Introduction
Osteoarthritis (OA) is the most common joint
disorder among the elderly presenting with joint pain and
deformities. OA is considered a major public health issue causing
chronic disability worldwide in the increasing number of aging
humans (1). OA is characterized
by qualitative and quantitative changes in the architecture and
composition of joint structures (2,3).
OA is a multifactorial disease of the cartilage, and aging is one
of the important risk factor for OA (4). However, the mechanisms, such as
inflammation, apoptosis, and degradation of major extracellular
matrix (ECM) components (including type II collagen and aggrecan)
are commonly involved in the cartilage degradation in OA (5).
Glucosamine (GlcN), a naturally occurring amino
monosaccharide, is present in connective and cartilage tissues as a
component of glycosaminoglycans (GAGs), and contributes to
maintaining the strength, flexibility and elasticity of these
tissues. Thus, GlcN has been widely used to treat OA for more than
three decades in humans (6–9).
In fact, several short-term and long-term clinical trials in OA
have shown the significant symptom-modifying effect of GlcN
(10–12). We previously revealed that GlcN
can induce hyaluronic acid (HA) production by synovial cells and
chondrocytes (13). Furthermore,
the balance between the synthesis and degradation of ECM components
in the cartilage is important for the maintenance of articular
metabolism, and the disturbance of this balance leads to the
progressive destruction of cartilage in OA (14–17). Thus, GlcN is expected to exert a
protective effect on the balance between the synthesis and
degradation of ECM components in the cartilage. However, the
effects of GlcN on the expression of the genes related to cartilage
metabolism are not fully understood.
In the present study, therefore, to further
elucidate the chondroprotective action of GlcN, we examined the
effect of GlcN on the expression of genes related to cartilage
metabolism, such as type II collagen and the matrix
metalloproteinases (MMPs).
Materials and methods
Reagents
D-Glucosamine hydrochloride (GlcN) was purchased
from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Penicillin-streptomycin mixed solution, dimethyl sulfoxide (DMSO)
and dithiothreitol (DTT) were purchased both from Nacalai Tesque,
Inc. (Kyoto, Japan). EX527 [a sirtuin 1 (SIRT1) inhibitor] was
purchased from Selleckchem (Houston, TX, USA).
Cells
Human chondrocytes (SW 1353) were purchased from the
American Type Culture Collection (HTB-94, ATCC; Manassas, VA, USA).
SW 1353 cells were maintained in Leibovitz's L-15 medium
(Gibco-Invitrogen Life Technologies, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS; Biological Industries, Cromwell, CT,
USA), penicillin and streptomycin at 37°C under a humidified
atmosphere without CO2.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
SW 1353 cells (3.0×106 cells/flask) were
plated into T-75 cm2 flasks (Corning Inc., Corning, NY,
USA) overnight. The cells were incubated in the absence or presence
of GlcN (0.1, 1 and 10 mM) for 24 h, or incubated with GlcN (1 mM)
in the absence or presence of EX527 (1 µM) or DMSO (as a
solvent) for 6 h (18). After
incubation, the cells were washed twice with ice-cold
phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM
Na2HPO4, 1.5 mM KH2PO4,
pH 7.4) and collected by a cell scraper (Sumitomo Bakelite Co.,
Ltd., Tokyo, Japan). Then, total RNA was purified using an RNeasy
Plus Mini kit and QIAshredder (both from Qiagen, Hilden, Germany)
by removing contaminated DNA, according to the manufacturer's
instructions, and stored at −80°C. Reverse transcription was
performed using a ReverTra Ace® (Toyobo Co., Ltd.,
Osaka, Japan), and PCR amplification was performed with
GoTaq® Hot Start Green Master Mix (Promega, Madison, WI,
USA) in a thermal cycler (GeneAmp PCR System 9700; Applied
Biosystems, Foster City, CA, USA) for type II collagen
(COL2A1), MMP-1, MMP-2, MMP-9 MMP-13,
SIRT1-SIRT7 and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), according to the manufacturer's instructions. In
brief, cDNA was synthesized by reverse transcription using total
RNA (500 ng), ReverTra Ace® reverse transcriptase and
oligo(dT)20. To discriminate mRNA-derived PCR products
from genomic DNA-derived products, the intron-spanning PCR primers
were used with the annealing temperature and cycle number, as shown
in Table I. PCR products were
resolved by 1% agarose gel electrophoresis in 1X Tris-acetate-EDTA
buffer and stained with ethidium bromide. In our preliminary
experiments, we tried to semi-quantitatively detect mRNA by using
different cycle numbers of PCR. The results revealed that the
amounts of RT-PCR products increased dependently on the cycle
number. Thus, we decided to measure the mRNA levels by RT-PCR with
the cycle number indicated in Table
I. The expression of GAPDH was used as a standard. The
detected bands were quantified using BioDoc-it Imaging System (UVP
LLC, Upland, CA, USA) and quantified using Multi Gauge version 3.0
(Fujifilm, Tokyo, Japan).
 | Table IGene-specific PCR primers, annealing
temperature (°C) and the cycle number for PCR. |
Table I
Gene-specific PCR primers, annealing
temperature (°C) and the cycle number for PCR.
| Gene | Forward primer | Reverse primer | Annealing
temperature (°C) | Cycle nos. | Refs. |
|---|
| COL2A1 |
5′-atgacaatctggctcccaacactgc-3′ |
5′-gaccggccctatgtccacaccgaat-3′ | 52 | 41 | (19) |
| MMP-1 |
5′-gccagatttgccaagagcaga-3′ |
5′-cggcaaattcgtaagcagcttc-3′ | 55 | 34 | (20) |
| MMP-2 |
5′-ggccctgtcactcctgagat-3′ |
5′-ggcatccaggttatcgggga-3′ | 58 | 29 | (21) |
| MMP-9 |
5′-cggagcacggagacgggtat-3′ |
5′-tgaaggggaagacgcacagc-3′ | 58 | 35 | (21) |
| MMP-13 |
5′-gacttcacgatggcattgctg-3′ |
5′-gcatcaacctgctgaggatgc-3′ | 59 | 39 | (22) |
| SIRT1 |
5′-gggatggtatttatgctcgc-3′ |
5′-ctatgatttgtttgatggatagttc-3′ | 55 | 34 | (23) |
| SIRT2 |
5′-agcaaggcacccctctccacc-3′ |
5′-ggtttctccctctctgttgtc-3′ | 57 | 31 | (24) |
| SIRT3 |
5′-tgagagagtgtcgggcatccctg-3′ |
5′-tcatcctatttgtctggtccatcaa-3′ | 55 | 33 | (25) |
| SIRT4 |
5′-accctgagaaggtcaaagagttac-3′ |
5′-ttccccacaatccaagcac-3′ | 55 | 33 | (26) |
| SIRT5 |
5′-ccgagtgtgagacccggctgggca-3′ |
5′-ttgtaattctcagccacaactcc-3′ | 54 | 31 | (27) |
| SIRT6 |
5′-ccaagttcgacaccaccttt-3′ |
5′-cggacgtactgcgtcttaca-3′ | 55 | 31 | (28) |
| SIRT7 |
5′-gggagtacgtgcgggtgttcgatg-3′ |
5′-ggccgccggctagggggcttggtc-3′ | 60 | 33 | (27) |
|
GAPDHa |
5′-accacagtccatgccatcac-3′ |
5′-tccaccaccctgttgctgta-3′ | 60 | 20 | |
Western blot analysis
SW1353 cells (3.0×106 cells/flask) were
plated into T-75 cm2 flasks overnight. The cells were
incubated in the absence or presence of GlcN (0.1, 1 and 10 mM) for
24 h. After incubation, the cells were washed twice with ice-cold
PBS and collected by a cell scraper (Sumitomo Bakelite Co., Ltd.).
For the detection of COL2A1, the cells were added together with 100
µl of RIPA buffer [50 mM Tris-HCl pH 7.6, 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)]
with a protease inhibitor (Complete Protease Inhibitor Cocktail
set; Roche Diagnostics, Mannheim, Germany), sonicated (Model UD-201
ultrasonic disruptor, output 20 W, duty 10 for 3 times; Tomy
Digital Biology, Co., Ltd., Tokyo, Japan) and placed on ice for 15
min. After centrifugation (8,000 × g, 4°C, 15 min), the
supernatants were used for SDS-polyacrylamide gel electrophoresis
(PAGE). For the detection of SIRT1, the cells were added together
with 100 µl of lysis buffer (50 mM Tris-HCl pH 7.6, 150 mM
NaCl, 1 mM MgCl2, 1% NP-40, 10% glycerol, 1 mM DTT) with
a protease inhibitor (Roche Diagnostics), sonicated (Model UD-201
ultrasonic disruptor, output 20 W, duty 10 for 5 times) and placed
on ice for 15 min. After centrifugation (8000 × g, 4°C, 15 min),
the supernatants were used for SDS-PAGE. Cell lysates (20 µg
protein/lane for COL2A1 and 10 µg protein/lane for SIRT1)
were subjected to SDS-PAGE and transferred to polyvinylidene
fluoride membranes (Immobilon-P; Millipore, Billerica, MA, USA).
The membranes were blocked overnight with Blocking One (Nacalai
Tesque, Inc.) at 4°C, washed with PBS containing 0.1% Tween-20
(PBST) at room temperature, and then probed with rabbit anti-human
COL2A1 antibody (sc-28887; 1,000-fold dilution) or rabbit
anti-human SIRT1 antibody (sc-15404; 1,000-fold dilution) (both
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed
by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(AQ132P; 5,000-fold dilution; Chemicon International, Inc.,
Temecula, CA, USA). Signals were detected with
SuperSignal® West Pico Chemiluminescent Substrate
(Pierce Biotechnology, Inc., Rockford, IL, USA), and quantified
using LAS-3000 luminescent image analyzer and Multi Gauge (both
from Fujifilm). The expression of GAPDH was analyzed with mouse
anti-human GAPDH antibody (MAB374; 30,000-fold dilution; Chemicon
International, Inc.) and HRP-conjugated goat anti-mouse IgG/IgM
(115-035-044; 5,000-fold dilution; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) as an internal
standard.
Statistical analysis
Data are shown as mean ± SD of three separate
experiments, and were analyzed for significant difference by a
Student's t-test in Excel analysis. Differences were considered
statistically significant at p<0.05.
Results
Effect of GlcN on the genes related to
cartilage metabolism in chondrocytes
First, the effect of GlcN on the expression of genes
related to cartilage metabolism, such as COL2A1 and
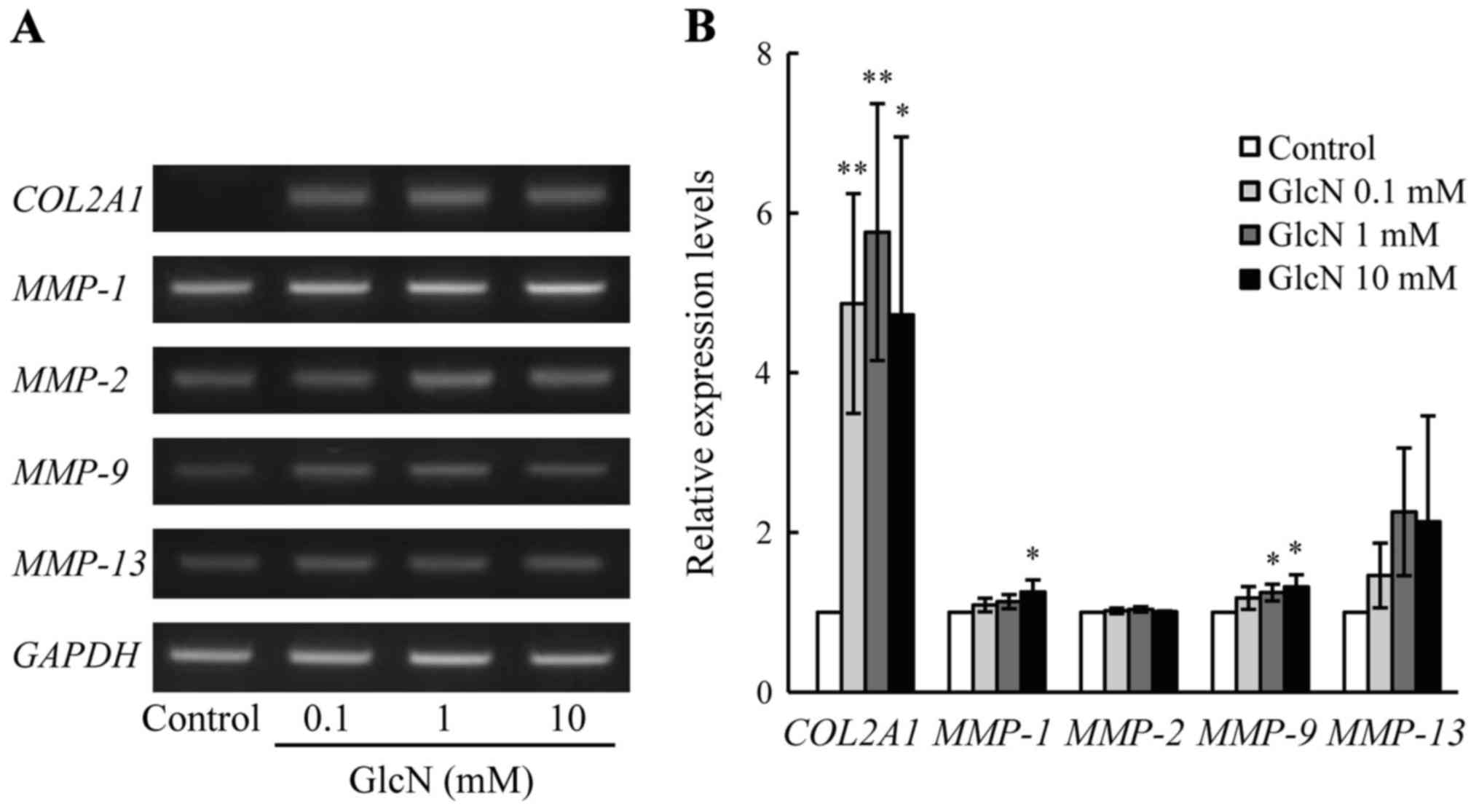
MMPs, was evaluated. As shown in Fig. 1, the expression of COL2A1
mRNA was significantly (>5-fold) increased by GlcN (0.1, 1 and
10 mM) compared with the expression noted in the control without
GlcN (p<0.05). Furthermore, the expression of MMP-1 mRNA
was significantly but only 1.2-fold increased by GlcN (10 mM)
(p<0.05). Moreover, the expression of MMP-9 mRNA was
significantly but only 1.2-fold increased by GlcN (1 and 10 mM). By
contrast, the expression of MMP-2 and MMP-13 mRNAs
was not essentially changed by GlcN, although the expression of
MMP-13 mRNA was slightly increased without statistically
significant difference.
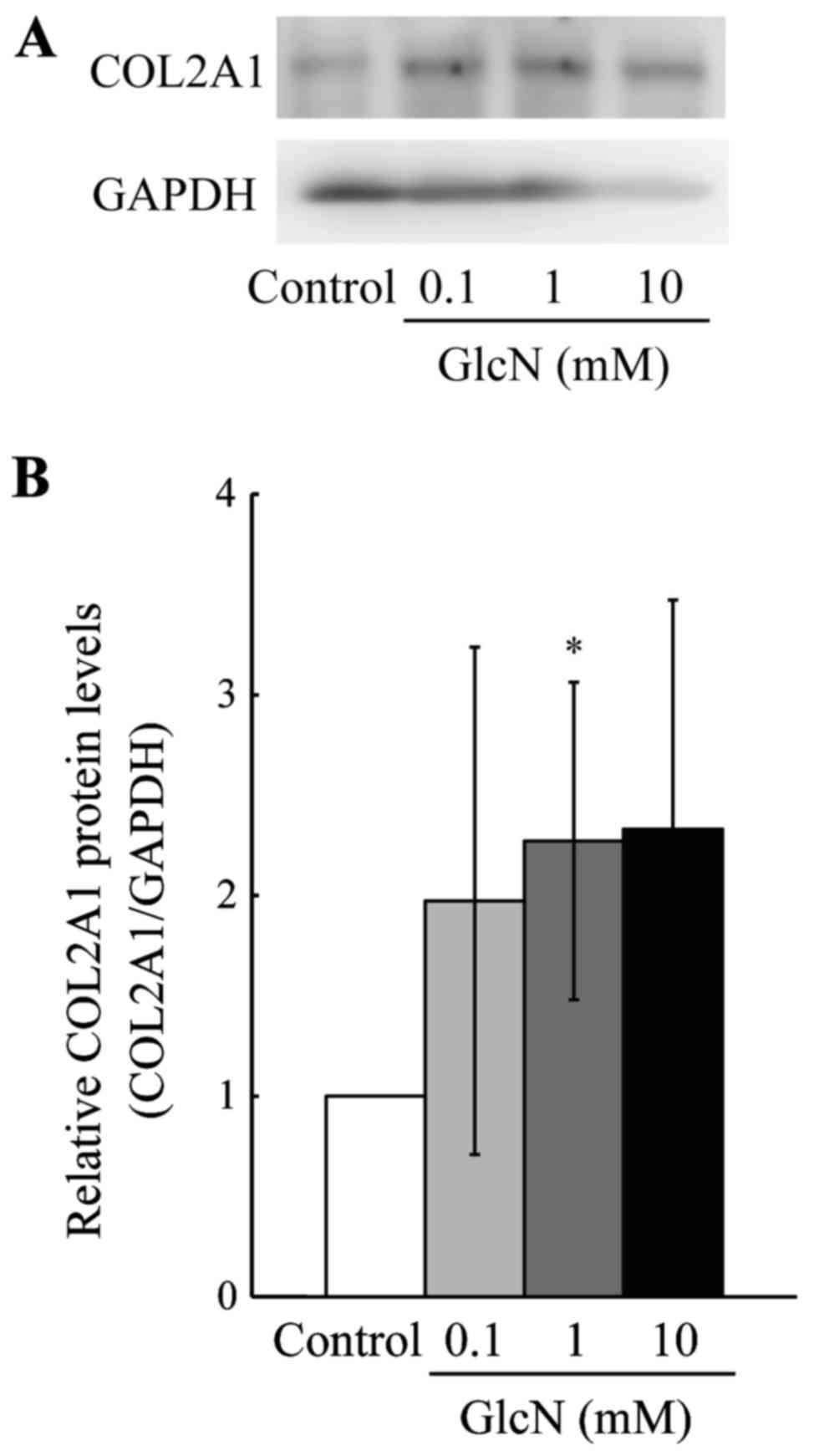
Second, the protein level of COL2A1 was evaluated,
since the expression of COL2A1 mRNA was greatly increased by
GlcN (Fig. 1). As shown in
Fig. 2, the protein level of
COL2A1 was significantly (>2-fold) increased by GlcN (1 mM)
compared with this level in the control without GlcN.
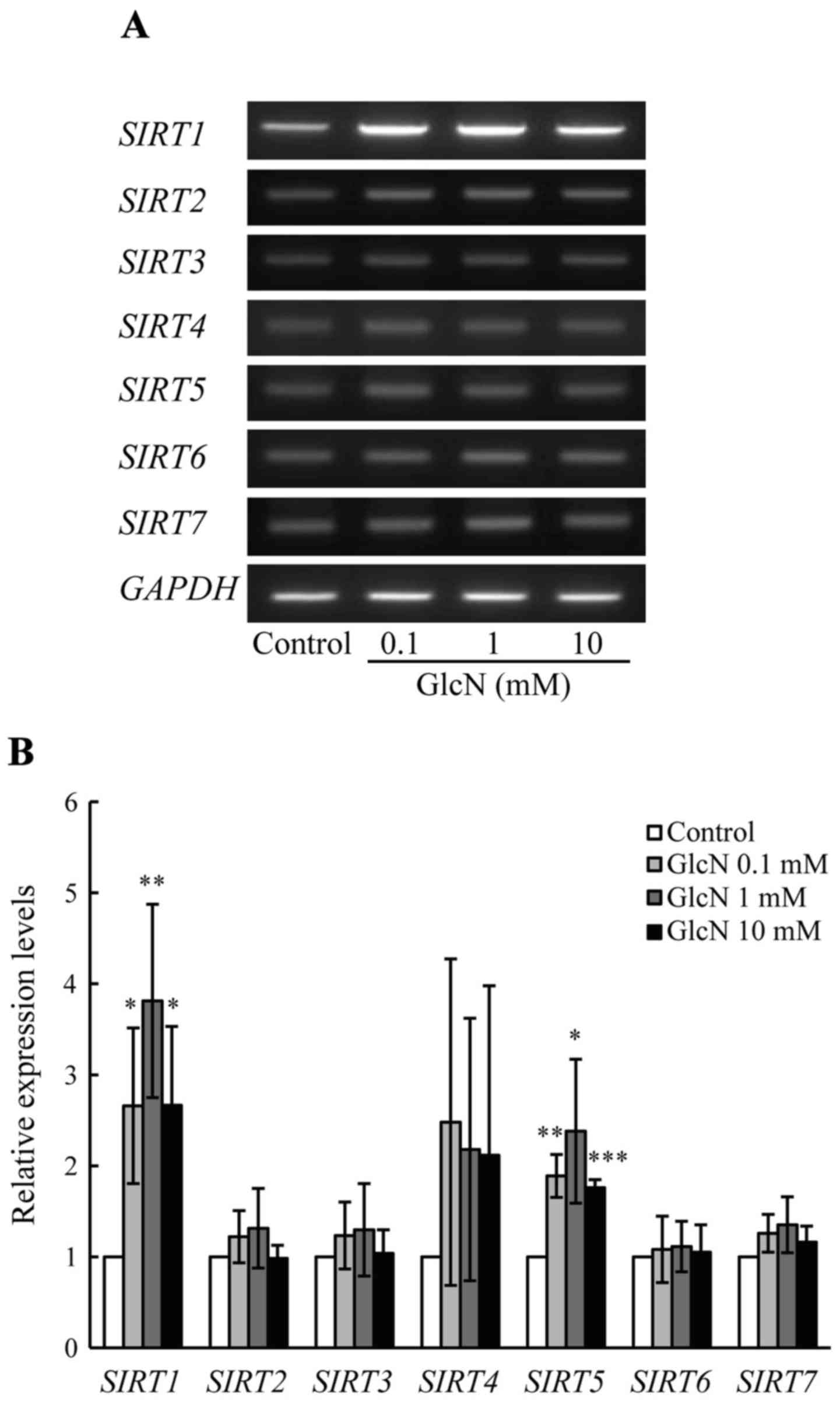
Effect of GlcN on SIRT gene expression in
chondrocytes
The expression of COL2A1 mRNA was
significantly increased by GlcN (Fig.
1). Thus, the effect of GlcN on the expression of SIRT1,
an upstream-regulating gene of COL2A1 (29), was evaluated. As shown in Fig. 3, the expression of SIRT1
mRNA was significantly (3- to 4-fold) increased by GlcN (0.1, 1 and
10 mM) compared with that noted in the control without GlcN
(p<0.05). In humans, the SIRT gene family consists of 7
genes, SIRT1-SIRT7 (30);
thus, the effect of GlcN on the expression of SIRT2-SIRT7
genes was evaluated. As shown in Fig.
3, the expression of SIRT5 mRNA was significantly but
only slightly (~2-fold) increased by GlcN (p<0.05). By contrast,
the mRNA expression of SIRT2, SIRT3, SIRT4,
SIRT6 and SIRT7 was not essentially changed by GlcN.
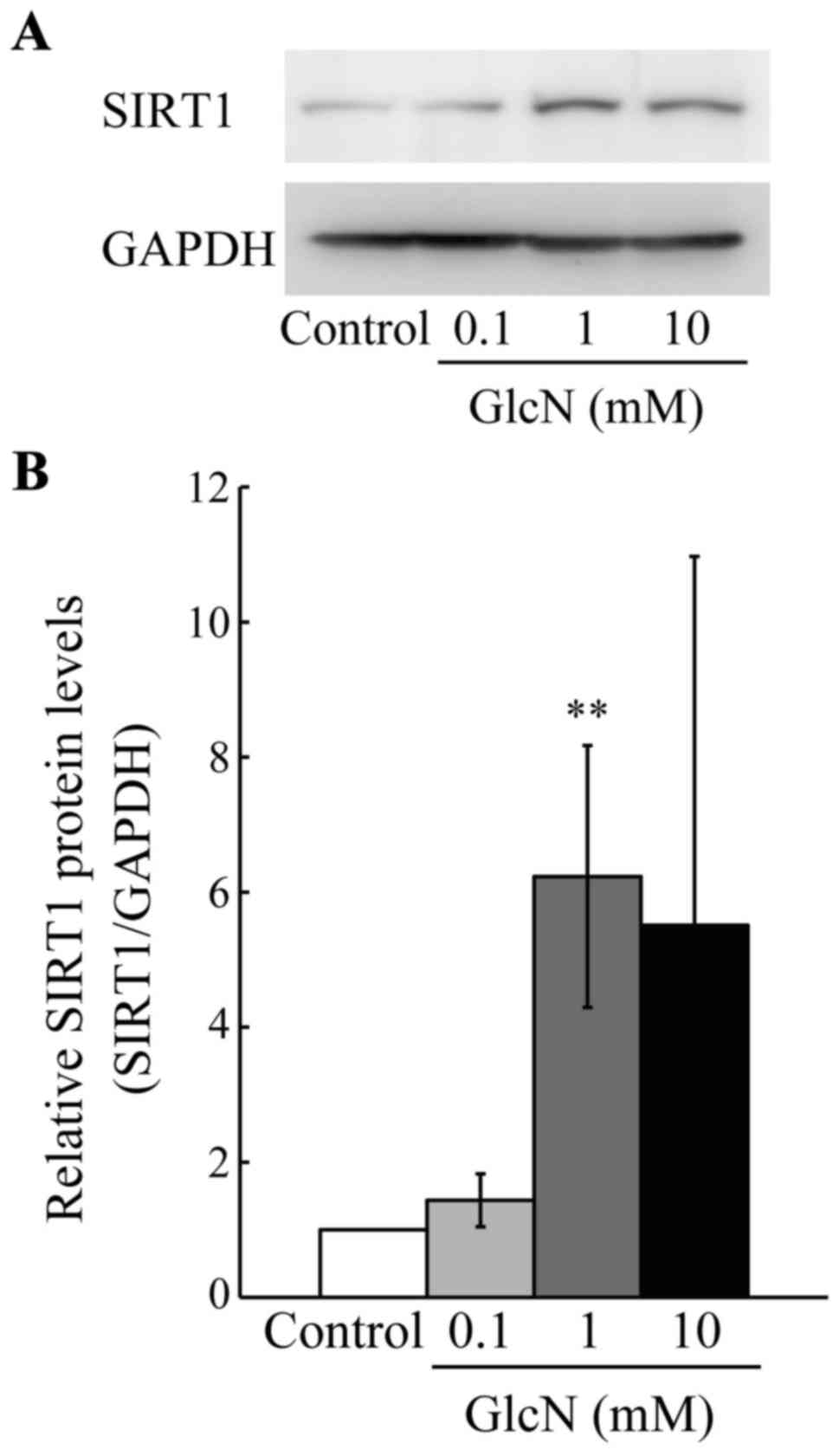
Moreover, the protein level of SIRT1 was evaluated, since the
expression of SIRT1 mRNA was found to be greatly increased
among the SIRT family genes by GlcN. As shown in Fig. 4, the protein level of SIRT1 was
significantly (5- to 6-fold) increased by GlcN (1 mM) compared with
this level in the control without GlcN (p<0.01).
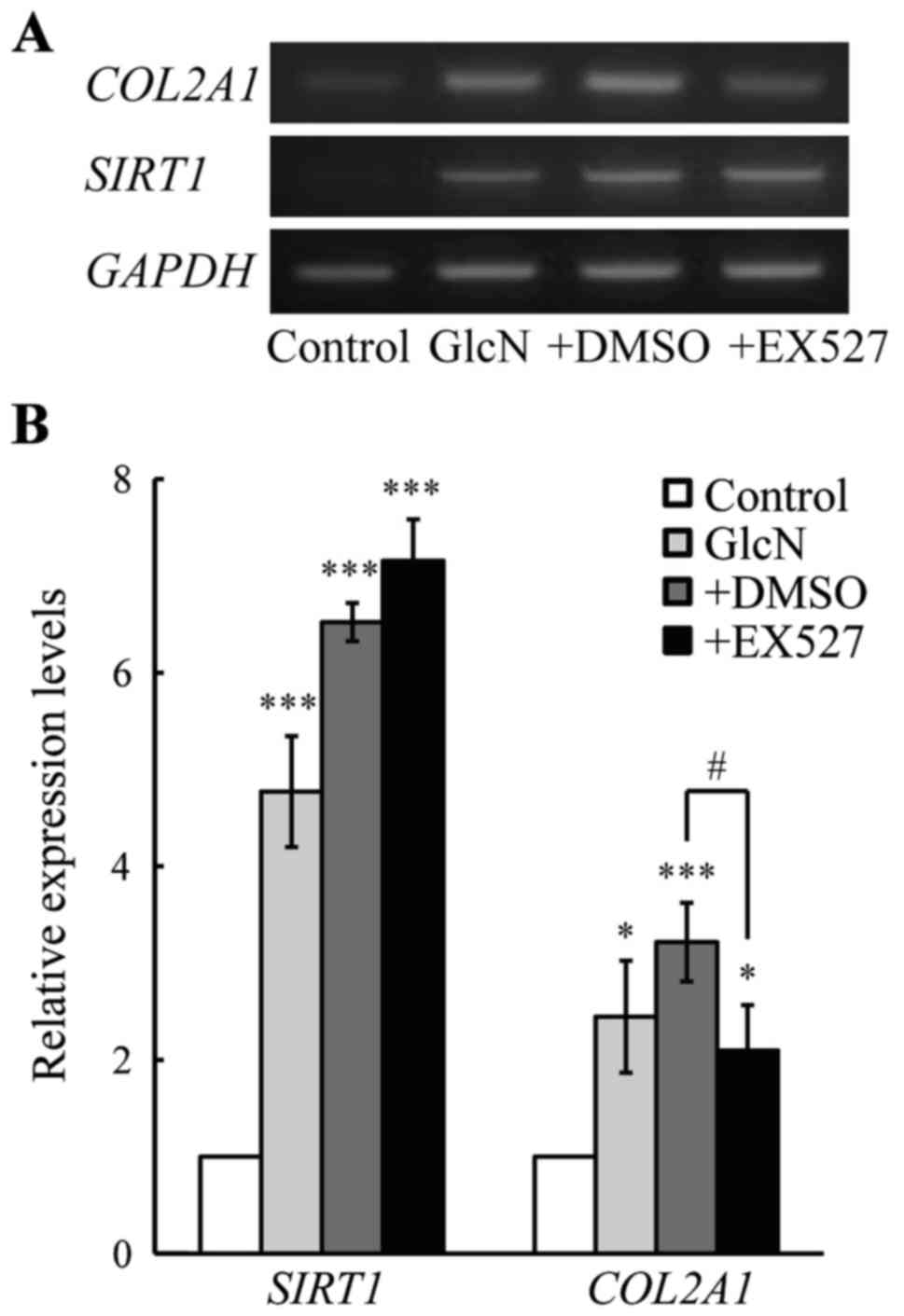
Effect of a SIRT1 inhibitor on COL2A1 and
SIRT1 gene expression in chondrocytes
As mentioned above, COL2A1 and SIRT1 expression
levels were increased by GlcN. Thus, we aimed to ascertain whether
the GlcN-induced COL2A1 gene expression is mediated by SIRT1
gene expression. For this purpose, the effect of EX527, a specific
SIRT1 inhibitor (31), on the
expression of COL2A1 gene expression was evaluated.
Importantly, the GlcN-induced COL2A1 gene expression was
significantly suppressed by EX527, although the GlcN-induced
SIRT1 gene expression was not affected by EX527 (Fig. 5). These observations obviously
indicate that the GlcN-induced COL2A1 gene expression was
regulated by the SIRT1 gene expression.
Discussion
GlcN exhibits a symptom-modifying effect on OA
(6–8), and has been used to relieve the
symptoms of OA in humans (32).
Previously, we demonstrated that GlcN can induce HA production by
synovial cells and chondrocytes (13). However, the effects of GlcN on
genes related to cartilage metabolism are not fully understood.
Collagens and proteoglycans are the major components
in the ECM (33). COL2A1 is the
most abundant collagen in articular cartilage, and provides
cartilage tissue with tensile strength (34,35). In this study, we revealed that
GlcN obviously enhanced the mRNA and protein levels of COL2A1 in
the chondrocytes (Figs. 1 and
2), although GlcN has been
previously reported to increase type II collagen synthesis
(36–39). By contrast, GlcN only slightly
increased the mRNA expression of the MMPs (Fig. 1). These observations suggest that
GlcN upregulates the expression of anabolic genes (such as
COL2A1) rather than catoblic genes (such as MMPs),
thereby possibly exhibiting chondroprotective actions on
degenerative joint diseases such as OA.
SIRT1 is known as an upstream-regulating gene
of COL2A1 in chondrocytes (29) and exerts chondroprotective action
in OA (40–42). In this context, it is important to
note that adult heterozygous Sirt1-knockout mice and
chondrocyte-specific Sirt1-conditional knockout mice
presented with reduced intensity of Safranin-O staining and
increased OA progression compared with wild-type mice (40,41); Sirt1 point mutation-knockin
mice were found to exhibit OA progression (42). Furthermore, SIRT1 promotes
cartilage-specific gene expression, such as COL2A1 (29) in chondrocytes, and protects
chondrocytes from senescence (43). Moreover, SIRT1 was found to
inhibit the apoptosis of human chondrocytes (44,45), whereas knockdown of SIRT1
led to osteoarthritic gene expression in human chondrocytes
(46). In addition,
overexpression of SIRT1 inhibited interleukin (IL)-1β- or tumor
necrosis factor (TNF)-α-induced expression of cartilage-degrading
enzymes (such as MMPs) by modulating the nuclear factor (NF)-κB
pathway (47,48). These observations suggest that
SIRT1 exhibits a chondroprotective action on OA by upregulating
cartilage-specific gene expression (such as COL2A1) but
inhibiting cartilage-degrading enzymes (such as MMPs) in
chondrocytes. Notably, the present results demonstrated that GlcN
markedly enhanced the mRNA and protein levels of SIRT1 as well as
COL2A1 in chondrocytes among the SIRT genes examined (Figs. 3 and 4). Importantly, the GlcN-induced
COL2A1 gene expression was significantly suppressed by
EX527, a SIRT1-specific inhibitor (Fig. 5). Together these observations
suggest the possibility that GlcN exhibits protective actions on
chondrocytes by upregulating COL2A1 expression via SIRT1
expression.
On the other hand, it has been noted that the
O-linked N-acety lglucosamine (O-GlcNAc)
modification of target proteins modulates cellular functions, such
as nuclear transport, transcription, translation, cell signaling,
apoptosis and cell shape, and the modification is mediated via the
addition of O-GlcNAc to the hydroxy group in a serine or
threonine residue by O-GlcNAc transferase (49,50). In this context, it has been
demonstrated that several transcription factors are modified by
O-GlcNAc, and such modification regulates their
transcriptional activities of genes (51–55). Moreover, we previously reported
that O-GlcNAc modification is increased by GlcN in
endothelial cells and synovial cells (56–58). Thus, elucidation of the
involvement of GlcN-mediated O-GlcNAc modification in the
transcriptional regulation of SIRT1 in chondrocytes in the future
is warranted.
In conclusion, the present study revealed that GlcN
upregulates COL2A1 and SIRT1 expression in chondrocytes. Moreover,
a SIRT1 inhibitor suppressed the GlcN-induced upregulation of
COL2A1 gene expression. Together these observations suggest
that GlcN enhances the mRNA expression and protein level of SIRT1
and its downstream gene COL2A1 in chondrocytes, thereby
possibly exhibiting chondroprotective action. However, the present
study has a limitation, as we only evaluated the effects of GlcN on
the expression of COL2A1, MMPs and SIRTs using a chondrocyte cell
line SW 1353. Thus, it remains to be elucidated whether GlcN
modulates the expression of these genes in articular
chondrocytes.
Acknowledgments
This study was supported by the Institute for
Environmental and Gender-Specific Medicine, Juntendo University and
JSPS KAKENHI (grant no. 23580183).
References
|
1
|
Hunter DJ and Felson DT: Osteoarthritis.
BMJ. 332:639–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gabriel SE, Crowson CS, Campion ME and
O'Fallon WM: Direct medical costs unique to people with arthritis.
J Rheumatol. 24:719–725. 1997.PubMed/NCBI
|
|
3
|
March LM and Bachmeier CJ: Economics of
osteoarthritis: a global perspective. Baillieres Clin Rheumatol.
11:817–834. 1997. View Article : Google Scholar
|
|
4
|
Loeser RF: Age-related changes in the
musculoskeletal system and the development of osteoarthritis. Clin
Geriatr Med. 26:371–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB: The role of cytokines as
inflammatory mediators in osteoarthritis: lessons from animal
models. Connect Tissue Res. 40:1–11. 1999. View Article : Google Scholar
|
|
6
|
Crolle G and D'Este E: Glucosamine
sulphate for the management of arthrosis: a controlled clinical
investigation. Curr Med Res Opin. 7:104–109. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drovanti A, Bignamini AA and Rovati AL:
Therapeutic activity of oral glucosamine sulfate in osteoarthrosis:
a placebo-controlled double-blind investigation. Clin Ther.
3:260–272. 1980.PubMed/NCBI
|
|
8
|
Tapadinhas MJ, Rivera IC and Bignamini AA:
Oral glucosamine sulphate in the management of arthrosis: report on
a multi-centre open investigation in Portugal. Pharmatherapeutica.
3:157–168. 1982.PubMed/NCBI
|
|
9
|
Lopes Vaz A: Double-blind clinical
evaluation of the relative efficacy of ibuprofen and glucosamine
sulphate in the management of osteoarthrosis of the knee in
out-patients. Curr Med Res Opin. 8:145–149. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McAlindon TE, LaValley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: a systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: a randomised, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: a 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Igarashi M, Kaga I, Takamori Y, Sakamoto
K, Miyazawa K and Nagaoka I: Effects of glucosamine derivatives and
uronic acids on the production of glycosaminoglycans by human
synovial cells and chondrocytes. Int J Mol Med. 27:821–827.
2011.PubMed/NCBI
|
|
14
|
Loeser RF: Molecular mechanisms of
cartilage destruction: mechanics, inflammatory mediators, and aging
collide. Arthritis Rheum. 54:1357–1360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abramson SB, Attur M and Yazici Y:
Prospects for disease modification in osteoarthritis. Nat Clin
Pract Rheumatol. 2:304–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attur M, Krasnokutsky-Samuels S, Samuels J
and Abramson SB: Prognostic biomarkers in osteoarthritis. Curr Opin
Rheumatol. 25:136–144. 2013. View Article : Google Scholar :
|
|
18
|
Li Y, Tsun A, Gao Z, Han Z, Gao Y, Li Z,
Lin F, Wang Y, Wei G, Yao Z, et al: 60-kDa Tat-interactive protein
(TIP60) positively regulates Th-inducing POK (ThPOK)-mediated
repression of eomesodermin in human CD4+ T cells. J Biol
Chem. 288:15537–15546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattori T, Kubota S, Yutani Y, Fujisawa T,
Nakanishi T, Takahashi K and Takigawa M: Change in cellular
localization of a rheumatoid arthritis-related antigen (RA-A47)
with downregulation upon stimulation by inflammatory cytokines in
chondrocytes. J Cell Physiol. 186:268–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neumann C, Yu A, Welge-Lüssen U,
Lütjen-Drecoll E and Birke M: The effect of TGF-beta2 on elastin,
type VI collagen, and components of the proteolytic degradation
system in human optic nerve astrocytes. Invest Ophthalmol Vis Sci.
49:1464–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SO and Kim MR: [6]-Gingerol prevents
disassembly of cell junctions and activities of MMPs in invasive
human pancreas cancer cells through ERK/NF-κB/Snail signal
transduction pathway. Evid Based Complement Alternat Med.
2013:7618522013. View Article : Google Scholar
|
|
22
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah ZH, Ahmed SU, Ford JR, Allison SJ,
Knight JRP and Milner J: A deacetylase-deficient SIRT1 variant
opposes full-length SIRT1 in regulating tumor suppressor p53 and
governs expression of cancer-related genes. Mol Cell Biol.
32:704–716. 2012. View Article : Google Scholar :
|
|
24
|
Ji S, Doucette JR and Nazarali AJ: Sirt2
is a novel in vivo downstream target of Nkx2.2 and enhances
oligodendroglial cell differentiation. J Mol Cell Biol. 3:351–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grubisha O, Rafty LA, Takanishi CL, Xu X,
Tong L, Perraud AL, Scharenberg AM and Denu JM: Metabolite of SIR2
reaction modulates TRPM2 ion channel. J Biol Chem. 281:14057–14065.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song R, Xu W, Chen Y, Li Z, Zeng Y and Fu
Y: The expression of sirtuins 1 and 4 in peripheral blood
leukocytes from patients with type 2 diabetes. Eur J Histochem.
55:e102011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orecchia A, Scarponi C, Di Felice F,
Cesarini E, Avitabile S, Mai A, Mauro ML, Sirri V, Zambruno G,
Albanesi C, et al: Sirtinol treatment reduces inflammation in human
dermal microvascular endothelial cells. PLoS One. 6:e243072011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanfi Y, Shalman R, Peshti V, Pilosof SN,
Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, et
al: Regulation of SIRT6 protein levels by nutrient availability.
FEBS Lett. 582:543–548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dvir-Ginzberg M, Gagarina V, Lee EJ and
Hall DJ: Regulation of cartilage-specific gene expression in human
chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J
Biol Chem. 283:36300–36310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gray SG and Ekström TJ: The human histone
deacetylase family. Exp Cell Res. 262:75–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gertz M, Fischer F, Nguyen GTT,
Lakshminarasimhan M, Schutkowski M, Weyand M and Steegborn C:
Ex-527 inhibits sirtuins by exploiting their unique
NAD+-dependent deacetylation mechanism. Proc Natl Acad
Sci USA. 110:E2772–E2781. 2013. View Article : Google Scholar
|
|
32
|
Nagaoka I, Igarashi M and Sakamoto K:
Biological activities of glucosamine and its related substances.
Adv Food Nutr Res. 65:337–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhosale AM and Richardson JB: Articular
cartilage: structure, injuries and review of management. Br Med
Bull. 87:77–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eyre DR: Collagens and cartilage matrix
homeostasis. Clin Orthop Relat Res. 427(Suppl): S118–S122. 2004.
View Article : Google Scholar
|
|
35
|
Eyre DR, Weis MA and Wu JJ: Articular
cartilage collagen: an irreplaceable framework? Eur Cell Mater.
12:57–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Block JA, Oegema TR, Sandy JD and Plaas A:
The effects of oral glucosamine on joint health: is a change in
research approach needed? Osteoarthritis Cartilage. 18:5–11. 2010.
View Article : Google Scholar
|
|
37
|
Varghese S, Theprungsirikul P, Sahani S,
Hwang N, Yarema KJ and Elisseeff JH: Glucosamine modulates
chondrocyte proliferation, matrix synthesis, and gene expression.
Osteoarthritis Cartilage. 15:59–68. 2007. View Article : Google Scholar
|
|
38
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthritis Cartilage.
15:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stoppoloni D, Politi L, Leopizzi M,
Gaetani S, Guazzo R, Basciani S, Moreschini O, De Santi M,
Scandurra R and Scotto d'Abusco A: Effect of glucosamine and its
peptidyl-derivative on the production of extracellular matrix
components by human primary chondrocytes. Osteoarthritis Cartilage.
23:103–113. 2015. View Article : Google Scholar
|
|
40
|
Gabay O, Oppenhiemer H, Meir H, Zaal K,
Sanchez C and Dvir-Ginzberg M: Increased apoptotic chondrocytes in
articular cartilage from adult heterozygous SirT1 mice. Ann Rheum
Dis. 71:613–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsuzaki T, Matsushita T, Takayama K,
Matsumoto T, Nishida K, Kuroda R and Kurosaka M: Disruption of
Sirt1 in chondrocytes causes accelerated progression of
osteoarthritis under mechanical stress and during ageing in mice.
Ann Rheum Dis. 73:1397–1404. 2014. View Article : Google Scholar
|
|
42
|
Gabay O, Sanchez C, Dvir-Ginzberg M,
Gagarina V, Zaal KJ, Song Y, He XH and McBurney MW: Sirtuin 1
enzymatic activity is required for cartilage homeostasis in vivo in
a mouse model. Arthritis Rheum. 65:159–166. 2013. View Article : Google Scholar
|
|
43
|
Hong EH, Lee SJ, Kim JS, Lee KH, Um HD,
Kim JH, Kim SJ, Kim JI and Hwang SG: Ionizing radiation induces
cellular senescence of articular chondrocytes via negative
regulation of SIRT1 by p38 kinase. J Biol Chem. 285:1283–1295.
2010. View Article : Google Scholar :
|
|
44
|
Takayama K, Ishida K, Matsushita T, Fujita
N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, et
al: SIRT1 regulation of apoptosis of human chondrocytes. Arthritis
Rheum. 60:2731–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gagarina V, Gabay O, Dvir-Ginzberg M, Lee
EJ, Brady JK, Quon MJ and Hall DJ: SirT1 enhances survival of human
osteoarthritic chondrocytes by repressing protein tyrosine
phosphatase 1B and activating the insulin-like growth factor
receptor pathway. Arthritis Rheum. 62:1383–1392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fujita N, Matsushita T, Ishida K, Kubo S,
Matsumoto T, Takayama K, Kurosaka M and Kuroda R: Potential
involvement of SIRT1 in the pathogenesis of osteoarthritis through
the modulation of chondrocyte gene expressions. J Orthop Res.
29:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moon MH, Jeong JK, Lee YJ, Seol JW,
Jackson CJ and Park SY: SIRT1, a class III histone deacetylase,
regulates TNF-α-induced inflammation in human chondrocytes.
Osteoarthritis Cartilage. 21:470–480. 2013. View Article : Google Scholar
|
|
48
|
Matsushita T, Sasaki H, Takayama K, Ishida
K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M and
Kuroda R: The overexpression of SIRT1 inhibited osteoarthritic gene
expression changes induced by interleukin-1β in human chondrocytes.
J Orthop Res. 31:531–537. 2013. View Article : Google Scholar
|
|
49
|
Wells L, Whelan SA and Hart GW: O-GlcNAc:
a regulatory post-translational modification. Biochem Biophys Res
Commun. 302:435–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Love DC and Hanover JA: The hexosamine
signaling pathway: deciphering the 'O-GlcNAc code'. Sci STKE.
2005:re132005.
|
|
51
|
Chou TY, Dang CV and Hart GW:
Glycosylation of the c-Myc transactivation domain. Proc Natl Acad
Sci USA. 92:4417–4421. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Han I and Kudlow JE: Reduced O
glycosylation of Sp1 is associated with increased proteasome
susceptibility. Mol Cell Biol. 17:2550–2558. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang MS and Hart GW: A subpopulation of
estrogen receptors are modified by O-linked N-acetylglucosamine. J
Biol Chem. 272:2421–2428. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang WH, Kim JE, Nam HW, Ju JW, Kim HS,
Kim YS and Cho JW: Modification of p53 with O-linked
N-acetylglucosamine regulates p53 activity and stability. Nat Cell
Biol. 8:1074–1083. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fujiki R, Hashiba W, Sekine H, Yokoyama A,
Chikanishi T, Ito S, Imai Y, Kim J, He HH, Igarashi K, et al:
GlcNAcylation of histone H2B facilitates its monoubiquitination.
Nature. 480:557–560. 2011.PubMed/NCBI
|
|
56
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Glucosamine, a naturally occurring amino monosaccharide
modulates LL-37-induced endothelial cell activation. Int J Mol Med.
22:657–662. 2008.PubMed/NCBI
|
|
57
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulation of TNF-alpha-induced endothelial cell
activation by glucosamine, a naturally occurring amino
monosaccharide. Int J Mol Med. 22:809–815. 2008.PubMed/NCBI
|
|
58
|
Someya A, Sakamoto K and Nagaoka I:
Glucosamine induces the O-N-acetylglucosamine modification of
transcription factor Sp1. Glucosamine Res. 9:48–52. 2013.
|