Introduction
General anaesthetics are commonly employed in
operative settings for surgery and/or for pain relief. Volatile
anaesthetics such as isoflurane and sevoflurane are widely used in
adults as well in infants and young children for surgical
procedures and other medical treatments that require anaesthesia
(1). Previous findings have
demonstrated that prolonged exposure to the anaesthetics isoflurane
and sevoflurane leads to apoptotic neurodegeneration in developing
animal brains and also affects learning and memory (2–7).
Liang et al (8) reported
that isoflurane anaesthesia induced more robust apoptosis of cells
in the neonatal rat brain than sevoflurane when administered at an
eqivalent dose. Further clinical retrospective studies have
demonstrated that children <4 years of age exposed to
anaesthesia are possibly at a higher risk of developing learning
disabilities (9,10).
The increased expression of activated caspase-3, the
principal enzyme in the cellular apoptotic pathway, and β-amyloid
peptide (Aβ) levels were observed in murine brains following
isoflurane exposure (11,12). Studies have also suggested that
inflammation may possibly be involved in anaesthetic-mediated
neurodegeneration, particularly cognitive dysfunction (13,14). Wu et al (15) observed that isoflurane increased
pro-inflammatory cytokine levels in both in vitro and in
vivo experiments. Inflammation involving microglial activation
and increased levels of pro-inflammatory cytokines, particularly
tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 in the
brain, may lead to cognitive impairment (16–21).
It is well documented that the nuclear factor-κB
(NF-κB)-dependent signalling pathway is required for cytokine gene
transcription (22). The NF-κB
family of transcription factors play essential roles in
inflammation and innate immunity, and regulate many genes,
particularly those involved in inflammation, injury and stress
(23). Isoflurane has been
reported to induce neuronal apoptotic degeneration via
[Ca2+]i overload through gamma-aminobutyric
acid (GABA)A receptor-mediated synaptic
voltage-dependent calcium channels (VDCCs). The excessive release
of Ca2+ from the endoplasmic reticulum through the
activation of inositol-1,4,5-trisphosphate (IP3) receptors
(24,25), activates the NF-κB signalling
pathway (26–28), eventually leading to increased
levels of pro-inflammatory cytokines (27,29).
In addition, the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) pathway is widely expressed in the
central nervous system and affected by growth factors, such as
nerve growth factor, brain-derived neurotrophic factor and glial
cell line-derived neurotrophic factor, as well as cytokines and
neurotransmitters. The pathway plays a crucial role in neuronal
survival (30). Akt, a
serine/threonine kinase, regulates neuronal survival and is
activated by growth factors (31). On activation, Akt inhibits
apoptosis by phosphorylating and inactivating Bad and glycogen
synthase kinase 3β (GSK-3β) (32–34). Another target modulated by Akt is
NF-κB (35). Thus, targeting the
PI3/Akt pathway may significantly modulate neuronal cell survival
and inflammation.
The flavonoid naringenin is found in oranges,
grapefruits and tomatoes (36).
It has been proven to possess health benefits such as
anti-inflammatory, antioxidant and free radical scavenging
properties (37,38) as well as to reduce lipid levels
(39) and to prevent dyslipidemia
(40). Taking into consideration
the biological effects of naringenin, in the present study, we
examined the ability of naringenin to inhibit isoflurane-induced
neuroapoptosis and to ameliorate inflammation-induced cognitive
dysfunction.
Materials and methods
Experimental animals
The study was approved by the Institutional Animal
Care Committee of Nanchang University (Nanchang, China) and was
performed in accordance with the National Institutes of Health
Guidelines for the Use of Laboratory Animals. Pregnant
Sprague-Dawley rats (Guangdong Medical Laboratory Animal Center,
Guangzhou, China) were housed separately in sterile cages under
animal room conditions maintained at 22±1°C on a 12-h light/dark
cycle. The rats were provided with standard pelleted diet and water
ad libitum and closely monitored for the date of birth of
the pups, that was noted as postnatal day 0 (P0). The pups were
maintained carefully with free access to water with their
littermates.
Chemicals and reagents
Isoflurane (0.75%) and naringenin were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The following antibodies were
used for expression analysis: antibodies against cleaved caspase-3
(#9661), Bcl-2 (mouse mAb #15071), Bad (rabbit mAb #9268), Bcl-xL
(rabbit mAb #2764), Bax (rabbit mAb #5023), Akt (rabbit mAb #4691),
phosphorylated (p-)Akt (rabbit mAb #4060), GSK-3β (rabbit mAb
#9315), p-GSK-3β (rabbit mAb #9322), phosphatase and tensin homolog
(PTEN; rabbit mAb #9188), the inhibitors of apoptosis proteins
(IAP) xIAP (rabbit mAb #2045), cIAP-1 (rabbit mAb #3130), survivin
(rabbit mAb #2808), TNF-α [rabbit mAb (mouse specific) #11948] and
β-actin (mouse mAb #3700) (all from Cell Signalling Technology,
Beverly, MA, USA); NF-κB p65 (#sc-8008) and p-IκBα (#sc-8404) (both
from Santa Cruz Biotechnology, Santa Cruz, CA, USA); and IL-6
(ab100712) and IL-1β (ab197742) (both from Abcam, Cambridge, MA,
USA). All other chemicals used were of analytical grade and
purchased from Sigma-Aldrich, unless specified otherwise.
Anaesthetic exposure and dosing
Sixty pups were used and 12 pups were randomly
allocated to each group. Separate groups of pups were administered
naringenin [25, 50 or 100 mg/kg body weight (b.wt], orally from P3
until P21 along with a standard diet. On P7, pups weighing about
16–20 g were exposed to 0.75% isoflurane [approximately 0.3 minimum
alveolar concentration (MAC)] (41) in 30% oxygen or air in a
temperature controlled chamber for 6 h (42,43). P7 rats were selected on the basis
of previous studies suggesting that this period was most vulnerable
to anaesthesia-induced neuronal damage (2). The control rats received no
anaesthesia or naringenin. At the end of 6 h of isoflurane
exposure, the rat pups were sacrificed and brain tissues were
excised in order to perform protein expression analysis and the
TUNEL assay. Briefly, P7 rat pups were anaesthetized with
isoflurane and transcardially perfused with ice-cold saline
followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The
brain tissues were excised and embedded in paraffin. The rat pups
not exposed to either anaesthesia or treated with naringenin were
segregated as control rats. The isoflurane-exposed pups not
administered naringenin were grouped as anaesthetic control pups.
The animals were anesthetised with pentobarbital (40 mg/kg body
weight) and were intracardially perfused with 0.01M PBS. The brains
were immediately excised and the hippocampi were isolated. The
brain tissues were kept at −80°C until required for use in
subsequent experiments.
Assessment of neuroapoptosis by TUNEL
assay
Following 6 h of exposure to isoflurane,
neuroapoptosis was assessed by the TUNEL assay as previously
described by Li et al (44). Three sections 5 μm thick
were sliced (200 μm apart) on the same plane of the
hippocampus from each rat pup and were further analysed. Apoptosis
was assessed using a DeadEnd™ fluorometric TUNEL System kit
(Promega, Madison, WI, USA). The slides were carefully protected
from exposure to direct light during the experiment. The Hoechst
stain was used to stain nuclei. The TUNEL-positive cells in the
regions of hippocampal CA1, CA3 and dentate gyrus (DG) were further
analysed using NIS-Elements Basic Research (BR) imaging processing
and analysis software (Nikon Corporation, Tokyo, Japan).
Immunohistochemistry (IHC) staining
Cleaved caspase-3 expression was used as a marker of
apoptosis and cell death. Immunohistochemical analysis was
performed to assess caspase-3 expression in the hippocampi of
isoflurane-exposed rats as previously described by Li et al
(42,44). Briefly, the brain sections were
incubated with anti-cleaved caspase-3 primary antibody overnight at
4°C and further incubated with a secondary antibody (Santa Cruz
Biotechnology) for 40 min. Following treatment with
avidin-biotinylated peroxidase complex (Vectastain ABC-kit; Vector
Laboratories, Burlingame, CA, USA) for 40 min, the tissue sections
were treated with diaminobenzidine and were analysed with
NIS-Elements BR imaging processing and analysis software. The
density of cleaved caspase-3-positive cells in CA1, CA3 and DG
regions was calculated by dividing the number of caspase-3-positive
cells by the area of that brain region.
Western blot analysis
Protein expression in the hippocampi of rat pups was
examined in order to determine the effect of naringenin on the
outcome of isoflurane exposure according to previously described
procedures (42,43). Briefly, the tissues were
homogenised and proteins were isolated. Protein concentrations in
the tissue samples were determined using a BCA protein assay
(Bio-Rad, Hercules, CA, USA). Sixty micrograms of each protein
sample were subjected to SDS-PAGE separation and blotted on to
polyvinylidene difluoride membranes. The blots were incubated with
primary antibodies overnight at 4°C and washed and further
incubated with appropriate secondary antibodies. The immunoreactive
bands were visualized and the images were scanned using an
ImageMaster II scanner (GE Healthcare, Milwaukee, WI, USA). The
densities of the bands were further analysed by ImageQuant TL
software v2003.03 (GE Healthcare). Protein expression was
normalized to β-actin.
Memory and learning studies
Isoflurane induction has been documented to cause
cognitive dysfunction affecting learning and memory (2). In the present study, the effects of
naringenin on the behaviour and learning of rat pups were
assessed.
Open-field test
An open-field test was performed to assess the
emotional response of the rats to a novel environment. P42 rats
exposed to isoflurane on P7 were subjected to an open-field test as
previously described (45). The
response was noted as the movement of the rats in the open field.
The total distance travelled in meters over 10 min was recorded.
The movement of the animals was monitored and analysed using a
computerized video tracking system (SMART; Panlab S.L., Barcelona,
Spain).
Elevated plus-maze test
The elevated plus-maze test was performed to
evaluate anxiety-related behaviour in rodents. The test was
conducted according to the procedure described by Satoh et
al (45). The apparatus
consisted of two open (25×5 cm) and two closed arms. The arms were
placed at 50 cm above the floor. The responses of the animals were
recorded using a computerized video tracking system (SMART). The
behaviour of P42 rats was observed for a period of 10 min. The
percentage of time spent in the open arm was noted and this was
considered as an anxiety index.
Y-maze test
This study was performed to assess spatial working
memory and was performed as previously described by Satoh et
al (45). The symmetrical,
acrylic Y-shaped maze consisted of three arms (25×5 cm) that were
separated by 15-cm-high transparent walls. The rats were placed at
the centre of the maze and each rat was allocated 8 min to freely
explore the maze. The total number of arms entered and sequence of
entry were noted too. The calculations were performed as described
by Kodama et al (46).
Fear conditioning test
The P49 rats were subjected to a fear response test.
The fear conditioning test is a reliable, sensitive test used to
assess the effect of a hippocampal-dependent and
hippocampal-independent conditioned stimulus-unconditioned stimulus
pair that were separated by 1 min each. The unconditioned stimulus
consisted of 1 mA foot shock of a 1 sec duration whereas the
conditioned stimulus consisted of 80 dB of white noise for 20 sec.
The unconditioned stimulus was delivered during the last few
seconds of the conditioned stimulus. A contextual fear test was
performed in the conditioning chamber for a period of 5 min in the
absence of white noise 24 h after conditioning. A cued test (for
the same set of rats) was conducted by presenting a cue (80 dB
white noise, 3-min duration) while presenting distinct visual and
tactile cues. The movement of the animals was monitored using the
computerized video tracking system (SMART). The freezing response
rate of the rats, which was identified as the absence of movement
in any part of the body for 1 sec, was scored automatically and the
data were analysed and used as a measure of fear memory (4).
Morris water maze (MWM) test
The effect of naringenin on isoflurane-induced
alterations in memory and learning, spatial reference memory and
learning were evaluated using the MWM and experiments were
performed as described previously by Li et al (44).
The P7 rat pups that were exposed to isoflurane
and/or naringenin were trained for a period of 4 days between P31
and P35 in the MWM. A platform 10 cm in diameter was submerged in a
circular pool (200 cm diameter, 60 cm depth) that was filled with
warm water (23±2°C). The rats were subjected to 2 training sessions
a day. The rats were allowed 60 sec to locate the hidden platform
in the pool. If the animals were unable to locate the platform
within 60 sec, they were gently guided. The performance of the rats
and swim paths were recorded using the ANY-maze video tracking
system (Stoelting Co., Wood Dale, IL, USA). The tracking system
records and measures the time taken (latency) by each rat to find
the platform(s), and also information on other behaviours.
P35 rats were subjected to cued trials to test for
any non-cognitive performance impairments such as visual
impairments and/or swimming difficulties. The circular pool was
covered by a white cloth in order to conceal the visual cues. The
rats were subjected to 4 trials per day. During each trial, the
animals were placed in a specific position in the swimming pool and
allowed to swim to the platform which had a rod attached, that
served as the cue. The rod was placed approximately at 20 cm above
water level in any one of the four quadrants of the swimming pool.
The rats were given a time of 60 sec to locate the submerged
platform with the help of the cue and a time of 30 sec to sit on
the platform. The rats that was unable to locate the platform in
the given period of time, were gently guided and allowed to remain
there for 30 sec. The time taken by each animal to find the cued
platform was recorded.
Place trials were also performed. The curtains that
surrounded the pool for the cued trials were removed and the same
set of rats were assessed for their ability to learn the spatial
relationship between cues and the submerged platform (with no cue
rod), that was kept in one of the four quadrants. The platform was
placed in the same position throughout the place trials. The rats
were placed at random starting points and the time taken to reach
the platform was noted.
Furthermore, the rats were subjected to probe trials
in order to assess memory. The probe trials were conducted 24 h
after place trials. The submerged platform with no cue rod was
removed from the target quadrant (the quadrant in which the
platform was placed throughout the place trials). Rats were allowed
to swim for 60 sec and the time that each rat spent in the target
quadrant looking for the submerged platform was recorded. The data
are presented as the percentage of time spent in the quadrants. The
time spent in the target quadrant by the rats in comparison with
the time spent in other quadrants is used as an indication of
memory retention.
Statistical analysis
The results are presented as the means ± SD, taken
from three or six independent experiments. The data were subjected
to statistical analysis using the SPSS statistical package (22.0).
The values at p<0.05 were considered to indicate a statistically
significant difference as determined by one-way analysis of
variance (ANOVA) at p<0.05 followed by Duncan's multiple range
test (DMRT) for post hoc analysis.
Results
Naringenin reduces isoflurane-induced
neuroapoptosis
Previous studies have demonstrated that isoflurane
exposure induces neuroapoptosis (5,47).
In agreement with the findings of earlier studies, in our study, an
increase (p<0.05) in apoptotic cell counts was observed
following 6 h of isoflurane exposure in the CA1, CA3 and the DG
regions or the rat hippocampi (Fig.
1). However, the administration of naringenin caused a marked
decline in TUNEL-positive cell counts indicating that it
effectively provided protection to the neuronal cells exposed to
anaesthesia. Naringenin at 100 mg exhibited maximum protective
effects as compared with lower doses of 25 and 50 mg.
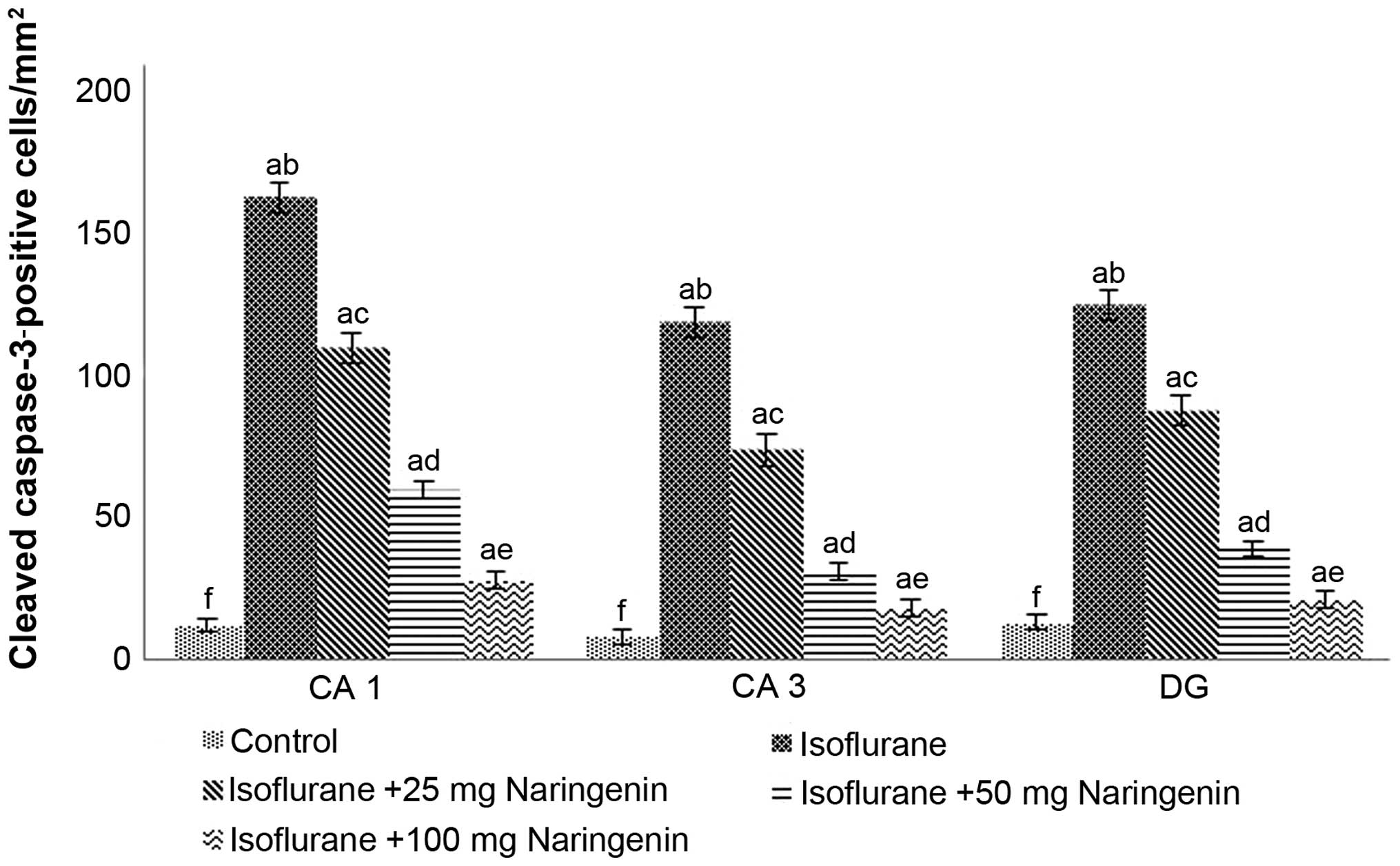
Cleaved caspase-3 expression is considered as a
significant marker of apoptosis (2,6).
To determine whether naringenin modulated the expression of
caspase-3 in the hippocampus, we assessed the expression of cleaved
caspase-3 by IHC staining. An increase in the number of
caspase-positive cells was observed in the hippocampus of rats
exposed to isoflurane (Fig. 2).
Analysis of cleaved caspase-3 protein expression by western blot
analysis also revealed significantly enhanced expression
(p<0.05) following 6 h of isoflurane exposure (Fig. 3). Elevated caspase-3 expression
may have contributed to the enhanced apoptotic counts evaluated by
the TUNEL assay. Notably, naringenin at doses of 25, 50 and 100 mg
downregulated caspase-3 protein expression and caspase-3-positive
cell counts which was in agreement with the TUNEL-positive cell
counts. Furthermore, caspase-3 expression was found to be
dose-dependent with a 100 mg dose of naringenin producing the
greatest decline in caspase-3 expression.
 | Figure 3Naringenin modulates the expression
of apoptosis pathway proteins. (A) Quantitative analysis of
relative protein expression. Naringenin effectively regulated the
expression of anti-apoptotic and pro-apoptotic proteins; naringenin
enhanced the expression of Bcl-2 and Bcl-xL,and markedly
downregulated the expression of Bax, Bad and caspase-3. (B)
Representative western blots of the proteins. Values are presented
as the means ± SD, n=6. a, p<0.05 compared with the control;
b–f, represent mean values within the same group that differ from
each other at p<0.05 as determined by one-way ANOVA followed by
Duncan's multiple range test analysis. (L1, control; L2,
isoflurane; L3, isoflurane +25 mg naringenin; L4, isoflurane +50 mg
naringenin; L5, isoflurane +100 mg naringenin). |
Naringenin effectively modulates the
expression of apoptotic pathway proteins
Isoflurane has been reported to cause robust
neurodegeneration and apoptosis in the developing brain (5). The balance between Bcl-2
pro-apoptotic proteins (Bax and Bad) and anti-apoptotic proteins
(Bcl-2 and Bcl-xL) regulates mitochondrial membrane integrity and
the release of apoptogenic factors that critically affect cell
survival and death (48).
Isoflurane exposure for 6 h in P7 rat pups caused a significant
increase in the expression of Bax and Bad with a decrease in the
expression of Bcl-2 and Bcl-xL (Fig.
3). The administration of naringenin at doses of 50 and 100 mg
induced a marked downregulation of the expression of Bax and Bad
and significantly (p<0.05) elevated the expression of the
anti-apoptotic proteins Bcl-xL and Bcl-2. Although a 25 mg dose of
naringenin modulated protein expression, the higher doses produced
more pronounced effects. Taken together, these findings suggest
that naringenin effectively regulates the expression of the
apoptotic pathway proteins in a dose-dependent manner.
Naringenin potentially activates the
PI3K/Akt pathway
Pro-survival pathways such as the PI3K/Akt pathway
may be inactivated during the apoptotic process (33). The pathway is widely expressed in
developing brains. In our study, isoflurane anaesthesia suppressed
the pathway as the levels of Akt, p-Akt, GSK-3β and p-GSK-3β were
reduced in the rat pups exposed to anaesthesia and not treated with
naringenin. Treatment with naringenin significantly upregulated
(p<0.05) the protein levels of Akt, p-Akt, and p-GSK-3β.
However, GSK-3β expression was not markedly altered by the
administration of naringenin (Fig.
4). PTEN, the negative regulator of the pathway, was increased
by isoflurane. Naringenin caused a significant decline in the
expression of PTEN in a dose-dependent manner with maximal effects
observed at a dose of 100 mg naringenin.
Naringenin significantly regulates the
NF-κB signalling pathway
The commonly used anaesthetic isoflurane is known to
induce neuro-inflammation (15).
It has been reported that the isoflurane-induced elevated cytosolic
Ca2+ levels activate the NF-κB signalling pathway
(28,29). Activated NF-κB is capable of
further activating and regulating various genes involved in
inflammatory responses (11). We
observed significantly high expression (p<0.05) of NF-κB p65,
p-IκBα, TNF-α, IL-1β and IL-6 following isoflurane exposure.
However, the levels of xIAP and cIAP were reduced (Fig. 5) which contributes to increased
apoptosis. Naringenin at all the tested doses caused marked
increases in the expression of xIAP and cIAP. However, naringenin
suppressed the activation of NF-κB and subsequently inhibited the
expression of TNF-α, IL-1β and IL-6.
Effects of naringenin on the behaviour
and memory of rats exposed to inhalation anaesthesia
The behaviour of the animals exposed to isoflurane
was assessed. In the present study, the rat pups exposed to
isoflurane on P7 were subjected to an open-field test which
involved observing the behaviour of the pups in a novel
environment. Isoflurane only-exposed pups did not exhibit
noticeable behavioural disturbances; the distance they moved across
the new field was comparatively less (p<0.05) than that in the
rats exposed to anaesthesia and treated with naringenin. The rats
that received 50 and 100 mg doses of naringenin exhibited behaviour
similar to the control rats that received no anaesthesia or
naringenin (Fig. 6A). In order to
assess anxiety-related behaviour, an elevated plus-maze test was
performed. The percentage of time spent in the open arms was noted.
Considerable differences were observed between the behaviour of the
animals treated with naringenin compared with that of the animals
in the isoflurane control groups (Fig. 6B).
Working memory is involved in holding information
temporarily to perform cognitive tasks that are complex and it
involves both the hippocampus and prefrontal cortex (49,50). To determine whether exposure to
isoflurane affected spatial working memory, a Y-maze test was
conducted. The tasks examined whether the rats remembered the arm
selected in the preceding choice. Rat pups exposed to isoflurane
exhibited significantly indifferent behaviour as compared with the
control rats not exposed to isoflurane. Naringenin at all the three
tested doses considerably improved the performance of the animals
(Fig. 6C).
The freezing responses of the animals exposed to
isoflurane were significantly (p<0.05) reduced in both the
contextual and cued conditioning tests (Fig. 6D). The freezing responses
exhibited by the animals treated with naringenin were substantially
higher than those of the animals administered with isoflurane
alone.
Assessment of learning and memory of
animals using MWM tests
To further evaluate the effect of isoflurane
exposure on potential learning and memory deficits, the animals
were subjected to the MWM test. The MWM is the most frequently used
and reliable measure of hippocampus-dependent spatial navigation
and reference memory assessment (51). The rats exposed to isoflurane
anaesthesia on P7 were trained to explore the swimming pool and
reach the hidden submerged platform. The time taken by each animal
to find and reach the platform was recorded as the escape latency.
The latency time recorded was found to decrease with each training
session in all the animals irrespective of whether they were
supplemented with naringenin or not (Fig. 7A). Nevertheless, the isoflurane
only-treated group exhibited slight variations from the animals
that received naringenin and isoflurane exposure on P7.
The cued trials were conducted by hiding the visual
cues in order to evaluate swimming and visual abilities. The P35
rats exposed to isoflurane anaesthesia on P7 took considerably
longer to reach the submerged platform with the rod compared with
the control group of rats that had received no anaesthesia.
Naringenin treatment at doses of 50 and 100 mg was observed to
improve the performance of the rats; however, the results were not
significant at a dose of 25 mg naringenin. The animals that
received naringenin (50 and 100 mg) took less time to reach the
platform compared with the rats that had received isoflurane alone.
Furthermore, 100 mg naringenin produced the maximum improvement in
the performance of the rats (Fig.
7B). Place and probe trials were conducted to assess the
ability of the rats to discover and remember the location of the
submerged platform without the cue rod. In the place trials, the
naringenin-treated rats exhibited a significant improvement
(p<0.05) in performance. The rats took significantly less time
to reach the platform.
Memory retention was assessed by removing the
platform from the target quadrant and placing it in a random
quadrant other than the target quadrant. The ability of the rats to
look for the platform in the target quadrant was observed.
Isoflurane exposure was found to have a significant impact on the
memory of the rats, as animals exposed to isoflurane spent
significantly less time (p<0.05) in the target quadrant in
comparison with the control group not exposed to anaesthesia
(Fig. 7B). The observed results
suggest that isoflurane induced memory and learning impairments.
Naringenin administration markedly improved the memory of the rats
as evidenced by the longer duration of the time spent by the rats
in the target quadrant looking for the platform, indicating the
effectiveness of naringenin in improving the memory of rats. A 100
mg dose of naringenin improved performance more effectively than
lower doses of 25 and 50 mg.
Discussion
Isoflurane is a widely used volatile anaesthetic
that has been reported to induce neurodegeneration and cognitive
impairments in developing brains (2,5,6,43).
The hippocampus is the most sensitive region to isoflurane-induced
neurotoxicity (52) and earlier
studies have demonstrated that neuroapoptosis occurs in the
hippocampi following exposure to isoflurane (8,53).
In agreement with the findings of previous studies, we observed
robust apoptosis in the hippocampi of P7 rats following exposure to
isoflurane anaesthesia. It has been demonstrated that the
developing brain is particularly vulnerable to anaesthestic-induced
neurotoxicity during the period of neurogenesis and synaptogenesis
and the P7 rats have been found to be extremely sensitive to
neurotoxic challenge (2). In our
study, IHC and western blot analysis revealed enhanced cleaved
caspase-3 expression in the hippocampal tissues of anaesthetic
only-exposed rats. Caspase-3 expression is often used as a potent
indicator of apoptosis (2,6)
and is also one of the final steps in apoptosis (54). Protein expression analysis and IHC
data revealed that naringenin effectively inhibited caspase-3
expression in a dose-dependent manner, suggesting that this
downregulation contributed to the decrease in apoptotic cell counts
in the hippocampi.
Furthermore, naringenin was also capable of
modulating the expression of the Bcl-2 family proteins that
regulate mitochondrial membrane integrity and control the release
of apoptogenic factors from mitochondria (48). Isoflurane exposure for 6 h was
found to upregulate Bad and Bax proteins and downregulate
anti-apoptotic proteins, namely Bcl-xL and Bcl-2. Studies have
reported that Bcl-xL enhances cell survival and is widely expressed
in the brain. In addition, Bcl-xL maintains mitochondrial membrane
integrity and inhibits cytochrome c release together with Bax
(48,55), thus inhibiting apoptosis. Earlier
studies of isoflurane have also shown similar results (56). Naringenin upregulated the
expression of Bcl-xL and Bcl-2 and also caused significant
downregulation of Bad and Bax protein expression. These
observations indicate that naringenin is effective at inhibiting
neurodegeneration.
The results of in vitro experiments have
demonstrated the critical role of the PI3K/Akt pathway in neurite
initiation, growth and stability (57–59), as well as in regulating the
branching of dendrites (60).
Propofol exerted neuroprotective effects by activating the PI3/Akt
pathway (61). In our study,
isoflurane was found to inhibit the phosphorylation of Akt, and to
eventually reduce the level of p-GSK-3β. Akt plays critical roles
in regulating and controlling cell survival and apoptosis.
Activation of the PI3K/Akt pathway potentially deactivates the
pro-apoptotic factors and activates the anti-apoptotic proteins
(62). Isoflurane-induced
downregulation of Akt and p-Akt and p-GSK-3β suggests the
activation of pro-apoptotic proteins. Activated Akt phosphorylates
and inactivates Bad causing the release of the anti-apoptotic
protein Bcl-xL that blocks apoptosis by binding to Bax (55,63). In our study, the administration of
naringenin significantly induced the phosphorylation of Akt and
GSK-3β. Moreover, the isoflurane-induced enhanced expression of
PTEN was suppressed by naringenin leading to the activation of the
PI3K/Akt signalling cascade. PTEN possesses lipid phosphatase
activity and is the negative regulator of the PI3K/Akt pathway
(64).
Accumulating evidence has shown that
neuro-inflammation is critically involved in anaesthetic-induced
neuroapoptosis and cognitive deficits (65–67). Isoflurane induced the release of
calcium from the endoplasmic reticulum (68,69), elevated cytosolic calcium levels
and this has been suggested to activate the NF-κB signalling
pathway which is implicated in inflammatory processes (28,70). This activation increased the
expression of TNF-α, IL-1 β and IL-6 (15,66,71). Our results also showed the
enhanced expression of NF-κB p65, p-IκBα, TNF-α, IL-1β and IL-6
suggesting activation of the NF-κB pathway. In addition, the
expression of NF-κB target genes - IAPs - xIAP, cIAP-1 and survivin
(72,73) were suppressed by isoflurane, thus
enhancing apoptosis. Naringenin, however, modulated the pathway by
effectively suppressing TNF-α, IL-1β and IL-6 suggesting the
involvement of the NF-κB signalling pathway in naringenin-mediated
neuroprotection.
Cognitive impairments in neonatal rats exposed to
isoflurane have been well documented. Moreover, it has been
reported that the period of synaptogenesis in the developing brain
is highly vulnerable to anaesthetic-induced neurotoxicity (2). Head et al (74) demonstrated that isoflurane causes
synaptic injury. In this study, tests for working memory as well as
long-term memory were performed to assess whether naringenin was
capable of ameliorating isoflurane-induced cognitive deficits. We
observed minor alterations in the general behaviour of the animals
as indicated in the open field and elevated maze test. However, the
spatial working memory was greatly affected following anaesthetic
exposure. Isoflurane induced profound alterations in the working
memory of the animals as evidenced in the Y-maze test. Working
memory is concerned with the cognitive functions that are
responsible for synchronized temporary storage and also with the
analysis and manipulation of information that is required in order
to execute intricate cognitive tasks (75).
Working memory is also involved in higher order
cognitive functions such as planning and sequential behaviour.
Impairment of the working memory is directly associated with
behavioural deficits and involves the hippocampus (76). Thus, isoflurane-induced
neurodegeneration in the hippocampi may have directly contributed
to the deficits in learning and memory. Notably, we observed a
marked improvement in the behaviour and memory of the animals
treated with naringenin. The rats exhibited a much improved
performance in the Y-maze tests indicating a significant
improvement in working memory. A naringenin-induced reduction in
hippocampal apoptosis may be in part responsible for the
significant improvement. Sanders et al (52) have demonstrated the
neuroprotective effects of dexmedetomidine. Dexmedetomidine reduced
isoflurane-induced hippocampal neuroapoptosis and also prevented
hippocampal-dependent neurocognitive impairment (52).
The MWM test was conducted in order to evaluate the
cognition and behaviour of the P35 rats. It is a reliable test for
assessing hippocampal-dependent spatial working and reference
memory (51). Jevtovic-Todorovic
et al (2) reported that
the period of neurogenesis is extremely sensitive to anaesthetic
challenge. Active neurogenesis is critical for
hippocampal-dependent learning and memory and any negative impact
affects the process (77,78). The escape latency time of the rats
that received isoflurane alone was significantly longer than the
controls. More importantly, neuro-inflammation also mediates
isoflurane-induced cognitive impairment (66,71). Nevertheless, we observed a marked
improvement in the working memory of the rats treated with
naringenin. At all the given doses, naringenin ameliorated memory
retention and the cognitive behaviour of the rats. These
observations suggest that naringenin may improve long-term
memory.
Thus, the marked reduction in neurodegeneration and
inhibition of inflammation observed following naringenin
administration may have contributed to the improvement in the
performance of the rats in the MWM tests. In conclusion, naringenin
effectively reduced neuronal apoptosis and neuro-inflammation by
modulating the NF-κB signalling pathway and also by activateing the
PI3K/Akt pathway. Thus, further studies of naringenin are warranted
in order to examine the molecular events and targets involved which
may aid in developing efficient therapeutic strategies against
anaesthetic-induced neuronal toxicity.
Acknowledgments
The present study was supported by the fund of the
Jiangxi Provincial Department of Science and Technology (no.
20142BBG70067) and the fund of the Jiangxi Provincial Department of
Education (no. 81539603).
References
|
1
|
Istaphanous GK and Loepke AW: General
anesthetics and the developing brain. Curr Opin Anaesthesiol.
22:368–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
3
|
Rizzi S, Carter LB, Ori C and
Jevtovic-Todorovic V: Clinical anesthesia causes permanent damage
to the fetal guinea pig brain. Brain Pathol. 18:198–210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takßishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brambrink AM, Evers AS, Avidan MS, Farber
NB, Smith DJ, Zhang X, Dissen GA, Creeley CE and Olney JW:
Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque
brain. Anesthesiology. 112:834–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kong F, Xu L, He D, Zhang X and Lu H:
Effects of gestational isoflurane exposure on postnatal memory and
learning in rats. Eur J Pharmacol. 670:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Istaphanous GK, Howard J, Nan X, Hughes
EA, McCann JC, McAuliffe JJ, Danzer SC and Loepke AW: Comparison of
the neuroapoptotic properties of equipotent anesthetic
concentrations of desflurane, isoflurane, or sevoflurane in
neonatal mice. Anesthesiology. 114:578–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalkman CJ, Peelen L, Moons KG, Veenhuizen
M, Bruens M, Sinnema G and de Jong TP: Behavior and development in
children and age at the time of first anesthetic exposure.
Anesthesiology. 110:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilder RT, Flick RP, Sprung J, Katusic SK,
Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL and
Warner DO: Early exposure to anesthesia and learning disabilities
in a population-based birth cohort. Anesthesiology. 110:796–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie Z, Culley DJ, Dong Y, Zhang G, Zhang
B, Moir RD, Frosch MP, Crosby G and Tanzi RE: The common inhalation
anesthetic isoflurane induces caspase activation and increases
amyloid beta-protein level in vivo. Ann Neurol. 64:618–627. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin D, Cao L, Wang Z, Li J, Washington JM
and Zuo Z: Lidocaine attenuates cognitive impairment after
isoflurane anesthesia in old rats. Behav Brain Res. 228:319–327.
2012. View Article : Google Scholar :
|
|
13
|
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN,
Culley DJ, Crosby G, Marcantonio ER, Tanzi RE and Xie Z:
Anesthetics isoflurane and desflurane differently affect
mitochondrial function, learning, and memory. Ann Neurol.
71:687–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu
Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE and Xie Z: The
inhalation anesthetic isoflurane increases levels of
proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging.
33:1364–1378. 2012. View Article : Google Scholar
|
|
16
|
Wilson CJ, Finch CE and Cohen HJ:
Cytokines and cognition - the case for a head-to-toe inflammatory
paradigm. J Am Geriatr Soc. 50:2041–2056. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perry VH: The influence of systemic
inflammation on inflammation in the brain: implications for chronic
neurodegenerative disease. Brain Behav Immun. 18:407–413. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goshen I, Kreisel T, Ounallah-Saad H,
Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E and Yirmiya R: A
dual role for interleukin-1 in hippocampal-dependent memory
processes. Psychoneuroendocrinology. 32:1106–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rudolph JL, Ramlawi B, Kuchel GA,
McElhaney JE, Xie D, Sellke FW, Khabbaz K, Levkoff SE and
Marcantonio ER: Chemokines are associated with delirium after
cardiac surgery. J Gerontol A Biol Sci Med Sci. 63:184–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teeling JL and Perry VH: Systemic
infection and inflammation in acute CNS injury and chronic
neurodegeneration: underlying mechanisms. Neuroscience.
158:1062–1073. 2009. View Article : Google Scholar
|
|
21
|
Patanella AK, Zinno M, Quaranta D, Nociti
V, Frisullo G, Gainotti G, Tonali PA, Batocchi AP and Marra C:
Correlations between peripheral blood mononuclear cell production
of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in
multiple sclerosis patients. J Neurosci Res. 88:1106–1112.
2010.
|
|
22
|
Combs CK, Karlo JC, Kao SC and Landreth
GE: β-Amyloid stimulation of microglia and monocytes results in
TNFalpha-dependent expression of inducible nitric oxide synthase
and neuronal apoptosis. J Neurosci. 21:1179–1188. 2001.PubMed/NCBI
|
|
23
|
Gilmore TD and Wolenski FS: NF-κB: Where
did it come from and why? Immunol Rev. 246:14–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Liang G, Chen Q, Joseph DJ, Meng
Q, Eckenhoff RG, Eckenhoff MF and Wei H: Anesthetic-induced
neurodegeneration mediated via inositol 1,4,5-trisphosphate
receptors. J Pharmacol Exp Ther. 333:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao YL, Xiang Q, Shi QY, Li SY, Tan L,
Wang JT, Jin XG and Luo AL: GABAergic excitotoxicity injury of the
immature hippocampal pyramidal neurons' exposure to isoflurane.
Anesth Analg. 113:1152–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Z and Yenari MA: Post-ischemic
inflammation: molecular mechanisms and therapeutic implications.
Neurol Res. 26:884–892. 2004. View Article : Google Scholar
|
|
27
|
Zheng Z, Kim JY, Ma H, Lee JE and Yenari
MA: Anti-inflammatory effects of the 70 kDa heat shock protein in
experimental stroke. J Cereb Blood Flow Metab. 28:53–63. 2008.
View Article : Google Scholar
|
|
28
|
Vexler ZS and Yenari MA: Does inflammation
after stroke affect the developing brain differently than adult
brain? Dev Neurosci. 31:378–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meffert MK, Chang JM, Wiltgen BJ, Fanselow
MS and Baltimore D: NF-kappa B functions in synaptic signaling and
behavior. Nat Neurosci. 6:1072–1078. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and-independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Staal SP: Molecular cloning of the akt
oncogene and its human homologues AKT1 and AKT2: Amplification of
AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci
USA. 84:5034–5037. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo HR, Hattori H, Hossain MA, Hester L,
Huang Y, Lee-Kwon W, Donowitz M, Nagata E and Snyder SH: Akt as a
mediator of cell death. Proc Natl Acad Sci USA. 100:11712–11717.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HK, Kumar P, Fu Q, Rosen KM and
Querfurth HW: The insulin/Akt signaling pathway is targeted by
intracellular beta-amyloid. Mol Biol Cell. 20:1533–1544. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmad A, Biersack B, Li Y, Kong D, Bao B,
Schobert R, Padhye SB and Sarkar FH: Targeted regulation of
PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their
derivatives: mechanistic details and biological implications for
cancer therapy. Anticancer Agents Med Chem. 13:1002–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vallverdú-Queralt A, Odriozola-Serrano I,
Oms-Oliu G, Lamuela-Raventós RM, Elez-Martínez P and Martín-Belloso
O: Changes in the polyphenol profile of tomato juices processed by
pulsed electric fields. J Agric Food Chem. 60:9667–9672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Renugadevi J and Prabu SM: Naringenin
protects against cadmium-induced oxidative renal dysfunction in
rats. Toxicology. 256:128–134. 2009. View Article : Google Scholar
|
|
38
|
Cavia-Saiz M, Busto MD, Pilar-Izquierdo
MC, Ortega N, Perez-Mateos M and Muñiz P: Antioxidant properties,
radical scavenging activity and biomolecule protection capacity of
flavonoid naringenin and its glycoside naringin: a comparative
study. J Sci Food Agric. 90:1238–1244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke JY, Kliewer KL, Hamad EM, Cole RM,
Powell KA, Andridge RR, Straka SR, Yee LD and Belury MA: The
flavonoid, naringenin, decreases adipose tissue mass and attenuates
ovariectomy-associated metabolic disturbances in mice. Nutr Metab
(Lond). 12:12015. View Article : Google Scholar
|
|
40
|
Mulvihill EE, Allister EM, Sutherland BG,
Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA and Huff
MW: Naringenin prevents dyslipidemia, apolipoprotein B
overproduction, and hyperinsulinemia in LDL receptor-null mice with
diet-induced insulin resistance. Diabetes. 58:2198–2210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Orliaguet G, Vivien B, Langeron O,
Bouhemad B, Coriat P and Riou B: Minimum alveolar concentration of
volatile anesthetics in rats during postnatal maturation.
Anesthesiology. 95:734–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Liu C, Zhao Y, Hu K, Zhang J, Zeng
M, Luo T, Jiang W and Wang H: Sevoflurane induces short-term
changes in proteins in the cerebral cortices of developing rats.
Acta Anaesthesiol Scand. 57:380–390. 2013a. View Article : Google Scholar
|
|
43
|
Li Y, Wang F, Liu C, Zeng M, Han X, Luo T,
Jiang W, Xu J and Wang H: JNK pathway may be involved in
isoflurane-induced apoptosis in the hippocampi of neonatal rats.
Neurosci Lett. 545:17–22. 2013b. View Article : Google Scholar
|
|
44
|
Li Y, Liang G, Wang S, Meng Q, Wang Q and
Wei H: Effects of fetal exposure to isoflurane on postnatal memory
and learning in rats. Neuropharmacology. 53:942–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Satoh Y, Endo S, Ikeda T, Yamada K, Ito M,
Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, et al:
Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show
deficits in long-term memory; ERK2 has a specific function in
learning and memory. J Neurosci. 27:10765–10776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kodama M, Satoh Y, Otsubo Y, Araki Y,
Yonamine R, Masui K and Kazama T: Neonatal desflurane exposure
induces more robust neuroapoptosis than do isoflurane and
sevoflurane and impairs working memory. Anesthesiology.
115:979–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei H, Kang B, Wei W, Liang G, Meng QC, Li
Y and Eckenhoff RG: Isoflurane and sevoflurane affect cell survival
and BCL-2/BAX ratio differently. Brain Res. 1037:139–147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity. J
Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jones MW: A comparative review of rodent
prefrontal cortex and working memory. Curr Mol Med. 2:639–647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saxe MD, Battaglia F, Wang JW, Malleret G,
David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER,
Santarelli L, et al: Ablation of hippocampal neurogenesis impairs
contextual fear conditioning and synaptic plasticity in the dentate
gyrus. Proc Natl Acad Sci USA. 103:17501–17506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hsu SY, Kaipia A, Zhu L and Hsueh AJ:
Interference of BAD (Bcl-xL/Bcl-2-associated death
promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms
and P11. Mol Endocrinol. 11:1858–1867. 1997.PubMed/NCBI
|
|
56
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Atwal JK, Massie B, Miller FD and Kaplan
DR: The TrkB-Shc site signals neuronal survival and local axon
growth via MEK and P13-kinase. Neuron. 27:265–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sanchez S, Sayas CL, Lim F, Diaz-Nido J,
Avila J and Wandosell F: The inhibition of
phosphatidylinositol-3-kinase induces neurite retraction and
activates GSK3. J Neurochem. 78:468–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dijkhuizen PA and Ghosh A: BDNF regulates
primary dendrite formation in cortical neurons via the PI3-kinase
and MAP kinase signaling pathways. J Neurobiol. 62:278–288. 2005.
View Article : Google Scholar
|
|
60
|
Jaworski J, Spangler S, Seeburg DP,
Hoogenraad CC and Sheng M: Control of dendritic arborization by the
phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin
pathway. J Neurosci. 25:11300–11312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang HY, Wang GL, Yu YH and Wang Y: The
role of phosphoinositide-3-kinase/Akt pathway in propofol-induced
postconditioning against focal cerebral ischemia-reperfusion injury
in rats. Brain Res. 1297:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koh PO: Nicotinamide attenuates the
ischemic brain injury-induced decrease of Akt activation and Bad
phosphorylation. Neurosci Lett. 498:105–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo
N, Chui DH and Guo XY: Activation of the canonical nuclear
factor-κB pathway is involved in isoflurane-induced hippocampal
interleukin-1β elevation and the resultant cognitive deficits in
aged rats. Biochem Biophys Res Commun. 438:628–634. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cao L, Li L, Lin D and Zuo Z: Isoflurane
induces learning impairment that is mediated by interleukin
learning impairment in rodents. PLoS One. 7:e514312012. View Article : Google Scholar
|
|
67
|
Shu Y, Zhou Z, Wan Y, Sanders RD, Li M,
Pac-Soo CK, Maze M and Ma D: Nociceptive stimuli enhance
anesthetic-induced neuroapoptosis in the rat developing brain.
Neurobiol Dis. 45:743–750. 2012. View Article : Google Scholar
|
|
68
|
Liang G, Wang Q, Li Y, Kang B, Eckenhoff
MF, Eckenhoff RG and Wei H: A presenilin-1 mutation renders neurons
vulnerable to isoflurane toxicity. Anesth Analg. 106:492–500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang H, Liang G, Hawkins BJ, Madesh M,
Pierwola A and Wei H: Inhalational anesthetics induce cell damage
by disruption of intracellular calcium homeostasis with different
potencies. Anesthesiology. 109:243–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim YJ, Hwang SY, Oh ES, Oh S and Han IO:
IL-1beta, an immediate early protein secreted by activated
microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK
and NF-kappaB pathways. J Neurosci Res. 84:1037–1046. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gyrd-Hansen M and Meier P: IAPs: From
caspase inhibitors to modulators of NF-kappaB, inflammation and
cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Head BP, Patel HH, Niesman IR, Drummond
JC, Roth DM and Patel PM: Inhibition of p75 neurotrophin receptor
attenuates isoflurane-mediated neuronal apoptosis in the neonatal
central nervous system. Anesthesiology. 110:813–825. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Baddeley A: Working memory. Science.
255:556–559. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Madsen TM, Kristjansen PE, Bolwig TG and
Wörtwein G: Arrested neuronal proliferation and impaired
hippocampal function following fractionated brain irradiation in
the adult rat. Neuroscience. 119:635–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rola R, Raber J, Rizk A, Otsuka S,
VandenBerg SR, Morhardt DR and Fike JR: Radiation-induced
impairment of hippocampal neurogenesis is associated with cognitive
deficits in young mice. Exp Neurol. 188:316–330. 2004. View Article : Google Scholar : PubMed/NCBI
|