Introduction
It has been proposed that adipose-derived stem cells
(ASCs) can be used for the treatment of chronic wounds (1). Several preclinical studies have
demonstrated that ASCs secrete soluble factors that promote
angiogenesis and keratinocyte re-epithelization, and protect
against apoptosis (2–4). Furthermore, studies have
demonstrated that the culture of ASCs under hypoxic conditions
enhances their angiogenic properties (2,5);
however, the mechanisms through which hypoxia modulates the ASC
properties relevant to re-epithelialization have not yet been fully
elucidated.
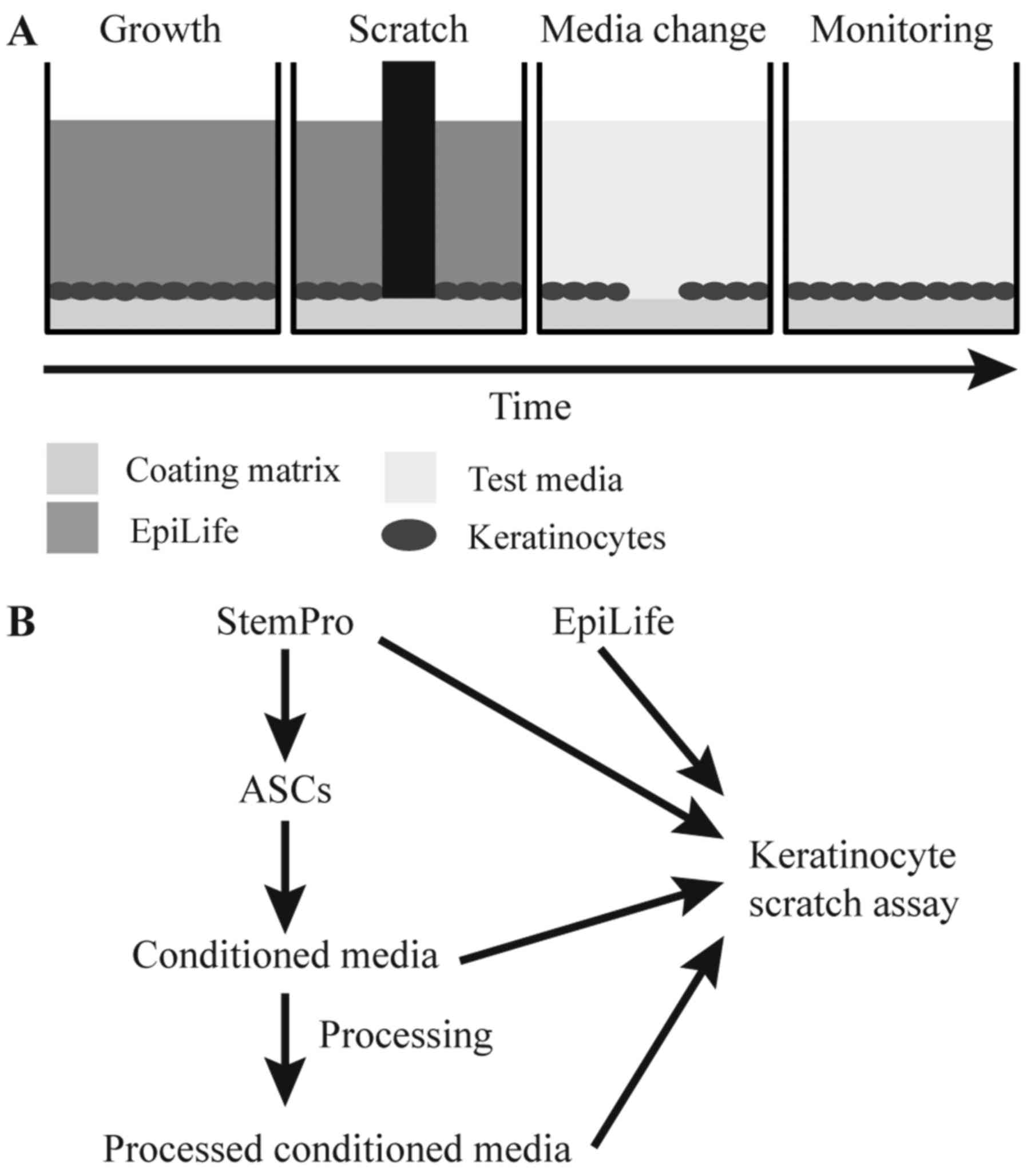
A commonly used in vitro model to assess
wound healing is the scratch assay, which is based on creating a
small scratch in a confluent monolayer cell culture and monitoring
the closure of the scratch by migration/growth of the cells
(Fig. 1A). To mimic the wound
healing process of cutaneous wounds, keratinocytes are the most
relevant cells, as re-epithelization steps include sequential
keratinocyte proliferation, migration and differentiation
converging in stratification (6).
Previously, when assessing the wound healing effect
of ASCs and other mesenchymal stem cells on keratinocytes in a
scratch assay, a combination of conditioned media from stem cells
and the spontaneously transformed aneuploid immortal keratinocyte
cell line, HaCaT, has often been preferred (3,7,8).
However, as transformed cells often display an altered response to
growth factors and cytokines compared to their non-cancerous
counterparts (9), and may respond
in a hyperactive manner to hypoxia-induced factors, such as
epidermal growth factor (EGF) and basic fibroblast growth factor
(bFGF) (9–11), this cell line may not be the best
option to predict in vivo responses. Another difference
between primary keratinocytes and the HaCaT cell line is that
primary keratinocytes are sensitive to calcium concentrations
>90 µM (12), which
induces differentiation and the cessation of proliferation, whereas
HaCaT cells are relatively insensitive to variations in calcium
concentration (13). The
calcium-sensitivity of primary keratinocytes may provide an
explanation for the favored use of HaCaT cells when evaluating the
effects of conditioned media from ASCs on wound healing, as ASCs
routinely are grown in media with a calcium concentration of 1 mM,
far surpassing what is tolerated by keratinocytes (14).
In this study, we describe how the ASC-keratinocyte
scratch assay can be modified to better mimic in vivo
re-epithelialization. This modification entails dialysis of
conditioned media from ASCs prior to testing on primary
keratinocytes. Furthermore, we demonstrate that the modified assay
can be used to explore the effects of hypoxia on the wound healing
properties of ASCs.
Materials and methods
Cell culture
Human primary keratinocytes from three donors; HEKa
lot #1249380, HEKa lot #1352914, HEKn lot #1030422 (Thermo Fisher
Scientific, Frederick, MD, USA) were used for all the experiments.
They were maintained in EpiLife, composed of EpiLife®
basal medium (Gibco™/Thermo Fisher Scientific, Taastrup, Denmark)
supplemented with 1X Human Keratinocyte Growth Supplement
(Gibco™/Thermo Fisher Scientific), 100 U/ml penicillin, 0.1 mg/ml
streptomycin (Invitrogen™/Thermo Fisher Scientific, Taastrup,
Denmark). The keratinocytes were maintained as prescribed by the
manufacturer, and cultured on tissue culture polystyrene (TCP)
flasks (Greiner Bio-One, Fredensborg, Denmark) coated with Coating
Matrix kit. For subcultivation, TrypLE™ (both from Gibco/Thermo
Fisher Scientific) was used.
The ASCs used in this study (ASC21) have previously
been isolated and extensively characterized in our laboratory
(2,15–19). The cells were obtained from the
adipose tissue of a healthy donor using a protocol that was
approved by the Regional Committee on Biomedical Research Ethics of
Northern Jutland, Denmark (project no. VN 2005/54). The ASCs were
cultured in StemPro, composed of StemPro® MSC SFM
XenoFree (Gibco/Thermo Fisher Scientific) supplemented with 200 mM
L-glutamine and 100 U/ml penicillin, 0.1 mg/ml streptomycin (both
from Gibco/Thermo Fisher Scientific) and cultured on cultured on
TCP flasks (Greiner Bio-One), which were coated with CellStart™
CTS™ according to the manufacturer instructions. For
subcultivation, TrypLE™ (both from Gibco/Thermo Fisher Scientific)
was used.
Comparison of ASC and keratinocyte
morphology and growth with varying concentrations of calcium
To compare the effects of the different calcium
concentrations in EpiLife and StemPro on keratinocytes and ASCs,
the cells were seeded at a density of 50,000 and 8,000
cells/cm2, respectively in 96-well plates and cultured
until 80% confluent. At this point, the cells were washed with
phosphate-buffered saline (PBS; Gibco™/Thermo Fisher Scientific)
and supplied with either EpiLife, EpiLife with increasing levels of
CaCl2 from 60 µM to 2 mM, or StemPro, and
incubated for 24 h. To assess cell morphology and cell number, the
cells were washed with PBS and fixed in 4% formaldehyde, stained
with Hoechst 33342 (1 µg/ml; Molecular Probes®,
Eugene, OR, USA), permeabilized using 0.1% Triton X-100
(Sigma-Aldrich), and stained with BODIPY® 558/568
phalloidin (1:40; Molecular Probes). The stained cells were kept in
PBS at 4°C until analysis. Fluorescence images were obtained using
the AxioVision software package with a Zeiss AxioObserver.Z1
microscope equipped with an AxioCam MRm camera and a motorized
stage (Carl Zeiss, Oberkochen, Germany). To quantify the cell
number, the number of nuclei was counted using ImageJ 1.47v
(National Institutes of Health, Bethesda, MD, USA).
To evaluate the effect on keratinocytes of lowering
the calcium concentration of StemPro, the medium was dialyzed
against EpiLife basal medium. For dialysis, 10 ml StemPro were
injected into a Slide-A-Lyzer Dialysis Cassette, 2 MWCO
(Pierce™/Thermo Fisher Scientific) and dialyzed in 1.25 liters
EpiLife at 4°C for 2 h after which the EpiLife was exchanged with
new EpiLife (1.25 liters) for continuing dialysis overnight. After
dialysis, the low-calcium StemPro was tested on keratinocyte
morphology as described above.
Effect of combining EpiLife and StemPro
on keratinocyte morphology
To determine whether diluting StemPro into EpiLife
improves the compatibility with keratinocytes, different ratios of
EpiLife vs. StemPro were tested on the keratinocytes. The
keratinocytes were seeded at 20,000 cells/cm2 in 96-well
plates and incubated overnight, after which, the cells were washed
with PBS and cultured for 24 h in either EpiLife, StemPro, or
EpiLife and StemPro at the ratios 3:1, 1:1 and 1:3. The morphology
of the cells was evaluated by phase contrast using the IncuCyte
ZOOM® system (Essen BioScience, Hertfordshire, UK) and
IncuCyte™ High Definition Imaging Mode.
To evaluate whether EpiLife supplemented with the
protein fraction from conditioned StemPro supports normal
keratinocyte morphology, conditioned StemPro was concentrated in
3,000 NMWL Amicon Ultra-15 centrifugal filter units (Merck
Millipore, Darmstadt, Germany) and reconstituted in EpiLife to 50,
75 or 100% of the original concentration and tested on the
keratinocytes as described above.
Preparation of conditioned media
For the production of conditioned media, the ASCs
were seeded in T175 culture flasks at a density of 8,000
cells/cm2, and incubated in a standard incubator at
37°C, 20% O2, 5% CO2. When the cells were 80%
confluent, they were washed twice in PBS and 30 ml fresh StemPro
were added to each flask. Half of the flasks were left in the
standard incubator for normoxic conditioning of the media and the
other half were transferred to a BioSpherix clove box (Xvivo;
BioSpherix, Redfield, NY, USA) and cultured at 37°C, 1%
O2, 5% CO2 for hypoxic conditioning. After 24
h of incubation the media were harvested, centrifuged to pellet
debris, and the supernatant kept at −80°C until further
analysis.
Scratch assay
To investigate the wound healing effect of ASCs on
human primary keratinocytes, the keratinocytes were seeded with
50,000 cells/cm2 in 24- or 96-well plates. When the
cells formed a confluent monolayer, they were scratched using the
Wounding Pin Tool (V&P Scientific, Inc., San Diego, CA, USA) or
the WoundMaker™ (Essen BioScience) and washed in PBS to remove cell
debris. StemPro, dialyzed StemPro, conditioned StemPro, dialyzed
normoxic/hypoxic conditioned StemPro, or EpiLife were added to the
cells (Fig. 1B). Wound healing
was monitored by time-lapse photography taking images every hour
using a PeCon Incubator system including a CTI-controller 3700
digital and a Tempcontrol 37-2 digital, connected to an AxioVision
software package with a Zeiss AxioObserver.Z1 microscope equipped
with an AxioCam MRm camera and a motorized stage (Carl Zeiss) or
using the IncuCyte ZOOM® system (Essen BioScience). The
relative wound size at each time point was analyzed using the
TScratch software (20) or the
IncuCyte™ Scratch Wound Cell Migration Software Module (Essen
BioScience). The media was tested on three primary keratinocyte
cultures in two separate experiments (n=6), each in technical
triplicates.
Statistical analysis
Statistical analysis was performed using SigmaPlot
12.0 (Systat Software, Erkrath, Germany). The normal distribution
of each group was assessed by means of the Shapiro-Wilk test.
Additionally, variance was tested using an Equal Variance test.
Data are presented as the mean ± standard error of the mean (SEM).
A p-value <0.05 was considered to indicate a statistically
significant difference. For the comparison of two groups, a paired
t-test was used. For the comparison of more than two groups, a
one-way repeated measures analysis of variance (ANOVA) with
Bonferroni's post hoc test was used.
Results
Comparison of the effect of varying
calcium concentrations on the morphology and growth of
keratinocytes and ASCs
EpiLife and StemPro were evaluated for their
compatibility for the culture of both cell types (Fig. 2A). It was evident that the
kera-tinocytes cultured in StemPro lost the typical
cobblestone-like appearance of basal keratinocytes and displayed a
more mature phenotype with morphology and rearranged cytoskeleton
with cortical actin bundles resembling those of differentiated
keratinocytes (21). On the other
hand, the ASCs retained their morphology when cultured in EpiLife;
however, since the number of cells after 24 h in EpiLife was
significantly lower than that in StemPro, it suggested that the
growth conditions were sub-optimal. To confirm that these
observations were a result of the cellular response to differences
in calcium concentrations, we performed a calcium dose escalation
experiment with EpiLife medium. For the keratinocytes, a gradual
change in cell morphology and cytoskeleton pattern was observed
with the increasing calcium concentrations already after 24 h
(Fig. 2B). The cells appeared
more clustered and the actin fibers were more diffuse. These
morphological changes were slightly noticeable when 120 µM
calcium were supplemented to the media, and were obviously
prominent at concentrations of 480 µM and above. No apparent
morphological or cytoskeletal effects of changing the calcium
concentration were observed for the ASCs. Despite the dramatic
effects on keratinocyte morphology, changes in the calcium
concentration did not affect cell numbers during short-term
exposure (Fig. 2C). This is in
sharp contrast to the ASCs, where the use of EpiLife decreased the
cell number. This inhibition was attenuated by supplementing
EpiLife with calcium in the range of 860 µM to 1.4 mM
(Fig. 2D).
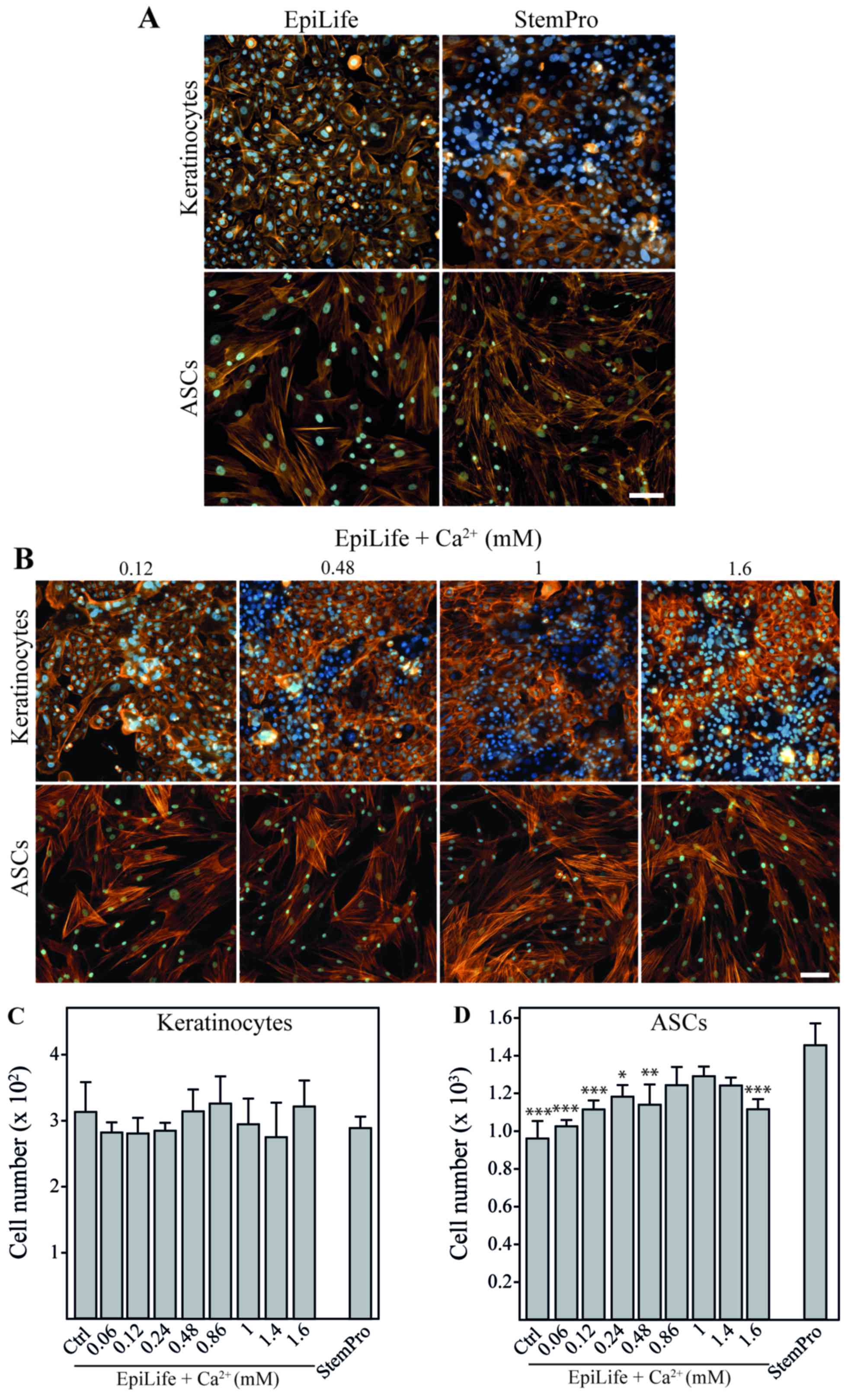 | Figure 2The effect of calcium on morphology
and cell number of keratinocytes and adipose-derived stem cells
(ASCs). Keratinocytes and ASCs were cultured until 80% confluent,
followed by 24 h of incubation in EpiLife, EpiLife supplemented
with calcium, or StemPro. (A) Representative images of
keratinocytes and ASCs showing morphology and cell density after
incubation in EpiLife or StemPro. To assess cell morphology and
cell number, cytoskeleton were visualized by phalloidin-Bodipy
558/568 staining (orange) and nuclei were counterstained with
Hoechst 33342 (blue). The scale bars denote 100 µm. (B)
Representative images of keratinocytes and ASCs showing morphology
and cell density after incubation in EpiLife supplemented with
increasing concentrations of calcium. Assessment of morphology was
performed as described for (A). (C) Number of keratinocytes after
incubation in EpiLife (Ctrl), EpiLife supplemented with increasing
concentrations of calcium, or StemPro. Values are represented as
the means and SEM; no statistically significant differences were
found (n=6). (D) Number of ASCs after incubation in EpiLife,
EpiLife supplemented with increasing concentrations of calcium, or
StemPro. Values are represented as mean and SEM (n=6). The data
from the EpiLife-based media were compared to those from StemPro
using a one-way ANOVA followed by a multiple comparisons vs.
control, *p<0.05, **p<0.01 and
***p<0.001. |
Development of keratinocyte-compatible
media based on StemPro
To circumvent the media incompatibility issue, we
cultured ASCs in StemPro for the production of conditioned media.
Consequently, for the subsequent use of conditioned media on
keratinocytes, we evaluated different methods to reduce the calcium
concentration while maintaining the ASC secreted proteins.
A simple solution of culturing the keratinocytes at
3:1, 1:1 and 1:3 ratios of EpiLife to StemPro was tested (Fig. 3A). The evaluation of the
morphology revealed that even in the highest dilution of StemPro
(with approximately 300 µM of calcium), the cultures
revealed the presence of clustered keratinocytes, which had lost
their cobblestone appearance.
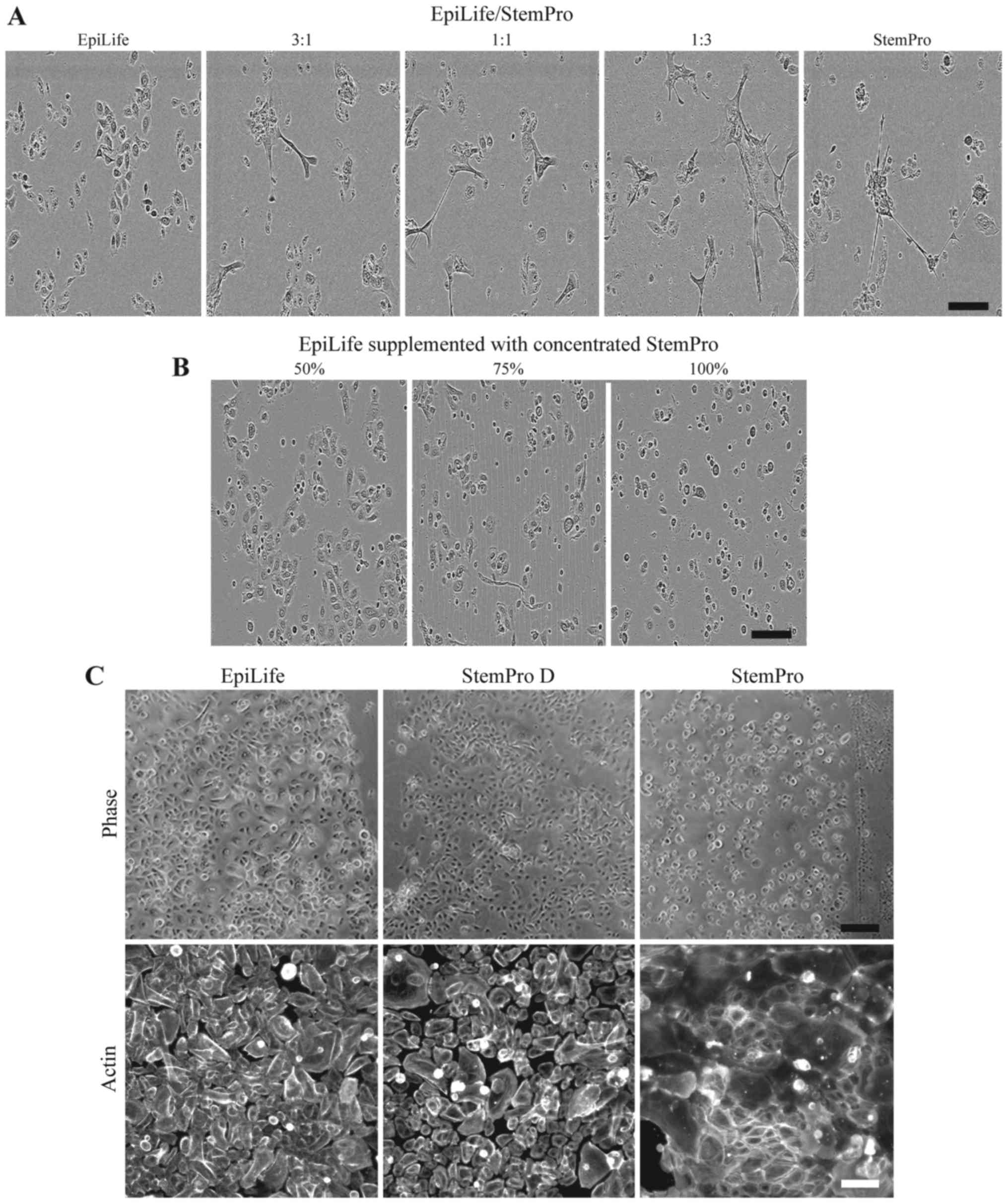 | Figure 3Effect on keratinocyte morphology of
24 h culture in combinations of EpiLife and StemPro. (A)
Keratinocytes were cultured in either EpiLife, StemPro, or
combinations of EpiLife and StemPro in the ratios of 3:1, 1:1 and
1:3, after which the morphology was assessed by phase contrast
microscopy. The scale bars denote 150 µm. (B) The protein
fraction from conditioned StemPro was concentrated on spin columns,
reconstituted in EpiLife at 50, 75 and 100% of the original
concentration, and added to keratinocytes. The morphology was
assessed by phase contrast microscopy as above. (C) Keratinocytes
were cultured in either EpiLife, StemPro dialyzed against EpiLife
(StemPro D), or StemPro. The morphology was assessed by phase
contrast microscopy. The scale bars denote 200 µm. To assess
the cytoskeleton, actin fibers were visualized by fluorescence
microscopy using a phalloidin-Bodipy 558/568 staining. The scale
bars denote 100 µm. |
Another method tested was concentrating the protein
fraction of StemPro using spin filter columns and afterwards
resuspending it in EpiLife in volumes, such that the concentration
of proteins derived from StemPro were 50, 75 and 100% of the
original (Fig. 3B). The cells did
not display the morphology associated with a high calcium content;
however, the cells appeared more round and smaller. In particular,
the cells grown in the highest concentration of protein detached
before a scratch assay could be completed (data not shown).
The effect of dialyzing StemPro in EpiLife prior to
the culture of keratinocytes was examined. We observed that the
morphology of the keratinocytes cultured in dialyzed StemPro
closely resembled that of cells grown in EpiLife (Fig. 3C, upper panel). Additionally, the
visualization of the cyto-skeleton revealed that StemPro induced a
more peripheral arrangement of the actin fibers, and that this to a
high degree was avoided by using dialyzed StemPro that maintained
the diffuse pattern (Fig. 3C,
lower panel).
Evaluation of keratinocyte-compatible
media based on StemPro in a scratch assay
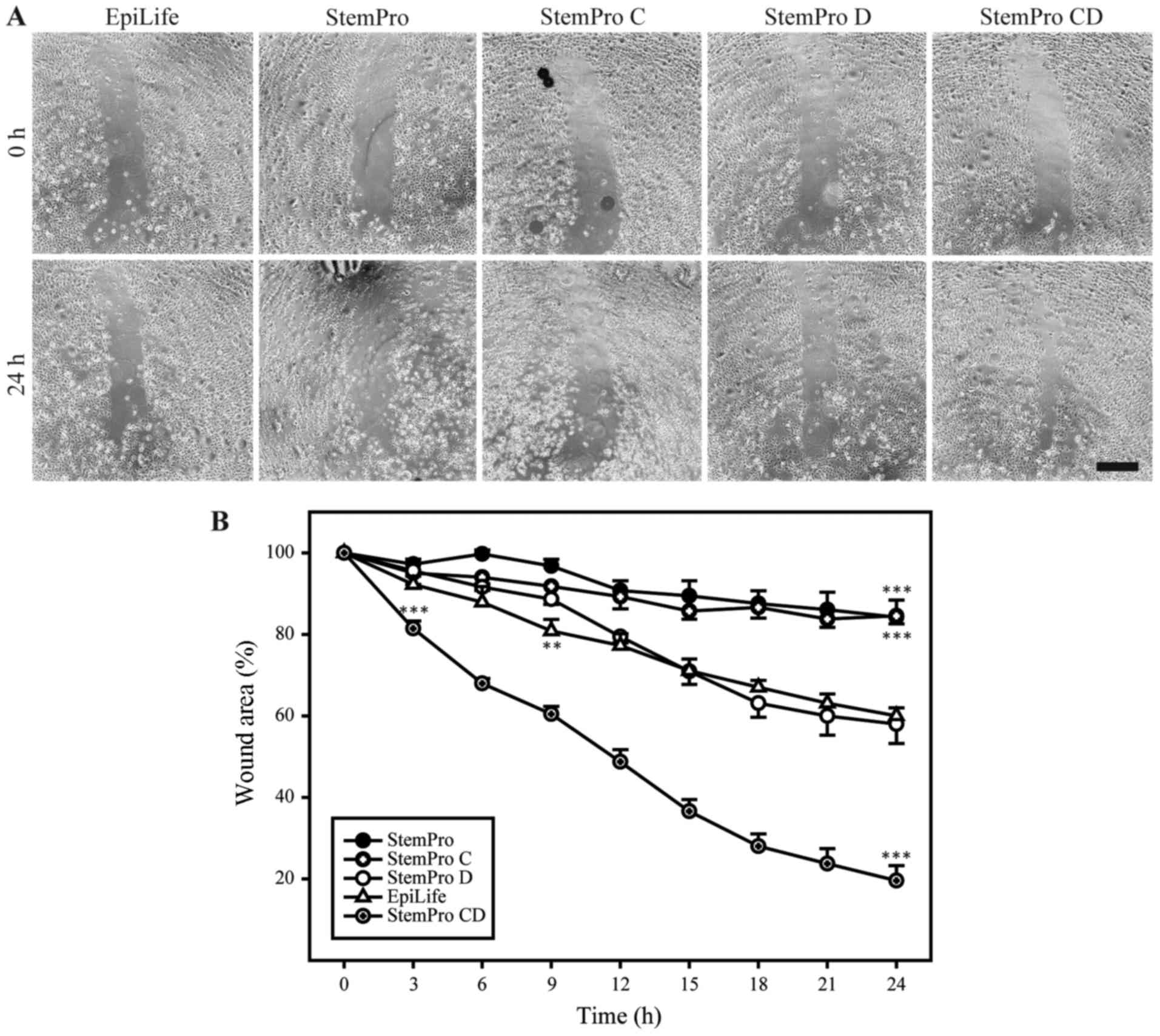
To functionally assess the keratinocyte-compatible
conditioned and dialyzed StemPro, this media was compared to
high-calcium media (StemPro and conditioned StemPro) and
low-calcium non-conditioned media (dialyzed StemPro and EpiLife) in
a scratch assay (Fig. 4). The
conditioned and dialyzed StemPro increased the closure rate of the
scratch when compared to the low-calcium non-conditioned media. The
two low-calcium non-conditioned media (dialyzed StemPro and
EpiLife) both supported healing of the scratch in a comparable
manner. Furthermore, the results from the non-dialyzed StemPro
media confirmed the unsuitability of using high-calcium media when
assessing growth and migration of primary keratinocytes.
Effect of hypoxia on the wound healing
properties of ASCs
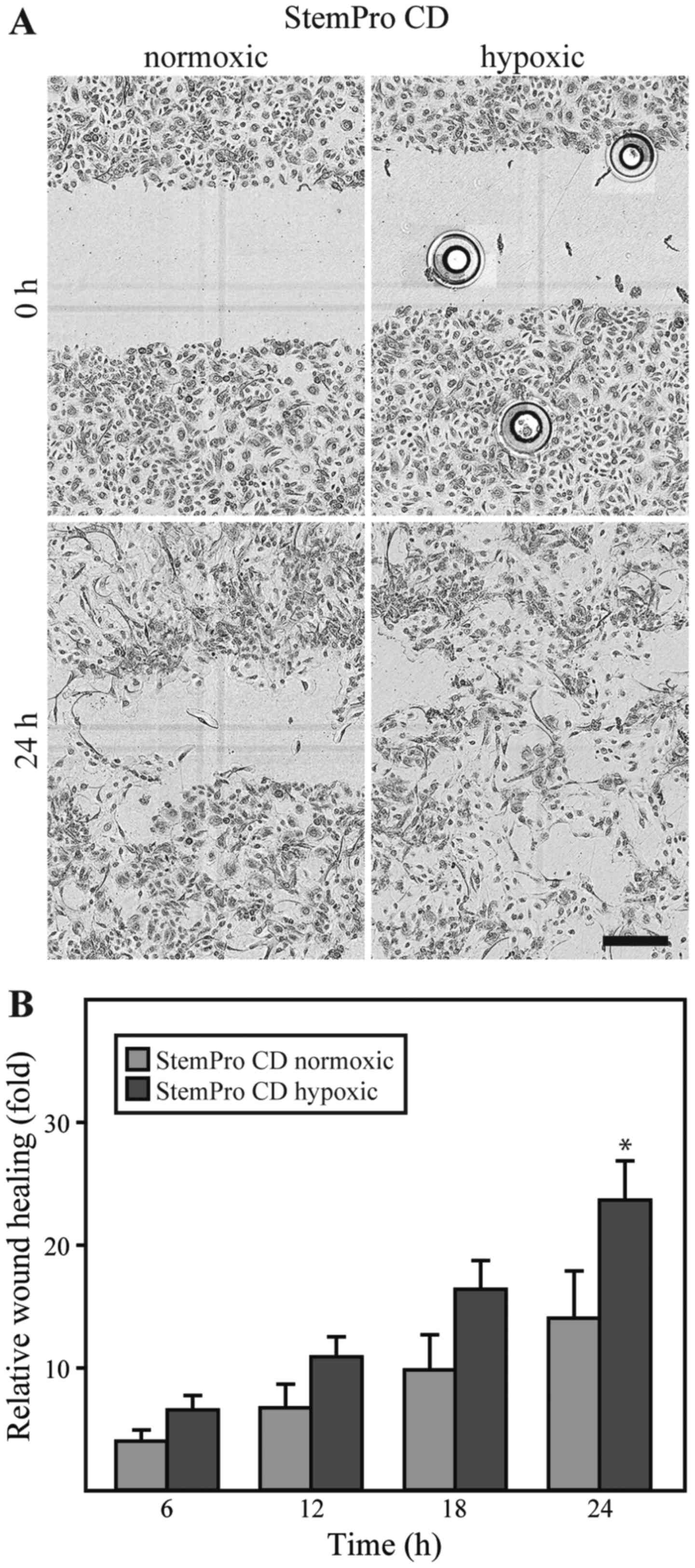
The dialyzed conditioned media derived from either
ASCs cultured under either hypoxic or normoxic conditions was
compared in the keratinocyte scratch assay. No differences between
the media were observed with respect to the effect on cell
morphology. However, it appeared that the closure of the scratch
was more complete in the keratinocytes exposed to the media from
ASCs cultured under hypoxic conditions (Fig. 5A). For both types of media, the
repopulation of the wound area occurred through cells migrating in
an individual random pattern, rather than as linear fronts of the
wound edge approximating one another. A quantification of the
keratinocyte wound closure in the conditioned media compared to the
closure in EpiLife, revealed that both normoxic and hypoxic
conditioned media promoted wound closure to a greater extent than
EpiLife (data not shown). Furthermore, it appeared that the media
from the ASCs cultured under hypoxic conditions was consistently
superior to that from the ASCs cultured under normoxic conditions,
and this difference was statistically significant after 24 h
(Fig. 5B).
Discussion
For the development of a treatment for chronic
wounds, it is apparent from animal experiments and small-scale
clinical trials that ASCs harbor a significant potential for use in
regenerative therapy (22). To
harness that potential, good in vitro models are warranted
that enable the systematic analysis of the various wound healing
properties of ASCs and how these properties may be enhanced.
In the present study, we described the modification
of a scratch assay, such that an evaluation of the ASC secretome on
wound healing properties of human primary keratinocytes could be
carried out. To create a scratch assay that is compatible with both
ASCs and primary keratinocytes, we tried a number of approaches.
First, we explored whether both ASCs and keratinocytes could be
cultured in either EpiLife or StemPro, such that the conditioned
medium from the ASCs could be use directly on the keratinocytes.
Since the ASCs did not proliferate in EpiLife, confirming previous
observations of ASCs in keratinocyte media (23) and the keratinocytes differentiated
in StemPro, this option was not viable. As the literature describes
keratinocyte media to be low and mesenchymal stem cell media to be
high in calcium (14), we
speculated that the failure of either cell type to thrive in the
medium developed for the other could be caused by different
requirements for calcium. A titration of calcium for both cell
types confirmed this. The primary keratinocytes differentiated when
exposed to even moderately higher calcium concentrations than those
found in EpiLife, in agreement with what is already known (24), and the ASCs cell number decreased
when the calcium concentration was lower than that found in
standard ASC media, possibly due to the influence of calcium on the
cell adhesion to the substrate.
Based on the incompatibility of the media with both
cell types, several approaches were explored to decrease the
calcium contribution from the conditioned StemPro. Simple dilution
of StemPro with EpiLife (up to 1:3) did not decrease the calcium
concentration sufficiently to avoid differentiation of the
keratinocytes. Further dilution was not attempted due to the risk
of attenuating the putative effect of the ASCs. Re-suspending a
concentrated protein fraction of the conditioned StemPro in EpiLife
was not a solution either, as the keratinocytes did not attach well
in this media, possibly due to increases in osmolarity. When
StemPro was dialyzed, a normal keratinocyte morphology was
maintained. Furthermore, we observed that dialyzed StemPro and
EpiLife supported keratinocyte migration in an equivalent manner.
Thus, it is possible to assess the wound healing effects of ASCs in
a scratch assay with primary keratinocytes, provided that the
conditioned media is dialyzed.
When applying conditioned media from ASCs cultured
under normoxic or hypoxic conditions, it was obvious that the ASCs
secreted factors that promoted primary keratinocyte wound healing
in vitro, and that this effect was enhanced by culturing the
ASCs under hypoxic conditions. Our results are thus consistent with
the findings of other studies, in that ASCs and other mesenchymal
stem cells promote keratinocyte-based wound healing significantly
(3,8). Furthermore, our findings indicated
that this effect is also present when studying the more relevant
primary human keratinocytes rather than immortalized cell lines. Of
note, the random migratory pattern of the cells during wound
closure could be an indication of the keratinocytes undergoing a
epithelial-to-mesenchymal transition (25).
In general, mesenchymal cells are known to provide a
microenvironment that maintains the progenitor status of basal
keratinocytes and augments epidermal proliferation, and mesenchymal
cells are therefore commonly used as feeder cells in keratinocyte
cultures (26). In connection to
this, ASCs, which are of mesenchymal origin, have been shown to
secrete a plethora of growth factors, such as keratinocyte growth
factor (KGF), EGF, bFGF, hepatocyte growth factor (HGF), and
insulin-like growth factor-1 (IGF-1) (27), which are key mediators of
migration of human primary keratinocytes (28). The secretion of these factors
could provide part of the explanation for the effect of the
conditioned medium on keratinocyte wound closure.
When using conditioned media from ASCs cultured
under hypoxic conditions, an even more pronounced effect on the
wound closure of the keratinocytes was observed. The exposure of
ASCs to hypoxia has been shown to increase the secretion of a wide
range of growth factors, signaling molecules and cytokines
(29). bFGF and IGF-1 are among
these factors (30,31), and it is possible that these
proteins play a role in the enhanced stimulatory effect of the
hypoxic-conditioned ASCs. The majority of studies on the ASC
secretome under hypoxic conditions have focused on a narrow range
of proteins mostly involved in angiogenesis (2,4,16),
and not on global discovery-based approaches. To begin to unravel
the molecular mechanisms underlying the wound healing effects of
ASCs, we recently performed a proteomic profiling of ASCs exposed
to hypoxia and normoxia, and found that several of the factors
differentially regulated by hypoxia were involved in extracellular
matrix (ECM) synthesis (32). It
will be interesting to determine whether the ECM-related proteins
secreted by ASCs are responsible for the observed effects on the
keratinocytes.
The establishment of good wound models will support
the translation of ASCs into clinical use, as they can play an
important role both for the development and validation of novel
protocols prior to embarking on large-scale clinical studies
(33). Additionally, the models
may be used post-translationally to predict treatment outcome, so
forth a correlation between the modeled parameter and the clinical
outcome can be verified. Good wound models may thus facilitate the
translation of regenerative ASC-based wound therapies into clinical
practice.
Abbreviations:
|
ASCs
|
adipose-derived stem cells
|
|
bFGF
|
basic fibroblast growth factor
|
|
EGF
|
epidermal growth factor
|
|
HGF
|
hepatocyte growth factor
|
|
IGF-1
|
insulin-like growth factor-1
|
|
KGF
|
keratinocyte growth factor
|
|
TCP
|
tissue culture polystyrene
|
Acknowledgments
The authors acknowledge the technical assistance
provided by O. Jensen and L. Sangenario. This study was supported
in part by funds from Lily Benthine Lunds fond (S.R.), Grosserer
L.F. Foghts Fond (V.Z.) and the Obelske family foundation (T.F.).
The funding sources had no influence on either the study design,
collection, analysis, interpretation of the data, the writing of
the study, or on the decision to submit the study for publication.
R.N., D.K., S.B. and M.V. are regular employees of ThermoFisher
Scientific and have not received any financial gains. They hold
some stocks of ThermoFisher Scientific as employees of ThermoFisher
Scientific.
References
|
1
|
Hassan WU, Greiser U and Wang W: Role of
adipose-derived stem cells in wound healing. Wound Repair Regen.
22:313–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rasmussen JG, Frøbert O, Pilgaard L,
Kastrup J, Simonsen U, Zachar V and Fink T: Prolonged hypoxic
culture and trypsinization increase the pro-angiogenic potential of
human adipose tissue-derived stem cells. Cytotherapy. 13:318–328.
2011. View Article : Google Scholar
|
|
3
|
Lee SH, Jin SY, Song JS, Seo KK and Cho
KH: Paracrine effects of adipose-derived stem cells on
keratinocytes and dermal fibroblasts. Ann Dermatol. 24:136–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsiao ST, Lokmic Z, Peshavariya H,
Abberton KM, Dusting GJ, Lim SY and Dilley RJ: Hypoxic conditioning
enhances the angiogenic paracrine activity of human adipose-derived
stem cells. Stem Cells Dev. 22:1614–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastar I, Stojadinovic O, Yin NC, Ramirez
H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR and
Tomic-Canic M: Epithelialization in Wound Healing: A comprehensive
review. Adv Wound Care (New Rochelle). 3:445–464. 2014. View Article : Google Scholar
|
|
7
|
Miranda JP, Filipe E, Fernandes AS,
Almeida JM, Martins JP, De la Fuente A, Abal M, Barcia RN, Cruz P,
Cruz H, et al: The human umbilical cord tissue-derived MSC
population UCX(®) promotes early motogenic effects on keratinocytes
and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when
transplanted in vivo. Cell Transplant. 24:865–877. 2015. View Article : Google Scholar
|
|
8
|
Walter MN, Wright KT, Fuller HR, MacNeil S
and Johnson WE: Mesenchymal stem cell-conditioned medium
accelerates skin wound healing: An in vitro study of fibroblast and
keratinocyte scratch assays. Exp Cell Res. 316:1271–1281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Antonyak MA, Singh G and Cerione
RA: A mechanism for the upregulation of EGF receptor levels in
glioblastomas. Cell Reports. 3:2008–2020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmad I, Iwata T and Leung HY: Mechanisms
of FGFR-mediated carcinogenesis. Biochim Biophys Acta.
1823:850–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turunen A and Syrjänen S: Extracellular
calcium regulates keratinocyte proliferation and HPV 16 E6 RNA
expression in vitro. APMIS. 122:781–789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Micallef L, Belaubre F, Pinon A,
Jayat-Vignoles C, Delage C, Charveron M and Simon A: Effects of
extracellular calcium on the growth-differentiation switch in
immortalized keratinocyte HaCaT cells compared with normal human
keratinocytes. Exp Dermatol. 18:143–151. 2009. View Article : Google Scholar
|
|
14
|
Conrad DR: Calcium in Cell Culture.
(Technical note). Sigma-Aldrich. http://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/calcium.html.
|
|
15
|
Zachar V, Rasmussen JG and Fink T:
Isolation and growth of adipose tissue-derived stem cells. Methods
Mol Biol. 698:37–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasmussen JG, Riis SE, Frøbert O, Yang S,
Kastrup J, Zachar V, Simonsen U and Fink T: Activation of
protease-activated receptor 2 induces VEGF independently of HIF-1.
PLoS One. 7:e460872012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Pilgaard L, Chase LG, Boucher S,
Vemuri MC, Fink T and Zachar V: Defined xenogeneic-free and hypoxic
environment provides superior conditions for long-term expansion of
human adipose-derived stem cells. Tissue Eng Part C Methods.
18:593–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fink T, Rasmussen JG, Lund P, Pilgaard L,
Soballe K and Zachar V: Isolation and expansion of adipose-derived
stem cells for tissue engineering. Front Biosci (Elite Ed). 3. pp.
256–263. 2011, View
Article : Google Scholar
|
|
19
|
Prasad M, Zachar V, Fink T and Pennisi CP:
Moderate hypoxia influences potassium outward currents in
adipose-derived stem cells. PLoS One. 9:e1049122014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gebäck T, Schulz MMP, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
21
|
Vespa A, Darmon AJ, Turner CE, D'Souza SJA
and Dagnino L: Ca2+-dependent localization of
integrin-linked kinase to cell junctions in differentiating
keratinocytes. J Biol Chem. 278:11528–11535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cerqueira MT, Pirraco RP and Marques AP:
Stem cells in skin wound healing: Are we there yet? Adv Wound Care
(New Rochelle). 5:164–175. 2016. View Article : Google Scholar
|
|
23
|
Seo BF, Kim KJ, Kim MK and Rhie JW: The
effects of human keratinocyte coculture on human adipose-derived
stem cells. Int Wound J. 13:630–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bikle DD, Xie Z and Tu CL: Calcium
regulation of keratinocyte differentiation. Expert Rev Endocrinol
Metab. 7:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreno-Bueno G, Peinado H, Molina P,
Olmeda D, Cubillo E, Santos V, Palacios J, Portillo F and Cano A:
The morphological and molecular features of the
epithelial-to-mesenchymal transition. Nat Protoc. 4:1591–1613.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie. 95:2222–2228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peplow PV and Chatterjee MP: A review of
the influence of growth factors and cytokines in in vitro human
keratinocyte migration. Cytokine. 62:1–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zachar V, Duroux M, Emmersen J, Rasmussen
JG, Pennisi CP, Yang S and Fink T: Hypoxia and adipose-derived stem
cell-based tissue regeneration and engineering. Expert Opin Biol
Ther. 11:775–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Gao J, Yuan Y, Chang Q, Liao Y and
Lu F: Hypoxia preconditioned human adipose derived mesenchymal stem
cells enhance angiogenic potential via secretion of increased VEGF
and bFGF. Cell Biol Int. 37:551–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An HY, Shin HS, Choi JS, Kim HJ, Lim JY
and Kim YM: Adipose mesenchymal stem cell secretome modulated in
hypoxia for remodeling of radiation-induced salivary gland damage.
PLoS One. 10:e01418622015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Riis S, Stensballe A, Emmersen J, Pennisi
CP, Birkelund S, Zachar V and Fink T: Mass spectrometry analysis of
adipose-derived stem cells reveals a significant effect of hypoxia
on pathways regulating extracellular matrix. Stem Cell Res Ther.
7:522016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riis S, Zachar V, Boucher S, Vemuri MC,
Pennisi CP and Fink T: Critical steps in the isolation and
expansion of adipose-derived stem cells for translational therapy.
Expert Rev Mol Med. 17:e112015. View Article : Google Scholar : PubMed/NCBI
|