Introduction
Chemical-induced corneal injury usually leads to
extensive damage to the entire anterior segment of the eye, and
remains a major cause of corneal diseases, which severely affects
visual function and is not easily cured by conservative treatment
(1). It has been well documented
that acute oxidative stress, inflammation and corneal
neovascularization play a crucial role in chemical burn-induced
corneal damage and even loss of vision (2). Chemical burns stimulate the
production of reactive oxygen species (ROS) in the corneas
(3). When ROS production is low
or intermediate, oxidative stress is prevented by intracellular
antioxidant systems, such as superoxide dismutase (SOD), catalase,
glutathione peroxidases and peroxiredoxins. ROS actively
participate in various cellular processes, such as cell
proliferation, differentiation and inflammation (4). However, if ROS production overwhelms
the cellular antioxidant capacity, the high level of oxidative
stress induces the perturbation of the mitochondrial permeability
transition pore and disrupts electron transfer, eventually leading
to apoptosis or necrosis. It has been demonstrated that high-level
oxidative stress contributes to the pathogenesis of several
diseases, such as diabetes (5,6),
age-related macular degeneration (7) and chemical injury of the cornea
(8). On the other hand, ROS act
as second messengers in triggering inflammation through the
activation of nuclear factor-κB (NF-κB), and subsequently stimulate
the release of inflammatory cytokines (9), the expression of vascular
endothelial growth factor (VEGF) (10) and matrix metalloproteinases (MMPs)
(11). The inflammatory
responses, and VEGF and MMPs, play an important role in pathologic
corneal neovascularization (CNV) after chemical burns. In general,
ROS are mainly generated through the mitochondrial electron
transport chain and enzymes, such as NADPH oxidases (Noxs).
Although the contribution of ROS to chemical burn-induced
inflammation and pathological CNV has been previously recognized,
the role of Noxs in chemical burn-induced corneal damage and CNV
remains to be elucidated.
There are 7 identified isoforms in the Nox family,
including Nox1, Nox2, Nox3, Nox4, Nox5, and dual oxidase 1 and 2
(DUOX1 and DUOX2) (12).
According to a model of activation, the 7 members of the Nox family
have been classified as constitutively active and acutely activated
enzymes. Nox2 was first described in neutrophils and macrophages
(13), and is also the member
which has received the most scholarly attention (14). Nox2 is located on the plasma
membrane, and forms a complex with the p22phox subunit. The
activation of Nox2 requires combination with p47phox, p67phox,
p40phox and Ras-related C3 botulinum toxin substrate 1 (Rac1)
(15). By contrast, Nox4 is the
single constitutively active member of the Nox family. Nox4 was
originally identified in the kidneys (16), and is mainly expressed in vascular
endothelial cells and smooth muscle cells (17,18). ROS are generated by Noxs through
the transfer of electrons across biological membranes (13). It has previously been demonstrated
that Noxs contribute to oxidative injury in endothelial cells in
diabetic rats in vivo (19), as well as in the migration and
proliferation of endothelial cells (20). Nox-mediated oxidative stress also
plays a crucial role in the activation of the NF-κB signaling
pathway (21) and MMPs (22), which contributes to inflammatory
responses and cell migration. In a previous study, it was found
that the inhibition of Nox4 activity effectively decreased VEGF
expression and retinal vascular premeability in db/db mice
(23), suggesting that Nox4 is
involved in retinal neovascularization. In addition, the expression
of Noxs has been detected in corneal epithelial and stromal cells
(24,25). However, the expression of Noxs in
corneas after chemical burns and its role in corneal damage,
inflammation and pathologic CNV remain unclear.
In the present study, we established a mouse model
of alkali burn-induced corneal injury, and examined the expression
of Noxs in human and mouse corneas after alkali burns. It was found
that the expression of Nox2 and Nox4 was significantly increased in
human and mouse corneas after alkali burns. Furthermore, ROS
production, oxidative stress, inflammatory cytokine release and CNV
were increased after alkali burns. The Nox inhibitors,
diphenyleneiodonium (DPI), or apocynin (Apo) effectively attenuated
alkali burn-induced oxidative stress, the activation of the
inflammatory response and pathological CNV in the corneas.
Therefore, our data indicate the vital role which Noxs play in
alkali burn-induced injury to the corneas.
Materials and methods
Human corneal tissues
Normal human corneas and corneas which had been
affected by alkali burns were obtained from the Affiliated Eye
Hospital of Nanchang University, Nanchang, China. Normal corneas
were obtained from the archives, and the corneas affected by alkali
burns were obtained after penetrating keratoplasty. All experiments
involving patients were approved by the Ethics Committee of the
Affiliated Eye Hospital of Nanchang University and were performed
in accordance with the principles of the Declaration of Helsinki.
The corneal tissues were conventionally fixed with 4%
paraformaldehyde. The slices of the cornea which were embedded in
optimal cutting temperature (OCT) compound (Sakura Finetek, Inc.,
Torrance, CA, USA) were subsequently prepared at 7 µm
thickness.
Animals
C57BL/6 mice (n= 68, 6–8 weeks of age) were
purchased from Hunan SJA Laboratory Animal Co., Ltd., (Hunan,
China) and all experiments involving animals were approved by the
Shanghai Animal Institution, Chinese Academy of Sciences (Shanghai,
China). The animal experiments were conducted in accordance with
the ARVO statement for the Use of Animals in Ophthalmic and Vision
Research.
Establishment of the mouse model of
alkali burn-induced corneal injury and treatment with Nox
inhibitors
The animal model of alkali burn-induced corneal
injury was established as previously described (26). Briefly, the mice were anesthetized
by an intraperitoneal injection of 10% chloral hydrate (0.2 ml/100
g). Subsequently, 0.4% oxybuprocaine hydrochloride (Santen, Tokyo,
Japan) was applied topically to the corneal surface. Filter paper
(2.0 mm in diameter) soaked in 2 µl of 1 N NaOH was placed
on the central cornea of the right eye for 40 sec under a surgical
microscope, and the eyes were then rinsed with 10 ml saline buffer.
To examine the role of Noxs in alkali burn-induced corneal injury,
two commonly used Nox inhibitors, DPI and Apo (Sigma-Aldrich, St.
Louis, MO, USA), were administered by eye drops at concentrations
previously described (23,27).
The stock solution of DPI was prepared at 3 mM in DMSO, and diluted
to 0.1 µM in PBS for use. Apo was prepared at 500 mM and
diluted to 500 µM for use. The final DMSO concentration in
each eye drop was <0.1%. Immediately after corneal injury, DPI
or Apo were administered by eye drops and applied to the mouse
corneas 4 times a day for 7 or 14 days. Saline with an equivalent
concentration of DMSO was used as the control for treatment.
Immunofluorescence staining
The mice were sacrificed by cardiac injection of PBS
before the eyes were removed for immunofluorescence staining. A
total of 9 mice was used for this experiment. The eyes were removed
7 days after treatment, and were immediately frozen in OCT
compound. The OCT-embedded slices of the cornea were prepared at 7
µm thickness for immunofluorescence staining. The corneal
sections were fixed in ice-cold acetone for 20 min and washed in
PBS. After blocking in 1% BSA, immunofluorescence staining was
performed with the following primary antibodies: rabbit anti-Nox4
(1:100; sc-30141; Santa Cruz Biotechnology, Santa Cruz CA, USA),
mouse anti-gp91-phox (Nox2) (1:100; sc-130543; Santa Cruz
Biotechnology), mouse anti-3-nitrotyro-sine (3-NT) (1:200; ab1392;
Abcam, Cambridge, MA, USA), rat anti-CD11b (1:10; M1/70.15.11.5.2;
Hybridoma Bank, Iowa City, IA, USA). The secondary antibodies
included the following: Alexa Fluor® 488 donkey
anti-rabbit IgG (H+L) (1:200; A-21206; Invitrogen, Carlsbad, CA,
USA), Alexa Fluor® 488 donkey anti-mouse IgG (H+L)
(1:200; A-21202; Invitrogen), Alexa Fluor® 594 rabbit
anti-mouse IgG (H+L) (1:200; A27027; Invitrogen), Alexa
Fluor® 594 donkey anti-rat IgG (H+L) (1:200; A-21202;
Invitrogen). After 3 washes with TBST, the sections were further
incubated with mounting medium with DAPI (H-1200; Vector
Laboratories, Burlingame, CA, USA). Fluorescence signals were
detected under a fluorescence microscope (Olympus, Tokyo,
Japan).
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted from the corneas of 24 mice
using ice-cold TRIzol reagent (Invitrogen) and 500 ng total RNA was
reverse transcribed for thye synthesis of double-stranded cDNA
using TransScript® (AE301; Beijing Transgen Biotech Co.,
Ltd., Beijing, China). Real-time (quantitative) amplification was
performed using SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories,
Hercules, CA, USA) in a CFX connect real-time PCR system (Bio-Rad
Laboratories). PCR reactions were carried out at 25°C for 10 min
and 42°C for 30 min, 95°C for 30 sec, and then 40 cycles at 95°C
for 5 sec, 60°C for 5 sec. The primers used for qPCR were as
follows: Nox1, 5′-TGG CTAAATCCCATCCAGTC-3′ (forward) and 5′-CCCAA
GCTCTCCTCTGTTTG-3′ (reverse); Nox2, 5′-TCGCTGG AAACCCTCCTATG-3′
(forward) and 5′-GGATACCTTGG GGCACTTGA-3′ (reverse); Nox4,
5′-ACTTTTCATTGGG CGCTC-3′ (forward) and 5′-AGAACTGGGTCCACAG CAGA-3′
(reverse); peptidylprolyl isomerase A (PPIA),
5′-AATGCTGGACCAAACACAAA-3′ (forward) and 5′-TTC
CACAATGTTCATGCCTT-3′ (reverse); tumor necrosis factor-α (TNF-α),
5′-CAGCCTCTTCTCATTCCTGCTTG-3′ (forward) and
5′-GGGTCTGGGCCATAGAACTGA-3′ (reverse); interleukin (IL)-1β,
5′-CTCCATGAGCTTTGTA CAAGG-3′ (forward) and 5′-TGCTGATGTACCAGTT
GGGG-3′ (reverse); IL-6, 5′-CAAAGCCAGAGTCCT TCAGA-3′ (forward) and
5′-GATGGTCTTGGTCCTTAGCC-3′ (reverse); VEGF,
5′-TTACTGCTGTACCTCCACC-3′ (forward) and 5′-ACAGGACGGCTTGAAGATG-3′
(reverse); VEGF receptor (VEGFR)1, 5′-GTGATCAGCTCCAGGTTTGA CTT-3′
(forward) and 5′-GAGGAGGATGAGGGTGTCTA TAGGT-3′ (reverse); VEGFR2,
5′-CTGTGAACGCTTGCC TTAT-3′ (forward) and 5′-CAACATCTTGACGGCTACTG-3′
(reverse); MMP2, 5′-CCCCGATGCTGATACTGA-3′ (forward) and
5′-CTGTCCGCCAAATAAACC-3′ (reverse); MMP9,
5′-CAGCCAACTATGACCAGGAT-3′ (forward) and 5′-CTG CCACCAGGAACAGG-3′
(reverse); MMP13, 5′-GTGTGG AGTTATGATGATGT-3′ (forward) and
5′-TGCGATTAC TCCAGATACTG-3′ (reverse).
Measurement of corneal ROS
production
Corneal ROS production was measured using CellROX
Green reagent (Invitrogen) according to the manufacturer's
instructions. In brief, the fresh corneal sections were washed with
PBS and permeabilized in 0.5% Triton-X for 10 min. To detect ROS
levels in the corneas, 5 µM CellROX reagent wre added to the
sections followed by incubation for 30 min at 37°C. Followng
incubation, the sections were washed 3 times with PBS. Fluorescence
signals of ROS were detected with excitation and emission
wavelengths at 485/530 nm under a fluorescence microscope
(Olympus), and fluorescence intensities were analyzed using ImageJ
software (Broken Symmetry Software).
Assessment of CNV
To observe CNV following alkali burn-induced injury
in our time course experiments, the mouse corneas from 15 mice were
examined under a dissecting microscope (SM200L; Olympus) and
photographed. In addition, FITC-dextran corneal angiography was
used to quantify the area of CNV. Briefly, the mice were sacrificed
14 days after being subjected to alkali burns by a cardiac
injection of FITC-dextran (Sigma-Aldrich). The eyes were removed
and fixed in 4% paraformaldehyde at 4°C for 2 h. The corneas were
excised and processed for whole-mount preparation. The CNV signal
was detected under a fluorescence microscope (Olympus), and the
positive CNV area was quantified using cellSens software
(Olympus).
Western blot analysis
The whole lysate of the dissected corneal tissues
from 20 mice was prepared using radio immunoprecipitation assay
(RIPA) lysis buffer. Proteins in whole lysate were separated by
7.5% SDS-PAGE and transferred onto nitrocellulose membranea
(HATF00010; Millipore, Bellerica, MA, USA). The membranes were
blocked in 5% skim milk in TBST buffer for 1 h at room temperature,
and then immunoblotted for 2 h at room temperature with the
following primary antibodies: rabbit anti-Nox4 (sc-30141; Santa
Cruz Biotechnology), mouse anti-gp91-phox (sc-130543; Santa Cruz
Biotechnology), mouse anti-3-NT (ab1392; Abcam) and mouse anti
β-actin (sc-47778; Santa Cruz Biotechnology). Following 3 washes
with TBST, the membranes were further incubated with a horseradish
peroxidase-conjugated secondary antibody (ZB-2310; Zhongshan
Jinqiao, Beijing, China). Chemiluminescence assays were carried out
with enhanced chemiluminescence reagents (SuperSignal®
West Dura Extended Duration Substrate; Thermo Fisher Scientific,
Waltham, MA, USA). The immunoblot signal was detected using
ChemiFast (G:BOX Chemi XT4; Syngene International Ltd., San Jose,
CA, USA), and the signal density of each band was analyzed using
ImageJ software (Broken Symestry Software).
Statistical analysis
The quantitative data are presented as the means ±
SD. Statistical analysis was performed using SPSS 17.0 software.
Data were analyzed using one-way ANOVA or a Student's t-test to
perform comparisons between 2 groups, and a P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Noxs in human and mouse
corneas following alkali burn-induced corneal injury
It has been reported that alkali burns increase ROS
generation, and trigger inflammatory responses and
neovascularization in corneas (3). Noxs are an important source of ROS.
Thus, to investigate the possible role of Noxs in alkali
burn-induced oxidative stress and injury to corneas, the expression
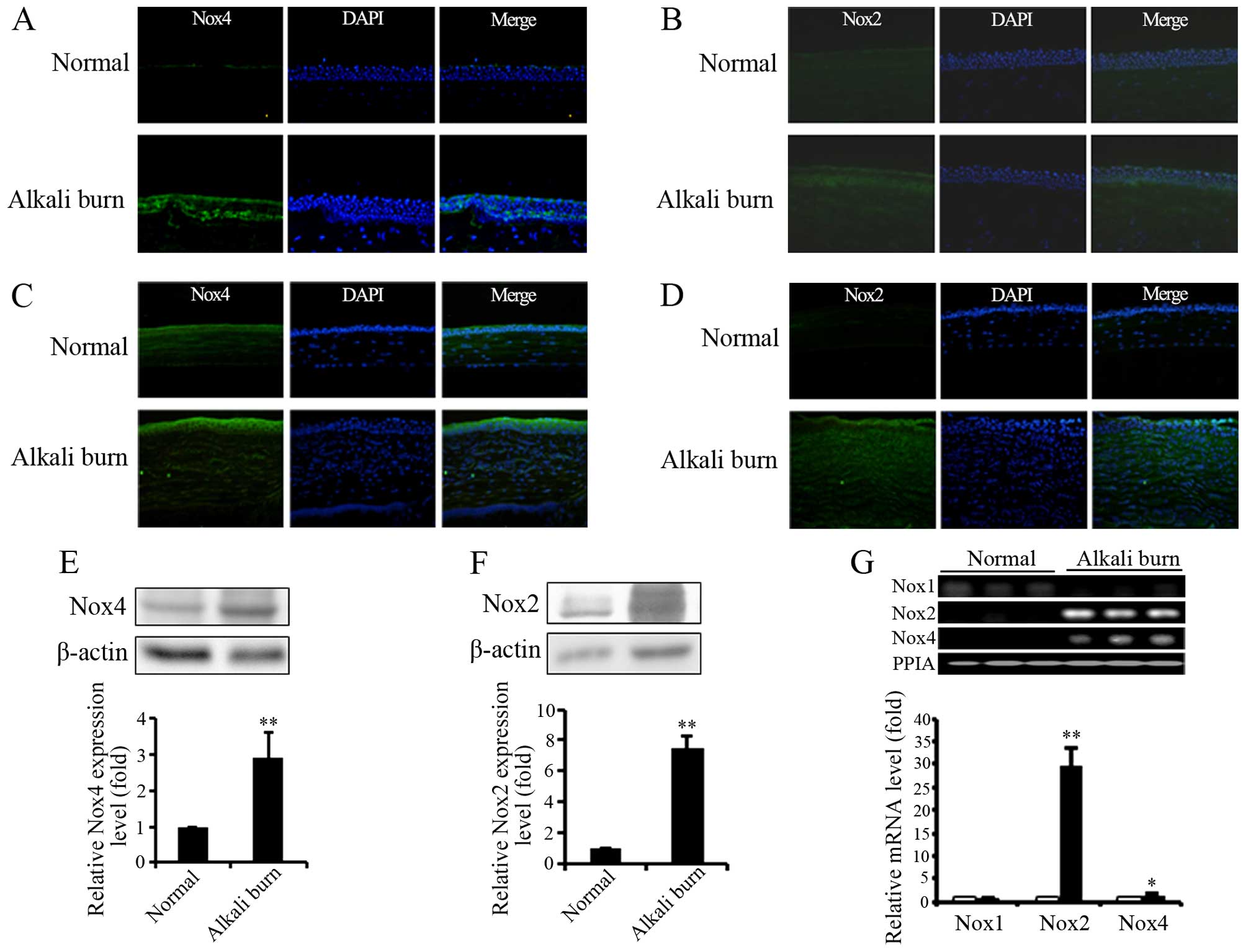
of Nox isoforms was examined. Immunofluorescence staining
demonstrated that the expression of Nox2 and Nox4 was upregulated
in both the human and mouse corneas affected by alkali burns
(Fig. 1A–D). The results of
western blot analysis also revealed the upregulation of Nox2 and
Nox4 in the mouse corneas following alkali burn-induced injury
(Fig. 1E and F). In addition, the
mRNA level of Nox isoforms was measured by RT-qPCR. As shown in
Fig. 1G, alkali burns induced the
transcription of Nox2 and Nox4, but decreased the mRNA levels of
NNox1 in mouse corneas. These results indicate that alkali burns
selectively stimulate the transcription and expression of Nox2 and
Nox4 in both human and mouse corneas, and we thus suggest that Nox2
and Nox4 are involved in alkali burn-induced oxidative stress and
corneal damage.
Alkali burn-induced oxidative stress is
attenuated by Nox inhibitors
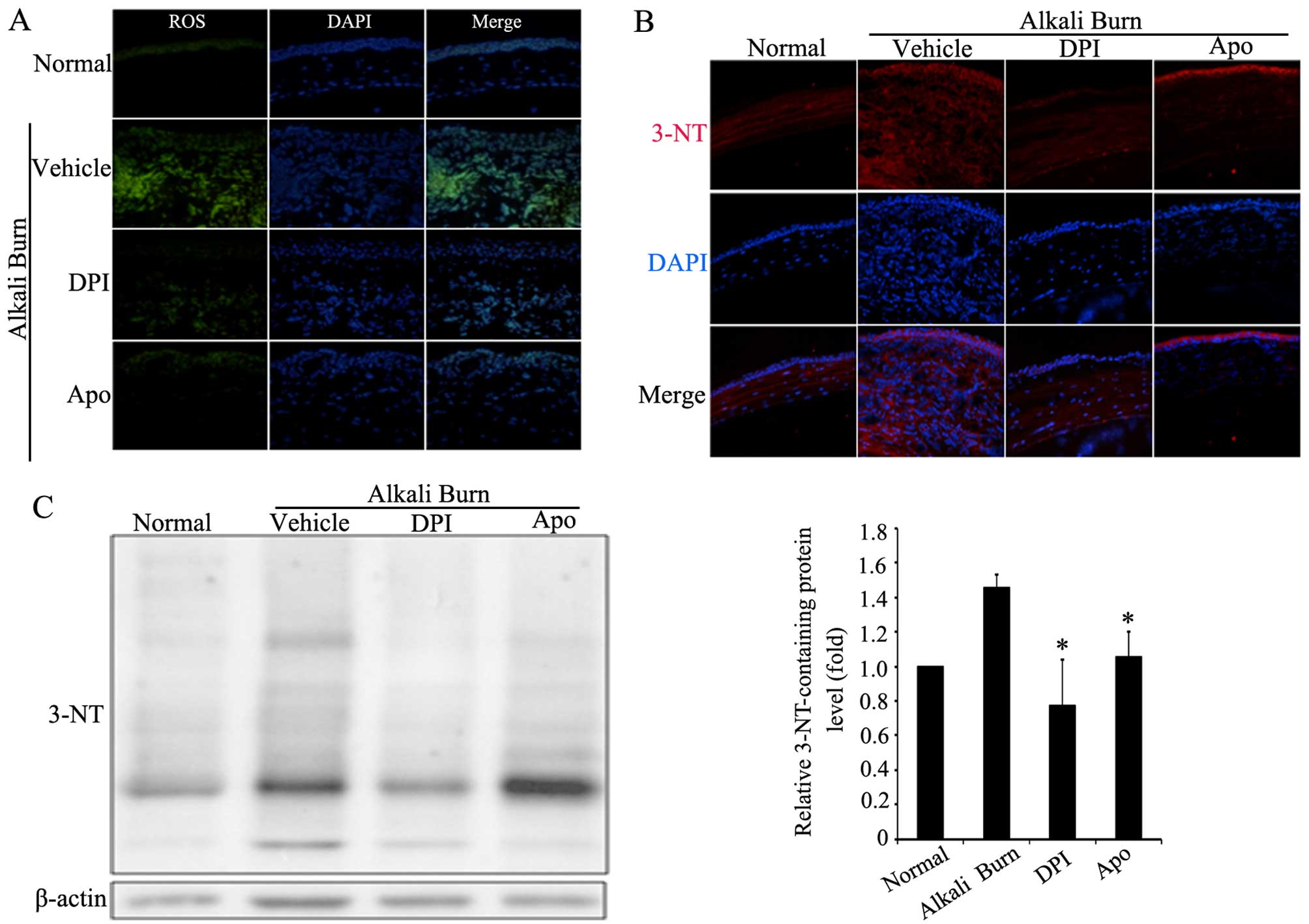
In the present study, CellROX Green reagent was used
to measure ROS levels in the corneas. To elucidate the role of Noxs
in alkali burn-induced corneal oxidative stress, two common Nox
inhibitors, DPI and Apo, were administered by eye drops. As shown
in Fig. 2A, the ROS levels in the
corneas were markedly increased after the alkali burns. By
contrast, the Nox inhibitors, DPI or Apo, effectively reduced the
levels of alkali burn-induced ROS in corneas. On the other hand,
3-NT was used to monitor peroxynitrite (ONOO-) formation. The
appearance of 3-NT-containing proteins is a biomarker of oxidative
stress, indicating the development of reactive nitrogen species
(RNS) (28). Thus, the effect of
Nox inhibitors on the level of 3-NT-containing proteins was further
examined in the corneas. The results of immunofluorescence staining
and western blot analysis revealed that the Nox inhibitors, DPI or
Apo, significantly decreased the levels of 3-NT-containing proteins
(Fig. 2B and C). Taken together,
these results suggest that Noxs play an important role in alkali
burn-induced oxidative stress in corneas.
Effect of Nox inhibitors on corneal
inflammatory responses after alkali burns
It is well known that alkali burns trigger acute
inflammation in the corneas, and ROS play an important role in the
inflammatory response (9). As
mentioned above, the expression of Noxs and oxidative stress were
increased in corneas after alkali burns. Therefore, the effect of
Nox inhibitors on the alkali burn-induced corneal inflammatory
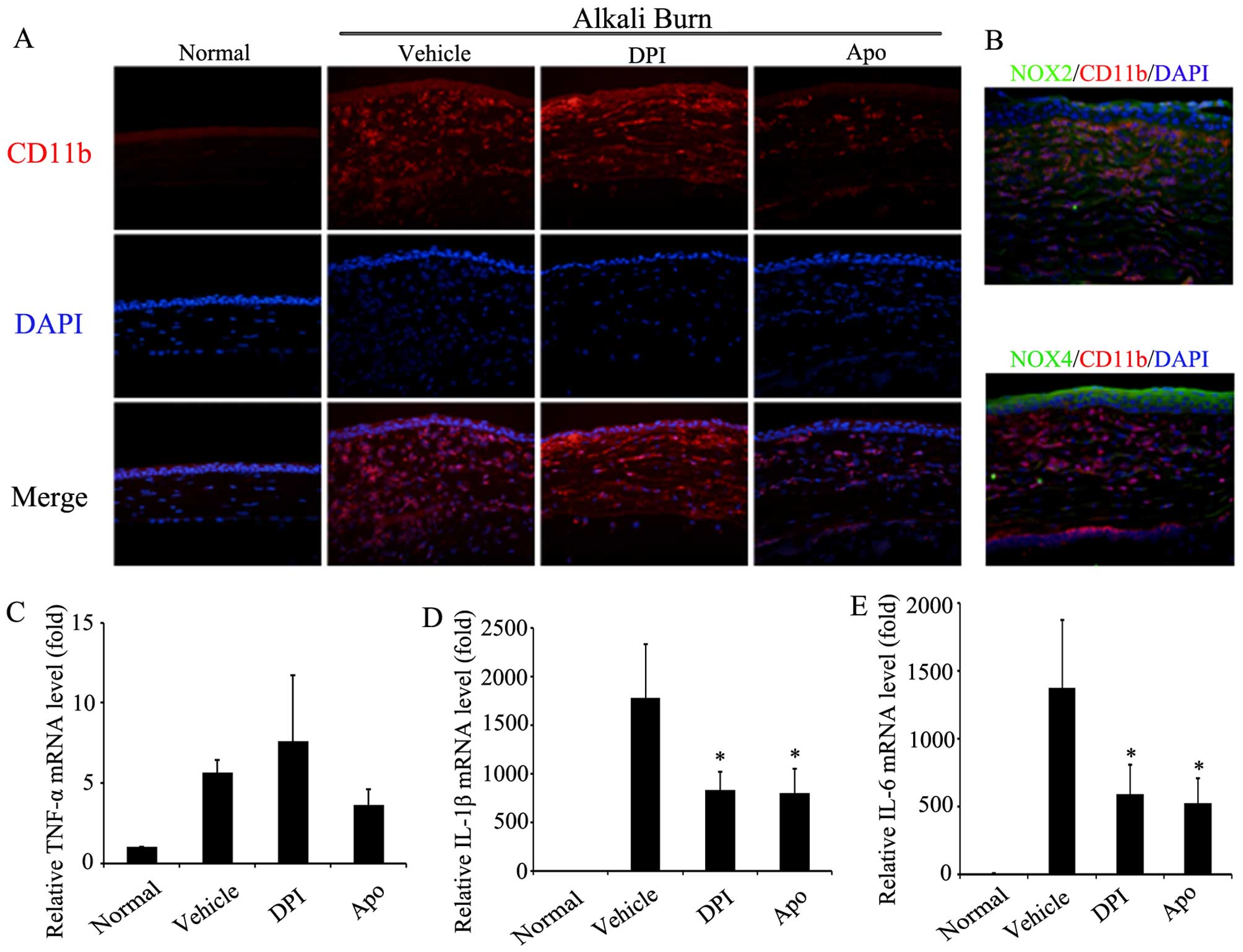
response was further examined. Firstly, the infiltration of
inflammatory cells into the corneas was assessed using CD11b
immunofluorescence staining. As shown in Fig. 3A, alkali burns induced the marked
infiltration of CD11b-positive inflammatory cells into the corneal
stroma. The administration of DPI and Apo by eye drops markedly
reduced the infiltration of CD11b-positive inflammatory cells. As
shown in Fig. 3B, CD11b/Noxs
double-immunofluorescence staining revealed the partial
co-localization of CD11b with Nox2 and Nox4, which suggests the
involvement of Nox2 and Nox4 in the infiltration of inflammatory
cells in corneas after alkali burns. Moreover, RT-qPCR revealed
that the mRNA levels of pro-inflammatory cytokines, namely IL-6,
IL-1β and TNF-α, were increased in the corneas on day 7 after
alkali burns. The administration of DPI or Apo by eye drops
significantly attenuated the alkali burn-induced increment of the
mRNA levels of IL-6 and IL-1β, but had no significant effect on the
TNF-α levels (Fig. 3C). These
results suggest that Noxs participate in the corneal inflammatory
response through the regulation of inflammatory cell infiltration
and the release of pro-inflammatory cytokines after alkali
burns.
Inhibition of Noxs effectively attenuates
alkali burn-induced CNV
Inflammation resulting from infection, aberrant
immune responses, or chemical burns usually disrupts the balance
between angiogenic and anti-angiogenic factors and eventually
triggers the development of CNV (29). In the present study, we monitored
the development of CNV after alkali burns. As shown in Fig. 4A, CNV occurred on day 3, and it
affected 50% of the whole area of the cornea on day 7, and on day
14, CNV coverage of the cornea reached maximum levels. DPI and Apo
effectively suppressed the length and density of CNV on day 7 after
alkali burns. To quantify the exact area of CNV after alkali burns,
FITC-dextran corneal angiography was conducted. As shown in
Fig. 4B, both DPI and Apo
significantly reduced the alkali burn-induced CNV area on day 14
after alkali burns. To further illustrate the role of Noxs in CNV,
the mRNA levels of angiogenic factors, namely VEGF, VEGFR1 and
VEGFR2 and MMPs in the corneas after alkali burns were measured by
RT-qPCR. The results revealed that alkali burns markedly increased
the mRNA levels of VEGF, VEGFR1, VEGFR2, MMP2, MMP9 and MMP13 in
the corneas. By contrast, administration of DPI or Apo by eye drops
significantly reduced the alkali burn-induced transcription of
these angiogenic factors, namely VEGF, VEGFR1/2, MMP2 and MMP13
(Fig. 4C and D). These results
suggest that Noxs play an important role in chemical burn-induced
CNV through the upregulation of angiogenic factors.
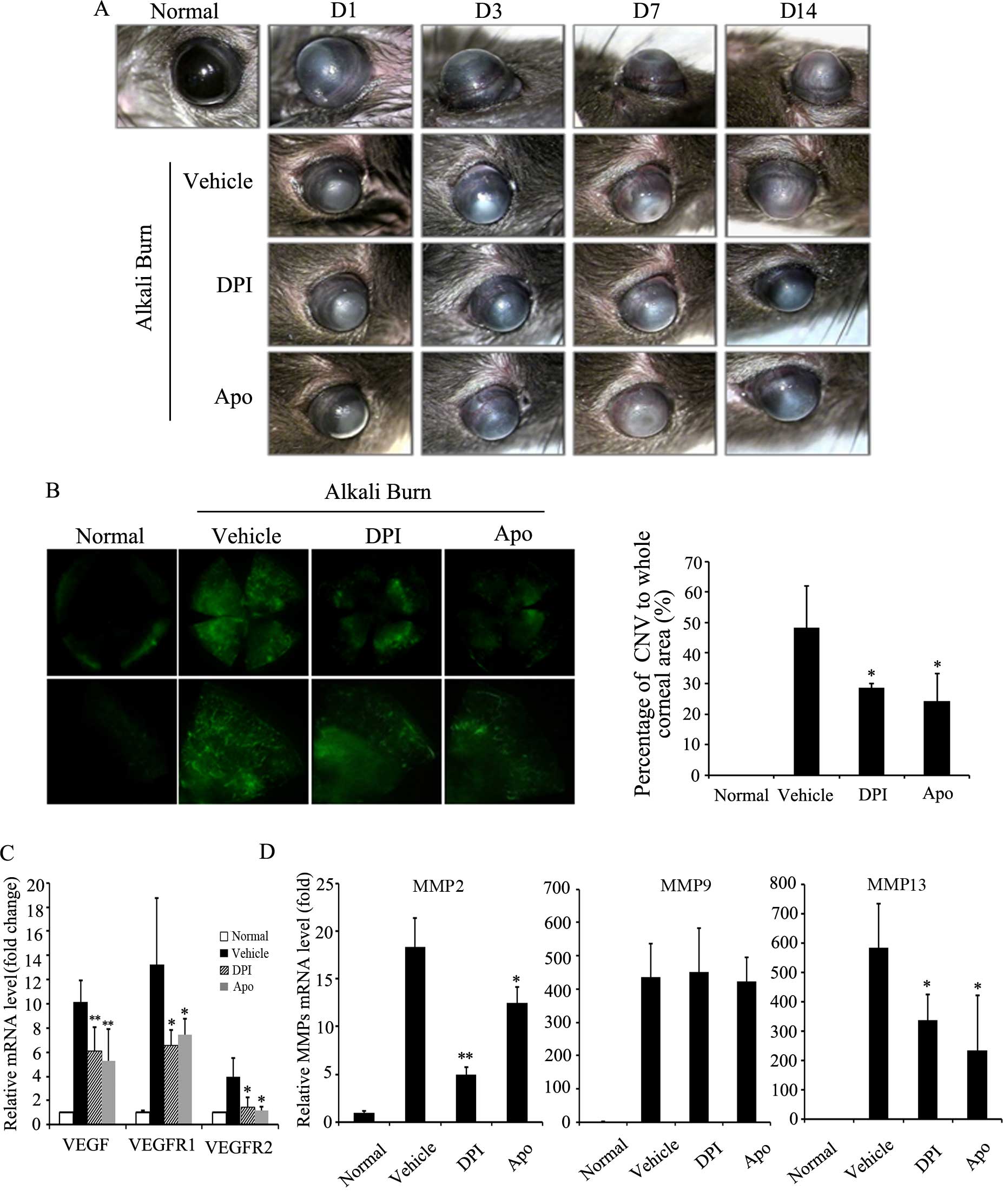 | Figure 4Effect of NADPH oxidase (NOX)
inhibitors on corneal neovascularization after alkali burns. (A)
Effect of diphenyleneiodonium (DPI) or apocynin (Apo) on the
development of corneal neovascularization (CNV) after alkali burns.
Corneal neovascularization was observed under a dissecting
microscope and photographed over the time course of our study.
Representative images of CNV at 1, 3, 7 and 14 days after alkali
burns are shown. D, day. (B) Effect of DPI and Apo on CNV area
after alkali burns. At 14 days after alkali burns, mice were
sacrificed by a cardiac injection of FITC-dextran. The corneas were
excised, and CNV was evaluated by FITC-dextran corneal angiography
under fluorescence microscope (original magnification, x4). The
graph shows quantification of CNV area in each condition.
*P<0.05 vs. vehicle. (C and D) Effect of NOX
inhibitors on the mRNA level of angiogenic factors in corneas after
alkali burns. RT-qPCR was applied to evaluate the transcription of
angiogenic factors, namely vascular endothelial growth factor
(VEGF), VEGF receptor 1/2 (VEGFR1/2), matrix metalloproteinase
(MMP)2, MMP9 and MMP13. Three independent experiments for each
condition were carried out, *P<0.05,
**P<0.01 vs. vehicle. |
Discussion
Chemical burns remain an important cause of corneal
damage. The extensive damage to corneas caused by chemical burns
usually leads to visual impairment or loss of vision (30). It is well known that oxidative
stress plays a crucial role in chemical burn-induced corneal
damage, and oxidative stress is characterized by increased ROS
production (31). As previously
demonstrated, Noxs are an important source of ROS, which induces
cellular oxidative stress, inflammation, tissue injuries and
diseases (32). In the present
study, we first examined the levels of Noxs in human and mouse
corneas after alkali burns. The transcription and expression of
Nox2 and Nox4 were significantly upregulated after alkali burns
(Fig. 1). In addition, Nox2 and
Nox4 were partially co-localized with CD11b-positive inflammatory
cells in the corneal stroma (Fig.
3B). These results suggest that Nox2 and Nox4 are involved in
the infiltration of inflammatory cells after alkali burns.
Importantly, the topical administration of the Nox inhibitors, DPI
or Apo, effectively attenuated alkali burn-induced oxidative stress
and the infiltration of CD11b-positive inflammatory cells into the
corneas (Figs. 2 and 3A). These results suggest the
involvement of Noxs in alkali burn-induced oxidative stress and
inflammatory responses in the cornea.
The inflammatory cells which infiltrate into the
corneal stroma after chemical burns usually trigger the
inflammatory responses through the release of inflammatory
cytokines (1). In a previous
study (33), the expression of
IL-1, IL-6, IL-10 and TNF-α was detected in alkali-burned corneas.
IL-1 and IL-6 levels were markedly upregulated at the early stages
of alkali burn-induced injury, and the production peak occurred on
days 3 and 7. By contrast, the production of IL-10 and TNF-α was
not significantly increased after alkali burns. However, in the
present study, we noted that the mRNA levels of IL-1β, IL-6 and
TNF-α were all elevated after alkali burns (Fig. 3C–E). The differences in results
between the two studies are possibly due to the different
evaluation methods used. Moreover, we noted that the administration
of the Nox inhibitors, DPI or Apo, effectively reduced the increase
in IL-1β and IL-6 levels, but had no significant effect on TNF-α
after alkali burns (Fig. 3C–E).
These results suggest that the inhibition of Noxs effectively
suppresses the release of inflammatory cytokines in corneas after
alkali burns.
Under physiological conditions, the cornea is a
transparent tissue. However, the inflammation induced by chemical
burns, infection and aberrant immune responses often leads to
pathological neovascularization in the corneas. CNV is one of the
severe sequelae of alkali burns, and it greatly influences corneal
transparence and results in permanent vision loss (1). In a previous study, it was reported
that ROS derived from Nox upregulated angiogenesis-related factors
and enhanced the migration and proliferation of endothelial cells
(34). Consistent with this
previous study, we found that alkali burns markedly increased the
area of CNV. The inhibition of Noxs by DPI or Apo significantly
suppressed alkali burn-induced CNV (Fig. 4A and B). Moreover, DPI or Apo
effectively attenuated the alkali burn-induced upregulation of the
angiogenesis-related factors, VEGF, VEGFR1/2, MMP2 and MMP13
(Fig. 4C and D). These
angiogenesis-related factors likely stimulate the proliferation,
migration and tube formation of endothelial cells to promote
neovascularization in corneas primarily through the VEGF receptors
(35).
In conclusion, in the present study, we found that
alkali burns upregulated the transcription and expression of Nox2
and Nox4 in corneas. The inhibition of Noxs by the administration
of DPI or Apo effectively suppressed oxidative stress, inflammatory
responses and neovascularization in the corneas after alkali burns.
Although the role of Noxs in non-ophthalmological diseases has been
previously described (36-39),
our data demonstrate for the first time (to the best of our
knowledge) that Noxs contribute to oxidative stress, inflammation
and neovascularization in corneas after alkali burns. Thus, we
suggest that the inhibition of Nox activity is a potential strategy
for preventing corneal damage after chemical burns.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300786) and the
Jiangxi Science and Technology Department (grant no.
20132BAB205024) and the research grant from Jiangxi Education
Department (grant no. GJJ13175), and supported partially by the
National Natural Science Foundation of China (grant nos. 31360241
and 81472371).
References
|
1
|
Wagoner MD: Chemical injuries of the eye:
current concepts in pathophysiology and therapy. Surv Ophthalmol.
41:275–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kubota M, Shimmura S, Kubota S, Miyashita
H, Kato N, Noda K, Ozawa Y, Usui T, Ishida S, Umezawa K, et al:
Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced
angiogenesis in a mouse corneal alkali-burn model. Invest
Ophthalmol Vis Sci. 52:427–433. 2011. View Article : Google Scholar
|
|
3
|
Conners MS, Urbano F, Vafeas C, Stoltz RA,
Dunn MW and Schwartzman ML: Alkali burn-induced synthesis of
inflammatory eicosanoids in rabbit corneal epithelium. Invest
Ophthalmol Vis Sci. 38:1963–1971. 1997.PubMed/NCBI
|
|
4
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mokini Z, Marcovecchio ML and Chiarelli F:
Molecular pathology of oxidative stress in diabetic angiopathy:
role of mitochondrial and cellular pathways. Diabetes Res Clin
Pract. 87:313–321. 2010. View Article : Google Scholar
|
|
6
|
Hakim FA and Pflueger A: Role of oxidative
stress in diabetic kidney disease. Med Sci Monit. 16:RA37–RA48.
2010.PubMed/NCBI
|
|
7
|
Blasiak J, Petrovski G, Veréb Z, Facskó A
and Kaarniranta K: Oxidative stress, hypoxia, and autophagy in the
neovascular processes of age-related macular degeneration. BioMed
Res Int. 2014:7680262014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shoham A, Hadziahmetovic M, Dunaief JL,
Mydlarski MB and Schipper HM: Oxidative stress in diseases of the
human cornea. Free Radic Biol Med. 45:1047–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saika S, Miyamoto T, Yamanaka O, Kato T,
Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW, Sato M, et al:
Therapeutic effect of topical administration of SN50, an inhibitor
of nuclear factor-kappaB, in treatment of corneal alkali burns in
mice. Am J Pathol. 166:1393–1403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou AY, Bai YJ, Zhao M, Yu WZ and Li XX:
KH902, a recombinant human VEGF receptor fusion protein, reduced
the level of placental growth factor in alkali burn induced-corneal
neovascularization. Ophthalmic Res. 50:180–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carter RT, Kambampati R, Murphy CJ and
Bentley E: Expression of matrix metalloproteinase 2 and 9 in
experimentally wounded canine corneas and spontaneous chronic
corneal epithelial defects. Cornea. 26:1213–1219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumimoto H, Miyano K and Takeya R:
Molecular composition and regulation of the Nox family NAD(P)H
oxidases. Biochem Biophys Res Commun. 338:677–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sareila O, Kelkka T, Pizzolla A, Hultqvist
M and Holmdahl R: NOX2 complex-derived ROS as immune regulators.
Antioxid Redox Signal. 15:2197–2208. 2011. View Article : Google Scholar
|
|
15
|
Nisimoto Y, Diebold BA, Cosentino-Gomes D
and Lambeth JD: Nox4: a hydrogen peroxide-generating oxygen sensor.
Biochemistry. 53:5111–5120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geiszt M, Kopp JB, Várnai P and Leto TL:
Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl
Acad Sci USA. 97:8010–8014. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hilenski LL, Clempus RE, Quinn MT, Lambeth
JD and Griendling KK: Distinct subcellular localizations of Nox1
and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc
Biol. 24:677–683. 2004. View Article : Google Scholar
|
|
18
|
Kuroda J, Nakagawa K, Yamasaki T, Nakamura
K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y,
Sueishi K and Sumimoto H: The superoxide-producing NAD(P)H oxidase
Nox4 in the nucleus of human vascular endothelial cells. Genes
Cells. 10:1139–1151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellis EA, Grant MB, Murray FT, Wachowski
MB, Guberski DL, Kubilis PS and Lutty GA: Increased NADH oxidase
activity in the retina of the BBZ/Wor diabetic rat. Free Radic Biol
Med. 24:111–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petry A, Djordjevic T, Weitnauer M,
Kietzmann T, Hess J and Görlach A: NOX2 and NOX4 mediate
proliferative response in endothelial cells. Antioxid Redox Signal.
8:1473–1484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manea A, Tanase LI, Raicu M and Simionescu
M: Transcriptional regulation of NADPH oxidase isoforms, Nox1 and
Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells.
Biochem Biophys Res Commun. 396:901–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deem TL and Cook-Mills JM: Vascular cell
adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix
metalloproteinases: role of reactive oxygen species. Blood.
104:2385–2393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Wang JJ, Yu Q, Chen K, Mahadev K and
Zhang SX: Inhibition of reactive oxygen species by Lovastatin
down-regulates vascular endothelial growth factor expression and
ameliorates blood-retinal barrier breakdown in db/db mice: role of
NADPH oxidase 4. Diabetes. 59:1528–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Brien WJ, Krema C, Heimann T and Zhao H:
Expression of NADPH oxidase in rabbit corneal epithelial and
stromal cells in culture. Invest Ophthalmol Vis Sci. 47:853–863.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Brien WJ, Heimann T and Rizvi F: NADPH
oxidase expression and production of superoxide by human corneal
stromal cells. Mol Vis. 15:2535–2543. 2009.PubMed/NCBI
|
|
26
|
Ren S, Zhang F, Li C, Jia C, Li S, Xi H,
Zhang H, Yang L and Wang Y: Selection of housekeeping genes for use
in quantitative reverse transcription PCR assays on the murine
cornea. Mol Vis. 16:1076–1086. 2010.PubMed/NCBI
|
|
27
|
Matthiesen S, Lindemann D, Warnken M,
Juergens UR and Racké K: Inhibition of NADPH oxidase by apocynin
inhibits lipopolysaccharide (LPS) induced up-regulation of arginase
in rat alveolar macrophages. Eur J Pharmacol. 579:403–410. 2008.
View Article : Google Scholar
|
|
28
|
Ahsan H: 3-Nitrotyrosine: A biomarker of
nitrogen free radical species modified proteins in systemic
autoimmunogenic conditions. Hum Immunol. 74:1392–1399. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Benayoun Y, Casse G, Forte R, Dallaudière
B, Adenis JP and Robert PY: Corneal neovascularization:
epidemiological, physiopathological, and clinical features. J Fr
Ophtalmol. 36:627–639. 2013.In French. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamill CE, Bozorg S, Peggy Chang HY, Lee
H, Sayegh RR, Shukla AN and Chodosh J: Corneal alkali burns: a
review of the literature and proposed protocol for evaluation and
treatment. Int Ophthalmol Clin. 53:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cejkova J, Trosan P, Cejka C, Lencova A,
Zajicova A, Javorkova E, Kubinova S, Sykova E and Holan V:
Suppression of alkali-induced oxidative injury in the cornea by
mesenchymal stem cells growing on nanofiber scaffolds and
transferred onto the damaged corneal surface. Exp Eye Res.
116:312–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang F, Zhang Y and Dusting GJ: NADPH
oxidase-mediated redox signaling: roles in cellular stress
response, stress tolerance, and tissue repair. Pharmacol Rev.
63:218–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sotozono C, He J, Matsumoto Y, Kita M,
Imanishi J and Kinoshita S: Cytokine expression in the
alkali-burned cornea. Curr Eye Res. 16:670–676. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ushio-Fukai M: Redox signaling in
angiogenesis: role of NADPH oxidase. Cardiovasc Res. 71:226–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shakiba Y, Mansouri K, Arshadi D and
Rezaei N: Corneal neovascularization: molecular events and
therapeutic options. Recent Pat Inflamm Allergy Drug Discov.
3:221–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lassègue B and Griendling KK: NADPH
oxidases: functions and pathologies in the vasculature.
Arterioscler Thromb Vasc Biol. 30:653–661. 2010. View Article : Google Scholar :
|
|
37
|
Nayernia Z, Jaquet V and Krause KH: New
insights on NOX enzymes in the central nervous system. Antioxid
Redox Signal. 20:2815–2837. 2014. View Article : Google Scholar :
|
|
38
|
Kimura M, Rabbani ZN, Zodda AR, Yan H,
Jackson IL, Polascik TJ, Donatucci CF, Moul JW, Vujaskovic Z and
Koontz BF: Role of oxidative stress in a rat model of
radiation-induced erectile dysfunction. J Sex Med. 9:1535–1549.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spencer NY and Engelhardt JF: The basic
biology of redoxosomes in cytokine-mediated signal transduction and
implications for disease-specific therapies. Biochemistry.
53:1551–1564. 2014. View Article : Google Scholar : PubMed/NCBI
|