Introduction
Diabetic myopathy, characterized by a reduction in
muscle mass, strength and physical capacity (1,2),
is a serious, but often overlooked complication of diabetes that
contributes to an overall worsening of the diabetic condition.
Studies have reported that the skeletal muscle of individuals with
type 2 diabetes mellitus (T2DM) exhibits an increased number of
glycolytic fibers (3), muscle
atrophy (4) and a decrease in
capillary density (5). Functional
impairments are also evident, as demonstrated by a decline in
muscle strength and motor dysfunction (6). The pathogenesis of diabetic myopathy
is very complex.
Oxidative stress, induced by an abundance of
reactive oxygen species (ROS) or by the failure of the antioxidant
defense machinery, is considered a critical factor for the
pathogenesis of diabetes (7).
Manganese superoxide dismutase (MnSOD), also known as SOD2, is a
primary mitochondrial antioxidant that neutralizes mitochondrial
ROS through the conversion of super-oxide to hydrogen peroxide, and
ultimately to H2O by catalase (8). In humans, mutations in MnSOD are
associated with age-related disorders, such as cardiovascular
disease, insulin resistance and diabetes, indicating that upstream
signaling proteins that regulate MnSOD may also play a role in
these pathologies (9).
AMP-activated protein kinase (AMPK) is an ubiquitous
heterotrimeric serine/threonine protein kinase, which functions as
a fuel sensor in a number of tissues, including skeletal muscle
(10). The activation of AMPK
enhances the mRNA expression of peroxisome proliferator-activated
response-γ coactivator-1α (PGC1α) and MnSOD (11). PGC1α, as a pivotal factor for
mitochondrial function, has been shown to be essential for the
regulation of mitochondrial metabolism, biogenesis and oxidative
stress (12).
As a member of the sirtuin family, silent
mating-type information regulation 2 homolog 3 (SIRT3) is mainly
localized in the mitochondria and regulates several pivotal
oxidative pathways by targeting several enzymes involved in central
metabolism (13). It has been
demonstrated that SIRT3 functions as a downstream target of PGC1α,
which is directly regulated by AMPK and has multiple cellular
functions by deacetylating mitochondrial proteins, as well as MnSOD
(14–16). Thus, based on the data of previous
studies (17,18) indicating that the AMPK-PGC1α-SIRT3
pathway and MnSOD modulates mitochondrial biogenesis and oxidative
stress, we hypothesized that the levels of AMPK, PGC1α, SIRT3 and
MnSOD may be all decreased in the skeletal muscle of individuals
with diabetes, which may be responsible for increased oxidative
stress. Our findings demonstrated that the AMPK-PGC1α-SIRT3
signaling pathway was downregulated in the skeletal muscle of
diabetic patients.
Celastrol is a triterpenoid compound extracted from
the Chinese herb, Tripterygium wilfordii Hook.f., which is
known to have exert various biological effects, including
immunosuppressive, anti-inflammatory and antitumor effects
(19). Nowadays, it is widely
used in the treatment of diabetic nephropathy (20). Celastrol has been proven to exert
antioxidant effects, and has been shown to reduce ROS generation in
hypertensive rats and vascular smooth muscle cells (21). It also can promote the glutathione
(GSH) redox cycle by increasing the intracellular GSH content and
the GSH/GSSG ratio in macrophages (19). However, the antioxidant effect of
celastrol on skeletal muscle in individuals with diabetes has not
been fully investigated. Thus, in this study, we examined the
effects of celastrol on oxidative stress in the skeletal muscle of
diabetic rats, as well as the potential involvement of the
AMPK-PGC1α-SIRT3 signaling pathway. Our findings demonstrated that
celastrol exerted antioxidant effects in the skeletal muscle of
diatetic rats, and that these effects were partly mediated by the
activation of the AMPK-PGC1α-SIRT3 signaling pathway.
Materials and methods
Patients and sample collection
Approval for this study was obtained from the Ethics
Committee of the Metabolic Disease Hospital of Tianjin Medical
University, Tianjin, China and informed consent was obtained from
all participants prior to orthopedic surgery. This trial has been
verified by the Chinese Clinical Trial Registry, and its
registration number is ChiCTR-COC-15007025. All participants were
patients with lumbar disc herniation who required orthopedic
surgery. Among these patients, 10 patients suffered from T2DM and
the another 10 were non-diabetic patients. The patients with
diabetes had been previously diagnosed at the hospital. Patients
with cardiovascular disease, neoplastic disease, neurodegenerative
disease and acute inflammation were excluded from the study.
Blood samples were collected from the antecubital
vein at the first day after admission. All blood was drawn from the
patients at the same time in the morning (between 6 and 8 a.m.).
Laboratory test results were evaluated, including fasting plasma
glucose (FPG), blood urea nitrogen (BUN), creatinine, aspartate
aminotransferase (AST), alanine aminotransferase (ALT) and glycated
haemoglobin A1c (HbA1c), levels, and lipid profiles, including
total cholesterol (TC), high-density lipoprotein cholesterol
(HDL-C), low-density lipoprotein cholesterol (LDL-C) and
triglyceride (TG) levels. All the laboratory parameters were
assessed using conventional laboratory methods. The blood samples
were centrifuged for 15 min at 3,000 rpm (AU5400; Olympus, Tokyo,
Japan), and plasma was assayed for the laboratory parameters using
an automated analyzer (7600A-020; Hitachi, Tokyo, Japan)
Between 300 and 500 mg of paravertebral skeletal
muscle was obtained from patients undergoing lumbar vertebral disc
decompression discectomy. Dissections of skeletal muscle were
obtained within 10 min of delivery and snap-frozen in liquid
nitrogen and stored at −80°C until further analysis. Tissues were
also embedded in paraffin for histological analysis by hemotoxylin
and eosin (H&E) staining. For all surgerical procedures, a
spinal anesthesia and/or epidural were used. All muscle samples
were obtained at the time of surgery (between 8:30 a.m. and 13:00
p.m.). All patients were fasted overnight.
Animals and experimental design
Male Sprague-Dawley (SD) rats (n=90, 6 weeks old),
weighing 161±9 g, were purchased from Beijing Hua Fu Kang
Biotechnology Co., Ltd. (Beijing, China). Rats were given free
access to food and tap water and were caged individually under a
12-h light-dark cycle at a temperature of 22±3°C and humidity of
55±5%. All animal experiments were conducted in accordance with the
Principles of Laboratory Care, and approved by the Institutional
Animal Care and Use Committee, Metabolic Disease Hospital of
Tianjin Medical University.
The rats were randomly divided into the control (NC)
and the high energy diet (HED) groups. In the control group, the
animals received a standard chow diet, while the rats in the HED
group were fed with an additional high energy emulsion, as
previously described (22,23).
After 8 weeks on their respective diets, streptozotocin (STZ; 45
mg/kg; Sigma, St. Louis, MO, USA) dissolved in 0.1 mol/l citrate
buffer (pH 4.5) was injected into the caudal vein of the rats in
the HED group to establish a model of T2DM, while the rats in the
control group were injected with sodium citrate buffer. The rats
with blood glucose levels ≥16.7 mmol/l at 7 days after the STZ
injection were selected as the model of diabetes. On average, 80%
of the rats injected with STZ met these criteria. At 1 week
following the injection of STZ, the rats with successfully-induced
diabetes were randomly divided into the diabetes model (DM) group,
the celastrol low-dose group (1 mg/kg/day), the celastrol
middle-dose group (3 mg/kg/day) and the celastrol high-dose group
(6 mg/kg/day) (n=15 rats per group). The rats in the treatment
groups were administered celastrol by gavage, whereas the rats in
the NC and DM groups were administered an equal amount of distilled
water (2 ml). Following 8 weeks of the respective treatments, rats
were anesthetized with an intraperitoneal injection of sodium
pentobarbital (30 mg/kg body weight) and tissue samples were
collected for analysis. The paravertebral muscle was excised from
the rat bodies, and was cut perpendicularly along the longitudinal
axis and fixed in phosphate-buffered 20% formaldehyde. Histological
paraffin-embedded sections (5 µm) were then prepared for
H&E staining. The sections of paravertebral muscle were
snap-frozen in liquid nitrogen and stored at −80°C until further
analysis.
Measurement of biochemical and physical
parameters
Body weight and fasting blood glucose (FBG) levels
were measured each week. The overnight-fasted rats were weighed by
electronic balance (AM1100; Mettler-Toledo AG, Schwerzenbach,
Switzerland) at the same time in the morning (between 6 and 8
a.m.). Then blood samples were obtained from the caudal vein and
FBG levels were measured using a Accu-Chek Aviva glucometer (Roche
Diagnostics, Mannheim, Germany). After 8 weeks of treatment, the
rats were euthanized. Blood samples were obtained from the
retroorbital venous plexus by using plain microhematocrit capillary
tubes tubes (VWR, West Chester, PA, USA) and collected into tubes
containing EDTA at the time of sacrifice and were centrifuged at
3,000 × g/min for 15 min. Plasma was separated for measuring FPG,
BUN, serum creatinine (SCr), AST, ALT, TC and TG using an automatic
biochemistry analyzer (CD-1600CS; Abbott Labs, North Chicago, IL,
USA).
Measurements of mitochondrial oxidative
biochemical parameters
Mitochondrial-enriched supernatants were prepared
from frozen skeletal muscle samples as previously described
(24). The content of
malondialdehyde (MDA) and GSH was measured by enzyme-linked
immunosorbent assay (ELISA) using respective kits following the
manufacturer's instructions (Jiancheng Co. Ltd., Nanjing, China).
MnSOD activity was determined by spectrophotometry. Briefly,
reaction buffer (50 mM sodium phosphate, 0.1 mM EDTA, 0.01 mM
xanthine, and 0.01 mM cytochrome c, pH 7.8) was mixed with
0.005 U/ml xanthine oxidase and 2 mM sodium cyanide, and the
absorbance change at 550 nm was followed. Then sample was added
stepwise (in 20 µl increments at a concentration of 5–10
mg/ml), and the concentration of sample required to decrease the
rate of reaction by 50% (defined as 1 unit of MnSOD activity) was
calculated. Enzyme values are presented as units per milligram of
protein.
SIRT3 activity assays
Mitochondrial-enriched supernatants were prepared
from frozen skeletal muscle samples as described (24). SIRT3 enzyme activity in the
gastrocnemius mitochondria was assayed using a fluorometric kit
(Biomoles, Inc., Shoreline, WA, USA) as per the manufacturer's
instructions. Briefly, 25 µl distilled water, 5 µl
buffer, 5 µl fluorosubstrate peptide 5 µl NAD and 5
µl developer were added to each microtiter plate wells and
mixed well. Reactions were initiated by adding 5 µl samples
or buffer of samples or recombinant SIRT3 to matching well and
mixing thoroughly. SIRT3 activity was measured using a fluorimetric
microplate reader at 450 nm with an excitation of 350 nm. We
measured and calculated the rate of reaction, while the reaction
velocity remained constant. The enzyme activity was normalized to
the total protein content of each sample and the results are
expressed relative to the mean for the NC group.
Western blot analysis
Protein lysates were obtained by homogenizing
paravertebral muscle with lysis buffer. The protein concentration
was measured using the Bio-Rad protein assay (Bio-Rad, Richmond,
CA, USA). Approximately 50 µg protein was subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes. After
blocking with 5% dried milk in Tris-buffered saline (TBS)
containing 0.1% Tween-20 for 2 h, the membranes were subsequently
incubated overnight with primary antibodies diluted in 5% dried
milk-TBS containing 0.1% Tween-20. The primary antibodies were as
follows: anti-AMPK (#2532, 1:1,000), anti-phosphorylated (p-)AMPK
(:#2531, 1:1,000) (both from Cell Signaling Technology, Danvers,
MA, USA), anti-α-tubulin (sc-8035, 1;10,000), anti-β-actin
(sc-47778, 1:10,000) (both from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), anti-SIRT3 (ab86671, 1:500), anti-MnSOD
(ab13534, 1:2,000) and anti-PGC1α (ab54481, 1:1,000) (all from
Abcam Cambridge, MA, USA) antibodies. The membranes were then
incubated with appropriate horseradish-peroxidase-conjugated
secondary antibodies (sc-2005 and sc-2003, Santa Cruz
Biotechnology, Inc.). An enhanced chemiluminescence system was used
to visualize the bands. Densitometric analysis was performed using
a gel image analysis system (UVP Inc., San Gabriel, CA, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
The MnSOD, Sirt3 and PGC1α mRNA levels were
quantified by SYBR-Green Real-Time PCR (Invitrogen, Carlsbad, CA,
USA). RT-qPCR was performed as previously described (25). RNA was extracted from
paravertebral muscle using TRIzol reagent (Invitrogen). Reverse
transcription was performed using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The quantitative PCR (qPCR) measurement of individual cDNAs was
carried out using SYBR-Green dye to measure duplex DNA formation
with the LightCycler System (Roche Diagnostics). The data were
analyzed using Bio-Rad CFX manager software 1.6. The quantified
results were normalized to those of GADPH, using the 2-(ΔΔCT)
method. The nucleotide sequences of the PCR primers used were as
follows: MnSOD forward, 5′-ACTGAAGTTCAATGGTGGGG-3′ and reverse,
5′-GCTTGATAGCCTCCAGCAAC-3′; Sirt3 forward,
5′-TACAGAAATCAGTGCCCCGA-3′ and reverse, 5′-GGTGGACACAAGAACTGCTG-3′;
PGC1α forward, 5′-ATGAGAAGCGGGAGTCTGAA-3′ and reverse,
5′-GCGGTCTCTCAGTTCTGTCC-3′; GAPDH forward,
5′-TGCCACTCAGAAGACTGTGG-3′ and reverse,
5′-TTCAGCTCTGGGATGACCTT-3′.
Statistical analysis
All data are presented as the means ± SD.
Differences between groups were analyzed using the Student's t-test
and one-way analysis of variance (ANOVA) test using the statistical
software. SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Values of
p<0.05 were considered to indicate statistically significant
differences.
Results
Comparison between diabetic and
non-diabetic patients Comparison of physical and biochemical
parameters between diabetic and non-diabetic patients
There were no significant differences in gender, age
and body weight between the normal control and diabetic patients.
The FPG, HbA1c, TG, TC and LDL-C levels were significantly higher
in the diabetic patients compared to the normal controls (all
P<0.01). Although the HDL-C levels were decreased in the
diabetic patients, there were no significant differences between
the 2 groups (Table I).
 | Table IPhysical and biochemical parameters
of diabetic and non-diabetic patients. |
Table I
Physical and biochemical parameters
of diabetic and non-diabetic patients.
| Parameter | NC | DM | P-value |
|---|
| Gender (M/F) | 7/3 | 6/4 | P=0.64 |
| Age (years) | 60.80±6.02 | 62.10±5.80 | P=0.65 |
| BW (kg) | 77.05±5.37 | 79.40±5.84 | P=0.41 |
| FPG (mmol/l) | 5.13±0.42 | 8.70±1.01 | P<0.01 |
| HbA1c (%) | 5.54±0.24 | 7.55±0.57 | P<0.01 |
| TG (mmol/l) | 1.59±0.33 | 2.31±0.61 | P<0.01 |
| TC (mmol/l) | 4.73±0.36 | 5.44±0.35 | P<0.01 |
| HDL-C (mmol/l) | 1.34±0.09 | 1.28±0.10 | P=0.20 |
| LDL-C (mmol/l) | 2.58±0.33 | 3.47±0.29 | P<0.01 |
Changes in skeletal muscle of diabetic
patients
H&E staining of the paraspinal muscle of
non-diabetic patients was presented as normal (Fig. 1A and C), whereas the skeletal
muscle of the diabetic patients exhibited an irregular fiber
structure, with wide gaps, nuclei of diverse sizes, an increased
number of nuclei and some with an abnormal position, partly
inserted in the muscle fibers (Fig.
1B and D).
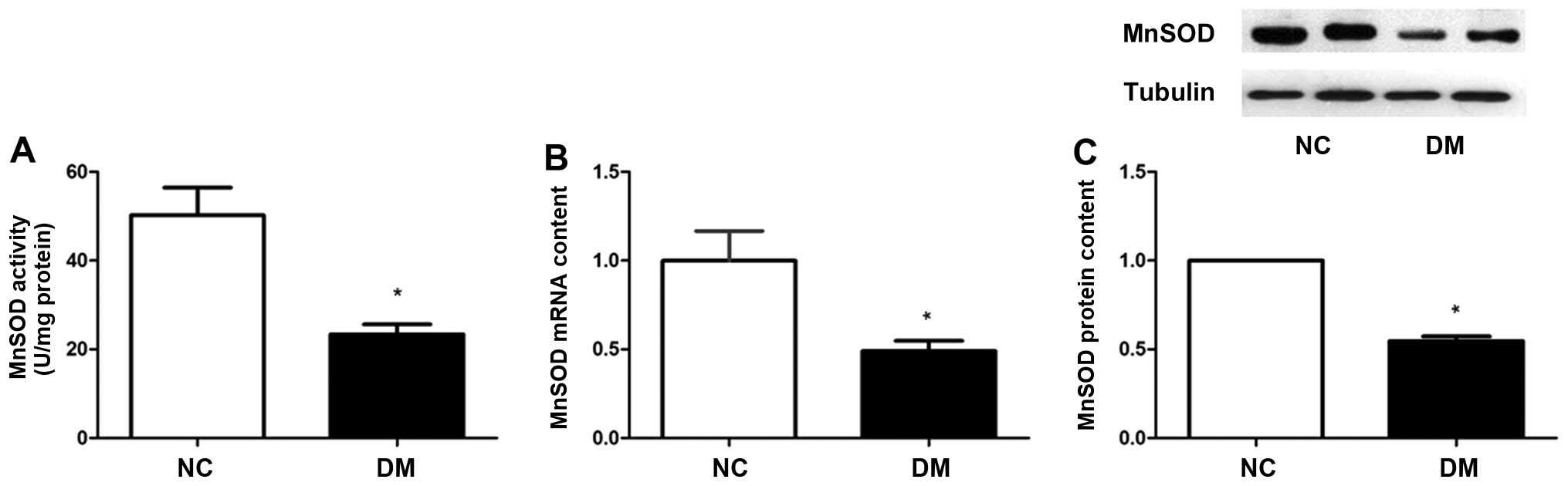
MnSOD enzyme activity is decreased in
skeletal muscle of diabetic patients
Both an increase in ROS production and a decline in
ROS clearance can lead to increased mitochondrial oxidative stress
(26). In the present study, we
focused on MnSOD, the primary antioxidative enzyme in mitochondria.
Compared to the control group, MnSOD enzyme activity in the
diabetes group was decreased by approximately 50% (Fig. 2A). We also found that the diabetic
patients had lower mRNA levels of MnSOD by approximately 50%
(Fig. 2B) and a decreased protein
content of MnSOD (Fig. 2C)
compared to the non-diabetic control group.
The AMPK-PGC1α-SIRT3 pathway is
downregulated in the skeletal muscle of diabetic patients
It has been demonstrated that SIRT3 and MnSOD
protein abundance is regulated in a signaling axis involving both
AMPK and PGC1α (27). Thus, in
this study, we compared the expression of the AMPK-PGC1α-SIRT3
signaling pathway between diabetic and non-diabetic patients. As
shown in Fig. 3, compared with
the non-diabetic patients, the PGC1α and SIRT3 mRNA levels
were decreased by almost 63 and 47%, respectively in the diabetic
patients (Fig. 3A and B). The
protein levels of PGC1α (Fig. 3D)
and SIRT3 (Fig. 3E) were similar
to those of the mRNA levels, and were decreased by 60 and 50% in
the diabetic patients, respectively. Western blot analysis revealed
an approximate 60% decrease in AMPK phosphorylation levels in the
diabetic patients, which was determined by the p-AMPK levels
normalized to the total AMPK levels (Fig. 3C).
Evaluation of the effects of celastrol on
the skeletal muscle of diabetic rats
According to the results of our clinical research,
we found that the levels of MnSOD were reduced in the skeletal
muscle of diabetic patients, and that the AMPK-PGC1α-SIRT3 pathway
was downregulated in the skeletal muscle of diabetic patients. In
view of the antioxidant effects of celastrol and that it is widely
used in the treatment of diabetic nephropathy, we examined the
antioxidant effects of this drug in the skeletal muscle of diabetic
rats. We preliminarily investigated whether the antioxidant effects
of celastrol are mediated through the AMPK-PGC1α-SIRT3 signaling
pathway, and compared the effects of different doses of celastrol
on this pathway.
Effects of celastrol on biochemical and
physical parameters of experimental rats
Table II shows
the biochemical results for each group. As expected, the FPG
concentrations were significantly higher, while body weight was
lower in the diabetic rats compared to the normal control rats
throughout the experimental period. However, there were no
significant differences in the plasma levels of ALT, AST, BUN and
SCr between the normal control rats and the diabetic rats. Of note,
the TG and TC content increased significantly in the diabetic rats
compared with the normal control rats due to the high fat diet
(HED). Although it has been reported that treatment with celastrol
significantly decreases body weight, blood glucose levels, and
plasma TC and TG levels (28), 8
weeks of treatment with various doses of celastrol had no
significant effect on body weight, the FPG level, and plasma TC and
TG levels in the present study.
 | Table IIEffects of celastrol on physical and
biochemical parameters of experimental animals. |
Table II
Effects of celastrol on physical and
biochemical parameters of experimental animals.
| Parameter | Week | NC | DM | DM + celastrol 1
mg/kg | DM + celastrol 3
mg/kg | DM + celastrol 6
mg/kg |
|---|
| BW (g) | 0 | 397.6±41.8a | 309.8±32.3 | 320.8±18.7 | 333.3±22.8 | 321.7±21.1 |
| 8 | 442.7±43.8a | 298.6±37.1 | 318.2±22.1 | 308.6±37.8 | 328.5±16.8 |
| FPG (mmol/l) | 0 | 6.2±0.2a | 24.2±8.6 | 26.7±2.1 | 23.9±4.1 | 28.8±2.8 |
| 8 | 6.0±0.2a | 28.2±3.0 | 25.8±6.9 | 27.6±5.7 | 29.0±3.1 |
| ALT (U/l) | 8 | 49.7±9.5 | 55.1±5.1 | 53.8±10.9 | 54.9±9.7 | 54.2±8.9 |
| AST (U/l) | 8 | 105.1±18.4 | 109.4±8.4 | 116.8±9.5 | 99.5±15.9 | 112.2±13.9 |
| BUN (mmol/l) | 8 | 10.9±2.0 | 10.7±1.6 | 10.9±1.3 | 10.0±1.9 | 10.2±1.2 |
| SCr
(µmol/l) | 8 | 32.8±4.4 | 31.3±3.3 | 32.8±3.5 | 31.1±4.1 | 31.5±3.8 |
| TG (mmol/l) | 8 | 1.5±1.2a | 4.4±1.8 | 4.0±2.2 | 3.7±1.8 | 3.5±1.4 |
| TC (mmol/l) | 8 | 2.2±1.2a | 6.9±0.9 | 6.6±2.1 | 6.4±2.2 | 6.2±2.0 |
Effects of celastrol on pathological
changes in the skeletal muscle of experimental rats
H&E staining of the paraspinal muscle of the
rats in the NC group exhibited a normal morphology (Fig. 4A and F). However, while the
skeletal muscle of the rats in the DM group exhibited an irregular
fiber structure, with wide gaps, nuclei of diverse sizes, an
increased number of nuclei and some with an abnormal position,
partly inserted in the muscle fibers (Fig. 4B and G). Following treatment with
celastrol, pathological damage was attenuated in varying degrees
compared with the rats in the DM group in a dose-dependent manner
(Fig. 4C–E and H–J).
Celastrol decreases the levels of markers
of oxidative stress
Markers of oxidative stress had been found to be
altered in mice with type 2 diabetes and in individuals with
diabetes (29,30). Thus, in this study, we analyzed
mitochondrial oxidative stress by measuring the levels of MDA, GSH
and MnSOD.
The level of MDA in the DM group was 2-fold higher
than that of the NC group. Following treatment with 3 and 6 mg/kg
celastrol, the levels of MDA were significantly decreased by 35.2
and 36.7% (P<0.05), respectively; while there was no significant
difference in the levels of MDA between the DM group and the group
treated with celastrol 1 mg/kg (Fig.
5A). By contrast, the level of GSH was decreased by 59.2% due
to the onset of diabetes. Treatment with 3 and 6 mg/kg celastrol
markedly restored the GSH level (P<0.05) to almost normal
levels. However, treatment with 1 mg/kg celastrol had no
significant effect compared with the DM group (P>0.05; Fig. 5B).
As an antioxidant defense mechanism, increased MnSOD
can partly indicate the attenuation of oxidative stress (31). In this study, we found that the
diabetic animals had 64.2 and 52.5% lower mRNA and protein levels
of MnSOD, respectively compared to the NC group. Treatment with 3
and 6 mg/kg celastrol significantly increased these levels. The
results of western blot analysis also indicated that the MnSOD
protein level was upregulated in the paravertebral muscle of rats
treated with 3 and 6 mg/kg celastrol (Fig. 6A and B). Furthermore, we analyzed
the enzyme activity of MnSOD. We also found that treatment with 3
and 6 mg/kg celastrol markedly enhanced the enzyme activity of
MnSOD (Fig. 6C). However, neither
the expression nor the enzyme activity of MnSOD exhibited a
significant increase following treatment with 1 mg/kg celastrol.
Taken together, these findings indicate that celastrol attenuates
oxidative stress in a dose-dependent manner.
Celastrol promotes the activation of the
AMPK-PGC1α-SIRT3 signaling pathway in skeletal muscle of rats with
diabetes
To determine whether celastrol ameliorates oxidative
stress by regulating SIRT3, we assessed the enzyme activity and
content of SIRT3 in the paravertebral muscle of experiment rats. As
expected, the mRNA and protein level of SIRT3 in the DM group was
decreased by 68.4 and 65.3%, respectively, compared to the NC
group. Treatment with 3 and 6 mg/kg celastrol significantly
increased both the mRNA and protein level of SIRT3 (Fig. 7A and D). Furthermore, we analyzed
the enzyme activity of SIRT3. A reduction of 41.9% in SIRT3 enzyme
activity was observed in the DM group compared with the NC group.
Treatment with 3 and 6 mg/kg celastrol markedly enhanced the enzyme
activity of SIRT3 (Fig. 7C).
Consistent with the results obtained for MnSOD, neither the
expression nor the enzyme activity of SIRT3 exhibited a marked
improvement following treatment with 1 mg/kg celastrol.
It has been found that SIRT3 functions as a
downstream target of PGC1α, which is directly regulated by AMPK
(14,15). Therefore, we examined the possible
role of the AMPK-PGC1α signaling pathway as a modulator of the
regulatory effects of celastrol on SIRT3. The mRNA and protein
levels of PGC1α were decreased in the DM group by 66.3 and 64.9%,
respectively compared to the NC group (Fig. 7B and E). Treatment with 3 and 6
mg/kg celastrol significantly increased the levels PGC1α. In
accordance with this upregulation, we also observed increased
p-AMPK (Fig. 7F) levels in the
paravertebral muscle of rats treated with 3 and 6 mg/kg celastrol.
Similarly, there was no significant increase in the levels of PGC1α
and p-AMPK in the group treated with 1 mg/kg celastrol. Taken
together, our results indicated that celastrol increased the
expression of p-AMPK and PGC1α in a dose-dependent manner in the
skeletal muscle of rats with diabetes. Celastrol activated the
AMPK-PGC1α signaling pathway in vivo.
Discussion
In this study, we firstly found that the expression
levels of AMPK, PGC1α, SIRT3 and MnSOD were all decreased in the
skeletal muscle of diabetic patients, accompanied by pathological
damage. We also demonstrated that celastrol attenuated the
pathological damage and oxidative stress, and activated the
AMPK-PGC1α-SIRT3 signaling pathway in the skeletal muscle of
diabetic rats.
Recently, ROS have been shown to play important
roles in the activation of different signaling pathways and in the
development of T2DM (7).
Antioxidants, such as MnSOD, catalase (CAT), glutathione peroxidase
(GPX) and GSH effectively form a defensive mechanism against the
onslaught of ROS, protecting cells from oxidative stress (32). As is known, MDA is the main
product of lipid peroxidation and its concentration usually
reflects the total level of lipid peroxidation (33). In this study, both the activity
and expression of MnSOD, as well as the level of GSH markedly
decreased, with a significant increase in the level of MDA in
diabetic rats, indicating that oxidative stress was enhanced during
the development of diabetes. Following treatment with the middle
and high dose (3 and 6 mg/kg/day) of celastrol, the activity and
the expression of MnSOD, coupled with the level of GSH and MDA,
returned towards their normal control values. Therefore it can be
proposed that the middle and high dose of celastrol may be able to
counteract oxidative stress-induced toxicity, whereas the low dose
of celastrol (1 mg/kg/day) did not have such an effect.
Furthermore, we explored the potential mechanisms of
the celastrol-induced ameliorative effects related to oxidative
stress in this study. SIRT3 is a mitochondrial sirtuin and
regulates energy homeostasis and oxidative metabolism (34,35). SIRT3 suppresses the cellular
production of deleterious ROS, via deacetylation and the activation
of MnSOD (36) and isocitrate
dehydrogenase 2 (IDH2) (37,38). Other studies have shown links
between SIRT3 and mitochondrial ROS production by targeting HIF-1α
and SOD2 under different pathological and physiological conditions
(38,39). Recently, a study verified that the
decreased level of SIRT3 in skeletal muscle in states of diabetes
is a major component of the pathogenesis of T2DM, which can lead to
altered mitochondrial function, increased ROS production and
oxidative stress, and finally results in insulin resistance; SIRT3
expression in skeletal muscle is altered in models of both type 1
and 2 diabetes (40). In the
present study, we found that SIRT3 expression was decreased in the
skeletal muscle of patients and rats with T2DM. Following treatment
with the middle and high dose of celastrol, SIRT3 expression was
increased in the skeletal muscle of diabetic rats in accordance
with the results obtained for MnSOD.
In this study, we also investigated the mechanism of
celastrol-induced SIRT3 activation. It has been demonstrated that
PGC1α, a nuclear transcriptional coactivator, increases SIRT3
expression at the mRNA and protein level (17). A previous study also reported that
PGC1α improved mouse SIRT3 activity in both hepatocytes and muscle
cells, indicating that PGC1α acts as an endogenous regulator of
SIRT3 (41). The normal
functioning of the PGC1α/SIRT3 axis has been reported as essential
for the regulation of mitochondrial metabolism, biogenesis and
oxidative stress (12). Abnormal
PGC1α levels have been linked to the development of DM (42). In addition, AMPK increases the
activity of PGC1α (43), at least
through two mechanisms. Firstly, PGC1α is phosphorylated and
activated by AMPK directly, which can then coactivate at its own
promoter to stimulate its expression (15,44). Next, AMPK increases the levels of
cellular NAD+, in turn activating SIRT1 to activate
PGC1α (45,46). What is more, in this study, we
found that the expression of AMPK and PGC1α was decreased in the
skeletal muscle of patients with T2DM. We presumed that celastrol
upregulated SIRT3 expression via the activation of the AMPK-PGC1α
axis. As expected, our results revealed that the expression levels
of AMPK and PGC1α increased following treatment with the middle and
high dose of celastrol. Thus, it can be concluded that celastrol
regulates SIRT3 expression at least partly through the activation
of the AMPK-PGC1α axis.
This study has some limitations. First, this study
did not employ special gene deficient mice, such as Sirt3
knockout mice, in order to provide more conclusive evidence of the
signaling pathway in question. Second, cell culture was not
conducted to verify the potential involvement of the
AMPK-PGC1α-SIRT3 signaling pathway following treatment with
celastrol in vitro.
In conclusion, the findings of the present study
indicated that celastrol attenuated oxidative stress in skeletal
muscle partial by regulating the AMPK-PGC1α-SIRT3 signaling
pathway. Our findings suggest a potential role for celastrol in the
treatment of the chronic complications of diabetes mellitus.
Further studies on this matter are warranted using specific gene
deficient mice and performing in vitro experiments to
confirm our findings.
Abbreviations:
|
T2DM
|
type 2 diabetes mellitus
|
|
ROS
|
reactive oxygen species
|
|
MnSOD
|
manganese superoxide dismutase
|
|
AMPK
|
AMP-activated protein kinase
|
|
PGC1α
|
peroxisome proliferator-activated
response-γ coactivator 1α
|
|
Sirt3
|
silent mating-type information
regulation 2 homolog 3
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
FPG
|
fasting plasma glucose
|
|
BUN
|
blood urea nitrogen
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
TBS
|
Tris-buffered saline
|
|
HbA1c
|
glycated haemoglobin A1c
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
SCr
|
serum creatinine
|
References
|
1
|
Andersen H, Schmitz O and Nielsen S:
Decreased isometric muscle strength after acute hyperglycaemia in
type 1 diabetic patients. Diabet Med. 22:1401–1407. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersen H, Gjerstad MD and Jakobsen J:
Atrophy of foot muscles: A measure of diabetic neuropathy. Diabetes
Care. 27:2382–2385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyholm B, Qu Z, Kaal A, Pedersen SB,
Gravholt CH, Andersen JL, Saltin B and Schmitz O: Evidence of an
increased number of type IIb muscle fibers in insulin-resistant
first-degree relatives of patients with NIDDM. Diabetes.
46:1822–1828. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang BK, Monu JU and Doumanian J:
Diabetic myopathy: MRI patterns and current trends. AJR Am J
Roentgenol. 195:198–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Souza DM, Al-Sajee D and Hawke TJ:
Diabetic myopathy: Impact of diabetes mellitus on skeletal muscle
progenitor cells. Front Physiol. 4(379)2013. View Article : Google Scholar
|
|
6
|
Park SW, Goodpaster BH, Strotmeyer ES, de
Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA and Newman AB:
Decreased muscle strength and quality in older adults with type 2
diabetes: The health, aging, and body composition study. Diabetes.
55:1813–1818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tao R, Vassilopoulos A, Parisiadou L, Yan
Y and Gius D: Regulation of MnSOD enzymatic activity by Sirt3
connects the mitochondrial acetylome signaling networks to aging
and carcinogenesis. Antioxid Redox Signal. 20:1646–1654. 2014.
View Article : Google Scholar :
|
|
9
|
Montano MA, Barrio Lera JP, Gottlieb MG,
Schwanke CH, da Rocha MI, Manica-Cattani MF, dos Santos GF and da
Cruz IB: Association between manganese superoxide dismutase (MnSOD)
gene polymorphism and elderly obesity. Mol Cell Biochem. 328:33–40.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kahn BB, Alquier T, Carling D and Hardie
DG: AMP-activated protein kinase: Ancient energy gauge provides
clues to modern understanding of metabolism. Cell Metab. 1:15–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kukidome D, Nishikawa T, Sonoda K, Imoto
K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T and
Araki E: Activation of AMP-activated protein kinase reduces
hyperglycemia-induced mitochondrial reactive oxygen species
production and promotes mitochondrial biogenesis in human umbilical
vein endothelial cells. Diabetes. 55:120–127. 2006. View Article : Google Scholar
|
|
12
|
Bell EL and Guarente L: The SirT3 divining
rod points to oxidative stress. Mol Cell. 42:561–568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwer B, North BJ, Frye RA, Ott M and
Verdin E: The human silent information regulator (Sir)2 homologue
hSIRT3 is a mitochondrial nicotinamide adenine
dinucleotide-dependent deacetylase. J Cell Biol. 158:647–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong X, Wang R, Xue Y, Liu X, Zhang H,
Chen Y, Fang F and Chang Y: Sirtuin 3, a new target of PGC-1α,
plays an important role in the suppression of ROS and mitochondrial
biogenesis. PLoS One. 5:e117072010. View Article : Google Scholar
|
|
15
|
Jäger S, Handschin C, St-Pierre J and
Spiegelman BM: AMP-activated protein kinase (AMPK) action in
skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl
Acad Sci USA. 104:12017–12022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Li L, Li CY, Pei Z, Zhou M and Li
N: SIRT3 protects cells from hypoxia via PGC-1α- and
MnSOD-dependent pathways. Neuroscience. 286:109–121. 2015.
View Article : Google Scholar
|
|
17
|
Rato L, Duarte AI, Tomás GD, Santos MS,
Moreira PI, Socorro S, Cavaco JE, Alves MG and Oliveira PF:
Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating
mitochondrial bioenergetics and oxidative stress. Biochim Biophys
Acta. 1837:335–344. 2014. View Article : Google Scholar
|
|
18
|
Hao J, Hao C, Zhang L, Liu X, Zhou X, Dun
Y, Li H, Li G, Zhao X, An Y, et al: OM2, a novel
oligomannuronate-chromium(III) complex, promotes mitochondrial
biogenesis and lipid metabolism in 3T3-L1 adipocytes via the
AMPK-PGC1α pathway. PLoS One. 10:e01319302015. View Article : Google Scholar
|
|
19
|
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y,
Meng G, Xie L, Wang J, Xiao Y, et al: Celastrol prevents
atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS
One. 8:e654772013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge Y, Xie H, Li S, Jin B, Hou J, Zhang H,
Shi M and Liu Z: Treatment of diabetic nephropathy with
Tripterygium wilfordii Hook F extract: A prospective, randomized,
controlled clinical trial. J Transl Med. 11:1342013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu X, Tao W, Jiang F, Li C, Lin J and Liu
C: Celastrol attenuates hypertension-induced inflammation and
oxidative stress in vascular smooth muscle cells via induction of
heme oxygenase-1. Am J Hypertens. 23:895–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rato L, Alves MG, Dias TR, Lopes G, Cavaco
JE, Socorro S and Oliveira PF: High-energy diets may induce a
pre-diabetic state altering testicular glycolytic metabolic profile
and male reproductive parameters. Andrology. 1:495–504. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ai J, Wang N, Yang M, Du ZM, Zhang YC and
Yang BF: Development of Wistar rat model of insulin resistance.
World J Gastroenterol. 11:3675–3679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frazier AE and Thorburn DR: Biochemical
analyses of the electron transport chain complexes by
spectrophotometry. Methods Mol Biol. 837:49–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brandauer J, Vienberg SG, Andersen MA,
Ringholm S, Risis S, Larsen PS, Kristensen JM, Frøsig C, Leick L,
Fentz J, et al: AMP-activated protein kinase regulates nicotinamide
phosphoribosyl transferase expression in skeletal muscle. J
Physiol. 591:5207–5220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mikhed Y, Daiber A and Steven S:
Mitochondrial oxidative stress, mitochondrial DNA damage and their
role in age-related vascular dysfunction. Int J Mol Sci.
16:15918–15953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brandauer J, Andersen MA, Kellezi H, Risis
S, Frøsig C, Vienberg SG and Treebak JT: AMP-activated protein
kinase controls exercise training- and AICAR-induced increases in
SIRT3 and MnSOD. Front Physiol. 6:852015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JE, Lee MH, Nam DH, Song HK, Kang YS,
Lee JE, Kim HW, Cha JJ, Hyun YY and Han SY: Celastrol, an NF-κB
inhibitor, improves insulin resistance and attenuates renal injury
in db/db mice. PLoS One. 8:e620682013. View Article : Google Scholar
|
|
29
|
Lodovici M, Bigagli E, Luceri C, Mannucci
E, Rotella CM and Raimondi L: Gender-related drug effect on several
markers of oxidation stress in diabetes patients with and without
complications. Eur J Pharmacol. 766:86–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng B, Yan XF, Xue JL, Xu L and Wang H:
The protective effects of α-lipoic acid on kidneys in type 2
diabetic Goto-Kakisaki rats via reducing oxidative stress. Int J
Mol Sci. 14:6746–6756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawler JM, Kwak HB, Kim JH and Suk MH:
Exercise training inducibility of MnSOD protein expression and
activity is retained while reducing prooxidant signaling in the
heart of senescent rats. Am J Physiol Regul Integr Comp Physiol.
296:R1496–R1502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Srinivasan M, Rajendra Prasad N and Menon
VP: Protective effect of curcumin on gamma-radiation induced DNA
damage and lipid peroxidation in cultured human lymphocytes. Mutat
Res. 611:96–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondi-aldehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar
|
|
34
|
Onyango P, Celic I, McCaffery JM, Boeke JD
and Feinberg AP: SIRT3, a human SIR2 homologue, is an NAD-dependent
deacetylase localized to mitochondria. Proc Natl Acad Sci USA.
99:13653–13658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwer B, North BJ, Frye RA, Ott M and
Verdin E: The human silent information regulator (Sir)2 homologue
hSIRT3 is a mitochondrial nicotinamide adenine
dinucleotide-dependent deacetylase. J Cell Biol. 158:647–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tao R, Coleman MC, Pennington JD, Ozden O,
Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et
al: Sirt3-mediated deacetylation of evolutionarily conserved lysine
122 regulates MnSOD activity in response to stress. Mol Cell.
40:893–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu X, Brown K, Hirschey MD, Verdin E and
Chen D: Calorie restriction reduces oxidative stress by
SIRT3-mediated SOD2 activation. Cell Metab. 12:662–667. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Someya S, Yu W, Hallows WC, Xu J, Vann JM,
Leeuwenburgh C, Tanokura M, Denu JM and Prolla TA: Sirt3 mediates
reduction of oxidative damage and prevention of age-related hearing
loss under caloric restriction. Cell. 143:802–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bell EL, Emerling BM, Ricoult SJ and
Guarente L: SirT3 suppresses hypoxia inducible factor 1α and tumor
growth by inhibiting mitochondrial ROS production. Oncogene.
30:2986–2996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jing E, Emanuelli B, Hirschey MD, Boucher
J, Lee KY, Lombard D, Verdin EM and Kahn CR: Sirtuin-3 (Sirt3)
regulates skeletal muscle metabolism and insulin signaling via
altered mitochondrial oxidation and reactive oxygen species
production. Proc Natl Acad Sci USA. 108:14608–14613. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park SJ, Ahmad F, Philp A, Baar K,
Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al:
Resveratrol ameliorates aging-related metabolic phenotypes by
inhibiting cAMP phosphodiesterases. Cell. 148:421–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Joseph AM, Joanisse DR, Baillot RG and
Hood DA: Mitochondrial dysregulation in the pathogenesis of
diabetes: potential for mitochondrial biogenesis-mediated
interventions. Exp Diabetes Res. 2012:6420382012. View Article : Google Scholar
|
|
43
|
Lin J, Handschin C and Spiegelman BM:
Metabolic control through the PGC-1 family of transcription
coactivators. Cell Metab. 1:361–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Handschin C, Rhee J, Lin J, Tarr PT and
Spiegelman BM: An autoregulatory loop controls peroxisome
proliferator-activated receptor gamma coactivator 1alpha expression
in muscle. Proc Natl Acad Sci USA. 100:7111–7116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cantó C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+
metabolism and SIRT1 activity. Nature. 458:1056–1060. 2009.
View Article : Google Scholar
|
|
46
|
Fulco M, Cen Y, Zhao P, Hoffman EP,
McBurney MW, Sauve AA and Sartorelli V: Glucose restriction
inhibits skeletal myoblast differentiation by activating SIRT1
through AMPK-mediated regulation of Nampt. Dev Cell. 14:661–673.
2008. View Article : Google Scholar : PubMed/NCBI
|