Introduction
Colon cancer is the second most common type of
cancer and the fourth leading cause of cancer-related mortality
worldwide (1,2). Indeed, the lifetime risk of
developing colon cancer in the United States is approximately 20%
(2), and in China the development
of colon cancer is increasing each year (3). Despite major advances in the
treatment of colon cancer, its prognosis remains very poor
(4,5). Frequent intraperitoneal metastases
and post-surgical recurrence are characteristics of colon cancer
and the major factors leading to poor prognosis (6,7).
Therefore, exploring the molecular mechanisms underlying the
pathogenesis of colon cancer may provide valuable insight and aid
in the development of novel and effective therapeutic approaches
for colon cancer.
MicroRNAs (miRNAs or miRs) are a specific type of
small non-coding RNA that modulate gene expression through the
suppression of mRNA translation. The aberrant expression of miRNAs
has been linked to tumor initiation, progression and prognosis
(8–10). Of these cancer-associated miRNAs,
miR-218 expression has been frequently observed to be decreased in
human colon cancer (11,12). Furthermore, it has been reported
that the miR-218 expression level is associated with the TNM stage,
and that miR-218 hypo-expression in sera and tissues is indicative
of a poor prognosis in patients with colon cancer (13). However, the mechanisms of action
of miR-218 in colon cancer progression remain elusive.
In addition to miRNAs the hyperactivation of the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling
pathway is frequently observed in various types of cancer (14). The involvement of this pathway in
the development of colon cancer and metastasis, including cell
proliferation, apoptosis and migration, has been extensively
investigated (15–18). Furthermore, the blockade of
PI3K/Akt activity in colon cancer cells has shown promising
anti-cancer effects (19–22). Recent studies have demonstrated
that the downstream factor of the PI3K/Akt pathway, the mammalian
target of rapamycin (mTOR), is a potential therapeutic target in
various types of cancer, including non-small cell lung cancer
(23), colorectal cancer
(24,25), renal carcinoma (26), non-Hodgkin’s lymphoma (27) and leukemia (28,29). In addition, the activation of the
PI3K/Akt pathway has been shown to correlate with a poor prognosis
in stage II colon cancer, and Akt phosphorylation is a prognostic
factor for disease-free survival (30). These findings confirm that the
PI3K/Akt pathway is essential for the development of colon cancer
and is a promising target for the treatment of colon cancer.
It has been previously reported that the
upregulation of miR-218 inhibits the proliferation of oral squamous
carcinoma cells by targeting Akt and mTOR (31), and increases the sensitivity of
gastrointestinal stromal tumor cells to imatinib through the
PI3K/Akt pathway (32). In the
present study, we also revealed that the PI3K/Akt signaling pathway
may be a potential target of miR-218 using gene target prediction
programs. Furthermore, the effect of miR-218 on the PI3K/Akt/mTOR
signaling pathway was explored, and it was shown that miR-218 is a
negative regulator of this pathway. Matrix metalloproteinase (MMP)9
was identified as a downstream factor of miR-218, and miR-218
inhibited MMP9 expression in colon cancer cells. Therefore, miR-218
was identified as a repressor of colon cancer development and
progression by targeting the PI3K/Akt/mTOR signaling pathway and
MMP9 expression. Consequently, miR-218 is a potential target in the
treatment of colon cancer treatment.
Materials and methods
Reagents
The miRNA extraction kit was from Tiangen Biotech
(Beijing) Co., Ltd. (Beijing, China). The TaqMan MicroRNA Assay
kit, and TaqMan® Universal PCR Master Mix were purchased
from Applied Biosystems (Foster City, CA, USA). TargetScan version
5.1 (http://www.targetscan.org/index.html) was used to find
potential targets for miR-218.
Cell lines and colon tissue
specimens
Human colon tissue specimens were obtained from
patients with surgical resections performed at the Affiliated
Cancer Hospital of Guangzhou Medical University (Guangdong, China).
A total of 4 tumor tissue samples and adjacent normal tumor tissue
samples were collected from 4 patients with colon cancer. The study
was approved by the Ethics Committee of the Affiliated Cancer
Hospital of Guangzhou Medical University, and legally effective
informed consent was obtained from all patients.
Cell transfection
Human LoVo cancer cells (obtained from the Cell Bank
of Sun Yat-Sen University) were seeded in 6-well plates at a final
density of 8×104 cells/well and cultured overnight at
37°C in a humidified atmosphere of 95% air and 5% CO2
prior to transfection. Subsequently, transfections were conducted
for miR-218 mimic, non-specific control (NC) and miR-218 inhibitor
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The culture
medium was changed from serum-free DMEM to DMEM containing 10%
fetal bovine serum (Gibco-BRL, Grand Island, NY, USA) 5 h after
transfection. Following culture for a further 48 h, total RNA and
cellular protein lysates were collected and used for reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis,
respectively.
MTT assay
LoVo cells were seeded into 96-well plates
(8×104 cells/well) and assessed by MTT assay. To
determine viability, the cells were then treated with 5 mg/ml MTT
for 4 h at 37°C and then the medium was carefully removed. The
resulting formazan crystals were dissolved in 150 μl
dimethyl sulfoxide (DMSO), and the absorbance at 570 nm was
determined using a plate reader.
Protein extraction and western blot
anlaysis
Total cellular proteins were extracted using lysis
buffer containing 150 mM NaCl, 10 mM Tris at pH 7.2, 0.1% SDS, 1.0%
Triton X-100, 1% deoxycholate and 5 mM EDTA. Subsequently, protein
levels were quantified using the Bio-Rad Protein Assay kit
(Bio-Rad, Hercules, CA, USA). A total of 30 μg protein was
used for 10% sodium dodecyl sulphate polyacrylamide gel
electrophoresis (SDS-PAGE) followed by transfer to PVDF membranes
(Bio-Rad). The PVDF membranes were then incubated in 5% milk
dissolved in 1X TBST buffer at room temperature for 1 h to block
the potential non-specific binding of primary antibodies.
Subsequently, the primary antibodies, including mouse monoclonal
anti-Akt (ab79360), anti-mTOR (ab134903) and anti-GAPDH (ab22556)
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
were added followed by incubation overnight at 4°C. After washing
with 1X TBST buffer, the corresponding secondary HRP-conjugated
antibody (LS-C170893; Santa Cruz Biotechnology, Inc.) was added.
After washing with 1X TBST buffer, the ECL chemiluminescent
detection system (Pierce Biotechnology, Inc., Rockford, IL, USA)
and X-ray films were used for protein detection. The blots were
then scanned and the band density was quantified using the
GeneGnome western blot imaging system (Syngene, Cambridge, UK)
using GeneSnap software.
RNA extraction and RT-qPCR
Total RNA was extracted from the cultured LoVo cells
using the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). The
isolated RNA was quantified by measuring its absorbance at 260 nm.
The expression level of matured miRNAs was analyzed by stem-loop
reverse transcription followed by quantitative PCR (qPCR). All
reagents for the stem-loop reverse transcription, including the
TaqMan MicroRNA Assay kit and the TaqMan Universal PCR Master Mix
were obtained from Applied Biosystems. miR-218 expression in the
human colon tissue samples was normalized to the endogenous
reference gene, GAPDH. For the detection of the mRNA expression of
PI3K, Akt and mTOR, reverse transcription was performed using the
PrimeScript™ RT reagent kit [Takara Biotechnology (Dalian) Co.,
Ltd., Liaoning, China]. qPCR was performed on a LightCycler 480
System (Roche, Basel, Switzerland) using SYBR Premix Ex Taq II
[Takara Biotechnology (Dalian) Co., Ltd.]. GAPDH was used as the
endogenous control. The 2−ΔΔCT method was used to
quantify the expression changes of target genes. Three independent
experiments were performed.
Wound healing assay
Wound healing assay was conducted 48 h after cell
transfection. An artificial homogeneous wound was created on the
monolayer using a sterized 200 μl micropipette tip when the
cells reached approximately 90% confluency. Cell debris was removed
by washing with DMEM twice. Wound closure via cell migration was
observed 12 h later by capturing images using an inverted
microscope with ×40 objective (Olympus Corp., Tokyo, Japan).
Invasion and migration assays and
immunofluorescence staining
Tumor invasion assay was performed as follows:
friefly, an 8-μm pore polycarbonate membrane filter was
inserted into each Transwell chamber (Corning, Inc., Corning, NY,
USA) and coated with 50 μl of Matrigel (Sigma, St. Louis,
MO, USA) with a final concentration of 4 mg/ml. A total of
5×103 transiently transfected LoVo cells was then seeded
into the upper chamber with 100 μl of serum-free medium, and
1 ml DMEM containing 20% FBS was added to the bottom chamber. The
cells were incubated at 37°C for a further 48 h. For
immunofluorescence staining, the LoVo cells that had invaded into
the lower surface of the filter were fixed with 4% paraformaldehyde
and stained with crystal violet. Cells from 3 random visual fields
per filter were captured using a microscope at ×100 magnification
and counted for quantification. Tumor cell motility assay was
performed similarly to the Matrigel invasion assay, but without the
Matrigel coating of the filter. For all assays, 3 independent
experiments were performed.
Data analysis
Data are presented as the means ± SD and analyzed
using SPSS 13.0 software (SAS Institute, Inc., Cary, NC, USA).
Significant differences/correlations between the different groups
were calculated using the Student’s t-test, χ2 test or
Pearson’s correlation. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Aberrant changes in miR-218 expression
and the PI3K/Akt/mTOR pathway in colon cancer tissues
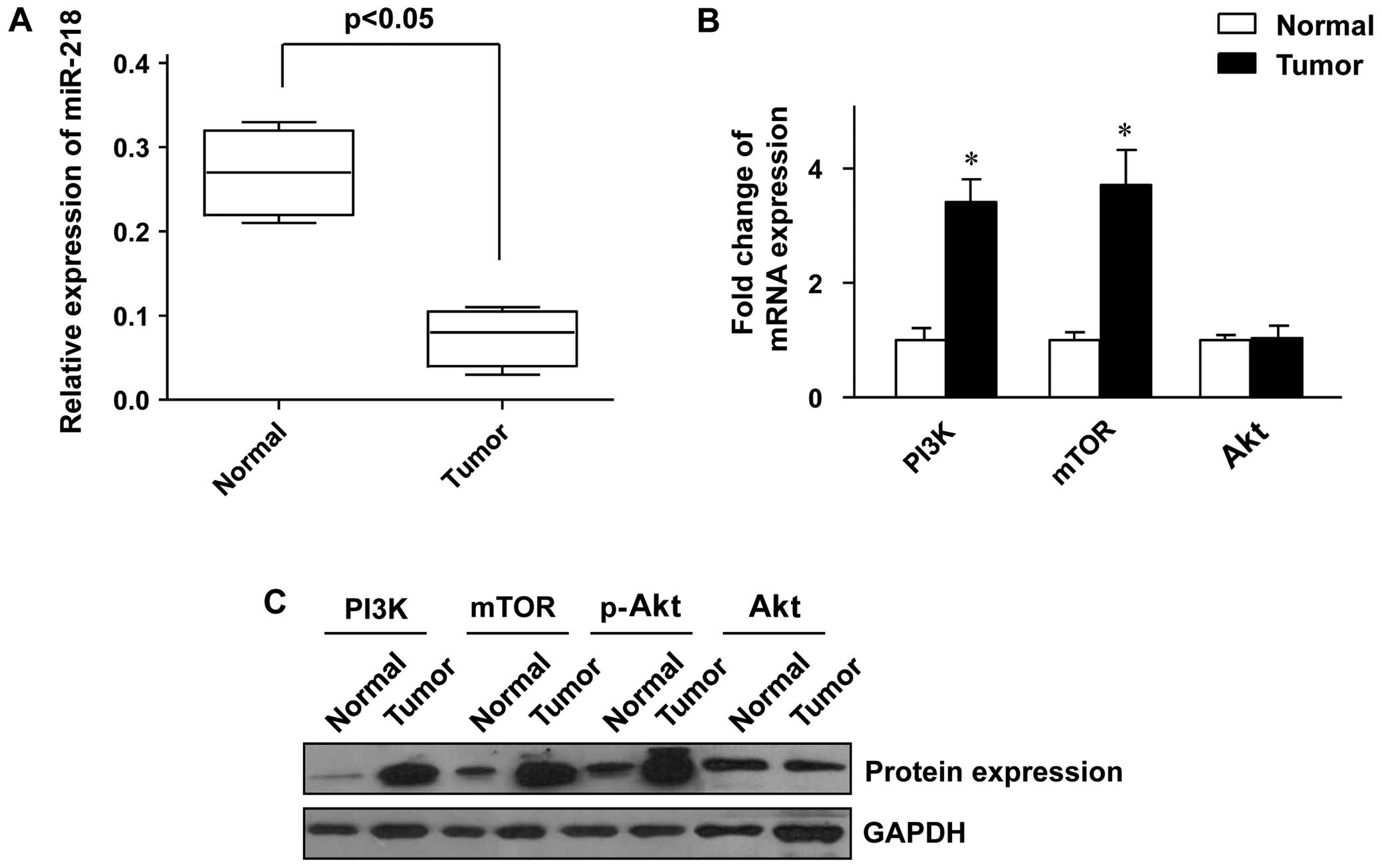
We first examined the miR-218 expression level in
the colon cancer and adjacent normal tissue samples. It was shown
that the expression level of miR-218 was significantly lower in the
tumor tissues than in the adjacent normal tissues (Fig. 1A). The status of the PI3K/Akt/mTOR
pathway in thetumor tissues was further analyzed, and the results
revealed that the expression of PI3K and mTOR at both the mRNA and
protein level was markedly increased in the tumor tissues compared
to the adjacent normal tissues (Fig.
1B and C). Whereas the expression of Akt was only slightly
altered at both the mRNA and protein level (Fig. 1B and C), the phosphorylation of
Akt protein was significantly enhanced in the tumor tissues
(Fig. 1C), which indicated that
the PI3K/Akt/mTOR pathway was aberrantly activated in the colon
cancer tissue samples.
Overexpression of miR-218 inhibits the
proliferation, migration and invasion of LoVo colon cancer
cells
As described above, miR-218 expression negatively
correlated with the development of colon cancer. To investigate the
causal role of miR-218 in the development of colon cancer, miR-218
mimics and inhibitors were transfected into the LoVo human colon
cancer cells to examine the effect of miR-218 overexpression or
inhibition on cell proliferation, migration and invasion. MTT assay
revealed that cell proliferation was significantly inhibited by
transfection with miR-218 mimics (24.7±5.5%), while it was
significantly promoted by transfection with miR-218 inhibitors
(Fig. 2A).
In addition to its effect on cell proliferation,
miR-218 was found to be critical for the migration and invasion
abilities of the LoVo colon cancer cells. In the migration assay,
the number of invading cells in the lower Transwell chamber was
significantly decreased in the miR-218 mimic-transfected group
(19.7±5.0%), while this number was increased in miR-218
inhibitor-transfected group (117.0±9.6%) (Fig. 2B). Similar phenomena were also
observed in the Matrigel invasion assay, in which the number of
invading cells was reduced to 28.3±7.6% by transfection with
miR-218 mimics and increased to 118.7±9.5% by transfection with
miR-218 inhibitors (Fig. 2C).
Furthermore, the rate of wound closure was slower in the miR-218
mimic-transfected group, while the rate was increased in the
miR-218 inhibitor-transfected group (Fig. 2D).
Regulation of the PI3K/Akt/mTOR signaling
pathway by miR-218
In order to investigate the molecular mechanisms
underlying the miR-218-mediated development of colon cancer, the
potential targets of miR-218 were predicted using the TargetScan
database. Potential miR-218 target sites were detected in the 3′
UTR of PIK3C2A (position 393–400 of PIK3C2A 3′ UTR) and PIK3R1
(position 3591–3598 of PIK3R1 3′ UTR) (Fig. 3A).
The effect of miR-218 on the PI3K/Akt/mTOR signaling
pathway was further investigated in the LoVo colon cancer cells. It
was shown that the expression levels of PI3K and mTOR at the mRNA
and protein level were upregulated by transfection with miR-218
inhibitor, while they were markedly inhibited by transfection with
miR-218 mimics (Fig. 3B, C and
E). Furthermore, the phosphorylation of Akt was inhibited by
transfection with miR-218 mimics, while it was promoted by
transfection with miR-218 inhibitor (Fig. 3E). However, the expression of Akt
at both the mRNA (Fig. 3D) and
protein level (data not shown) was only slighlty altered following
transfection with miR-218 mimics or inhibitor. These results
indicate that miR-218 is a negative regulator of the PI3K/Akt/mTOR
signaling pathway.
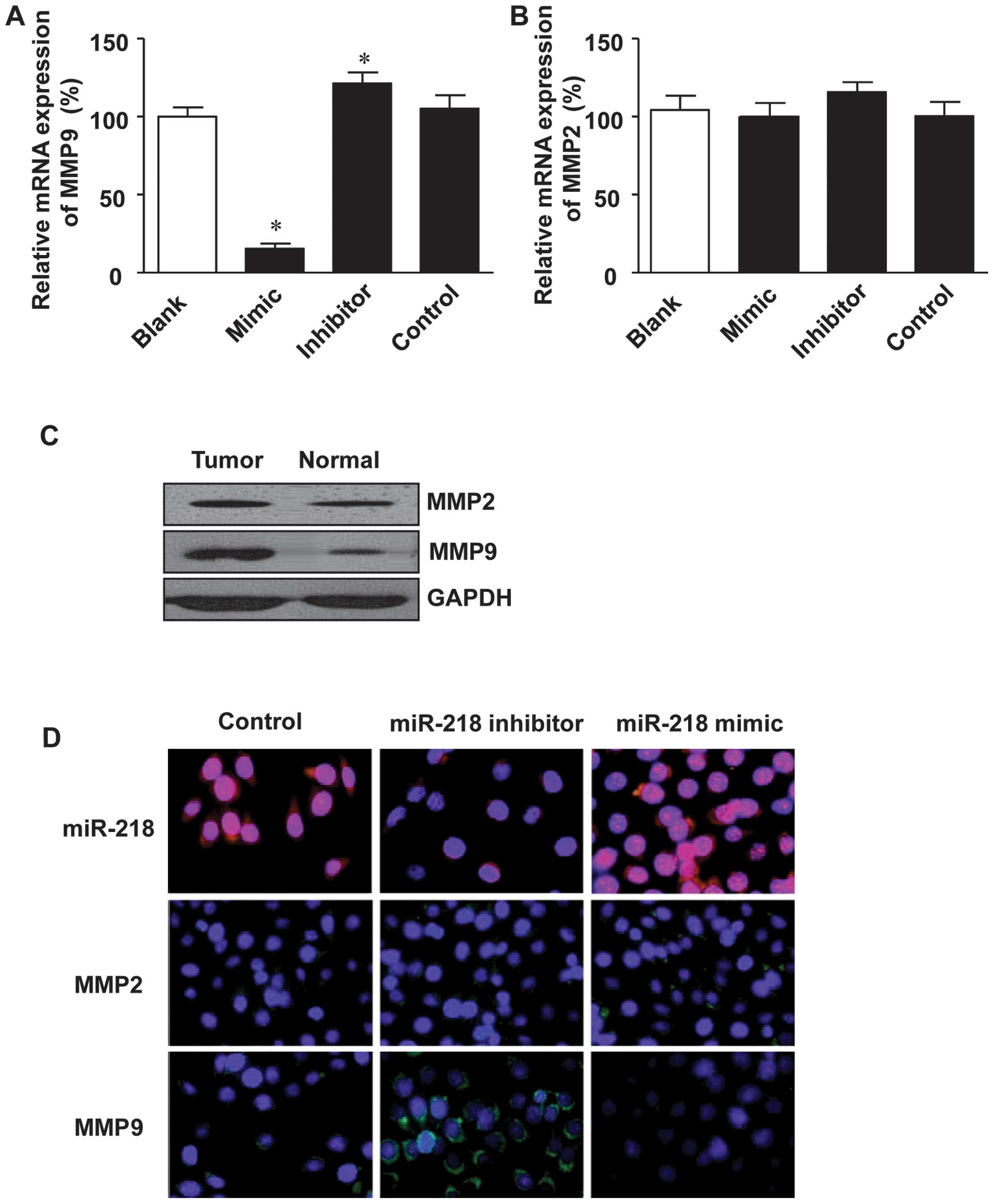
Expression of MMP9 is inhibited by
miR-218
The degradation of basement membranes and the
extracellular matrix is critical for tumor invasion and metastasis.
MMPs, MMP2 and 9 in particular, are the most vital enzymes for
extracellular matrix degradation in tumor invasion (33–35). This, in combination with the fact
that miR-218 was found to negatively correlate with tumor migration
and invasion, led us to investigate the effect of miR-218 on the
expression of MMP9 and MMP2. As shown in Fig 4A, transfection with miR-218 mimics
inhibited the expression of MMP9 at the mRNA level, while
transfection with miR-218 inhibitor restored the mRNA expression of
MMP9. However, MMP2 expression was only slightly altered in both
the miR-218 mimic- and the miR-218 inhibitor-transfected cells
(Fig. 4B).
Furthermore, the expression of MMP9 at the protein
level was analyzed in the colon cancer tissue specimens. It was
shown that MMP9 expression was significantly higher in the tumor
tissues as compared to the adjacent normal tissues (Fig. 4C). However, MMP2 expression was
quite similar between the colon cancer tissues and adjacent normal
tissues (Fig. 4C). In addition to
western blot anlaysis, immunofluorescence staining was employed to
examine the effect of miR-218 on the expression status of MMP9 and
MMP2 in the LoVo colon cancer cells, and the results revealed that
transfection with the miR-218 inhibitor enhanced the expression of
MMP9, while transfection with the miR-218 mimics inhibited MMP9
expression (Fig. 4D). However
miR-218 had little effect on MMP2 expression (Fig. 4D).
Discussion
The survival and prognosis of colon cancer is poor,
partly due to frequent tumor relapse and metastasis. Therefore,
clarification of the molecular pathogenesis of colon cancer is
crucial for developing effective therapeutic strategies to improve
prognosis. In the present study, we focused on the correlation
between miR-218 and the development of colon cancer. It was found
that miR-218 expression was downregulated in the colon cancer cells
and tissue samples. Furthermore, the inhibition of miR-218 in the
human colon cancer cell line enhanced the cell migration and
invasion ability, which was suppressed by the overexpression of
miR-218. Indeed miR-218 has also been found to inhibit invasion and
metastasis in other tumors, including gastric cancer (36,37), head and neck squamous cell
carcinoma (38) and cervical
squamous cell carcinoma (39).
These findings indicate that miR-218 is a potential tumor
suppressor in colon cancer.
As described above, miR-218 functions as a tumor
suppressor in several types of cancer, and multiple downstream
targets, such as the Slit-Robo1 pathway (36), the Wnt signaling pathway (40) and the TGFβ signaling pathway
(41), have been reported. In
this study, we found that miR-218 binds to the 3′ UTR of PIK3C2A
(position 393–400) and PIK3R1 (position 3591–3598), indicating PI3K
signaling as a potential downstream pathway of miR-218. We further
confirmed that miR-218 is an inhibitor of the PI3K/Akt/mTOR pathway
in human colon cancer cells. PI3K is a membrane protein related to
G protein-coupled receptors (42). PI3K activation triggers a series
of intracellular events leading to the activation of Akt and mTOR
(43–45), which thereafter induces the
expression of multiple target genes that regulate cell
proliferation, differentiation and other funtions (46,47). Our results revealed that the
PI3K/Akt/mTOR pathway was involved in the invasion and metastasis
of colon cancer cells, which was negatively regulated by
miR-218.
The results of the present study demonstrated that
cancer cell migration and invasion were inhibited by transfection
with miR-218 mimics, whereas transfection with miR-218 inhibitor
promoted the migration and invasion of colon cancer cells. These
results indicate that miR-218 plays an important role in
suppressing the metastasis of colon cancer cells. Furthermore,
there was an inverse correlation between PI3K/Akt/mTOR expression
and the miR-218 expression level in the colon cancer tissues, which
was consistent with our in vitro results. These data
confirmed that miR-218 inhibited colon cancer cell migration and
invasion through the downregulation of PI3K/Akt/mTOR.
The activation of the PI3K signaling pathway is also
associated with the hyper-expression of MMP, which accelerates
tumor migration and invasion (48–51). Our results revealed that the
activation of the PI3K/Akt/mTOR signaling pathway was enhanced and
the expression levels of MMP9 were significantly higher in thecolon
cancer tissues compared with the non-tumor tissues. Furthermore,
the overexpression of miR-218 inhibited MMP9 expression and tumor
aggressiveness. These results indicate that MMP9 is a downstream
factor of miR-218 in regulating cancer cell invasion and
migration.
In conclusion, our study demonstrated that miR-218
suppressed the invasion of colon cancer cells by inhibiting the
activation of the PI3K/Akt/mTOR signaling pathway and MMP9
expression. Furthermore, the miR-218 expression level inversely
correlated with both PI3K/Akt/mTOR and MMP9 in the resected colon
cancer tissues. These findings suggest that miR-218 is a potential
target in the treatment of advanced colon cancer.
Acknowledgments
This study was supported by the Guangdong Province
Natural Science Fund (no. S2013010016662), the Health Bureau of
Guangdong Province (nos. A2014224 and B2014196), the Science and
Technology Planning Project of Guangdong Province (no.
2013B021800284) and the National Natural Science Foundation of
China (nos. 81201932 and 81372493).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar :
|
|
3
|
Chen WQ, Zhang SW, Zou XN and Zhao P:
Cancer incidence and mortality in China, 2006. Chin J Cancer Res.
23:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simmonds PC, Primrose JN, Colquitt JL,
Garden OJ, Poston GJ and Rees M: Surgical resection of hepatic
metastases from colorectal cancer: A systematic review of published
studies. Br J Cancer. 94:982–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yokota M, Kojima M, Nomura S, Nishizawa Y,
Kobayashi A, Ito M, Ochiai A and Saito N: Clinical impact of
elastic laminal invasion in colon cancer: Elastic laminal
invasion-positive stage II colon cancer is a high-risk equivalent
to stage III. Dis Colon Rectum. 57:830–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glehen O, Osinsky D, Cotte E, et al:
Intraperitoneal chemohyperthermia using a closed abdominal
procedure and cytoreductive surgery for the treatment of peritoneal
carcinomatosis: Morbidity and mortality analysis of 216 consecutive
procedures. Ann Surg Oncol. 10:863–869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aghili M, Izadi S, Madani H and Mortazavi
H: Clinical and pathological evaluation of patients with early and
late recurrence of colorectal cancer. Asia Pac J Clin Oncol.
6:35–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medina-Villaamil V, Martínez-Breijo S,
Portela-Pereira P, Quindós-Varela M, Santamarina-Caínzos I,
Antón-Aparicio LM and Gómez-Veiga F: Circulating MicroRNAs in blood
of patients with prostate cancer. Actas Urol Esp. 38:633–639.
2014.In English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang M, Liu R, Sheng J, Liao J, Wang Y,
Pan E, Guo W, Pu Y and Yin L: Differential expression profiles of
microRNAs as potential biomarkers for the early diagnosis of
esophageal squamous cell carcinoma. Oncol Rep. 29:169–176.
2013.
|
|
10
|
Leite KR, Tomiyama A, Reis ST,
Sousa-Canavez JM, Sañudo A, Camara-Lopes LH and Srougi M: MicroRNA
expression profiles in the progression of prostate cancer – from
high-grade prostate intraepithelial neoplasia to metastasis. Urol
Oncol. 31:796–801. 2013. View Article : Google Scholar
|
|
11
|
He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan
FK, Sung JJ and Yu J: MicroRNA-218 inhibits cell cycle progression
and promotes apoptosis in colon cancer by downregulating BMI1
polycomb ring finger oncogene. Mol Med. 18:1491–1498.
2012.PubMed/NCBI
|
|
12
|
Heckmann D, Maier P, Laufs S, et al: The
disparate twins: A comparative study of CXCR4 and CXCR7 in
SDF-1α-induced gene expression, invasion and chemosensitivity of
colon cancer. Clin Cancer Res. 20:604–616. 2014. View Article : Google Scholar
|
|
13
|
Yu H, Gao G, Jiang L, Guo L, Lin M, Jiao
X, Jia W and Huang J: Decreased expression of miR-218 is associated
with poor prognosis in patients with colorectal cancer. Int J Clin
Exp Pathol. 6:2904–2911. 2013.PubMed/NCBI
|
|
14
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YZ, Wu K, Huang J, et al: The
PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in
the inhibitory effect of resveratrol on human colon cancer cell
proliferation. Int J Oncol. 45:104–112. 2014.PubMed/NCBI
|
|
16
|
Jiang QG, Li TY, Liu DN and Zhang HT:
PI3K/Akt pathway involving into apoptosis and invasion in human
colon cancer cells LoVo. Mol Biol Rep. 41:3359–3367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao ZM, Wang XY and Wang AM: Periostin
induces chemo-resistance in colon cancer cells through activation
of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. Dec
24–2013.Epub ahead of print. View
Article : Google Scholar
|
|
18
|
Josse C, Bouznad N, Geurts P, Irrthum A,
Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V and Oury C:
Identification of a microRNA landscape targeting the PI3K/Akt
signaling pathway in inflammation-induced colorectal
carcinogenesis. Am J Physiol Gastrointest Liver Physiol.
306:G229–G243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014.PubMed/NCBI
|
|
20
|
Li XX, Huang LY, Peng JJ, Liang L, Shi DB,
Zheng HT and Cai SJ: Klotho suppresses growth and invasion of colon
cancer cells through inhibition of IGF1R-mediated PI3K/AKT pathway.
Int J Oncol. 45:611–618. 2014.PubMed/NCBI
|
|
21
|
Nuvoli B, Santoro R, Catalani S,
Battistelli S, Benedetti S, Canestrari F and Galati R: CELLFOOD™
induces apoptosis in human mesothelioma and colorectal cancer cells
by modulating p53, c-myc and pAkt signaling pathways. J Exp Clin
Cancer Res. 33:242014. View Article : Google Scholar
|
|
22
|
Enayat S, Ceyhan MS, Başaran AA, Gürsel M
and Banerjee S: Anticarcinogenic effects of the ethanolic extract
of Salix aegyptiaca in colon cancer cells: Involvement of Akt/PKB
and MAPK pathways. Nutr Cancer. 65:1045–1058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trigka EA, Levidou G, Saetta AA, et al: A
detailed immunohistochemical analysis of the PI3K/AKT/mTOR pathway
in lung cancer: Correlation with PIK3CA, AKT1, K-RAS or PTEN
mutational status and clinicopathological features. Oncol Rep.
30:623–636. 2013.PubMed/NCBI
|
|
24
|
Banerjee N, Kim H, Talcott S and
Mertens-Talcott S: Pomegranate polyphenolics suppressed
azoxymethane-induced colorectal aberrant crypt foci and
inflammation: Possible role of miR-126/VCAM-1 and
miR-126/PI3K/AKT/mTOR. Carcinogenesis. 34:2814–2822. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through down-regulation of p53-dependent Bcl-2
expression and inhibition of Mcl-1 protein stability. PLoS One.
9:e955882014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zang C, Eucker J, Liu H, Müller A,
Possinger K and Scholz CW: Concurrent inhibition of PI3-kinase and
mTOR induces cell death in diffuse large B cell lymphomas, a
mechanism involving down regulation of Mcl-1. Cancer Lett.
339:288–297. 2013. View Article : Google Scholar
|
|
28
|
Kampa-Schittenhelm KM, Heinrich MC, Akmut
F, Rasp KH, Illing B, Döhner H, Döhner K and Schittenhelm MM: Cell
cycle-dependent activity of the novel dual PI3K-MTORC1/2 inhibitor
NVP-BGT226 in acute leukemia. Mol Cancer. 12:462013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Müller A, Zang C, Chumduri C, Dörken B,
Daniel PT and Scholz CW: Concurrent inhibition of PI3K and
mTORC1/mTORC2 overcomes resistance to rapamycin induced apoptosis
by down-regulation of Mcl-1 in mantle cell lymphoma. Int J Cancer.
133:1813–1824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malinowsky K, Nitsche U, Janssen KP, Bader
FG, Späth C, Drecoll E, Keller G, Höfler H, Slotta-Huspenina J and
Becker KF: Activation of the PI3K/AKT pathway correlates with
prognosis in stage II colon cancer. Br J Cancer. 110:2081–2089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uesugi A, Kozaki K, Tsuruta T, Furuta M,
Morita K, Imoto I, Omura K and Inazawa J: The tumor suppressive
microRNA miR-218 targets the mTOR component Rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res. 71:5765–5778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan R, Zhong J, Zheng S, Wang Z, Xu Y, Li
S, Zhou J and Yuan F: microRNA-218 increase the sensitivity of
gastrointestinal stromal tumor to imatinib through PI3K/AKT
pathway. Clin Exp Med. Apr 5–2014.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stellas D and Patsavoudi E: Inhibiting
matrix metalloproteinases, an old story with new potentials for
cancer treatment. Anticancer Agents Med Chem. 12:707–717. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xin SY, Feng XS, Zhou LQ, Sun JJ, Gao XL
and Yao GL: Reduced expression of circulating microRNA-218 in
gastric cancer and correlation with tumor invasion and prognosis.
World J Gastroenterol. 20:6906–6911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kinoshita T, Hanazawa T, Nohata N, et al:
Tumor suppressive microRNA-218 inhibits cancer cell migration and
invasion through targeting laminin-332 in head and neck squamous
cell carcinoma. Oncotarget. 3:1386–1400. 2012.PubMed/NCBI
|
|
39
|
Yamamoto N, Kinoshita T, Nohata N, Itesako
T, Yoshino H, Enokida H, Nakagawa M, Shozu M and Seki N: Tumor
suppressive microRNA-218 inhibits cancer cell migration and
invasion by targeting focal adhesion pathways in cervical squamous
cell carcinoma. Int J Oncol. 42:1523–1532. 2013.PubMed/NCBI
|
|
40
|
Hassan MQ, Maeda Y, Taipaleenmaki H, et
al: miR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo F, Carter DE and Leask A: miR-218
regulates focal adhesion kinase-dependent TGFβ signaling in
fibroblasts. Mol Biol Cell. 25:1151–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Knight ZA, Gonzalez B, Feldman ME, et al:
A pharmacological map of the PI3-K family defines a role for
p110alpha in insulin signaling. Cell. 125:733–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hales EC, Taub JW and Matherly LH: New
insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling
axis: Targeted therapy of γ-secretase inhibitor resistant T-cell
acute lymphoblastic leukemia. Cell Signal. 26:149–161. 2014.
View Article : Google Scholar
|
|
44
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim SM, Park JH, Kim KD, Nam D, Shim BS,
Kim SH and Ahn KS, Choi SH and Ahn KS: Brassinin induces apoptosis
in PC-3 human prostate cancer cells through the suppression of
PI3K/Akt/mTOR/S6K1 signaling cascades. Phytother Res. 28:423–431.
2014. View Article : Google Scholar
|
|
46
|
Jung KH, Yan HH, Fang Z, Son MK, Lee H,
Hong S and Hong SS: HS-104, a PI3K inhibitor, enhances the
anticancer efficacy of gemcitabine in pancreatic cancer. Int J
Oncol. 45:311–321. 2014.PubMed/NCBI
|
|
47
|
Slotkin EK, Patwardhan PP, Vasudeva SD, de
Stanchina E, Tap WD and Schwartz GK: MLN0128, an ATP-competitive
mTOR kinase inhibitor with potent in vitro and in vivo antitumor
activity, as potential therapy for bone and soft-tissue sarcoma.
Mol Cancer Ther. 14:395–406. 2015. View Article : Google Scholar
|
|
48
|
Yuan H, Yang P, Zhou D, Gao W, Qiu Z, Fang
F, Ding S and Xiao W: Knockdown of sphingosine kinase 1 inhibits
the migration and invasion of human rheumatoid arthritis
fibroblast-like synoviocytes by down-regulating the PI3K/AKT
activation and MMP-2/9 production in vitro. Mol Biol Rep.
41:5157–5165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014.PubMed/NCBI
|
|
50
|
Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S
and Fu S: Id1 enhances human ovarian cancer endothelial progenitor
cell angiogenesis via PI3K/Akt and NF-κB/MMP-2 signaling pathways.
J Transl Med. 11:1322013. View Article : Google Scholar
|
|
51
|
Li X, Yang Z, Song W, et al:
Overexpression of Bmi-1 contributes to the invasion and metastasis
of hepatocellular carcinoma by increasing the expression of matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor via the PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802.
2013.PubMed/NCBI
|