Introduction
Tribulus terrestris (T. terrestris) is
a herbal remedy that has a variety of uses in folk medicine. In
traditional medicine, the extract from T. terrestris has
been used to treat various diseases including hypertension,
coronary heart disease (1),
fungal diseases and infertility in both genders (2,3).
It has also been described as a highly valuable drug that can help
to restore decreased liver function, and it is used in the
treatment of diabetes and hyperlipidemia (4,5).
In traditional Chinese medicine, the fruit of T. terrestris
has been used to treat pruritus, edema, tracheitis and inflammation
(6). N-trans-ρ-caffeoyl
tyramine (CT) is one of the compounds isolated from T. terres-
tris (7). A previous study
reported that CT acts as an antioxidant and moderately inhibits
acetylcholinesterase in vitro and in vivo (8). However, the anti-inflammatory
effects of CT have not yet been completely elucidated.
Inflammation is a complex pathological process
mediated by diverse molecules involving a variety of immune cells,
such as leukocytes, macrophages and mast cells (9). Nitric oxide (NO) and prostaglandin
E2 (PGE2) are involved in various
pathophysiological processes, including inflammation, and inducible
NO synthase (iNOS) and cyclooxygenase-2 (COX-2) are mainly
responsible for the production of large quantities of these
mediators (10,11). NO produced by the constitutive
isoform of NO synthase (NOS) is a key regulator of homeostasis;
however, the generation of NO by iNOS plays a significant role in
inflammation (12). Activated
macrophages play a pivotal role in inflammatory diseases, as they
excessively produce pro-inflammatory cytokines, including tumor
necrosis factor-α (TNF-α) and inflammatory mediators, such as NO
and PGE2 (13,14). PGE2 is another
important inflammatory mediator and is produced from arachidonic
acid metabolites by the catalysis of COX-2 (15). PGE2 is related to the
pathogenesis of acute and chronic inflammatory states (16), and specific COX-2 inhibitors
decrease the symptoms of inflammation (17).
In the present study, we examined the
anti-inflammatory effects of CT isolated from T. terrestris
on lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Our
findings demonstrated that CT inhibited NO production and
suppressed the expression COX-2 and cytokines related to
inflammation in LPS-stimulated RAW 264.7 cells.
Materials and methods
Preparation of T. terrestris extract
The dried fruit of T. terrestris (Fructus
Tribuli) was purchased from the Gyeongdong oriental Herbal
Store, Seoul, Korea, in March 2012 and was formally identified by
Professor Joa Sub Oh (College of Pharmacy, Dankook University,
Cheonan, Korea). A voucher specimen (G46) was deposited at the
Natural Products Research Laboratory, Gyeonggi Institute of Science
and Technology Promotion, Suwon, Korea. The air-dried, crushed
fruits of T. terrestris (10 kg) were pulverized and the
extract was removed with 80% ethanol (EtOH; 3×18 liters) at room
temperature (twice each day for 2 days).
Extraction and isolation of CT
The 80% EtOH extract was filtered and concentrated
in vacuo at 40°C to yield 673.5 g of residue, and the
residue was then suspended in water and partitioned with hexane
(3×1.5 liters) to produce a hexane-soluble layer (40 g). The
aqueous layer was partitioned with CHCl3 to provide a
CHCl3-soluble residue (8.1 g). The CHCl3
layer was subjected to liquid chromatography [glass column (7×20
cm) packed with silica gel (230–400 mesh)] using
CHCl3:MeOH (100:0, 99:1, 98:2, 97:3, 96:4, 94:6, 92:8,
90:10, 80:20, 70:30, 60:40, 50:50; v/v) gradient mixtures as
eluents. The eluent fractions G46-51-(1–13)
were obtained from this initial liquid chromatographic separation.
The fractions F001-F011 were subjected to an in vitro
bioassay to evaluate their NO inhibitory activity. The fraction
G46-51-7 exhibited promising inhibitory activity against NO
production and was thus selected for further analysis. Column
chromatography of the CHCl3-soluble layer (8.1 g) on a
silica gel using MeOH, with increasing polarity, yielded 13
fractions, G46-51-(1–13). Fraction G46-51-7 (2.71 g) was
further applied to flash column chromatography on a sephadex LH-20
column using CHCl3:MeOH (1:1), and 21 fractions were
noted: G46-52-(1–21). Of these 21 fractions, CT (97.5 mg)
was isolated from fraction G46-52-12, which was precipitated with
CHCl3. 1H- and 13C-NMR spectra
were recorded on a Bruker Ascend 700 MHz spectrometer (Bruker,
Billerica, MA, USA) using CDCl3 as a solvent. Electrospray
ionization (ESI) mass spectra were obtained on an LTQ Orbitrap XL
(Thermo Scientific, Bremen, Germany) mass spectrometer.
N-trans-ρ-caffeoyl tyramine (CT)
Amorphous powder; 1H-NMR
(CD3OD, 700 MHz) δ: 7.40 (1H, d, J=15.4 Hz,
H-7′), 7.07 (2H, d, J=8.4 Hz, H-2, 6), 7.01 (1H, d,
J=1.4 Hz, H-2′), 6.92 (1H, dd, J=8.4, 2.1 Hz, H-6′),
6.78 (1H, d, J=8.4 Hz, H-5′), 6.74 (2H, d, J=8.4 Hz,
H-3, 5), 6.35 (1H, d, J=15.4 Hz, H-8′), 3.47 (1H, t,
J=7.0 Hz, H-7), 2.77 (1H, t, J=7.0 Hz, H-8);
13C-NMR (CD3OD, 175 MHz) δ 167.9 (C-9′),
155.5 (C-4), 147.3 (C-4′), 145.3 (C-3′), 140.8 (C-7′), 129.9
(C-1′), 129.3 (C-2, 6), 126.9 (C-1), 120.7 (C-6′), 117.0 (C-8′),
115.0 (C-5′), 114.8 (C-3, 5), 113.6 (C-2′), 41.1 (C-8), 34.4 (C-7);
ESI mass spectrometry (ESIMS; negative) m/z 298
[M-H]− (18). The
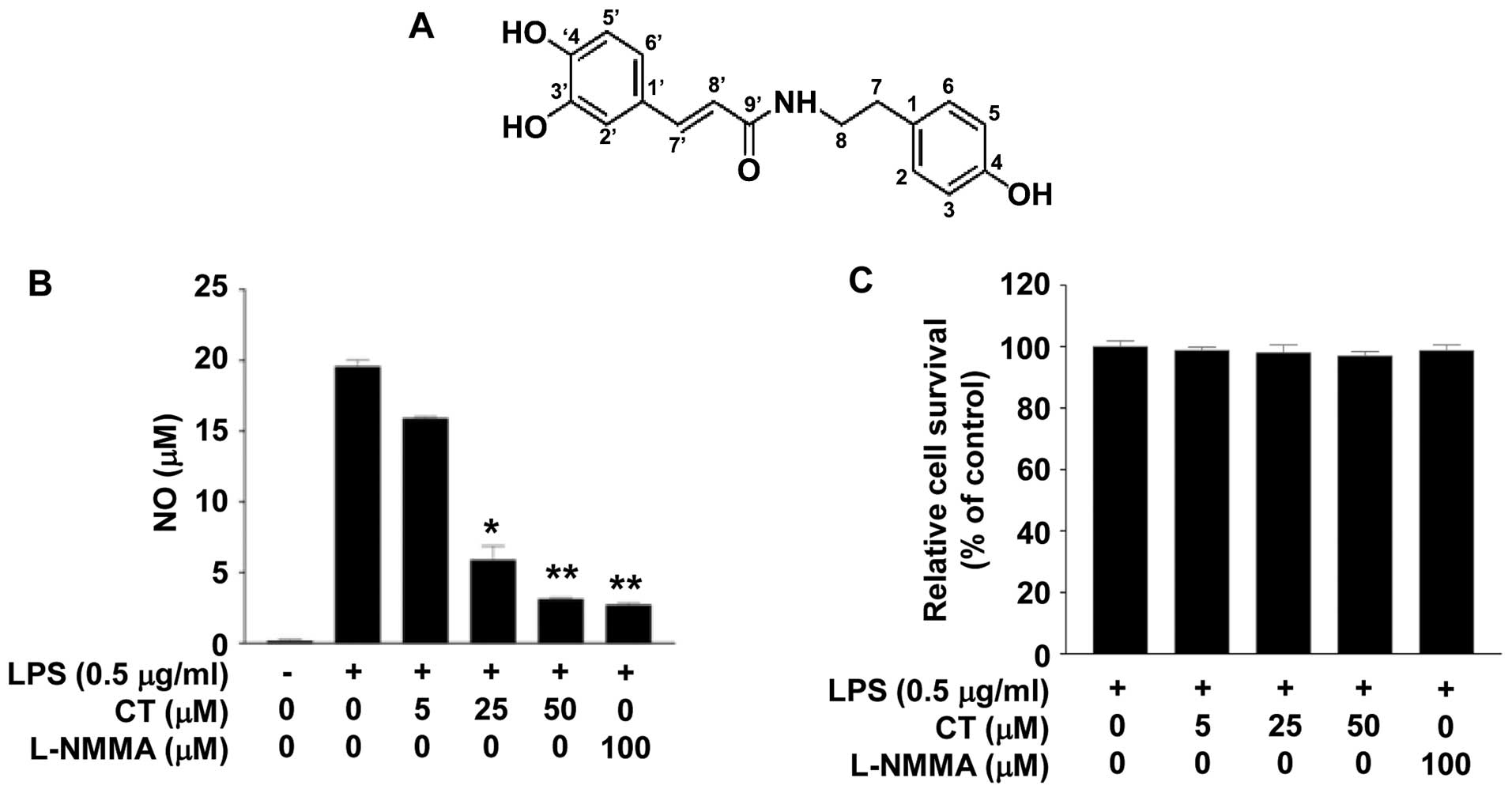
structure of CT is presented in Fig.
1A.
Reagents
The following pharmacological agents and antibodies
were purchased from commercial sources: LPS from Escherichia
coli serotype 0111:B4, celecoxib, NG-monom
ethyl-l-arginine (L-NMMA) and dexamethasone (all from
Sigma-Aldrich, St. Louis, MO, USA); anti-COX-2 (M-19; sc-1747),
anti-β-actin (13E5) and anti-GAPDH antibodies, and goat and mouse
IgG-horseradish peroxidase conjugates (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA); anti-c-Jun N-terminal
protein kinase (JNK; #9251) and anti-phospho-JNK (Thr183/Tyr185)
antibodies (both from Cell Signaling Technology, Beverly, MA,
USA).
Cell culture and NO assay
RAW 264.7 murine macrophages (TIB-71) were purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both
from Gibco® Life Technologies, Inc., Grand Island, NY,
USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin (both from
Gibco® Life Technologies, Inc.) in a humidified
atmosphere of 95% air with 5% CO2 at 37°C. On day 0, the
cells were seeded in 96 well plates. After 24 h, the cells were
stimulated with medium (0.5 μg/ml LPS in 10% FBS-DMEM) for 2
h, and then this medium was replaced with maintenance medium (10%
FBS-DMEM). The cells were treated with various concentrations of CT
(0–50 μM) for 24 h. We then measured the levels of nitrite,
a stable metabolite of NO, using Griess reagent (1% sulfanilamide
and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 2.5%
phosphoric acid; Sigma-Aldrich). Subsequently, the mixture was
incubated at room temperature for 10 min, and the absorbance was
measured at 540 nm. The quantity of nitrite was determined from a
standard curve for sodium nitrite (Sigma-Aldrich).
Cell cytotoxicity assay
The
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) assay was used for the determination of cell
viability in vitro in the RAW 264.7 cells. The cells were
plated at a density of 4×104 cells/well in 100 μl
culture medium. One day after plating, a time zero control plate
was made. Following stimulation of the cells with LPS for 2 h, CT
was applied directly, and the cells were incubated for 24 h in a
humidified atmosphere with 5% CO2 at 37°C. Cell culture
was then performed. MTT (5 mg/ml in PBS) was added to each well,
followed by incubation for 90 min. The medium was removed from the
wells by aspiration; subsequently, 0.1 ml of buffered dimethyl
sulfoxide (DMSO; Sigma-Aldrich) was added to each well, and the
plates were shaken. The absorbance was measured on a microtiter
plate reader at 540 nm.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed for the determination of the
levels of cytokines in vitro in the RAW 264.7 cells. The
cells were plated at a density of 4×104 cells/well in
100 μl culture medium. One day after plating, a time zero
control plate was made. Following stimulation of the cells with LPS
for 2 h, CT was applied directly and the cells were incubated for
24 h in a humidified atmosphere with 5% CO2 at 37°C.
Cell culture was then performed. The supernatants were harvested
and assayed for cytokines by ELISA. The concentrations of
interleukin (IL)-6, IL-10 and TNF-α in the culture medium were
quantified using a platinum ELISA kit (eBioscience, San Diego, CA,
USA), and the concentration of PGE2 in the culture
medium was quantified using a competitive enzyme ELISA kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
instructions, respectively.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using a total RNA extraction
kit (Ambion, Carlsbad, CA, USA). Five micrograms of RNA were used
as a template for each RT-PCR reaction using the SuperScript™ III
One-Step RT-PCR system (Invitrogen, Carlsbad, CA, USA). Newly
synthesized cDNA from the RAW 264.7 control cells and CT-treated
cells was amplified using specific primers and the
Accupower® Pfu PCR PreMix (Bioneer, Daejeon, Korea). The
sequences of the primers used for RT-PCR are shown in Table I.
 | Table IThe primer sequence used for
RT-PCR. |
Table I
The primer sequence used for
RT-PCR.
| Target | Primer
sequence | Accession no. |
|---|
| GAPDH | Sense:
5′-GTATGACTCCACTCACGGCAAA-3′ | |
| Antisense:
5′-GGTCTCGCTCCTGGAGAGATG-3′ | NM_008084 |
| IL-6 | Sense:
5′-CACTTCACAAGTCGGAGGCTT-3′ | |
| Antisense:
5′-GCAAGTGCATCATCGTTGTTC-3′ | NM_031168 |
| IL-10 | Sense:
5′-CCTGGTAGAAGTGATGCCCCAGGCA-3′ | |
| Antisense:
5′-CTATGCAGTTGATGAAGATGTCAAA-3′ | NM_010548 |
| COX-2 | Sense:
5′-GGAGAGACTATCAAGATAGTGATC-3′ | |
| Antisense:
5′-ATGGTCAGTAGACTTTTACAGCTC-3′ | NM_011198 |
| TNF-α | Sense:
5′-AGCCTGTAGCCCACGTCGTA-3′ | |
| Antisense:
5′-TCTTTGAGATCCATGCCGTTG-3′ | NM_013693 |
Western blot analysis
The cells were harvested and washed with PBS and
then collected by centrifugation at 13,000 rpm for 1 min at 4°C. To
obtain the cell lysate, the cells were lysed on ice for 30 min in
RIPA buffer [50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1% NP-40, 0.1%
sodium dodecyl sulfate (SDS), 1 mM dithiothreitol (DTT) and 1 mM
phenylmethanesulfonyl fluoride (PMSF)], which contained protease
inhibitors (Roche, Mannheim, Germany). Insoluble materials were
removed by centrifugation at 13,000 rpm for 10 min at 4°C. A total
of 50 mg of the supernatants was separated using a 10%
polyacrylamide gel containing 10% SDS, 1.5 M Tris-HCl, 0.035%
N,N,N′,N′-tetramethylenediamine and 7 mg ammonium
persulfate. The separated proteins were electrically transferred
onto a nitrocellulose membrane (Whatman, Dassel, Germany) at 36 mA
in a transfer buffer containing 39 mM glycine, 48 mM Tris base,
0.037% SDS and 20% MeOH. All western blot analyses were performed
at least in triplicate, and representative blots are shown.
Statistical analysis
Data are expressed as the means ± SD. The
statistical significance of the experimental results was analyzed
(Student's t-test and one-way ANOVA with a subsequent Dunnett's
multiple-range test). P-values <0.05 were considered to indicate
statistically significant differences.
Results
Effects of CT on NO production and
cytotoxicity in LPS-stimulated RAW 264.7 cells
The chemical structure of CT is illustrated in
Fig. 1A. To examine the effects
of CT on the inflammatory response, we measured the levels of NO
production following treatment of the LPS (0.5 μg/ml)-
stimulated RAW 264.7 cells with CT (0, 5, 25 or 50 μM) for
24 h. Treatment with CT induced a marked decrease in NO levels in
the LPS-stimulated cells in a dose-dependent manner. Treatment with
50 μM CT induced an 84.07% decrease in NO production. We
also confirmed that this result was similar to that achieved by
treatment with 100 μM L-NMMA (Fig. 1B), as also previously demonstrated
(19). To evaluate the
cytotox-icity of CT, we conducted an MTT assay. Treatment with 5,
25 or 50 μM CT did not have a marked cytotoxic effect on the
LPS-stimulated RAW 264.7 cells (Fig.
1C).
Effects of CT on the expression and
production of cytokines in LPS-stimulated RAW 264.7 cells
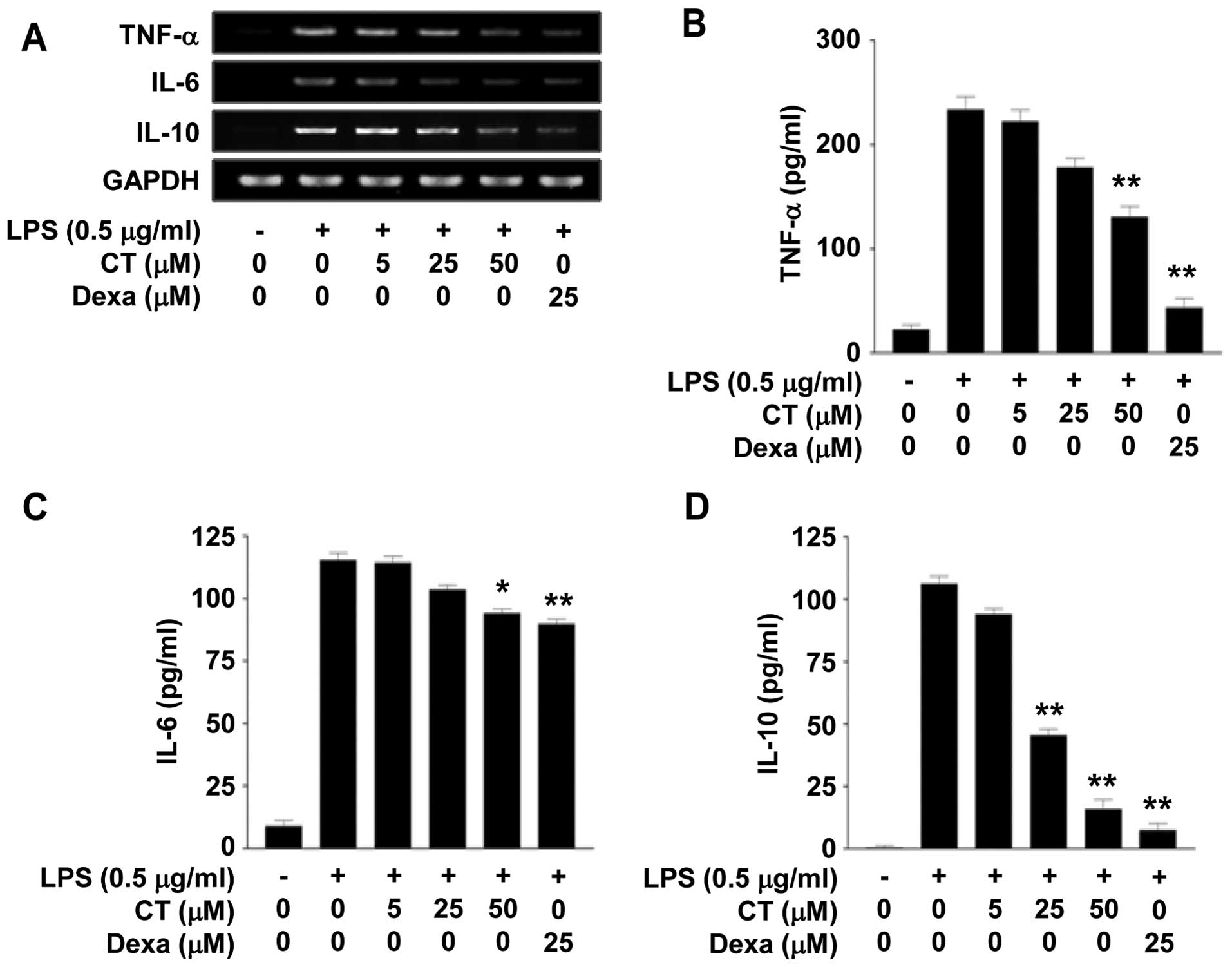
We investigated the effects of CT on the expression
of TNF-α, IL-6 and IL-10, which are pro-inflammatory cytokines, in
the LPS-stimulated RAW 264.7 cells. Firstly, we measured the mRNA
expression levels of TNF-α, IL-6 and IL-10 by RT-PCR following
treatment with 5, 25 or 50 μM CT. We observed that
treastment with CT suppressed the mRNA levels of TNF-α, IL-6 and
IL-10 in a dose-dependent manner (Fig. 2A). Treatment with dexamethasone
(25 μM), which is a potent synthetic member of the
glucocorticoid class of steroid drugs, also inhibited the mRNA
expression of TNF-α, IL-6 and IL-10 (Fig. 2A). We then confirmed the effects
of CT on TNF-α, IL-6 and IL-10 at the protein level by ELISA. The
protein levels of TNF-α, IL-6 and IL-10 in the conditioned medium
were decreased following treatment with 5, 25 or 50 μM CT.
In particular, treatment with 50 μM CT significantly
inhibited the release of TNF-α, IL-6 and IL-10 by up to 44.13,
18.38 and 84.99%, respectively (Fig.
2B–D).
Effects of CT on COX-2 expression and
phosphorylation of mitogen-activated protein kinase (MAPK) in
LPS-stimulated RAW 264.7 cells
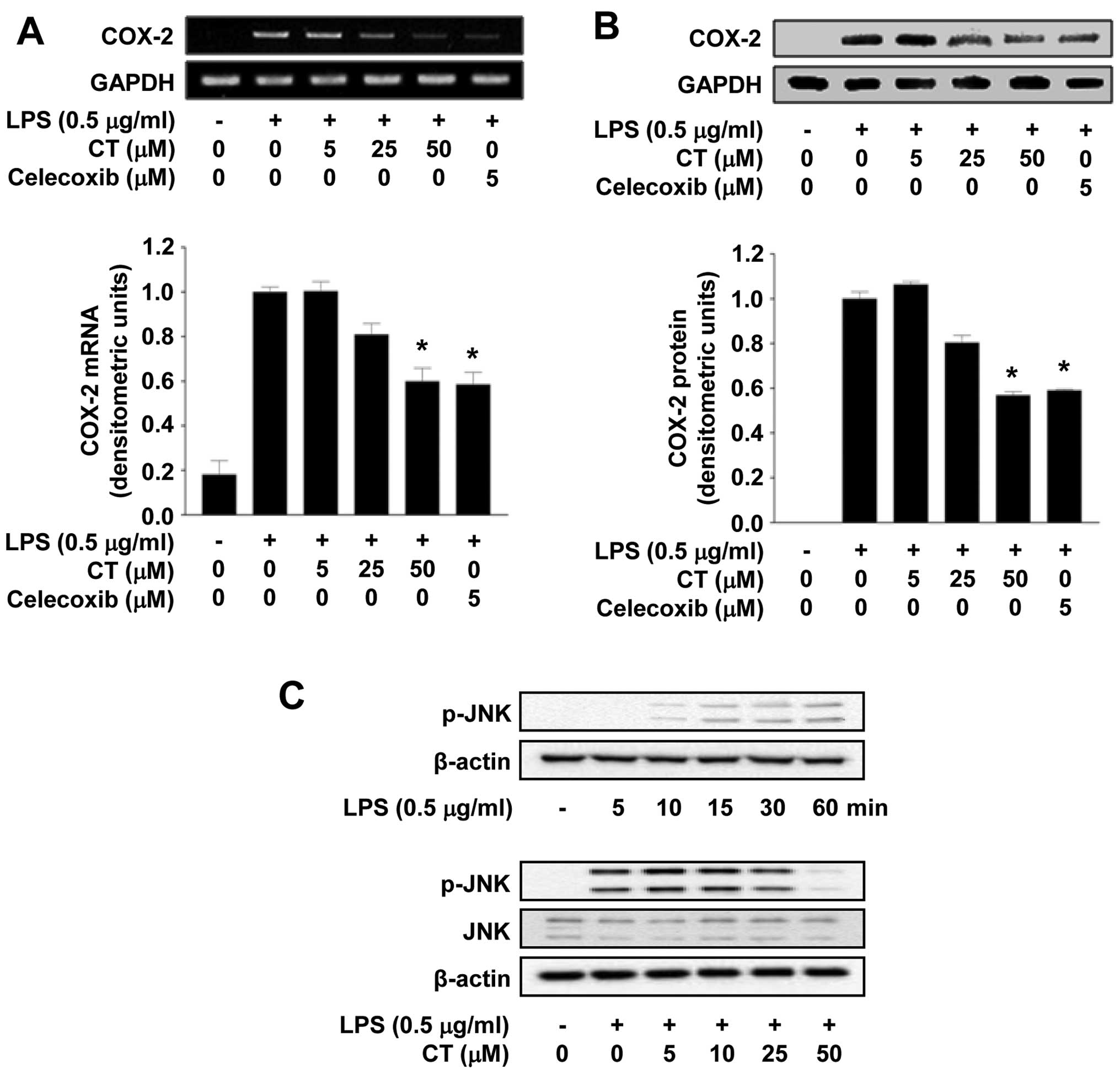
To determine the effects of CT on COX-2 expression,
we examined whether the expression of COX-2 is reduced at both the
mRNA and protein level in LPS-stimulated RAW 264.7 cells following
treatment with 5, 25 or 50 μM of CT. As shown in Fig. 3A, CT significantly inhibited COX-2
mRNA expression in a dose-dependent manner. Treatment with 5
μM of celecoxib, a well-known COX-2 inhibitor, significantly
inhibited COX-2 expression at the mRNA level. In addition,
treatment with 5, 25 or 50 μM CT also resulted in the
suppression of COX-2 expression at the protein level in a
dose-dependent manner, as evidenced by western blot analysis.
Treatment with celecoxib also significantly inhibited COX-2 protein
expression (Fig. 3B). Studies
have demonstrated that the LPS-induced phosphorylation of MAPKs
leads to the production of inflammatory cytokines (20,21). Thus, to determine whether the
activation of the MAPK pathway is regulated by CT, we measured the
phosphorylation levels of JNK. Treatment with CT (particularly with
50 μM CT) significantly inhibited the LPS-induced
phosphorylation of JNK, but did not affect the expression of JNK
(Fig. 3C).
Effects of CT on the PGE2
level in LPS-stimulated RAW 264.7 cells
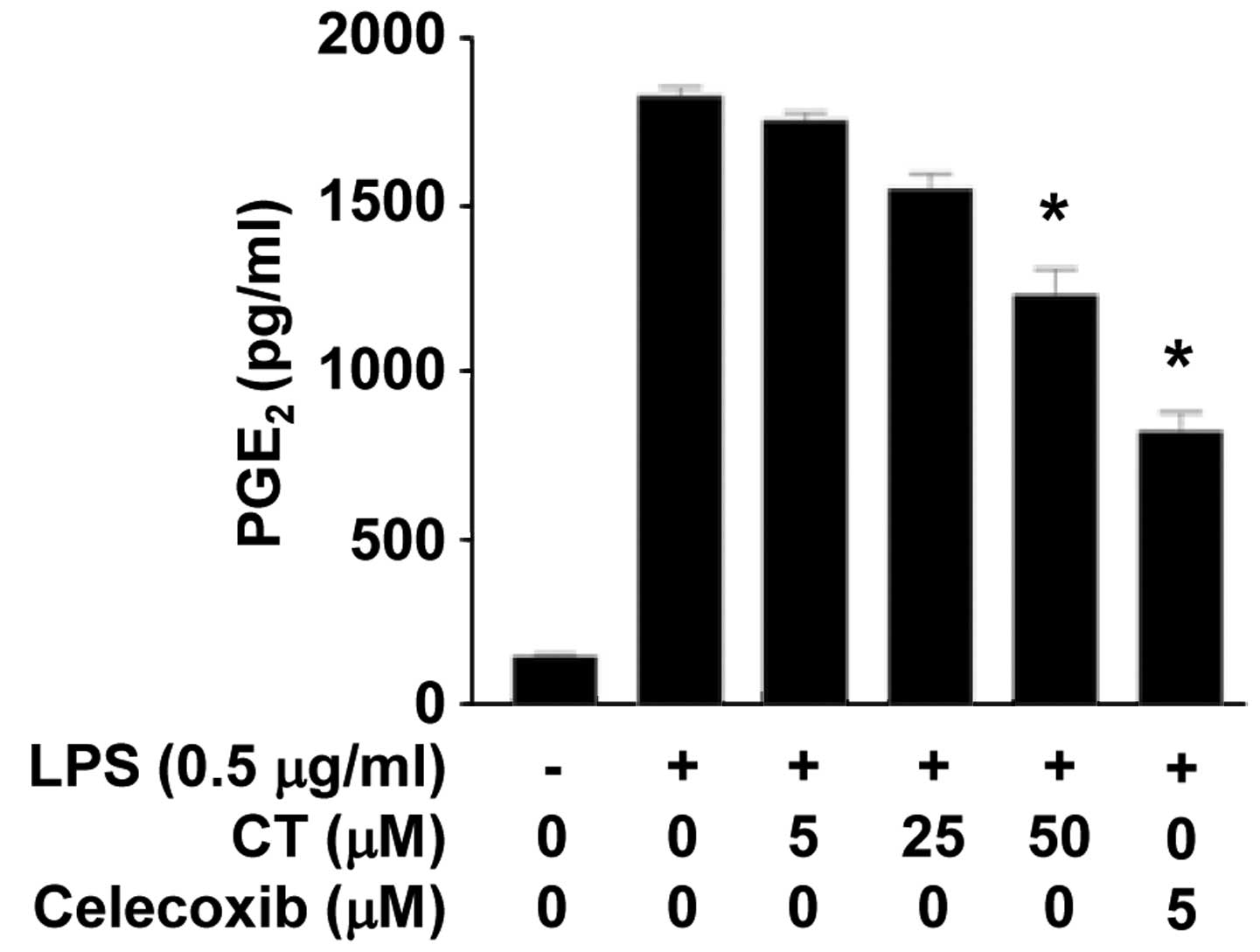
To confirm the effects of CT on PGE2, one
of the mediators produced by COX-2, we measured the secretion
levels of PGE2 following treatment of the LPS-stimulated
RAW 264.7 cells with CT (5, 25 or 50 μM) and celecoxib (5
μM). The conditioned media were collected and the
PGE2 content was measured by ELISA. As shown in Fig. 4, the levels of PGE2 in
the conditioned media were significantly decreased following
treatment with CT (50 μM) and celecoxib (5 μM).
Discussion
In this study, we demonstrated that CT isolated from
T. terrestris has a marked effect on the inflammatory
response and on the levels of related pro-inflammatory cytokines in
LPS-stimulated RAW 264.7 cells. We first examined the effects of an
80% ethanol extract of T. terrestris (EETT) on the
inflammatory response using an NO assay, and we observed the
dose-dependent suppression of NO production in the LPS-stimulated
RAW 264.7 cells (data not shown). A previous study demonstrated
that T. terrestris inhibited COX-2 expression using the
promoter assay (22). In the
present study, we isolated CT from the EETT, and we examined its
anti-inflammatory effects on RAW 264.7 murine macrophages. We
demonstrated that treatment with CT resulted in a decrease in NO
production in the LPS-stimulated macrophages and that it did not
cause cytotoxicity under our experimental conditions. We also
observed that treatment with 100 μM L-NMMA, a well-known NOS
inhibitor, decreased NO production in the LPS-stimulated
macrophages (Fig. 1B).
Macrophages are known to play a key role in the host
defense mechanism; they are activated by exposure to interferon-γ,
pro-inflammatory cytokines and bacterial LPS (10). NO is endogenously generated from
L-NMMA by NOS, and it plays an important role in the regulation of
a number of physiological processes (23). TNF-α, IL-6 and IL-10 are the most
important pro-inflammatory cytokines. The cytokines, TNF-α, IL-6
and IL-10, are produced mainly by activated monocytes or
macrophages (24). In the present
study, we noted that the LPS-stimulated cells exhibited increased
levels of expression and production of pro-inflammatory cytokines
compared to the unstimulated cells. Our data indicated that
treatment with CT reduced the expression of TNF-α, IL-6 and IL-10
at the mRNA level (Fig. 2A), and
it suppressed the secretion of TNF-α, IL-6 and IL-10 at the protein
level in the LPS-treated macrophages (Fig. 2B).
Glucocorticoids are a class of steroid hormones with
pleiotropic effects. At pharmacological concentrations,
glucocorticoids are used to prevent and suppress inflammation and
the activation of the immune system. Steroids exert their
anti-inflammatory effects mainly by modulating the transcription of
a variety of genes involved in controlling inflammatory processes
(25). Our results indicated that
treatment with dexamethasone, which is one of the glucocorticoids,
induced a decrease in the levels of TNF-α, IL-6 and IL-10 by up to
81.39, 22.19 and 93.13%, respectively (Fig. 2B–D). However, glucocorticoids are
known to have serious side-effects (26), and hence it was our aim to obtain
a drug from natural sources.
Prostaglandins (PGs) are key inflammatory mediators;
they are produced from the conversion of arachidonic acid by COX.
There are two isoforms of COX: COX-1 and COX-2 (27). COX-1 is the constitutively
expressed isoform under normal physiological conditions, whereas
COX-2 is expressed in response to inflammatory signals, such as
cytokines and the bacteria endotoxin LPS. Celecoxib, which is a
COX-2 selective inhibitor, is a useful drug for the treatment of
acute pain and chronic inflammatory diseases, particularly
arthritis (28); however, it is
known to cause various side-effects. In this study, we demonstrated
that treatment of the cells with 25 or 50 μM of CT, or 5
μM celecoxib, inhibited the expression of COX-2 at the mRNA
and protein level (Fig. 3A and
B). These findings suggest that CT isolated from T.
terrestris exerts a therapeutic effect and prevents
inflammatory responses by acting as a COX-2 selective inhibitor,
and may thus be a potentially safe naturally-derived drug which may
be used in the treatment of inflammatory diseases. Salvemini et
al reported that NO modulates the activity of COX-2 and plays a
role in the release of PGE2 by activating COX-2
(29). COX-2 produces large
amounts of PGE2 that induce an inflammatory response
(17). Therefore, the release of
the inflammatory mediator PGE2 is promoted by COX-2
activation. Our results demonstrated that treatment with CT (50
μM) induced a 32.70% decrease in PGE2 levels
(Fig. 4). These results suggest
that CT exerts an anti-inflammatory effect by suppressing COX-2
expression, which results in the inhibition of PGE2
synthesis.
In conclusion, in this study, we demonstrated that
CT can markedly inhibited macrophage-mediated inflammatory
responses through the suppression of the production of NO and
pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-10.
Moreover, CT inhibited the expression of COX-2, the phosphorylation
of JNK and PGE2 synthesis. These findings suggest that
CT has a therapeutic effect and may be used to prevent inflammatory
diseases. Thus, it can be considered as a potential drug candidate
for the treatment of arthritis and other inflammatory diseases,
functioning as a COX-2-specific inhibitor.
Acknowledgments
The present study was conducted by the research fund
of Dankook University in 2013.
References
|
1
|
Phillips OA, Mathew KT and Oriowo MA:
Antihypertensive and vasodilator effects of methanolic and aqueous
extracts of Tribulus terrestris in rats. J Ethnopharmacol.
104:351–355. 2006. View Article : Google Scholar
|
|
2
|
Adimoelja A: Phytochemicals and the
breakthrough of traditional herbs in the management of sexual
dysfunctions. Int J Androl. 23(Suppl 2): 82–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang JD, Cao YB, Xu Z, Sun HH, An MM, Yan
L, Chen HS, Gao PH, Wang Y, Jia XM and Jiang YY: In vitro and in
vivo antifungal activities of the eight steroid saponins from
Tribulus terrestris L. with potent activity against
fluconazole-resistant fungal pathogens. Biol Pharm Bull.
28:2211–2215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu S, Qu W, Pang X, Sun B and Huang X:
Effect of saponin from Tribulus terrestris on hyperlipidemia. Zhong
Yao Cai. 26:341–344. 2003.In Chinese. PubMed/NCBI
|
|
5
|
Amin A, Lotfy M, Shafiullah M and Adeghate
E: The protective effect of Tribulus terrestris in diabetes. Ann NY
Acad Sci. 1084:391–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiangsu New Medical College: Dictionary of
the Chinese Herbal Medicine. Shanghai People's Publishing House;
Shanghai: pp. 12741977
|
|
7
|
Lv AL, Zhang N, Sun MG, Huang YF, Sun Y,
Ma HY, Hua HM and Pei YH: One new cinnamic imide dervative from the
fruits of Tribulus terrestris. Nat Prod Res. 22:1013–1016. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Taweel AM, Perveen S, El-Shafae AM,
Fawzy GA, Malik A, Afza N, Iqbal L and Latif M: Bioactive phenolic
amides from Celtis africana. Molecules. 17:2675–2682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willeaume V, Kruys V, Mijatovic T and Huez
G: Tumor necrosis factor-alpha production induced by viruses and by
lipopolysac-charides in macrophages: similarities and differences.
J Inflamm. 46:1–12. 1996.
|
|
10
|
Xie QW, Whisnant R and Nathan C: Promoter
of the mouse gene encoding calcium-independent nitric oxide
synthase confers inducibility by interferon γ and bacterial
lipopolysaccharide. J Exp Med. 177:1779–1784. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vane JR, Mitchell JA, Appleton I,
Tomlinson A, Bishop-Bailey D, Croxtall J and Willoughby DA:
Inducible isoforms of cyclo-oxygenase and nitric-oxide synthase in
inflammation. Proc Natl Acad Sci USA. 91:2046–2050. 1994.
View Article : Google Scholar
|
|
12
|
Nathan C and Xie QW: Nitric oxide
synthases: roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon WJ, Ham YM, Yoo BS, Moon JY, Koh J
and Hyun CG: Oenothera Iaciniata inhibits lipopolysaccharide
induced production of nitric oxide, prostaglandin E2, and
proinflam-matory cytokines in RAW264.7 macrophages. J Biosci
Bioeng. 107:429–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reddy DB and Reddanna P: Chebulagic acid
(CA) attenuates LPS-induced inflammation by suppressing NF-kappaB
and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res
Commun. 381:112–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami A and Ohigashi H: Targeting NOX,
INOS and COX-2 in inflammatory cells: Chemoprevention using food
phytochemicals. Int J Cancer. 121:2357–2363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hinz B, Brune K and Pahl A: Prostaglandin
E(2) upregulates cyclooxygenase-2 expression in
lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem
Biophys Res Commun. 272:744–748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crofford LJ, Lipsky PE, Brooks P, Abramson
SB, Simon LS and van de Putte LB: Basic biology and clinical
application of specific cyclooxygenase-2 inhibitors. Arthritis
Rheum. 43:4–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen T, He J, Zhang J, Li X, Zhang H, Hao
J and Li L: The isolation and identification of two compounds with
predominant radical scavenging activity in hempseed (seed of
Cannabis sativa L.). Food Chem. 134:1030–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olken NM and Marletta MA:
NG-methyl-L-arginine functions as an alternate substrate
and mechanism-based inhibitor of nitric oxide synthase.
Biochemistry. 32:9677–9685. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uto T, Suangkaew N, Morinaga O, Kariyazono
H, Oiso S and Shoyama Y: Eriobotryae folium extract suppresses
LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB
and MAPK activation in murine macrophages. Am J Chin Med.
38:985–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu T, Lee YJ, Yang HM, Han S, Kim JH, Lee
Y, Kim C, Han MH, Kim MY, Lee J and Cho JY: Inhibitory effect of
Sanguisorba officinalis ethanol extract on NO and PGE2
production is mediated by suppression of NF-κB and AP-1 activation
signaling cascade. J Ethnopharmacol. 134:11–17. 2011. View Article : Google Scholar
|
|
22
|
Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA and
Lee SK: Evaluation of natural products on inhibition of inducible
cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured
mouse macrophage cells. J Ethnopharmacol. 83:153–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dawson TM, Dawson VL and Snyder SH: A
novel neuronal messenger molecule in brain: the free radical,
nitric oxide. Ann Neurol. 32:297–311. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walker G, Pfeilschifter J and Kunz D:
Mechanisms of suppression of inducible nitric-oxide synthase (iNOS)
expression in interferon (IFN)-gamma-stimulated RAW 264.7 cells by
dexa-methasone. Evidence for glucocorticoid-induced degradation of
iNOS protein by calpain as a key step in post-transcriptional
regulation. J Biol Chem. 272:16679–16687. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boumpas DT, Chrousos GP, Wilder RL, Cupps
TR and Balow JE: Glucocorticoid therapy for immune-mediated
diseases: basic and clinical correlates. Ann Intern Med.
119:1198–1208. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchell JA, Larkin S and Williams TJ:
Cyclooxygenase-2: regulation and relevance in inflammation. Biochem
Pharmacol. 50:1535–1542. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiba A, Mizuno M, Tomi C, Tajima R,
Alloza I, di Penta A, Yamamura T, Vandenbroeck K and Miyake S: A
4-trifluoro-methyl analogue of celecoxib inhibits arthritis by
suppressing innate immune cell activation. Arthritis Res Ther.
14:R92012. View
Article : Google Scholar
|
|
29
|
Salvemini D, Misko TP, Masferrer JL,
Seibert K, Currie MG and Needleman P: Nitric oxide activates
cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:7240–7244. 1993.
View Article : Google Scholar : PubMed/NCBI
|