Introduction
Endothelial progenitor cells (EPCs), which were
firstly identified in adult human peripheral blood (PB) in 1997
(1), exhibit the capacity for
endothelial differentiation (2)
and angiogenic growth factor and cytokine secretion (3,4).
As a novel therapeutic target for vascular diseases (5), they can incorporate into ischemic
tissue and play an important role in vasculogenesis for
physiological or pathological neovascularization (6-9).
In addition, EPCs have been reported to play an important role in
thrombosis resolution (10–13).
However, EPCs are present in very low numbers
(0.1–0.01% of mononuclear cells). Moreover, some risk factors of
vascular diseases, for example age, smoking, coronary artery
disease (CAD), atherosclerosis and diabetes mellitus (DM) (14–16), may reduce the number of EPCs in PB
(10). EPCs from CAD and DM
patients exhibit decreased migration as well as mobilization
capacity (16–18). Migration is essential for EPCs to
mobilize to the sites of ischemic tissue. These factors decrease
the numbers of EPCs in circulation and subsequently impair the
capacity of vascular endothelium repair. Thus, how to promote EPCs
to mobilize from the bone marrow (BM) niche and migrate into
special sites remains a current issue.
Metformin, an activator of AMPK and an inhibitor of
mTOR (19), is widely endorsed as
initial therapy for type 2 diabetes because of its low cost, safety
profile, and potential cardiovascular benefits (20). It has been demonstrated that
metformin improves endothelial function and enhances the
neovascularization of EPCs (21,22). At the same time, in our previous
study, metformin promoted EPC differentiation (23). But, the exact effect of metformin
on circulating EPC migration is still poorly understood. In this
study, we investigated the effect of metformin on the migration of
EPCs and explored the possible mechanisms.
Materials and methods
Antibodies and reagents
Antibodies for phospho(p)-AMPK (#4185), AMPK
(#2532), p-Akt (#9018), Akt (#9272), mTOR (#2972), LC3B (#2775)
were from Cell Signa ling Technology (Danvers, MA, USA). Antibodies
for p62 (ab56416), matrix metalloproteinase-2 (MMP-2; ab92536) and
MMP-9 (ab76003) were obtained from Abcam, Inc. (Cambridge, MA,
USA). Antibody for p-mTOR (sc-293132) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Metformin, AMPK
inhibitor compound C (CC), mitomycin C, and the antibody for
β-actin (A3854) were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Isolation and characterization of human
EPCs
Mononuclear cells were isolated from PB using the
Histopaque density centrifugation method. Fresh blood (50–100 ml)
was collected from volunteer donors by venipuncture and
anticoagulated with heparin. The Institutional Review Board at the
Second Affiliated Hospital of Soochow University approved all
protocols, and informed consent was obtained from all adult
donors.
Human mononuclear cells (MNCs) were isolated as
previously described (24).
Briefly, the anticoagulated blood was diluted 1:1 with
phosphate-buffered saline (PBS). Mononuclear cells, isolated by
density gradient centrifugation using Lymphocyte Separation
Medium-LSM™ (MP Biomedicals, Santa Ana, CA, USA), were seeded onto
collagen I-coated plates (Invitrogen, Carlsbad, CA, USA) at a
density of 2.5×106 cells/cm2 and cultured
with Endothelial Cell Growth Medium-2 (EGM-2MV; Lonza,
Walkersville, MD, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco, Carlsbad, CA, USA) and maintained in a 37°C/5%
CO2 incubator. Medium was changed daily for 7 days and
then every other day until the first passage. Cells within 10
passages were used for the following experiments.
EPC surface molecule analysis
EPC surface molecule analysis was performed as
previous described by flow cytometry (25,26). Cells were detached with EDTA and
labeled for 20 min at 4°C at manufacturer-recommended
concentrations with fluorescent antibodies, including
anti-VEGFR-2-PE (#560872), anti-CD45-FITC (#560976), anti-CD14-FITC
(#561710), anti-CD133-PE (#130-080-801), and anti-CD31-PE (#566125)
and anti-CD34-FITC (#560942). Fluorescent isotype-matched
antibodies were used as negative controls. All antibodies were
obtained from Becton-Dickinson (Franklin Lakes, NJ, USA), except
anti-CD133-PE (Miltenyi Biotec, Auburn, CA, USA). Cells were
washed, paraformaldehyde-fixed (Tosoumis, Rockville, MD, USA), and
analyzed on a FACSCalibur Instrument (Becton-Dickinson) with 10,000
events stored. Other commonly accepted criteria for identifying
EPCs, for example, uptake of DiI-acLDL and FITC-UEA-I binding, were
also performed.
To evaluate the effect of metformin, identified EPCs
were incubated with 10 mM metformin for 24 h. In the setting of
mechanism verification, AMPK inhibitor compound C was added at a
concentration of 10 µM.
Wound healing migration assay
A wound healing migration assay with EPCs was
performed following previously published methods (27). Briefly, EPCs (4×106
cells) with 10 mM metformin were resuspended and seeded into a
6-well plate, and grew until ~100% confluence. Cells were treated
with 10 µg/ml mitomycin C for 3 h to block cell
proliferation. A linear scratch was made by using a 10-µl
pipette tip. After washing with PBS twice, the cells were incubated
with serum free EGM-2MV medium for specific times. Images were
taken at 0 and 24 h at ×40 magnification and the wound size was
measured in 3 wells/group.
Matrigel invasion assay
EPC migration assay was performed in a Transwell
system as described previously (28). A Trans well chamber was used
(8-µm, 24-well plate). In the invasion assay, the insert
membranes were coated with diluted Matrigel. EPCs (2×105
cells) were resuspended in 200 µl serum-free EGM-2MV medium
and seeded into the upper chamber after treatment with 10
µg/ml mitomycin C for 3 h. The lower chamber was filled with
EGM-2MV medium supplemented with 10% FBS, vehicle control or 10 mM
metformin. After incubation at 37°C in 5% CO2 for 24 h,
the cells were stained with crystal violet and the cell number in
each well was counted in 3 randomly picked fields (magnification,
×200) under a light microscope. All the experiments were performed
in triplicate.
Real-time reverse transcription
polymerase chain reaction (RT-PCR)
After EPCs were treated as described above, total
cellular RNA was extracted using TRIzol reagent (Invitrogen).
Real-time RT-PCR was carried out using a SYBR®-Green
qPCR Mix (Thermo Scientific, MBI Fermentas, Waltham, MA, USA) and a
Roche LightCycler 480 (Roche, Basel, Switzerland). Expression of
the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was
assessed simultaneously in all samples as an internal control.
Relative gene expression was determined by the 2−ΔΔCq
method (29). The following
primers were used: MMP-2 sense, 5′-GGT TCC CCT GTT CAC TCT ACT TAG
C-3′ and antisense, 5′-CGG CTT GGT TTT CCT CCA T-3′; MMP-9 sense,
5′-CCC GGA GTG AGT TGA ACC A-3′ and antisense, 5′-AGG GCA CTG CAG
GAT GTC A-3′; GAPDH sense, 5′-GGT GGT CTC CTC TGA CTT CAA CA-3′ and
antisense, 5′-GTG GTC GTT GAG GGC AAT G-3′.
Western blot analysis
EPCs (1×106 cells) were lysed in RIPA
buffer, followed by high-speed centrifugation and bicinchoninic
acid quantification. Cellular proteins were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride membranes. After
blocking with 5% non-fat milk Tris-buffered saline-Tween-20
(TBS-T), the membranes were incubated with primary antibodies
against AMPK, p-AMPK, Akt, p-Akt, p-mTOR, mTOR, MMP-2, MMP-9, LC-3B
and p62. Appropriate horseradish peroxidase-conjugated secondary
antibodies were applied. β-actin (Sigma-Aldrich) was used as the
loading control. The protein bands were detected with Super Signal
West PicoChemiluminescent Substrate (Pierce, Rockford, IL, USA) on
X-ray film (Kodak, Tokyo, Japan).
SDS-PAGE gelatin zymography
The activities of MMP-2 and MMP-9 were determined by
gelatin zymography as previously described (30,31). Ten micrograms of each sample was
loaded onto a 8% SDS-polyacrylamide gel containing 1 mg/ml gelatin,
electrophoresed, renatured, and developed. Gels were stained with
0.25% Coomassie Brilliant Blue R-250 and destained in the same
solution without dye. Gelatinase activity was visualized as clear
bands against the blue-stained gelatin background and analyzed in a
computer system. Three individual experiments were conducted with
independent protein samples.
Statistical analyses
All statistical analyses were carried out using SPSS
v21 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ±
standard deviation (SD). Student's t-test or one way ANOVA was
utilized to examine differences between two groups or multiple
group comparison. P<0.05 was considered as statistically
significant.
Results
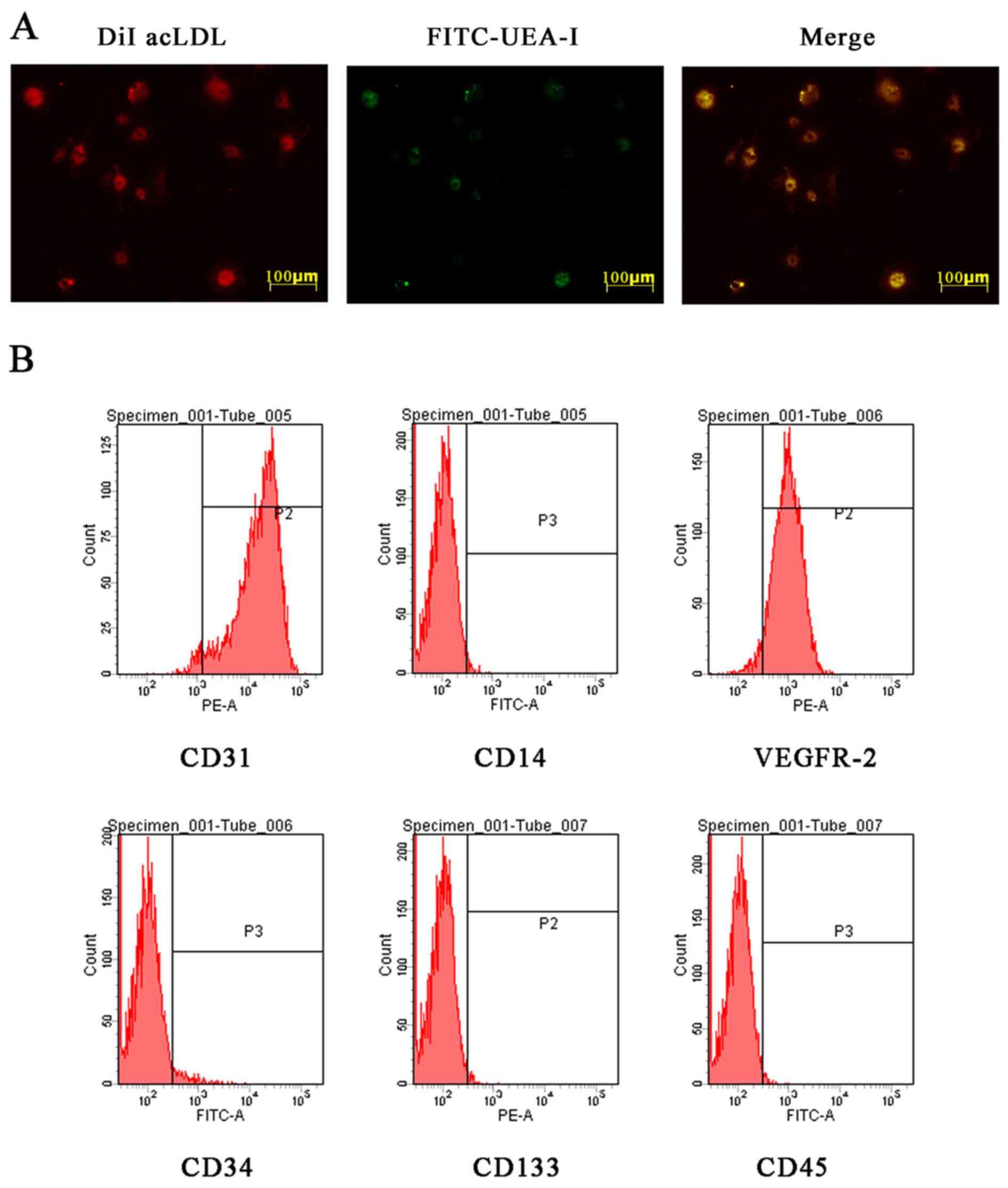
EPC characterization
Isolated MNCs were cultured in EGM-2MV medium
supplemented with 10% FBS and growth factors to be induced into
EPCs. EPCs were identified by morphology, fluorescence
double-staining and flow cytometry. Most adherent cells were double
stained by DiI-AcLDL and FITC-UEA-I (Fig. 1A). The flow cytometric analysis
matched with the previously described EPC phenotype (25,26) (Fig.
1B). The results of identification of these cells were
consistent with the characterization of late-outgrowth EPCs.
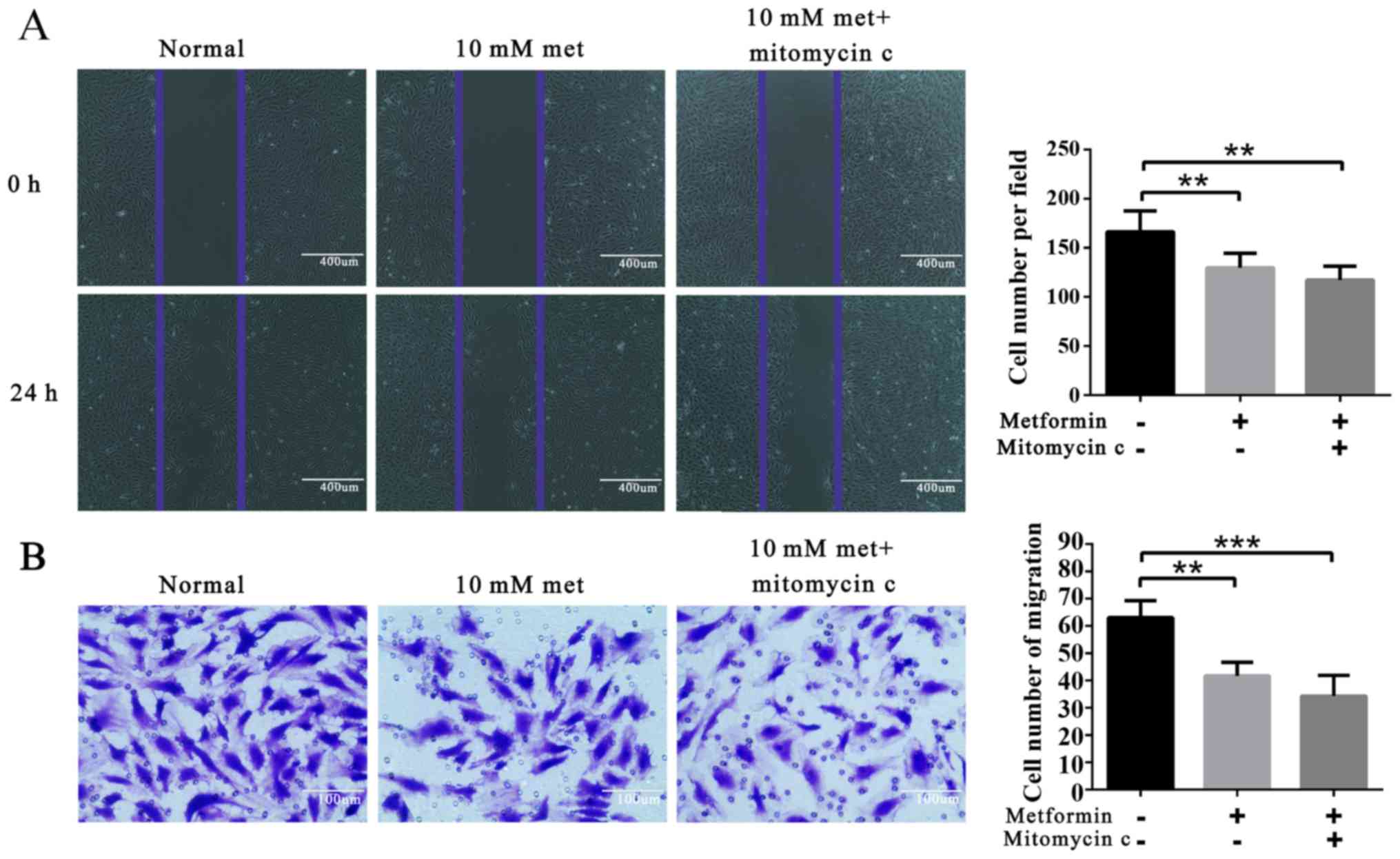
Metformin attenuates EPC migration
Wound healing migration and Matrigel invasion assays
were employed. The results showed that metformin treatment
significantly decreased EPC migration (Fig. 2).
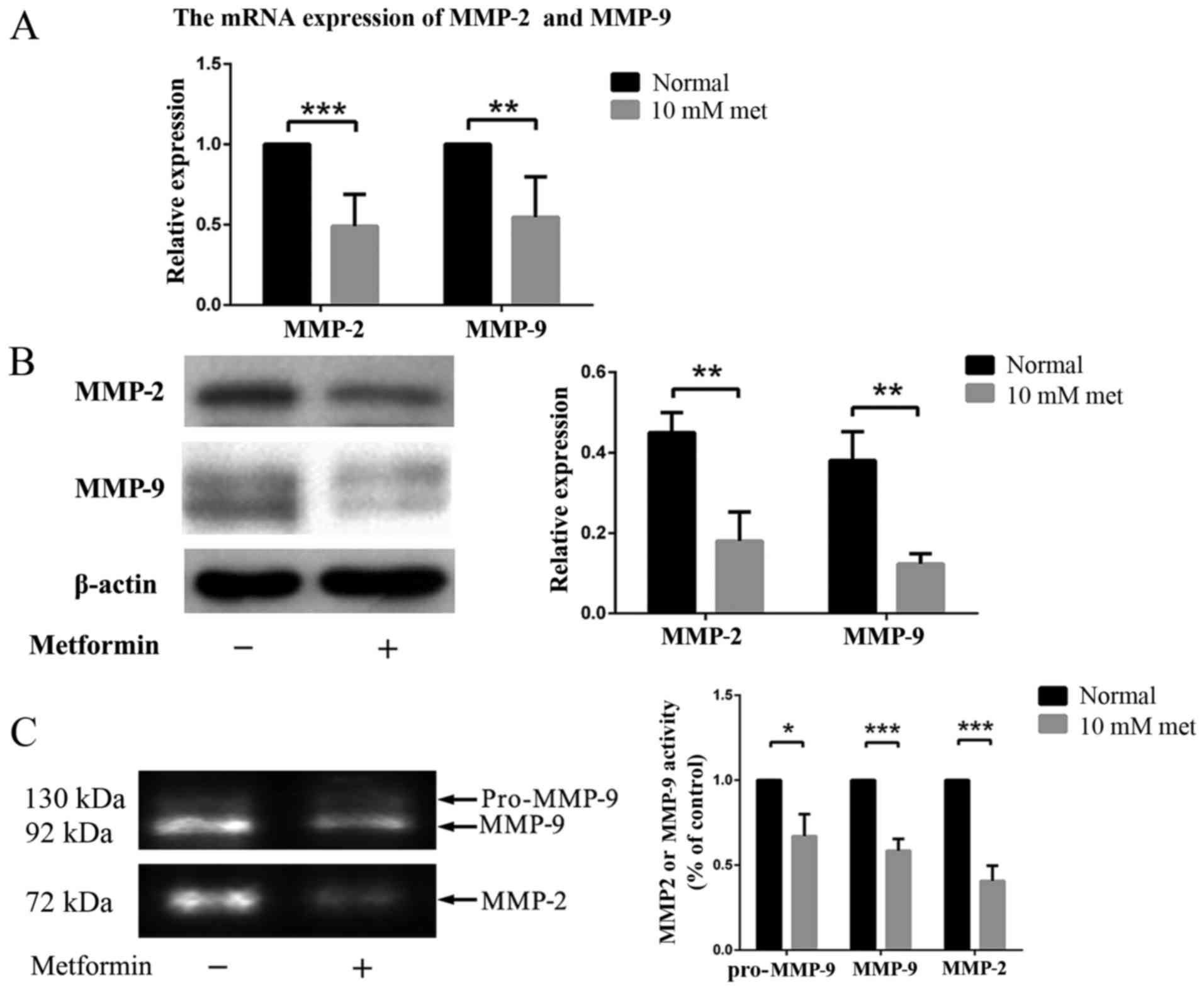
Metformin regulates EPC migration by
influencing MMP-2 and MMP-9
Moreover, we examined the expression levels of of
MMP-2 and MMP-9 in EPCs with or without metformin treatment via
RT-PCR and western blot analysis. The results revealed decreased
expression of MMP-2 and MMP-9 in EPCs treated with metformin
(Fig. 3A and B), indicating that
a decreased activity of gelatinase and fibrinolysis, may contribute
to this phenomenon.
The expression of MMP-2 and MMP-9 in EPCs at
different conditions was also examined by zymography. Three bands
were found in the gel, representing pro-MMP-9, active MMP-9 and
active MMP-2. The expression levels of both MMP-2 and MMP-9 were
significantly decreased in the EPCs after incubation with metformin
(Fig. 3C).
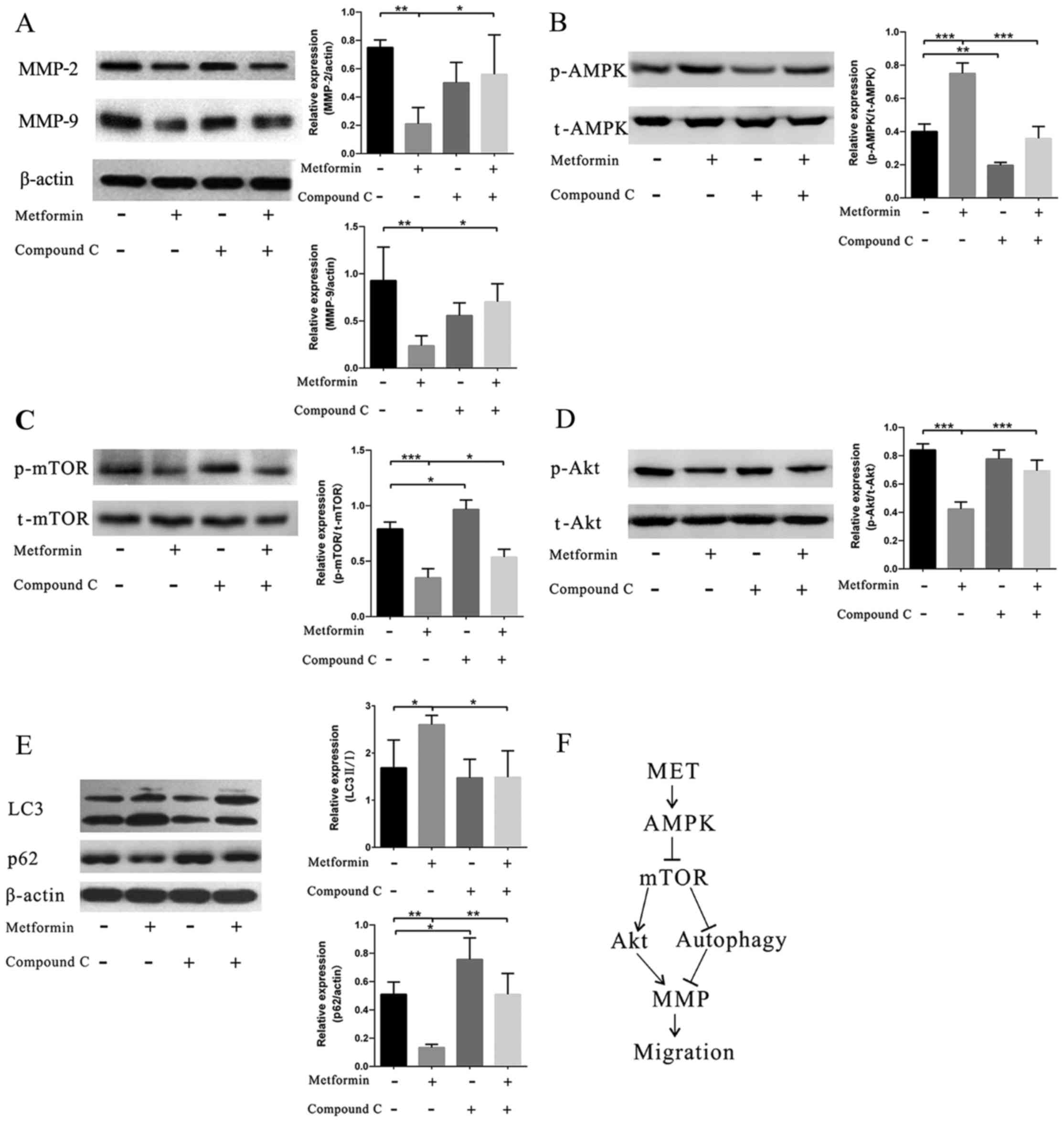
Metformin regulates migration via the
AMPK/mTOR/autophagy pathway
After metformin treatment, an increased level in
AMPK phosphorylation was observed (Fig. 4B), whereas the addition of AMPK
inhibitor CC into the system reversed the effect of metformin.
Correspondingly, MMP-2 and MMP-9 exhibited reversed changes
compared with the levels of AMPK phosphorylation (Fig. 4A). Moreover, we also found
decreased levels of mTOR and Akt phosphorylation (Fig. 4C and D). Furthermore, the
expression of autophagy components in the EPCs was tested. An
increased level of LC3B and a decreased level of p62 showed that
the autophagy pathway was involved in the effect of metformin
(Fig. 4E).
Discussion
EPCs, as promising therapeutic targets for vascular
diseases, have been verified to improve vascular function in
substantial animal and preliminary human studies (32,33). Migration is essential for EPCs to
mobilize to the sites of ischemic tissue. For example, in mice,
EPCs were increased 24 h after DVT induction, peaking 48 h
thereafter (12). However, the
number of EPCs in DM patients was decreased, which may result from
attenuated migration as well as mobilization capacity (34,35). Metformin is widely endorsed as
initial therapy for type 2 diabetes. In a study by Chen et
al, metformin enhanced the number of circulating EPCs (36). Yet, in another study, Esposito
et al (37) found no
effects on the number of circulating EPCs when newly diagnosed type
2 diabetes patients were treated with metformin. Thus, the exact
role of metformin on EPC migration has not been fully elaborated.
In addition, there are no studies in vitro to explore the
effect of metformin on EPC migration. Thus, in the present study,
we aimed to investigate the effect of metformin on the migration of
EPCs and explore the possible mechanisms. We found that metformin
treatment attenuated EPC migration via regulating the expression of
MMP-2 and MMP-9. In addition, the AMPK/mTOR/autophagy pathway was
found to be involved in the effect of metformin.
It has been demonstrated that metformin is an
agonist of AMPK (19), which is a
member of a metabolite-sensing protein kinase family and has the
capacity for the regulation of cell differentiation and migration
(38,39). Augmented AMPK activity inhibits
cell migration (39). This may be
related to the inhibition of MMPs regulated by AMPK (40,41). MMPs are reported to play an
important role in extracellular matrix degradation thereby
facilitating the migration of leukocytes, monocytes and other types
of cells in the setting of vascular diseases (42). In previous studies, it was found
that metformin could regulate endothelial cell and tumor cell
migration by inhibiting MMP expression. For example, metformin
inhibited the migration by attenuating the expression of MMP-2 and
MMP-9 in human umbilical vein endothelial cells partially through
an AMPK-dependent pathway (43).
Hwang et al (44) found
that metformin blocked the migration and invasion of tumor cells by
inhibition of MMP-9 activation. Fang et al (45) also reported that metformin reduced
A498 cell migration and invasion in vitro by decreasing
MMP-2. In the present study, we found that metformin inhibited EPC
migration by reducing the expression and impairing the activity of
MMP-2 and MMP-9, which was consistent with previous studies in
other cells. We also detected the expression of AMPK, mTOR as well
as Akt and found that metformin promoted AMPK phosphorylation but
attenuated mTOR and Akt phosphorylation. The latter two protein
kinases are involved in migration and MMP expression (46,47). Meanwhile, these changes in protein
kinases could result in an increased level of autophagy (Fig. 4F).
Metformin could induce autophagy by simultaneously
activating AMPK and inhibiting mTORC1 in both AMPK-independent and
AMPK-dependent approaches (48,49). According to previous research,
autophagy plays an important role in cell migration regulation but
in a controversial manner. On the one hand, autophagy enhances
production of various cytokines and thereby facilitates migration
and invasion of lung cancer cells (50). However, on the other hand,
autophagy inhibits cell migration and invasion by slowing down the
lysosomal degradation of certain molecules or proteinases, such as
MMPs and integrins, which are critical for cell migration (28,51). In our previous study (28), we found that the different roles
of autophagy in regulating cell migration may be associated with
the fundamental level of autophagy and the extent of autophagy
regulation.
There are a few limitations in the present study.
First, we only collected EPCs from normal healthy subjects to
examine the effect of metformin. The effect of metformin on the
EPCs derived from patients with DM or CAD has not yet been
examined. Second, the role of metformin in EPC migration under
hyperglycemia should be performed in the future. Appropriate animal
model studies should also be carried out to verify the functional
change of EPCs in vivo. Therefore, further studies are
needed to address these issues.
In conclusion, we here showed that metformin
treatment regulated the migration of EPCs. The mechanisms may be
associated with the AMPK/mTOR/autophagy-related pathway. The latter
attenuates the expression of MMP-2 and MMP-9 (Fig. 4F).
Acknowledgments
This study was supported by grants from the
Scientific and Technological Research Projects of Jiangsu Province
(no. BL2014043), Graduate Research and Innovation Program in
Colleges and Universities of Jiangsu Province (no.
KYLX15_1202).
References
|
1
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peichev M, Naiyer AJ, Pereira D, Zhu Z,
Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al:
Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells
identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2000.PubMed/NCBI
|
|
3
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamihata H, Matsubara H, Nishiue T,
Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi
E, et al: Implantation of bone marrow mononuclear cells into
ischemic myocardium enhances collateral perfusion and regional
function via side supply of angioblasts, angiogenic ligands, and
cytokines. Circulation. 104:1046–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Kawamoto A and Masuda H:
Concise review: Circulating endothelial progenitor cells for
vascular medicine. Stem Cells. 29:1650–1655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C,
Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, et al: Evidence
for circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
7
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi T, Kalka C, Masuda H, Chen D,
Silver M, Kearney M, Magner M, Isner JM and Asahara T: Ischemia-and
cytokine-induced mobilization of bone marrow-derived endothelial
progenitor cells for neovascularization. Nat Med. 5:434–438. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li WD and Li XQ: Endothelial progenitor
cells accelerate the resolution of deep vein thrombosis. Vascul
Pharmacol. 83:10–16. 2016. View Article : Google Scholar
|
|
11
|
Wang W, Li C, Li W, Kong L, Qian A, Hu N,
Meng Q and Li X: MiR-150 enhances the motility of EPCs in vitro and
promotes EPCs homing and thrombus resolving in vivo. Thromb Res.
133:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alessio AM, Beltrame MP, Nascimento MC,
Vicente CP, de Godoy JA, Silva JC, Bittar LF, Lorand-Metze I, de
Paula EV and Annichino-Bizzacchi JM: Circulating progenitor and
mature endothelial cells in deep vein thrombosis. Int J Med Sci.
10:1746–1754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuzzolo ER, Iachininoto MG and Teofili L:
Endothelial progenitor cells and thrombosis. Thromb Res.
129:309–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar
|
|
15
|
Michaud SE, Dussault S, Haddad P, Groleau
J and Rivard A: Circulating endothelial progenitor cells from
healthy smokers exhibit impaired functional activities.
Atherosclerosis. 187:423–432. 2006. View Article : Google Scholar
|
|
16
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menegazzo L, Albiero M, Avogaro A and
Fadini GP: Endothelial progenitor cells in diabetes mellitus.
Biofactors. 38:194–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C, et al: Circulating endothelial progenitor cells are
reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ
and McGuire DK: Metformin in patients with type 2 diabetes and
kidney disease: A systematic review. JAMA. 312:2668–2675. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mather KJ, Verma S and Anderson TJ:
Improved endothelial function with metformin in type 2 diabetes
mellitus. J Am Coll Cardiol. 37:1344–1350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Desouza CV: Does drug therapy reverse
endothelial progenitor cell dysfunction in diabetes? J Diabetes
Complications. 27:519–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li WD, Du XL, Qian AM, Hu N, Kong LS, Wei
S, Li CL and Li XQ: Metformin regulates differentiation of bone
marrow-derived endothelial progenitor cells via multiple
mechanisms. Biochem Biophys Res Commun. 465:803–809. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ingram DA, Mead LE, Tanaka H, Meade V,
Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D and Yoder
MC: Identification of a novel hierarchy of endothelial progenitor
cells using human peripheral and umbilical cord blood. Blood.
104:2752–2760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood 'endothelial progenitor cells' are derived from
monocyte/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
James MF, Beauchamp RL, Manchanda N,
Kazlauskas A and Ramesh V: A NHERF binding site links the betaPDGFR
to the cytoskeleton and regulates cell spreading and migration. J
Cell Sci. 117:2951–2961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WD, Hu N, Lei FR, Wei S, Rong JJ,
Zhuang H and Li XQ: Autophagy inhibits endothelial progenitor cells
migration via the regulation of MMP-2, MMP-9 and uPA under normoxia
condition. Biochem Biophys Res Commun. 466:376–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji Y, Strawn TL, Grunz EA, Stevenson MJ,
Lohman AW, Lawrence DA and Fay WP: Multifaceted role of plasminogen
activator inhibitor-1 in regulating early remodeling of vein bypass
grafts. Arterioscler Thromb Vasc Biol. 31:1781–1787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oku N, Sasabe E, Ueta E, Yamamoto T and
Osaki T: Tight junction protein claudin-1 enhances the invasive
activity of oral squamous cell carcinoma cells by promoting
cleavage of laminin-5 gamma2 chain via matrix metalloproteinase
(MMP)-2 and membrane-type MMP-1. Cancer Res. 66:5251–5257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Tanaka K, Chiba Y, Kimura T, Morioka
K, Uesaka T, Ihaya A, Sasaki M, Tsuda T and Yamada N: Role of MMPs
and plasminogen activators in angiogenesis after transmyocardial
laser revascularization in dogs. Am J Physiol Heart Circ Physiol.
284:H23–H30. 2003. View Article : Google Scholar
|
|
32
|
Xu S, Zhu J, Yu L and Fu G: Endothelial
progenitor cells: Current development of their paracrine factors in
cardiovascular therapy. J Cardiovasc Pharmacol. 59:387–396. 2012.
View Article : Google Scholar
|
|
33
|
Resch T, Pircher A, Kähler CM, Pratschke J
and Hilbe W: Endothelial progenitor cells: Current issues on
characterization and challenging clinical applications. Stem Cell
Rev. 8:926–939. 2012. View Article : Google Scholar
|
|
34
|
Loomans CJ, de Koning EJ, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar
|
|
35
|
Barthelmes D, Irhimeh MR, Gillies MC,
Karimipour M, Zhou M, Zhu L and Shen WY: Diabetes impairs
mobilization of mouse bone marrow-derived Lin(−)/VEGF-R2(+)
progenitor cells. Blood Cells Mol Dis. 51:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen LL, Liao YF, Zeng TS, Yu F, Li HQ and
Feng Y: Effects of metformin plus gliclazide compared with
metformin alone on circulating endothelial progenitor cell in type
2 diabetic patients. Endocrine. 38:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Esposito K, Maiorino MI, Di Palo C,
Gicchino M, Petrizzo M, Bellastella G, Saccomanno F and Giugliano
D: Effects of pioglitazone versus metformin on circulating
endothelial microparticles and progenitor cells in patients with
newly diagnosed type 2 diabetes - a randomized controlled trial.
Diabetes Obes Metab. 13:439–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Han Y, Pang W, Li C, Xie X, Shyy JY
and Zhu Y: AMP-activated protein kinase promotes the
differentiation of endothelial progenitor cells. Arterioscler
Thromb Vasc Biol. 28:1789–1795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan Y, Tsukamoto O, Nakano A, Kato H,
Kioka H, Ito N, Higo S, Yamazaki S, Shintani Y, Matsuoka K, et al:
Augmented AMPK activity inhibits cell migration by phosphorylating
the novel substrate Pdlim5. Nat Commun. 6:61372015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park SY, Jung CH, Song B, Park OJ and Kim
YM: Pro-apoptotic and migration-suppressing potential of EGCG, and
the involvement of AMPK in the p53-mediated modulation of VEGF and
MMP-9 expression. Oncol Lett. 6:1346–1350. 2013.PubMed/NCBI
|
|
41
|
Lee GR, Jang SH, Kim CJ, Kim AR, Yoon DJ,
Park NH and Han IS: Capsaicin suppresses the migration of
cholangiocarcinoma cells by downregulating matrix
metalloproteinase-9 expression via the AMPK-NF-κB signaling
pathway. Clin Exp Metastasis. 31:897–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Esfahanian N, Shakiba Y, Nikbin B, Soraya
H, Maleki-Dizaji N, Ghazi-Khansari M and Garjani A: Effect of
metformin on the proliferation, migration, and MMP-2 and -9
expression of human umbilical vein endothelial cells. Mol Med Rep.
5:1068–1074. 2012.PubMed/NCBI
|
|
44
|
Hwang YP and Jeong HG: Metformin blocks
migration and invasion of tumour cells by inhibition of matrix
metalloproteinase-9 activation through a calcium and protein kinase
Calpha-dependent pathway:
Phorbol-12-myristate-13-acetate-induced/extracellular
signal-regulated kinase/activator protein-1. Br J Pharmacol.
160:1195–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang Z, Xu X, Zhou Z, Xu Z and Liu Z:
Effect of metformin on apoptosis, cell cycle arrest migration and
invasion of A498 cells. Mol Med Rep. 9:2251–2256. 2014.PubMed/NCBI
|
|
46
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014.PubMed/NCBI
|
|
47
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tomic T, Botton T, Cerezo M, Robert G,
Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder
JM, et al: Metformin inhibits melanoma development through
autophagy and apoptosis mechanisms. Cell Death Dis. 2:e1992011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H and Liu Z: Autophagy facilitates TLR4- and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2014. View Article : Google Scholar
|
|
51
|
Tuloup-Minguez V, Hamaï A, Greffard A,
Nicolas V, Codogno P and Botti J: Autophagy modulates cell
migration and β1 integrin membrane recycling. Cell Cycle.
12:3317–3328. 2013. View Article : Google Scholar : PubMed/NCBI
|