characterization of a model
of persistent postoperative pain evoked by skin/muscle incision and
retraction (SMIR). Pain. 135:119–130. 2008. View Article : Google Scholar
|
|
8
|
Zhang ZJ, Dong YL, Lu Y, Cao S, Zhao ZQ
and Gao YJ: Chemokine CCL2 and its receptor CCR2 in the medullary
dorsal horn are involved in trigeminal neuropathic pain. J
Neuroinflammation. 9:1362012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo W, Wang H, Zou S, Dubner R and Ren K:
Chemokine signaling involving chemokine (CC motif) ligand 2 plays a
role in descending pain facilitation. Neurosci Bull. 28:193–207.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Covino B, Dubner R, Gybels J, et al:
Ethical standards for investigations of experimental pain in
animals. Pain. 9:141–143. 1980. View Article : Google Scholar
|
|
11
|
Hylden JL and Wilcox GL: Intrathecal
morphine in mice: a new technique. Eur J Pharmacol. 67:313–316.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dixon WJ: Staircase bioassay: the
up-and-down method. Neurosci Biobehav Rev. 15:47–50. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scholz J and Yaksh TL: Preclinical
research on persistent post-surgical pain: what we don’t know, but
should start studying. Anesthesiology. 112:511–513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao YJ, Zhang L, Samad OA, et al:
JNK-induced MCP-1 production in spinal cord astrocytes contributes
to central sensitization and neuropathic pain. J Neurosci.
29:4096–4108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarassishin L, Suh HS and Lee SC: LPS and
IL-1 differentially activate mouse and human astrocytes: Role of
CD14. Glia. 62:999–1013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nomura J, Busso N, Ives A, et al:
Febuxostat, an inhibitor of xanthine oxidase, suppresses
lipopolysaccharide-induced MCP-1 production via MAPK
phosphatase-1-mediated inactivation of JNK. PloS One. 8:e755272013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Julius D and Basbaum AI: Molecular
mechanisms of nociception. Nature. 413:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woolf CJ and Ma Q: Nociceptors - noxious
stimulus detectors. Neuron. 55:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu B, Linley JE, Du X, et al: The acute
nociceptive signals induced by bradykinin in rat sensory neurons
are mediated by inhibition of M-type K+ channels and
activation of Ca2+-activated Cl− channels. J
Clin Invest. 120:1240–1252. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ocaña M, Cendán CM, Cobos EJ, Entrena JM
and Baeyens JM: Potassium channels and pain: present realities and
future opportunities. Eur J Pharmacol. 500:203–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delmas P and Brown DA: Pathways modulating
neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci.
6:850–862. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liss B and Roeper J: Molecular physiology
of neuronal K-ATP channels (Review). Mol Membr Biol. 18:117–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inagaki N, Gonoi T, Clement JP IV, et al:
Reconstitution of IKATP: an inward rectifier subunit plus the
sulfonylurea receptor. Science. 270:1166–1170. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inagaki N, Gonoi T, Clement JP, et al: A
family of sulfonylurea receptors determines the pharmacological
properties of ATP-sensitive K+ channels. Neuron.
16:1011–1017. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawano T, Zoga V, McCallum J, et al:
ATP-sensitive potassium currents in rat primary afferent neurons:
biophysical, pharmacological properties, and alterations by painful
nerve injury. Neuroscience. 162:431–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodriguez AJ, Shenoy SM, Singer RH and
Condeelis J: Visualization of mRNA translation in living cells. J
Cell Biol. 175:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vit JP, Jasmin L, Bhargava A and Ohara PT:
Satellite glial cells in the trigeminal ganglion as a determinant
of orofacial neuropathic pain. Neuron Glia Biol. 2:247–257. 2006.
View Article : Google Scholar
|
|
28
|
Du X, Wang C and Zhang H: Activation of
ATP-sensitive potassium channels antagonize nociceptive behavior
and hyperexcitability of DRG neurons from rats. Mol Pain. 7:352011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Obata H, Eisenach JC, Hussain H, Bynum T
and Vincler M: Spinal glial activation contributes to postoperative
mechanical hypersensitivity in the rat. J Pain. 7:816–822. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ledeboer A, Sloane EM, Milligan ED, et al:
Minocycline attenuates mechanical allodynia and proinflammatory
cytokine expression in rat models of pain facilitation. Pain.
115:71–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Svensson CI, Fitzsimmons B, Azizi S,
Powell HC, Hua XY and Yaksh TL: Spinal p38β isoform mediates tissue
injury-induced hyperalgesia and spinal sensitization. J Neurochem.
92:1508–1520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao YJ and Ji RR: Targeting astrocyte
signaling for chronic pain. Neurotherapeutics. 7:482–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao YJ and Ji RR: Chemokines,
neuronal-glial interactions, and central processing of neuropathic
pain. Pharmacol Ther. 126:56–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scholz J and Woolf CJ: The neuropathic
pain triad: neurons, immune cells and glia. Nat neurosci.
10:1361–1368. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rossi DJ, Brady JD and Mohr C: Astrocyte
metabolism and signaling during brain ischemia. Nat Neurosci.
10:1377–1386. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peters CM and Eisenach JC: Contribution of
the chemokine (C-C motif) ligand 2 (CCL2) to mechanical
hypersensitivity after surgical incision in rats. Anesthesiology.
112:1250–1258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Figura M, Chilton L, Liacini A, et al:
Blockade of K(ATP) channels reduces endothelial hyperpolarization
and leukocyte recruitment upon reperfusion after hypoxia. Am J
Transplant. 9:687–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou F, Yao HH, Wu JY, Ding JH, Sun T and
Hu G: Opening of microglial K(ATP) channels inhibits
rotenone-induced neuroinflammation. J Cell Mol Med. 12:1559–1570.
2008. View Article : Google Scholar : PubMed/NCBI
|