Introduction
Inflammation is not only a physiological response
against deleterious stimuli, such as infection, but also a
contributing factor to the pathogenesis of various metabolic
disorders. For example, systemic inflammatory markers are
associated with the disease progression of type 2 diabetes and its
macrovascular complications (1).
The inflammation that accompanies metabolic syndrome usually
displays a unique characteristic. As it is not accompanied by
infection or massive tissue injury, it is often termed 'low-grade'
chronic inflammation (2).
Scientists have endeavoured to elucidate a mechanistic link between
inflammation and metabolic disorders.
The levels of free fatty acids are increased in
obesity, and saturated fatty acids have been nominated as
candidates that increase the inflammatory response in metabolic
syndrome (3). In particular,
palmitic acid levels in plasma and triglycerides correlate with
insulin resistance (4), and a
recent study revealed that palmitate activates inflammatory
pathways, leading to an impairment in insulin transcytosis
(5). Along with palmitate,
significant increases in plasma ceramide levels have also been
observed in patients with type 2 diabetes and in diabetic animals,
and these increases further correlate with the progression of
diabetes and the activation of inflammatory mediators, such as
tumour necrosis factor (TNF)-α (6,7).
Ceramide can be generated either by attaching fatty
acyl CoA to a long-chain base or by degrading pre-existing
sphingolipids, such as sphingomyelin (8). Six mammalian ceramide synthases
(CerS) determine the acyl chain length of ceramide (8,9).
CerS1 and CerS5-6 generate ceramides with long acyl chains (C18-
and C16-ceramides, respectively) (10–12). CerS4 generates C18-C20-ceramides
(13), and CerS2 produces
ceramides with very long acyl chains (C22-C24-ceramides) (14). CerS3 generates ceramides with even
longer acyl chains (C26-C34-ceramides) (15). The distribution of CerS and
ceramides with distinct fatty acyl chain lengths has been reported
to play different roles in various pathophysiologies (8,9).
The overexpression of CerS2 results in partial protection from
radiation-induced apoptosis, whereas the overexpression of CerS5
increases the apoptosis of HeLa cells (16). In addition to de novo
synthesis, ceramide can also be formed by sphingomyelinase (SMase)
activation. SMase degrades sphingomyelin into ceramide and
phosphorylcholine and can be either acidic (A-SMase) or neutral
(N-SMase), depending on the optimal pH needed for activation
(17).
The liver is a central organ in metabolism that
controls lipogenesis, gluconeogenesis and cholesterol. Liver
cirrhosis contributes to the fourth leading cause of mortality due
to diabetes (18). Considering
the important role of the liver in metabolic processes,
understanding inflammatory cytokine secretion in the liver would be
crucial for the elucidation of the underlying mechanisms of
inflammation in metabolic diseases. Therefore, in this study, we
examined the role of ceramide formation in hepatic inflammatory
cytokine production upon palmitate or lipopolysaccharide (LPS)
stimulation in vitro and upon high-fat diet (HFD) feeding
in vivo.
Materials and methods
Materials
The following materials were purchased: fumonisin B1
(FB1), LPS, palmitate, myriocin, GW4869, SB203580, SP600125,
anti-CerS2 antibody (HPA027262), anti-CerS4 antibody (SAB4301210),
anti-α-tubulin antibody (T9026) and anti-HA antibody (H6908) (all
from Sigma-Aldrich, St. Louis, MO, USA); PD98059 and anti-p38
(8690), anti-phosphorylated (phosphor)-p38 (Thr180/Tyr182) (4511),
anti-c-Jun N-terminal kinase (JNK) (9252), anti-phosphorylated
(p-)JNK (Thr183/Tyr185) (9255), anti-extracellular signal-regulated
kinase (ERK) (4695), anti-p-ERK (Thr202/Tyr204) (4370), anti-p65
(8242), anti-p-IκB (9246), and anti-Lamin A/C (4777) antibodies
(all from Cell Signalling Technology, Inc., Beverly, MA, USA);
pyrrolidinedithiocarbamate ammonium (PDTC) (BioVision, Inc.,
Mountain View, CA, USA); anti-CerS1 (H00010715-A01), anti-CerS5
(PAB13439) and anti-CerS6 (H00253782-A01) antibodies (Abnova,
Taipei, Taiwan); anti-mouse-HRP (horseradish
peroxidase)(115-036-003) and anti-rabbit-HRP (111-035-003)
antibodies (Jackson Laboratory, Ben Harbor, ME, USA).
Cell culture and transfection
Hep3B cells were purchased from the American Type
Culture Collection (ATCC, Rockville, MD, USA; HB8064) and were
grown in Dulbecco's modified Eagle's medium (HyClone, Logan, UT,
USA), which was supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin (HyClone). The Hep3B cells were transfected
with plasmids using Metafectene (Biontex Laboratories GmbH, Munich,
Germany), according to the manufacturer's instructions. In some
cases, the Hep3B cells were transfected with pCMV-empty vector, 2
or 5 μg of pCMV-CerS6-HA plasmid in 6-well plates. All
plasmids, including pCMV-empty vector, pCMV-CerS1-HA, CerS2-HA,
CerS4-HA, CerS5-HA and CerS6-HA were kindly provided by professor
Anthony H. Futerman (Weizmann Institute of Science, Rehovot,
Israel).
Animals and HFD feeding
Male C57BL/6J mice (6 weeks old) were purchased from
Orient Bio Inc. (Seoul, Republic of Korea), and housed under
specific pathogen-free conditions. All animal experiments and
procedures were approved by The Animal Ethics Committee at Ewha
Womans University College of Medicine (Seoul, Korea; ESM no.
14-0280), and all animals were treated in accordance with the
Animal Care Guidelines of Ewha Womans University. The mice were
housed in a controlled environment at 21–23°C and 51–54% humidity,
with a 12-h light-dark cycle, and were supplied with food and water
ad libitum. The mice were fed a HFD (D12492; Research Diets
Inc., New Brunswick, NJ, USA) for 6–24 weeks and hepatic CerS
levels were analyzed. Other mice were fed a HFD for 24 weeks and
administered an intraperitoneal injection of desipramine (10
mg/kg/day) and myriocin (0.15 mg/kg/day), as previously described
(19,20). Three mice were used in each group.
The mice were sacrificed by CO2 inhalation at 6, 12, 18
and 24 weeks after being fed the HFD. The mice not fed the HFD were
used as controls. The liver tissue was perfused with
phosphate-buffered saline through the inferior vena cava, and the
portal vein was cut using scissors to remove blood from the liver.
The livers were then collected, and snap-frozen in liquid nitrogen
for storage at −80°C.
Enzyme-linked immunosorbent assay
(ELISA)
At 24 h following CerS6-HA transfection or 5
μg/ml LPS treatment and a further 24 h of co-incubation with
various chemicals (2 μM desipramine, 10 μM Fumonisin
B1, 10 μM GW4869, 10 μM SB203580, 10 μM
SP600125, 10 μM PD98059, 10 μM PDTC, or 500 μM
palmitate) the levels of TNF-α, interleukin (IL)-1β and IL-6 in the
Hep3B cell culture medium were measured using respective ELISA kits
[TNF-α and IL-1β Human ELISA kits (Abfrontier, Seoul, Korea); and
the IL-6 Human ELISA kit (Abcam, Cambridge, MA, USA)], according to
the manufacturer's instructions. The TNF-α and IL-1β levels in the
livers of the HFD-fed mice that were co-treated with desipramine
(10 mg/kg/day) or myriocin (0.15 mg/kg/day) were analysed using a
Mouse TNF-α ELISA kit (Biolegend Inc., San Diego, CA, USA) and a
Mouse IL-1β ELISA kit (Abcam).
Western blot analysis
The Hep3B cells were lysed using RIPA buffer (50 mM
of Tris-Cl, pH 7.5, 150 mM of NaCl, 1% nonidet P-40, 0.5% sodium
deoxycholate and 0.1% SDS) containing protease and phosphatase
inhibitors (Sigma-Aldrich). The protein levels were then quantified
using Protein Assay Dye Reagent (Bio-Rad Laboratories, Hercules,
CA, USA). Fifty micrograms of proteins were separated by SDS-PAGE
on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes (Bio-Rad). The membranes were then blocked with 5% bovine
serum albumin (Sigma-Aldrich) in PBST (PBS with 0.1% Tween-20) for
1 h and incubated with primary antibodies overnight at 4°C.
Secondary antibodies were attached for 1 h at room temperature.
Protein bands were detected by the Chemidoc MP imaging system
(Bio-Rad), using ECL Western Blotting Detection Reagents (Amersham
Biosciences, Buckinghamshire, UK).
A-SMase activity assay
A-SMase activity was examined as previously
described (21,22). Briefly, the Hep3B cells were lysed
in 500 μl sodium acetate buffer (50 mM sodium acetate, pH
4.5). To initiate the reaction, C6-NBD-SM (1 nmol; Avanti Polar
Lipid, Alabaster, AL, USA) was added to the Hep3B cell lysates
followed by incubation at 37°C for 20 min. The reactions were
terminated by the addition of 3 volumes of chloroform:methanol
(2:1). NBD-ceramide was separated by thin-layer chromatography
using chloroform:methanol:9.8 mM aqueous CaCl2 (60:35:8;
v/v/v).
Separation of nuclear and cytoplasmic
fractions
The isolation of nuclear and cytoplasmic fractions
was performed as previously described (23). Briefly, the Hep3B cells were lysed
in fractionation buffer (50 mM HEPES, pH 7.4, 10 mM KCl, 1 mM EDTA,
1 mM EGTA, 1 mM dithiothreitol and 0.1% NP-40) containing protease
and phosphatase inhibitors (Sigma-Aldrich). Following 15 min of
incubation on ice, the lysates were centrifuged at 4,000 × g, for 5
min at 4°C; and the pellet was further resuspended in lysis buffer
(20 mM HEPES, pH 7.4, 150 mM NaCl, 12.5 mM glycerophosphate, 1.5 mM
MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM dithiothreitol, 1 mM
Na3VO4, 0.5% Triton X-100, protease and
phosphatase inhibitors) and sonicated to obtain the nuclear
fraction. The supernatant was centrifuged at 12,500 × g for 5 min
at 4°C to obtain the cytoplasmic fraction.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from the mouse livers was extracted using
RNeasy mini kits (Qiagen, Inc., Valencia, CA, USA), and cDNA from
the extracted RNA was synthesized using a Verso cDNA Synthesis kit
(Fisher Scientific, Hampton, NH, USA). qPCR was performed using the
SYBR-Green Real-Time PCR Master Mix (Life Technologies, Grand
Island, NY, USA) in an ABI PRISM 7500 Sequence Detection System
(Applied Biosystems Inc., Waltham, MA, USA), as previously
described (24). Relative gene
expression was calculated by using the 2−ΔΔCt method
(24). The primers used are
listed in Table I.
 | Table IPrimers used for qPCR. |
Table I
Primers used for qPCR.
| Gene | Primer
sequences | Refs. |
|---|
| TNF-α (mouse) | F:
5′-CTGTAGCCCTCGTAGC-3′
R: 5′-TTGAGATCCATGCCGTTG-3′ | (42) |
| IL-1β (mouse) | F:
5′-TGTAATGAAAGACGGCACACC-3′
R: 5′-TCTTCTTTGGGTATTGCTTGG-3′ | (42) |
| IL-6 (mouse) | F:
5′-TCCAGTTGCCTTCTTGGGAC-3′
R: 5′-GTACTCCAGAAGACCAGAGG-3′ | (43) |
| GAPDH (mouse) | F:
5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′
R: 5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′ | (42) |
Liquid chromatography-electrospray
ionization-tandem mass spectrometry (LC-ESI-MS-MS) analysis of
ceramide
Ceramide analyses by LC-ESI-MS-MS were conducted as
previously described (25,26)
with some modifications. Briefly, lipids extracted with
1×107 cells were injected into a HPLC (Agilent 1,200
series; Agilent Technologies, Inc., Santa Clara, CA, USA) and
separated through a reverse phase KINETEX C18 column (2.1×50 mm,
ID: 2.6 μm) (Phenomenex Inc., St. Louis, MO, USA). The HPLC
column effluent was then introduced into an API 3200 Triple
quadruple mass (AB Sciex, Toronto, ON, Canada) and analysed using
electrospray ionization in positive mode with multiple reaction
monitoring to select both parent and characteristic daughter ions
specific to each analyte simultaneously from a single injection.
Data were acquired using Analyst 1.4.2 software (Applied
Biosystems).
Statistical analysis
All experiments were repeated at least 3 times
independently, and values are presented as the means ± standard
error of the mean. Statistical significance was calculated using
the Student's t-test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Palmitate and CerS6 have synergistic
effects on TNF-α secretion via the phosphorylation of p38 MAPK
To examine the role of de novo ceramide
formation in inflammatory cytokine secretion from liver cells,
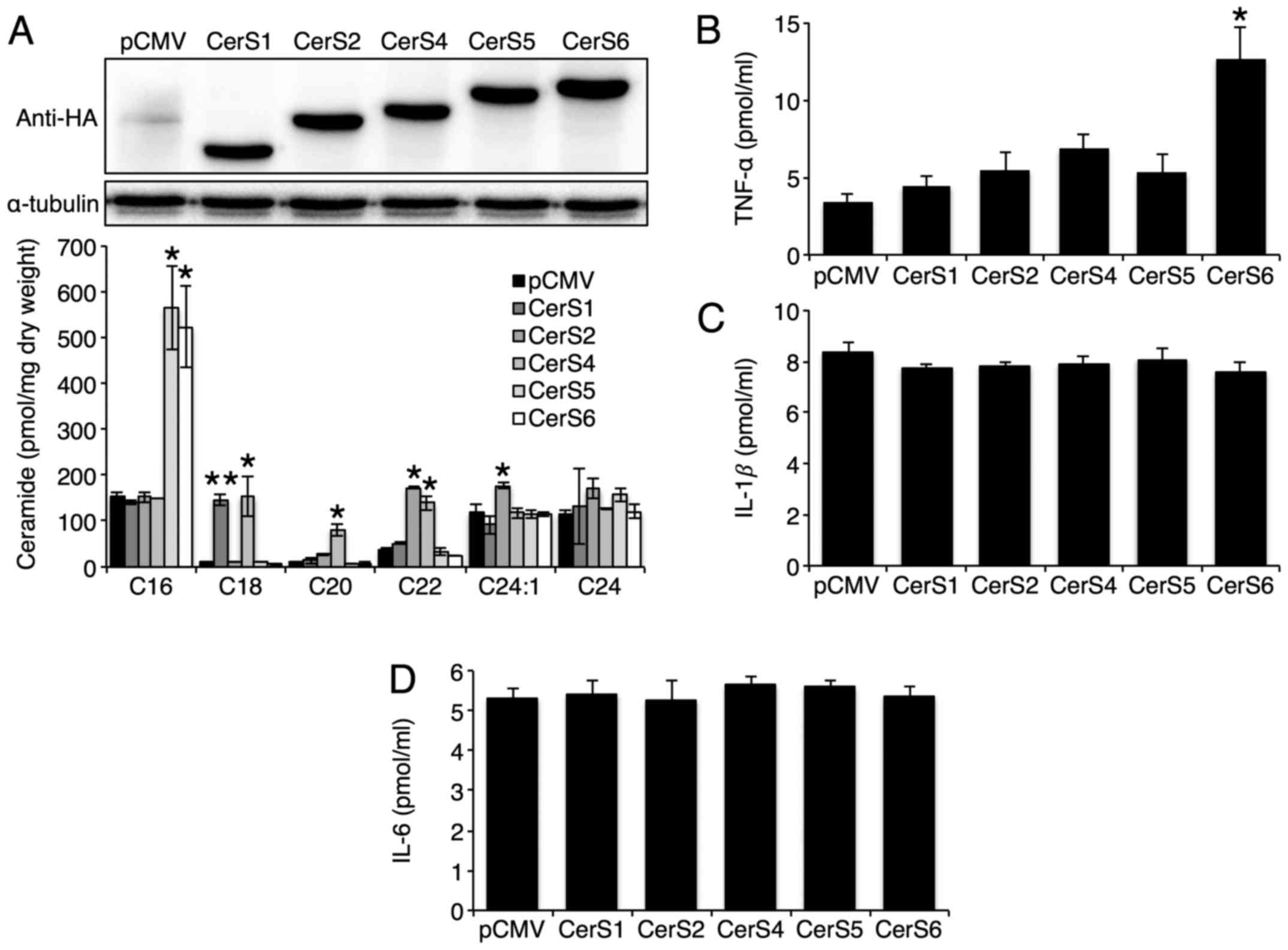
CerS1, CerS2, CerS4, CerS5, or CerS6 was overexpressed in Hep3B
cells and the overexpression of each CerS increased the levels of
ceramides with different acyl chain lengths successfully (Fig. 1A). CerS3 was excluded, as it was
previously shown to be hardly expressed in the liver (14). Only CerS6 overexpression increased
TNF-α secretion (Fig. 1B). The
overexpression of either CerS had no effect on IL-1β and IL-6
secretion (Fig. 1C and D). As
MAPK activation has been reported to be involved in TNF-α secretion
(27), we then examined the MAPK
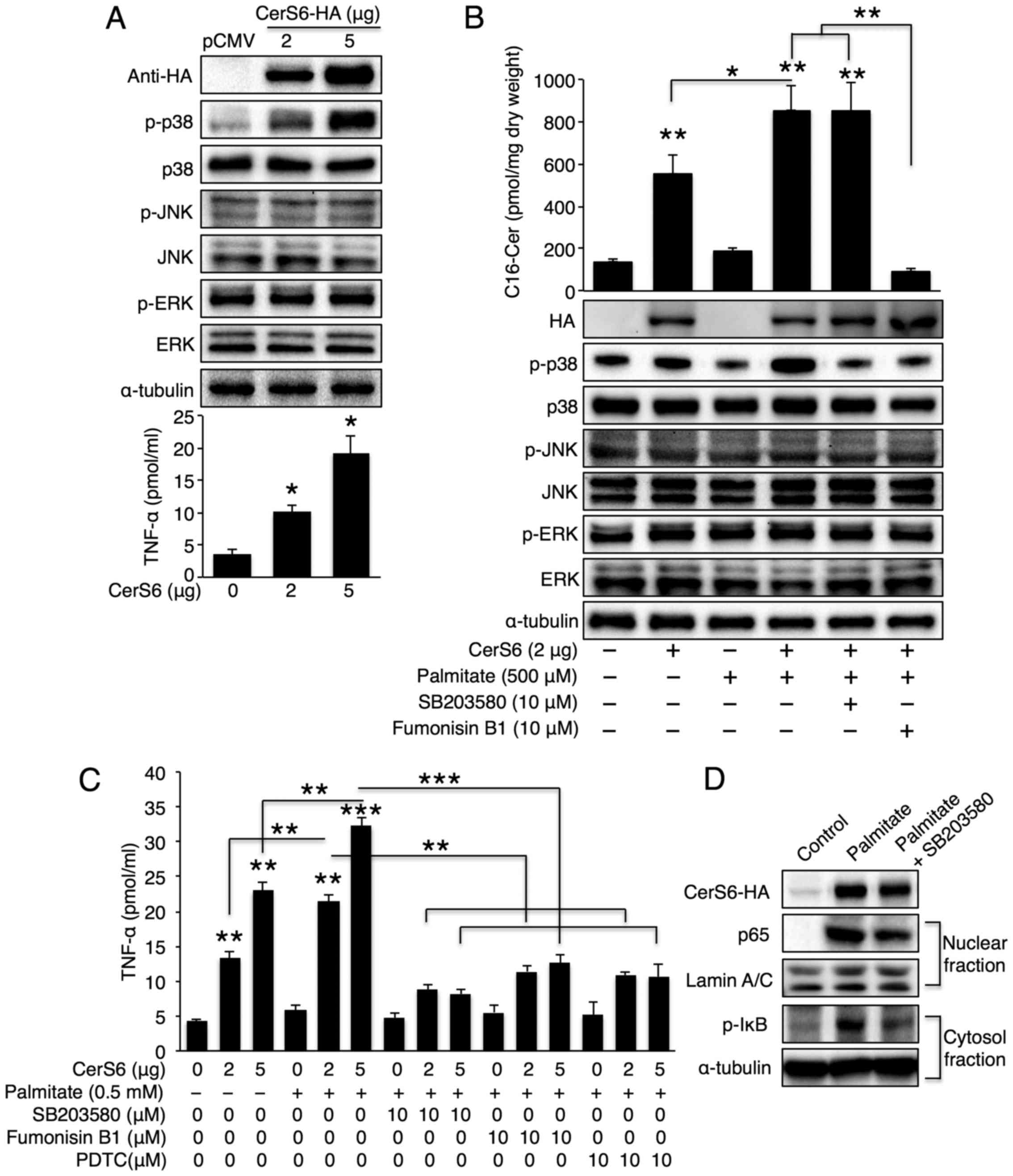
pathway following CerS6 overexpression. CerS6 overexpression
elevated p38 phosphorylation in a dose-dependent manner, although
neither JNK nor ERK was activated upon CerS6 overexpression
(Fig. 2A, upper panel). TNF-α
secretion increased as the CerS6 expression levels increased
(Fig. 2A, lower panel). Of note,
a synergistic effect was observed in the cells overexpressing CerS6
treated with palmitate as regards both C16-ceramide generation and
p38 phosphorylation (Fig. 2B). To
examine whether p38 is a direct target of CerS6 upon palmitate
treatment, the CerS6 overexpressing cells were co-treated with
palmitate and the CerS inhibitor, FB1. CerS inhibition reversed p38
phosphorylation; this effect was similar to that achieved with the
p38 inhibitor, SB203580, thus confirming that p38 is downstream of
CerS6 (Fig. 2B). In accordance
with p38 activation, we also observed a synergistic effect between
palmitate treatment and CerS6 overexpression as regards TNF-α
secretion (Fig. 2C); treatment
with SB203580 and FB1 also reversed the palmitate- and CerS6
overexpression-induced increase in TNF-α secretion (Fig. 2C). As the p38/NF-κB pathway is
important for TNF-α secretion (27), we further examined the NF-κB
pathway upon palmitate treatment in CerS6 overexpressing Hep3B
cells. As expected, palmitate treatment increased nuclear p65
translocation and IκB phosphorylation; both events were reversed by
p38 inhibition (Fig. 2D).
Similarly, TNF-α secretion was also inhibited by treatment with
PDTC (a NF-κB inhibitor) (Fig.
2C). Thus, we concluded that palmitate, which is a saturated
fatty acid that is increased in metabolic diseases, synergistically
increases TNF-α production with CerS6 in liver cells via the
CerS6/p38/NF-κB pathway.
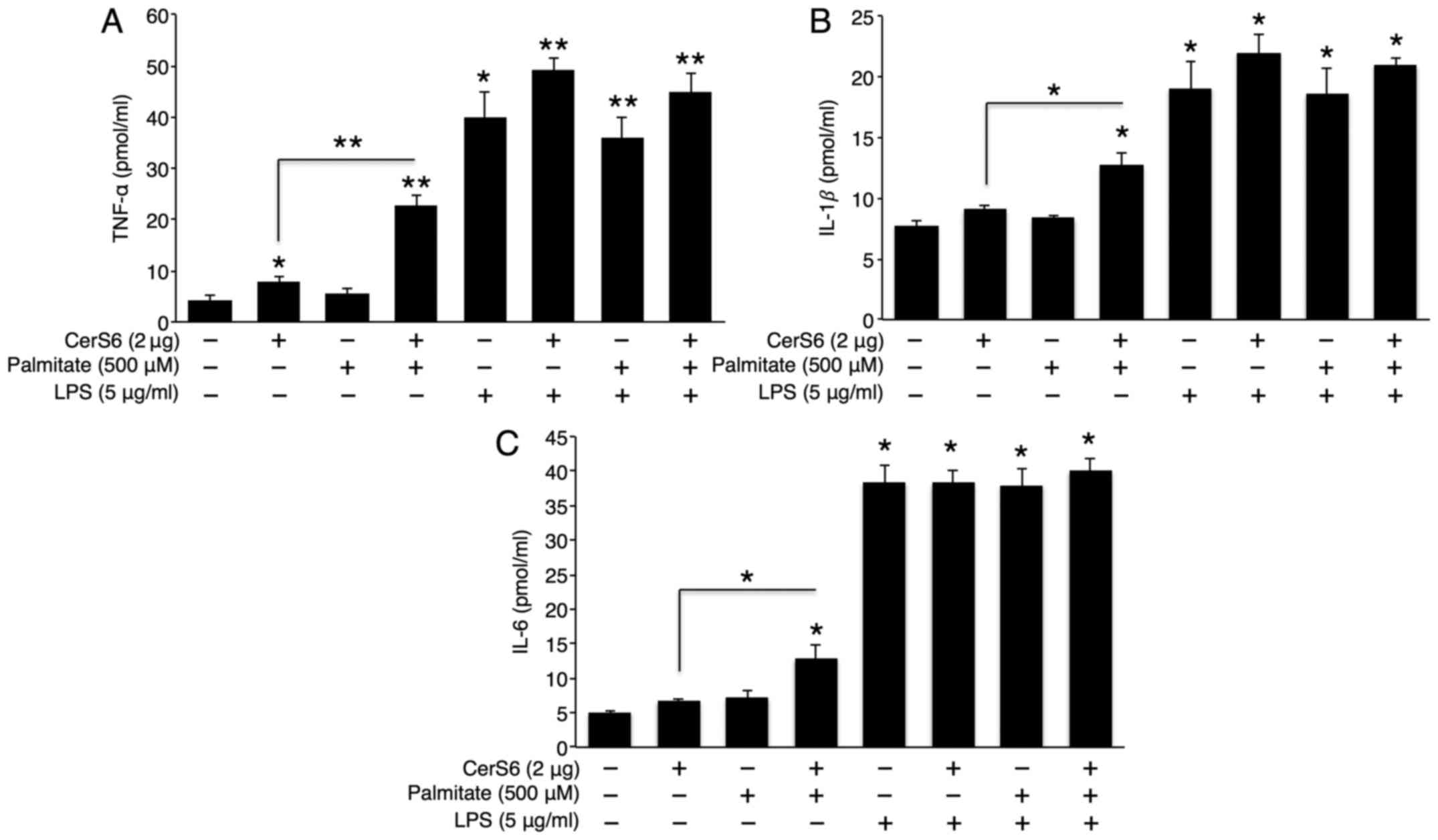
LPS increases TNF-α secretion via
sphingomyelin degradation, but not via CerS activation
LPS is the endotoxin that triggers strong
inflammatory responses in bacterial infection (28). To examine whether there is a
synergistic effect between CerS6 overexpression or palmitate
treatment and LPS in cytokine secretion, pCMV (empty
vector)-transfected or CerS6 overexpressing cells were co-treated
with palmitate and LPS. Although palmitate treatment alone did not
affect TNF-α, IL-1β and IL-6 secretion, palmitate synergistically
elevated TNF-α, IL-1β and IL-6 secretion in the CerS6
overexpressing Hep3B cells (Fig.
3). As expected, LPS treatment significantly increased TNF-α,
IL-1β and IL-6 secretion. However, no synergistic effect was
observed between LPS and palmitate treatment or CerS6
overexpression as regards cytokine secretion (Fig. 3).
As CerS6 did not affect LPS-induced cytokine
secretion, we examined whether ceramide generation via
sphingomyelin degradation is involved in LPS-induced cytokine
secretion. Treatment with LPS, but not palmitate, increased A-SMase
activity (Fig. 4A), and
co-incubation with the A-SMase inhibitor, desipramine, reduced
total ceramide and LPS-induced TNF-α, IL-1β and IL-6 secretion
(Fig. 4B–E). This result
demonstrated that A-SMase activity played a critical role in
LPS-induced cytokine secretion. Although N-SMase activity was not
altered upon LPS treatment (data not shown), co-incubation with the
N-SMase inhibitor, GW4869, also partially reduced total ceramide
and LPS-induced TNF-α, IL-1β and IL-6 secretion (Fig. 4B–E), suggesting that ceramide
generation via A-SMase and N-SMase is involved in LPS-induced
hepatic cytokine production. Co-incubation with FB1 did not
influence LPS-induced cytokine secretion (Fig. 4C–E). Therefore, CerS did not play
a critical role in LPS-induced hepatic cytokine secretion.
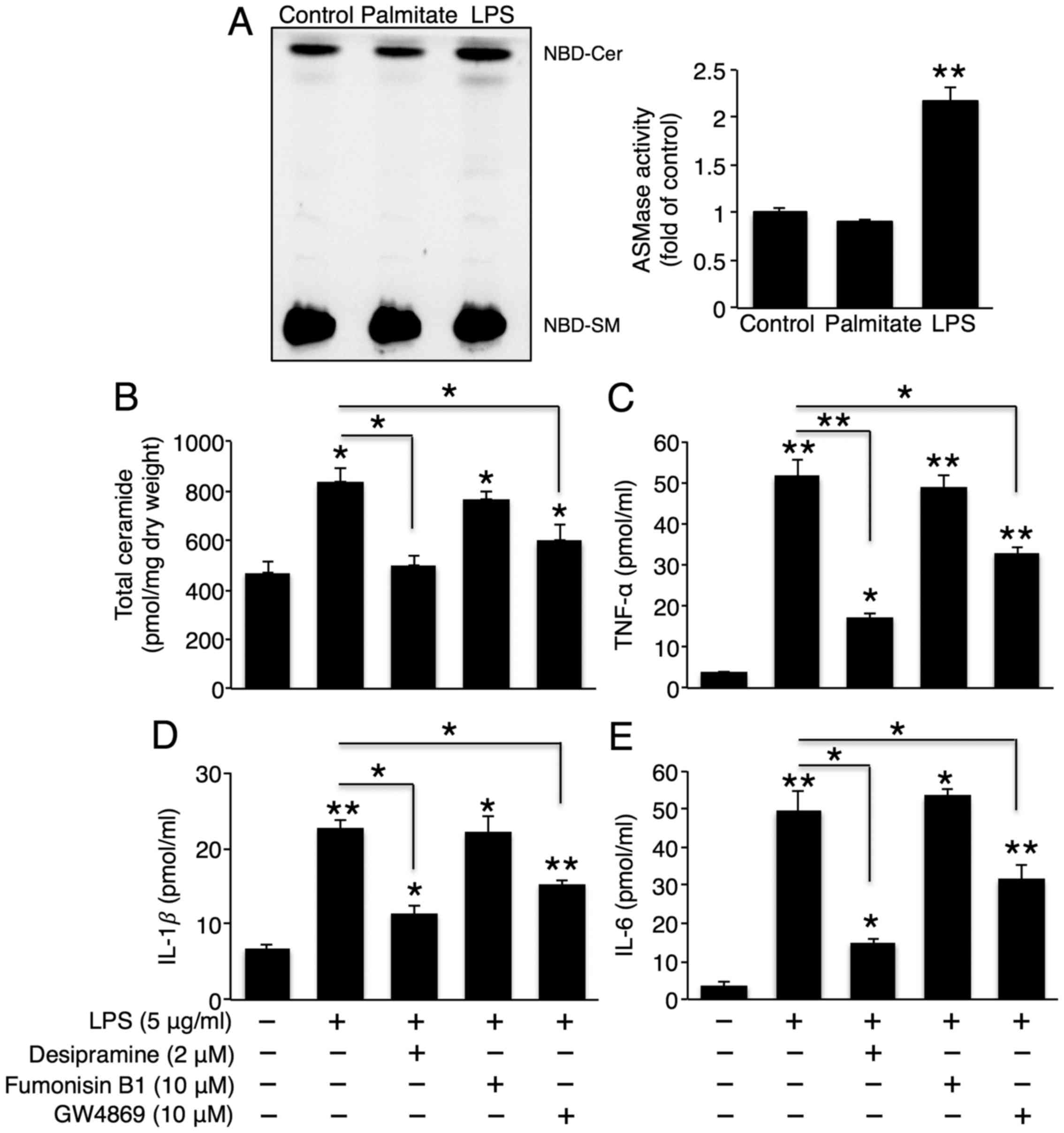 | Figure 4Sphingomyelin degradation plays an
important role in the lipopolysaccharide (LPS)-induced secretion of
tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6. (A)
Representative thin-layer chromatography of acidic sphingomyelinase
(A-SMase) activity upon treatment with 500 μM palmitate or 5
μg/ml LPS (left panel), as well as their quantification
(right panel) (n=3). Following co-treatment with LPS, desipramine
(A-SMase inhibitor), fumonisin B1 (CerS inhibitor), and GW4869
(neutral sphingomyelinase inhibitor), (B) total ceramide levels
were measured using ESI-MS-MS (n=3), and (C) TNF-α, (D) IL-1β, and
(E) IL-6 levels in Hep3B cell culture medium were measured using
ELISA kits (n=3). The values are expressed as the means ± standard
error of the mean. *P<0.05, **P<0.01.
Three independent experiments were performed. CerS, ceramide
synthases. |
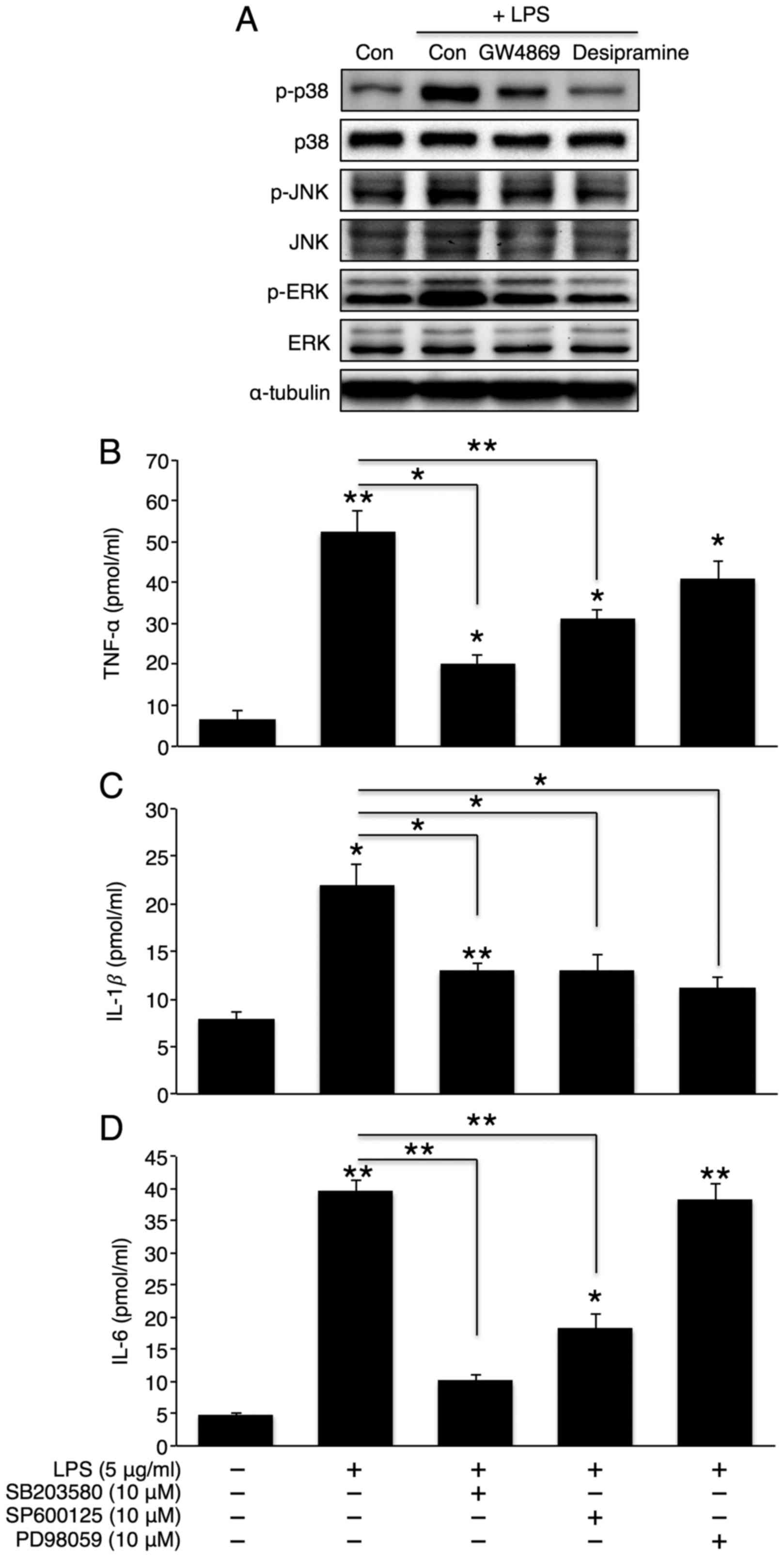
Activation of the p38 MAPK/NF-κB pathway
plays a critical role in hepatic inflammatory cytokine
secretion
MAPK activation plays an important role in
inflammatory cytokine production (29). Therefore, we examined whether the
inhibition of sphingomyelin degradation alters LPS-induced MAPK
activation. As expected, LPS elevated the phosphorylation levels of
all 3 MAPKs (p38, JNK, and ERK). Co-incubation with desipramine or
GW4869 reversed these phosphorylation levels (Fig. 5A), thus suggesting that the MAPK
pathway is downstream of sphingomyelin degradation. To further
investigate the direct role of MAPK in hepatic cytokine secretion,
we co-treated the cells with LPS and SB203580 (a p38 inhibitor),
SP600125 (a JNK inhibitor), or PD98059 (an ERK inhibitor). The
inhibition of p38 or JNK significantly attenuated the effects of
LPS on TNF-α, IL-1β and IL-6 secretion (Fig. 5B–D). However, the inhibition of
ERK only reduced IL-1β secretion. Similarly, the inhibition of p38
or JNK, but not ERK, markedly reduced the LPS-induced activation of
the NF-κB signalling pathway. However, the extent of NF-κB
inhibition was greater with p38 inhibition than with JNK inhibition
(Fig. 6A). Finally, we directly
examined the role of NF-κB in LPS-induced cytokine production.
Co-incubation with LPS and an NF-κB inhibitor, PDTC, significantly
reduced LPS-induced cytokine secretion (Fig. 6B–D). Of note, the direct NF-κB
inhibition further diminished the partial reduction in TNF-α and
IL-6 levels by JNK inhibition without affecting cytokine levels by
p38 inhibition (Fig. 6B–D). This
may be attributed to the complete inactivation of NF-κB by p38
inhibition alone.
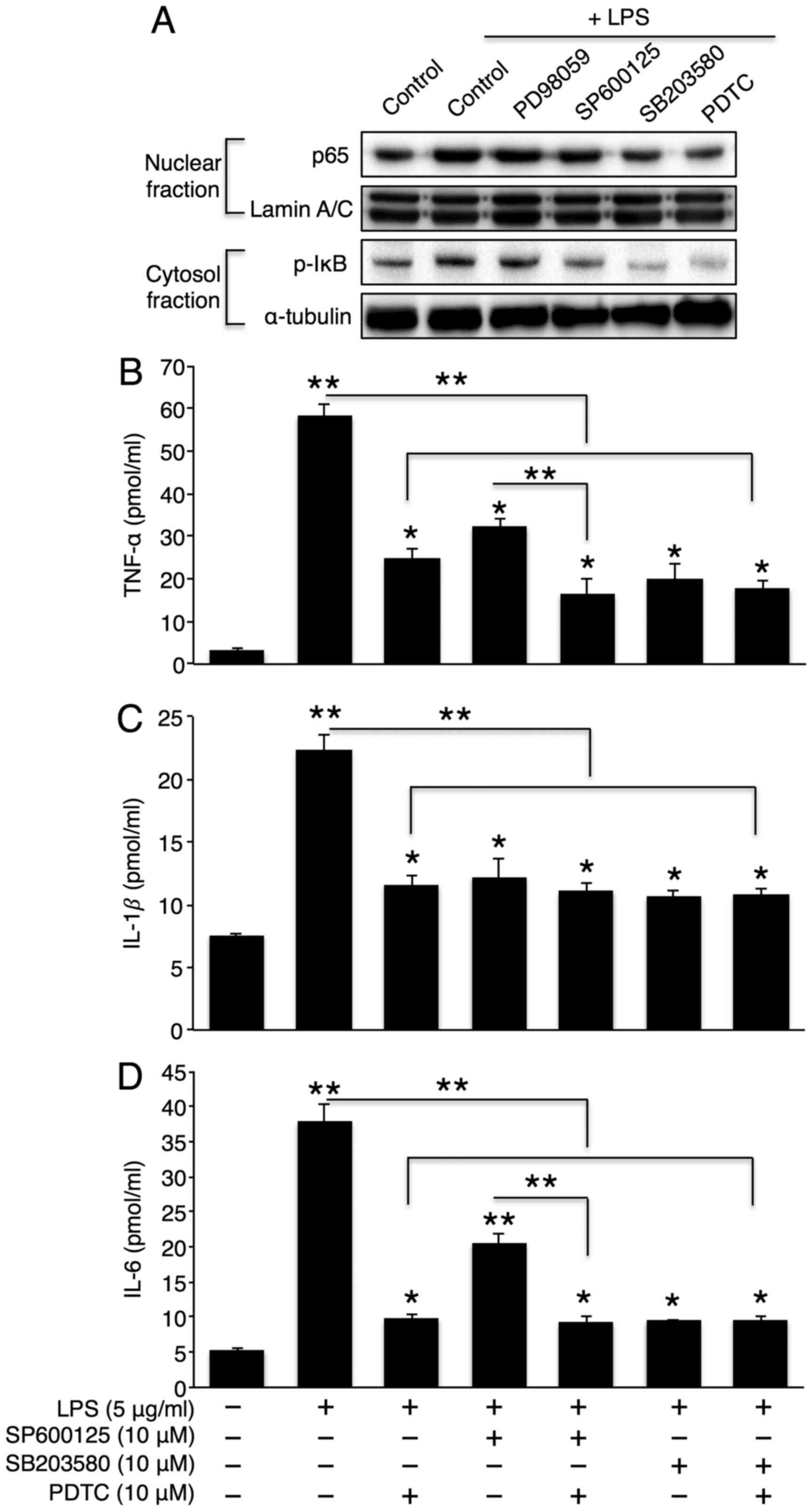 | Figure 6NF-κB activation plays a critical
role in lipopolysaccharide (LPS)- induced cytokine secretion. (A)
Representative western blots of the NF-κB pathway in LPS (5
μg/ml)-treated Hep3B cells that were co-incubated with 10
μM PD98059 (ERK inhibitor), 10 μM SP600125 (JNK
inhibitor), 10 μM SB203580 (p38 inhibitor), or 10 μM
PDTC (NF-κB inhibitor). Following co-treatment with LPS and
SB203580, SP600125, or PDTC, (B) tumour necrosis factor (TNF)-α,
(C) interleukin (IL)-1β, and (D) IL-6 levels in Hep3B cell culture
medium were measured using ELISA kits (n=3). The values are
expressed as the means ± standard error of the mean.
*P<0.05, **P<0.01. Three independent
experiments were performed. PDTC, pyrrolidinedithiocarbamate
ammonium. |
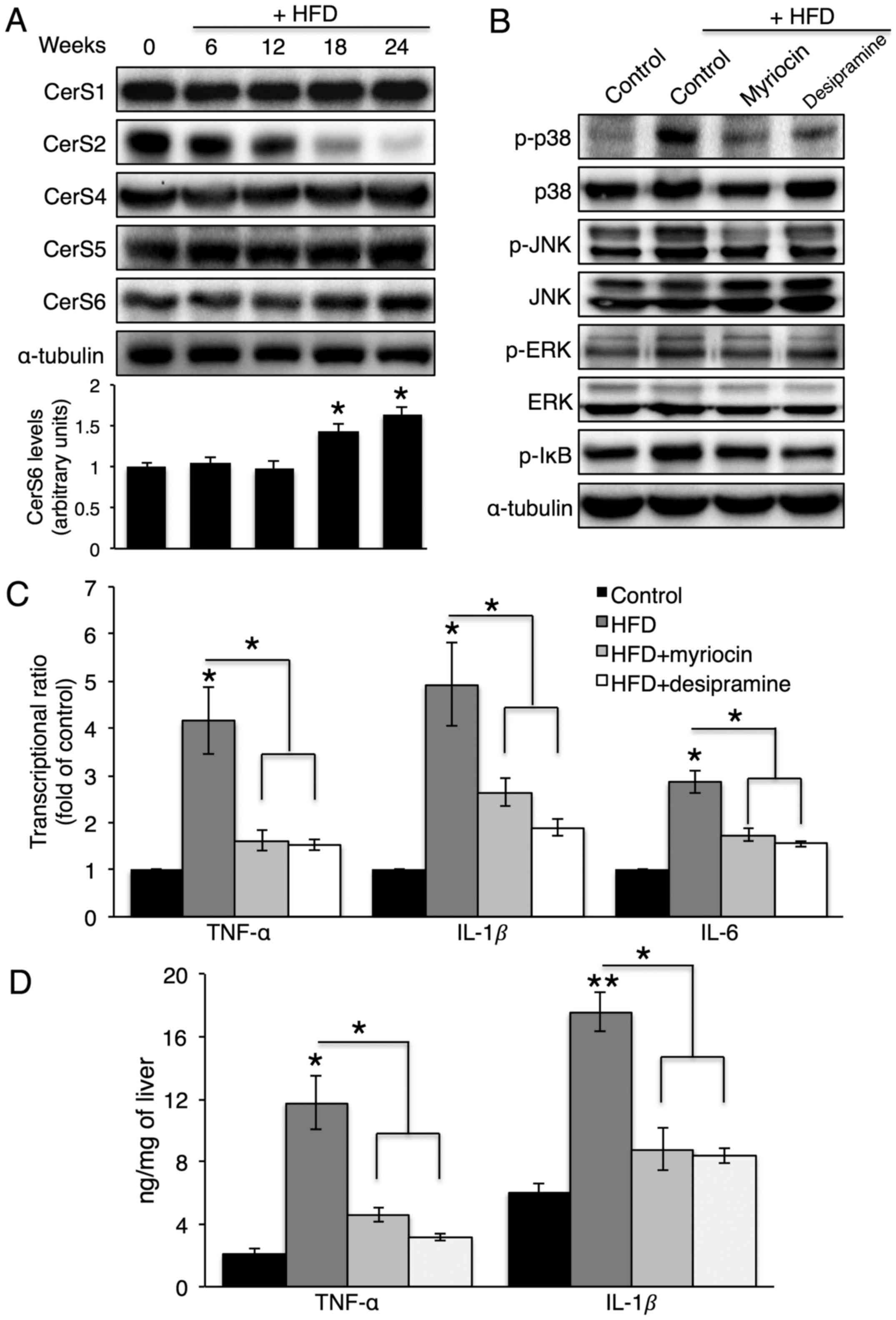
HFD feeding elevates hepatic cytokine
production, which is reduced by myriocin or desipramine
administration
To confirm whether A-SMase or CerS inhibition
affects hepatic cytokine production in vivo, mice were fed
an HFD for 24 weeks. Of note, the HFD increased CerS6 protein
expression and decreased CerS2 protein expression in the liver
(Fig. 7A), which caused a
concomitant increase in C16-ceramide and a decrease in C24-ceramide
(data not shown). Along with the HFD, desipramine or myriocin was
intraperitoneally injected to inhibit A-SMase or de novo
CerS, respectively. Due to the severe hepatotoxicity of FB1
treatment (30), an injection of
myriocin, which is an inhibitor of serine-palmitoyl transferase,
was used to inhibit de novo CerS. Desipramine or myriocin
injection not only reduced p38, JNK and IκB phosphorylation
(Fig. 7B), but also reduced
hepatic TNF-α, IL-1β and IL-6 production (Fig. 7C and D).
Discussion
Obesity, which usually accompanies chronic
inflammation (31), increases the
risk of mortality and complications caused by diabetes. Various
bioactive lipids, such as saturated fatty acids, cholesterol, and
sphingolipids, have been proposed as mechanisms to explain the
association between increased adiposity and the onset of these
pathologies (32). Among these,
ceramide has recently received attention as a key factor that is
involved in the development of metabolic disorders, as the
inhibition of ceramide formation ameliorates insulin resistance,
atherosclerosis and cardiomyopathy (32,33). In the present study, we further
investigated whether ceramide formation plays a role in hepatic
inflammation induced by palmitate, which is a saturated fatty acid,
and LPS, which is a powerful bacterial virulence factor (34).
Six mammalian CerS produce ceramides that carry
specific acyl chain lengths (8,9).
In this study, only CerS6 overexpression, which elevated
C16-ceramide, increased TNF-α secretion from Hep3B cells. This
confirmed a distinct role of ceramide that depended on the length
of the acyl chain. Although the C2-ceramide has been reported to
activate p38 MAPK and JNK in a T-cell line (35), in this study, CerS6 overexpression
in Hep3B cells only activated p38 MAPK. Thus, a ceramide may
activate different kinases depending on its acyl chain length. Of
note, CerS5 overexpression, which also increased the C16-ceramide,
did not affect TNF-α secretion. The difference between CerS5 and
CerS6 has not yet been fully disclosed, although recent studies
suggest that CerS5 and CerS6 play different roles (36,37). For example, CerS5, but not CerS6,
reduces fatty acid transport protein 5 expression. CerS6, but not
CerS5, diminishes fatty acid binding protein 1 expression in Hep3B
cells (36), and
myristate-induced cardiomyocyte hypertrophy is dependent on CerS5,
and not CerS6 (37). The exact
mechanisms behind the different roles of CerS5 and CerS6 require
further elucidation.
In this study, TNF-α generation was regulated by the
p38/NF-κB pathway, and both palmitate treatment in CerS6
overexpressing cells and LPS treatment elevated the phosphorylation
of p38 and the nuclear translocation of p65. However, the initial
steps differed. Treatment of the CerS6 overexpressing cells with
palmitate activated p38 MAPK via de novo ceramide
generation, whereas LPS stimulated p38 MAPK via A-SMase activation.
Similar to the present study, an increase in C16-ceramide, which
correlates with CerS6 elevation, has been shown to mediate
IFN-γ-induced TNF-α release in RAW 264.7 macrophages (38). Thus, our data confirmed the
general role of CerS6 in TNF-α generation.
LPS is found on the outer surface of Gram-negative
bacteria. It can stimulate Toll-like receptors and generate various
cytokines that stimulate the host response, and may lead to septic
shock (28). In this study, the
effect of LPS on the secretion of pro-inflammatory cytokines,
including TNF-α, IL-1β and IL-6 was robust. There was also no
synergistic effect observed between palmitate and LPS as regards
cytokine secretion by Hep3B cells. A previous study demonstrated
synergistic effects between palmitate and LPS on IL-6 generation in
macrophages (39). The
synergistic effects of palmitate and LPS may differ depending on
cell type, and these differences can be derived from distinct
sphingolipid metabolism upon palmitate and LPS treatment. In
macrophages, treatment with LPS and palmitate increases ceramide
biosynthesis via the de novo pathway (40) and sphingomyelin degradation
(39). However, in Hep3B cells,
treatment with palmitate alone activated de novo ceramide
generation. LPS only activated A-SMase, as FB1 treatment did not
reduce LPS-induced cytokine release.
Similar to previous reports (39,41), in this study, LPS activated
A-SMase in Hep3B cells, and ceramide generation via A-SMase played
a key role in cytokine secretion. Of note, the inhibition of
N-SMase also partially reduced cytokine generation by inhibiting
LPS-induced MAPK activation. This suggests that the activation of
both A-SMase and N-SMase, but not CerS, plays a role in LPS-induced
cytokine generation in the liver. Therefore, SMase inhibition may
be a good therapeutic target in LPS-induced liver inflammation.
In the present study, we demonstrated that CerS6
generated C16-ceramide, which increased TNF-α secretion in liver
cells. Furthermore, palmitate and CerS6 had synergistic effects on
TNF-α secretion via de novo ceramide biosynthesis. Unlike
palmitate stimulation, ceramide generation via sphingomyelin
degradation played a key role in LPS-induced inflammatory cytokine
production. Finally, the suppression of ceramide generation via
A-SMase inhibition or de novo CerS decreased HFD-induced
hepatic cytokine production in vivo. In conclusion,
regulating hepatic ceramide generation may prove to be an effective
therapeutic target for controlling hepatic inflammatory processes,
and specific targets should differ depending on the inflammatory
stimuli.
Abbreviations:
|
CerS
|
ceramide synthase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
IL
|
interleukin
|
|
JNK
|
c-Jun N-terminal kinase
|
|
SMase
|
sphingomyelinase
|
|
TNF-α
|
tumour necrosis factor-α
|
Acknowledgments
This study was supported by the Gachon University
research fund of 2015 (no. GCU-2015-5112), Gachon University Gil
Medical Center (no. GIL2012-01) and by grants from the Health
Technology R&D Project (no. HI14C2445) of the Ministry of
Health and Welfare, Republic of Korea.
References
|
1
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monteiro R and Azevedo I: Chronic
inflammation in obesity and the metabolic syndrome. Mediators
Inflamm. 2010:2896452010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boden G: Obesity, insulin resistance and
free fatty acids. Curr Opin Endocrinol Diabetes Obes. 18:139–143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ståhlman M, Pham HT, Adiels M, Mitchell
TW, Blanksby SJ, Fagerberg B, Ekroos K and Borén J: Clinical
dyslipidaemia is associated with changes in the lipid composition
and inflammatory properties of apolipoprotein-B-containing
lipoproteins from women with type 2 diabetes. Diabetologia.
55:1156–1166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pillon NJ, Azizi PM, Li YE, Liu J, Wang C,
Chan KL, Hopperton KE, Bazinet RP, Heit B, Bilan PJ, et al:
Palmitate-induced inflammatory pathways in human adipose
microvascular endothelial cells promote monocyte adhesion and
impair insulin transcytosis. Am J Physiol Endocrinol Metab.
309:E35–E44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haus JM, Kashyap SR, Kasumov T, Zhang R,
Kelly KR, Defronzo RA and Kirwan JP: Plasma ceramides are elevated
in obese subjects with type 2 diabetes and correlate with the
severity of insulin resistance. Diabetes. 58:337–343. 2009.
View Article : Google Scholar :
|
|
7
|
Brozinick JT, Hawkins E, Hoang Bui H, Kuo
MS, Tan B, Kievit P and Grove K: Plasma sphingolipids are
biomarkers of metabolic syndrome in non-human primates maintained
on a Western-style diet. Int J Obes. 37:1064–1070. 2013. View Article : Google Scholar
|
|
8
|
Park WJ and Park JW: The effect of altered
sphingolipid acyl chain length on various disease models. Biol
Chem. 396:693–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JW, Park WJ and Futerman AH: Ceramide
synthases as potential targets for therapeutic intervention in
human diseases. Biochim Biophys Acta. 1841:671–681. 2014.
View Article : Google Scholar
|
|
10
|
Venkataraman K, Riebeling C, Bodennec J,
Riezman H, Allegood JC, Sullards MC, Merrill AH Jr and Futerman AH:
Upstream of growth and differentiation factor 1 (uog1), a mammalian
homolog of the yeast longevity assurance gene 1 (LAG1), regulates
N-stearoyl-sphinganine (C18-(dihydro) ceramide) synthesis in a
fumonisin B1-independent manner in mammalian cells. J Biol Chem.
277:35642–35649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lahiri S and Futerman AH: LASS5 is a bona
fide dihydroceramide synthase that selectively utilizes
palmitoyl-CoA as acyl donor. J Biol Chem. 280:33735–33738. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizutani Y, Kihara A and Igarashi Y:
Mammalian Lass6 and its related family members regulate synthesis
of specific ceramides. Biochem J. 390:263–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riebeling C, Allegood JC, Wang E, Merrill
AH Jr and Futerman AH: Two mammalian longevity assurance gene
(LAG1) family members, trh1 and trh4, regulate dihydroceramide
synthesis using different fatty acyl-CoA donors. J Biol Chem.
278:43452–43459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laviad EL, Albee L, Pankova-Kholmyansky I,
Epstein S, Park H, Merrill AH Jr and Futerman AH: Characterization
of ceramide synthase 2: Tissue distribution, substrate specificity,
and inhibition by sphingosine 1-phosphate. J Biol Chem.
283:5677–5684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizutani Y, Kihara A and Igarashi Y: LASS3
(longevity assurance homologue 3) is a mainly testis-specific
(dihydro) ceramide synthase with relatively broad substrate
specificity. Biochem J. 398:531–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mesicek J, Lee H, Feldman T, Jiang X,
Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z and
Kolesnick R: Ceramide synthases 2, 5, and 6 confer distinct roles
in radiation-induced apoptosis in HeLa cells. Cell Signal.
22:1300–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goñi FM and Alonso A: Sphingomyelinases:
Enzymology and membrane activity. FEBS Lett. 531:38–46. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tolman KG, Fonseca V, Dalpiaz A and Tan
MH: Spectrum of liver disease in type 2 diabetes and management of
patients with diabetes and liver disease. Diabetes Care.
30:734–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roux JC, Dura E, Moncla A, Mancini J and
Villard L: Treatment with desipramine improves breathing and
survival in a mouse model for Rett syndrome. Eur J Neurosci.
25:1915–1922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn
CM, Rye KA, Stocker R, Jessup W and Garner B: Inhibition of
atherosclerosis by the serine palmitoyl transferase inhibitor
myriocin is associated with reduced plasma glycosphingolipid
concentration. Biochem Pharmacol. 73:1340–1346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali M, Fritsch J, Zigdon H, Pewzner-Jung
Y, Schütze S and Futerman AH: Altering the sphingolipid acyl chain
composition prevents LPS-GLN-mediated hepatic failure in mice by
disrupting TNFR1 internalization. Cell Death Dis. 4:e9292013.
View Article : Google Scholar
|
|
22
|
Halasiddappa LM, Koefeler H, Futerman AH
and Hermetter A: Oxidized phospholipids induce ceramide
accumulation in RAW 264.7 macrophages: Role of ceramide synthases.
PLoS One. 8:e700022013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong YM, Park WJ, Kim MK, Baek KJ, Kwon
NS, Yun HY and Kim DS: Leucine-rich glioma inactivated 3 promotes
HaCaT keratinocyte migration. Wound Repair Regen. 21:634–640. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Choi S, Kim JA, Kim TH, Li HY, Shin KO,
Lee YM, Oh S, Pewzner-Jung Y, Futerman AH and Suh SH: Altering
sphingolipid composition with aging induces contractile dysfunction
of gastric smooth muscle via K(Ca) 1.1 upregulation. Aging Cell.
14:982–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shaner RL, Allegood JC, Park H, Wang E,
Kelly S, Haynes CA, Sullards MC and Merrill AH Jr: Quantitative
analysis of sphingolipids for lipidomics using triple quadrupole
and quadrupole linear ion trap mass spectrometers. J Lipid Res.
50:1692–1707. 2009. View Article : Google Scholar :
|
|
27
|
Campbell J, Ciesielski CJ, Hunt AE,
Horwood NJ, Beech JT, Hayes LA, Denys A, Feldmann M, Brennan FM and
Foxwell BM: A novel mechanism for TNF-alpha regulation by p38 MAPK:
Involvement of NF-kappa B with implications for therapy in
rheumatoid arthritis. J Immunol. 173:6928–6937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Józefowski S, Czerkies M, Łukasik A,
Bielawska A, Bielawski J, Kwiatkowska K and Sobota A: Ceramide and
ceramide 1-phosphate are negative regulators of TNF-α production
induced by lipopolysaccharide. J Immunol. 185:6960–6973. 2010.
View Article : Google Scholar
|
|
29
|
El Alwani M, Wu BX, Obeid LM and Hannun
YA: Bioactive sphingolipids in the modulation of the inflammatory
response. Pharmacol Ther. 112:171–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soriano JM, González L and Catalá AI:
Mechanism of action of sphingolipids and their metabolites in the
toxicity of fumonisin B1. Prog Lipid Res. 44:345–356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dandona P, Aljada A and Bandyopadhyay A:
Inflammation: The link between insulin resistance, obesity and
diabetes. Trends Immunol. 25:4–7. 2004. View Article : Google Scholar
|
|
32
|
Bikman BT and Summers SA: Ceramides as
modulators of cellular and whole-body metabolism. J Clin Invest.
121:4222–4230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holland WL, Brozinick JT, Wang L-P,
Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky
A, et al: Inhibition of ceramide synthesis ameliorates
glucocorticoid-, saturated-fat-, and obesity-induced insulin
resistance. Cell Metab. 5:167–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blais DR, Vascotto SG, Griffith M and
Altosaar I: LBP and CD14 secreted in tears by the lacrimal glands
modulate the LPS response of corneal epithelial cells. Invest
Ophthalmol Vis Sci. 46:4235–4244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CL, Lin CF, Chang WT, Huang WC, Teng
CF and Lin YS: Ceramide induces p38 MAPK and JNK activation through
a mechanism involving a thioredoxin-interacting protein-mediated
pathway. Blood. 111:4365–4374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park WJ, Park JW, Merrill AH Jr, Storch J,
Pewzner-Jung Y and Futerman AH: Hepatic fatty acid uptake is
regulated by the sphingolipid acyl chain length. Biochim Biophys
Acta. 1841:1754–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Russo SB, Baicu CF, Van Laer A, Geng T,
Kasiganesan H, Zile MR and Cowart LA: Ceramide synthase 5 mediates
lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin
Invest. 122:3919–3930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schiffmann S, Ferreirós N, Birod K, Eberle
M, Schreiber Y, Pfeilschifter W, Ziemann U, Pierre S, Scholich K,
Grösch S and Geisslinger G: Ceramide synthase 6 plays a critical
role in the development of experimental autoimmune
encephalomyelitis. J Immunol. 188:5723–5733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin J, Zhang X, Lu Z, Perry DM, Li Y,
Russo SB, Cowart LA, Hannun YA and Huang Y: Acid sphingomyelinase
plays a key role in palmitic acid-amplified inflammatory signaling
triggered by lipopolysaccharide at low concentrations in
macrophages. Am J Physiol Endocrinol Metab. 305:E853–E867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schilling JD, Machkovech HM, He L, Sidhu
R, Fujiwara H, Weber K, Ory DS and Schaffer JE: Palmitate and
lipopolysaccharide trigger synergistic ceramide production in
primary macrophages. J Biol Chem. 288:2923–2932. 2013. View Article : Google Scholar :
|
|
41
|
Cuschieri J, Bulger E, Billgrin J, Garcia
I and Maier RV: Acid sphingomyelinase is required for lipid Raft
TLR4 complex formation. Surg Infect (Larchmt). 8:91–106. 2007.
View Article : Google Scholar
|
|
42
|
Liu G, Friggeri A, Yang Y, Park YJ,
Tsuruta Y and Abraham E: miR-147, a microRNA that is induced upon
Toll-like receptor stimulation, regulates murine macrophage
inflammatory responses. Proc Natl Acad Sci USA. 106:15819–15824.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawane K, Tanaka H, Kitahara Y, Shimaoka S
and Nagata S: Cytokine-dependent but acquired immunity-independent
arthritis caused by DNA escaped from degradation. Proc Natl Acad
Sci USA. 107:19432–19437. 2010. View Article : Google Scholar : PubMed/NCBI
|