Osteopontin (OPN) is a bone associated,
extracellular matrix glycosylated phosphoprotein which is produced
by several cell types, including osteoblasts, osteoclasts, immune
cells, endothelial cells, epithelial cells and extra-osseous cells
(skin, kidney and lung) (1–3).
Due to differences in post-translational modification (PTM)
(phosphorylation, glycosylation, sulfation and proteolysis) from
different cellular sources, OPN has a molecular weight ranging from
41 to 75 kDa, which may have a cell type-specific structure and
function (4–7). OPN plays a major role in various
normal physiological processes, including bone remodelling,
immune-regulation, inflammation and vascularisation (8,9).
In addition, OPN has also been shown to be involved in
carcinogenesis with multi-functional activities (10–12).
OPN is involved in a series of biological functions
through interactions with different integrins and CD44. Therefore,
OPN is classified as a member of the 'small integrin-binding ligand
N-linked glycoproteins' (SIBLINGs) together with other molecules,
including bone sialoprotein (BSP), dentin matrix protein 1 (DMP1),
dentin sialophosphoprotein (DSPP) and matrix extracellular
phosphoglycoprotein (MEPE) (13).
Two critical integrin binding sequences of OPN have been
identified: arginine-glycine-aspartic acid (RGD) and
serine-valine-valine-tyrosine-glutamate-leucine-arginine (SVVYGLR).
OPN interacts mainly with various αv (particularly αvβ1, αvβ3,
αvβ5) integrin receptors via the classical RGD sequence, and
interacts with α9β1, α4β1, α4β7 via SVVYGLR (14–16). In addition, it also interacts with
the CD44 splice variants, CD44v3, CD44v6 and CD44v7, via the
C-terminal fragment calcium binding site (17–20). These properties of OPN induce the
activation of signal transduction pathways, leading to cell
proliferation, adhesion, invasion and migration, which have been
demonstrated by both in vitro and in vivo models
(21–23). The binding of OPN to integrins and
CD44 initiates a downstream signalling cascade via the PI3K/AKT
signalling pathway leading to NF-κB mediated cell proliferation and
survival (24–26). In additon, through the
Ras/Raf/MEK/ERK signalling pathway, an OPN-integrin complex and
subsequent induction of AP-1-dependent gene expression,
urokinase-type plasminogen activator (uPA) and matrix
metalloproteinases (MMPs) confer a metastatic phenotype on some
cancer cell types (27–29). Induced by vascular endothelial
growth factor (VEGF), OPN and certain integrins are able to promote
angiogenesis through enhanced endothelial cell migration,
proliferation and the subsequent formation of capillaries, which
are all essential requirements for the process of angiogenesis
(30,31).
OPN has been shown to correlate with tumourigenesis,
as well as with the progression and metastasis of different
malignancies in both experimental and clinical studies (Table I). The upregulation of OPN
expression has been identified in a variety of human cancers,
including breast, prostate, lung, stomach, pancreatic and
colorectal cancer, glioma and melanoma (Table I).
In clinical investigations, elevated OPN levels have
been shown to correlate with increased tumour burden (stage, grade
and tumour size), a poor prognosis and reduced survival, although
discrepancies remain. For example, the increased expression of OPN
in plasma and tumour tissues has been identified in breast cancer
patients and has been shown to be associated with metastatic
disease and decreased survival (35). Similarly, in advanced gastric
cancer, patients with OPN-positive cancer (as determined by
immunohistochemistry) have a decrease in their 5-year survival,
when compared with those with OPN-negative cancer (40). In colorectal cancer (CRC)
patients, increased OPN mRNA levels have been shown to
significantly correlate with stage, lymph node metastasis and
lymphatic or venous invasion, as well as with lower disease-free
and overall survival rates (41,42). A study on patients with pancreatic
ductal adenocarcinoma (PDAC) demonstrated that serum levels of OPN
are elevated with advanced tumour grades (44). Elevated OPN levels have also been
strongly associated with increased stage, grade and tumour size in
melanoma patients (52). In early
stage non-small cell lung cancer (NSCLC) patients high OPN plasma
levels were observed which were reduced after surgical tumour
resection. However, in those patients which showed recurrence after
surgery OPN plasma levels re-elevated, indicating that OPN is not
only a potential diagnostic marker in NSCLC, but also has potential
as a tool for detecting tumour recurrence after treatment (53). Thus, OPN may be a useful biomarker
to monitor cancer progression and a significant predictor of poor
prognosis and survival rates.
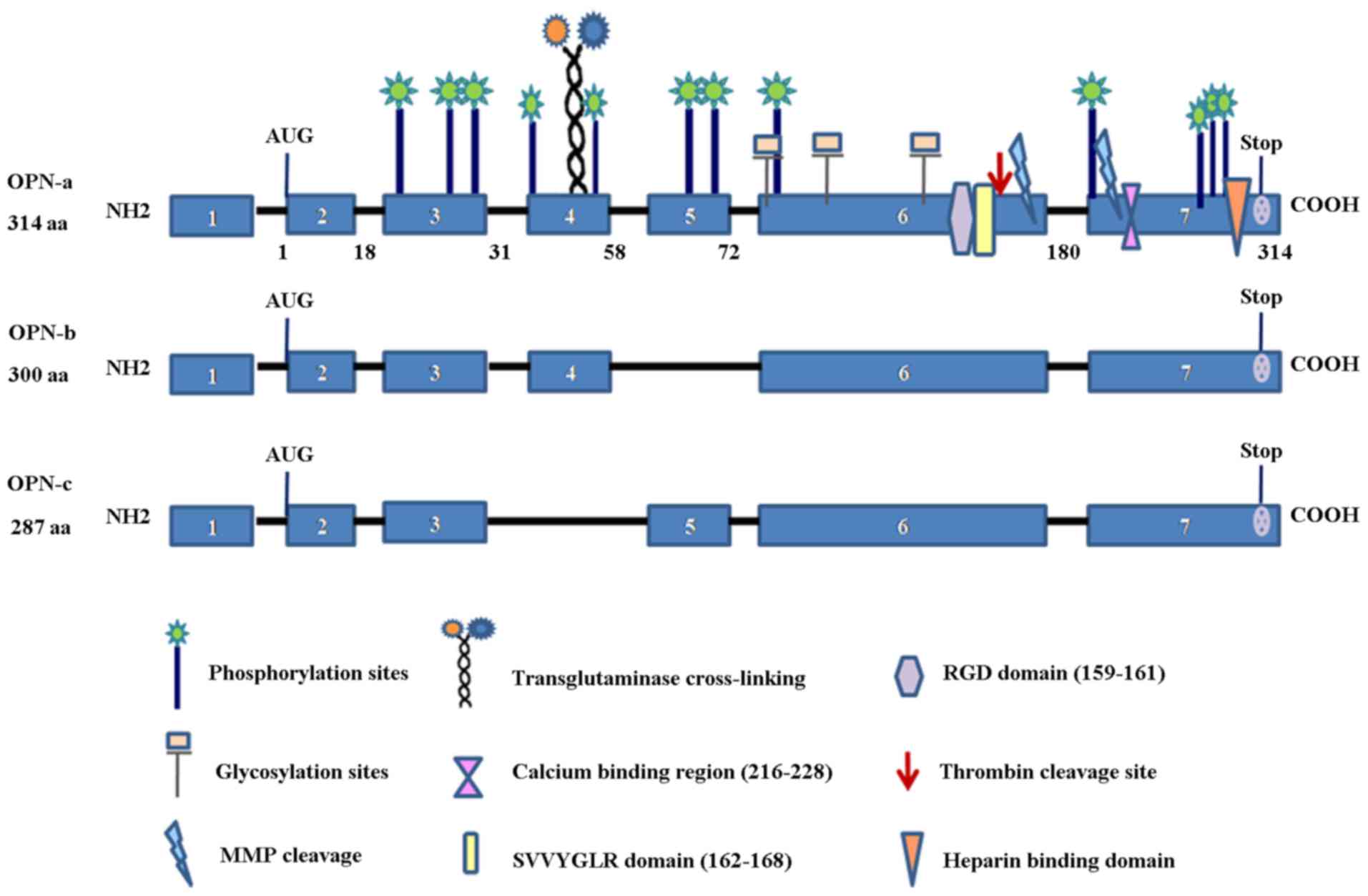
The alternative splice-generation of multiple mRNA
products from a single gene is a critical mechanism for generating
proteomic diversity. The OPN precusor-mRNA (pre-mRNA) is subject to
alternative splicing, which generates three splice variants, OPN-a
(consists of all exons), OPN-b (which lacks exon 5) and OPN-c
(which lacks exon 4) (Fig.
1).
Recent studies have demonstrated that the expression
of OPN splice variants in malignancies is cell-type/tissue specific
and may have functional heterogeneity (Table II). For example, Pang et
al reported that OPN-c is selectively expressed in breast
cancer tissue (57). In their
study on 170 breast cancer patients, OPN-c protein staining was
positive in 70% of all of the samples and was significantly higher
in tumour tissues compared to normal tissues. The study indicated
an inverse correlation between OPN-c and E-cadherin, an established
tumour suppressor. However, the observations by the same authors
that OPN-c also inversely correlated with β-catenin and that
E-cadherin positively correlated β-catenin are intriguing. Despite
β-catenin working with E-cadherin to form cell-cell adhesion
complexes, β-catenin has been classically regarded as an oncogenic
protein. Thus, the true link between OPN-c and the cell adhesion
complex requires further study and one has to delineate the
cellular location of β-catenin (namely membrane-associated,
cytoplasmic and nucleus) in this context. However, the study by
Pang has clearly demonstrated that high levels of OPN-c staining in
breast tumours are associated with TNM staging, nodal involvement,
recurrence and metastasis, and interestingly with the triple
negativity of the tumours and, thus is an independent prognostic
indicator for these patients (57). Another study by Sun et al
demonstrated that each of the three OPN splice variants was able to
increase the growth of breast tumours, in vivo (56). It is noteworthy that OPN-a appears
more effective in promoting tumour growth than the other two splice
forms.
Conflicting evidence also exists regarding the
function of OPN isoforms in hepatocellular carcinoma (HCC). It has
been previously demonstrated that tumour tissue predominantly
expressed OPN-a and OPN-b, and the ratio of OPN-a and OPN-b to
OPN-c increased substantially as the tumours progressed in SK-Hep1
cells and Hep3B cells (67).
Thus, it is possible that OPN-a and OPN-b may be associated with a
poor prognosis, and OPN-c may prevent both cell migration and
invasion in more migratory and invasive cells. By contrast, in
another study, Takafuji et al (68) reported that the increased
expression of OPN-c in Hep3B cells appeared to enhance cellular
invasion and metastatic potential due to the formation of OPN-c
fragment by MMP-9.
These studies emphasise that OPN splice variants may
have diverse expression patterns and different functional roles,
which may be cancer type-specific.
OPN has mainly been studied as a secreted protein,
but recent studies have shown that, in some cases, OPN is not
secreted and instead will be found in the cytoplasm and nucleus.
Mouse OPN mRNA has the canonical AUG translation initiation site
and an alternative translation initiation site. When translation
initiates from the canonical site, the peptide produced is targeted
to secretory vesicles. On the other hand, when translation starts
from the alternative translation initiation site, the peptide
produced is accompanied by deletion of a signal sequence and
localises in the cytoplasm (76,77). Although sOPN and iOPN are
generated from the same OPN mRNA species, the biological outcomes
mediated by the two isoforms may differ (78). The role of human sOPN in cell
physiology has been extensively studied in different cellular
contexts. Compared with sOPN, the biological roles of iOPN have
only begun to emerge over the past several years. Thus far, iOPN
has been found in calvarial cells, dendritic cells, macrophages and
nerve cells (77,79,80). It is involved in cell motility,
cytoskeletal rearrangement and mitosis by physical association with
polo-like kinase-1 (Plk-1) (81–83). Similarly, these two isoforms may
play distinct roles in cancer progression. Indeed, in patients with
various solid tumours, sOPN has been proposed as a diagnostic
marker to distinguish either resectable cases or predict survival
rates (43,84,85). However, cytoplasmic OPN expression
has appeared not to correlate with average tumour size, tumour
stage and nodal status (10,86). The same has been observed for OPN
splice variants. For example, in a study on expression patterns of
OPN variants and its functions on cell apoptosis and invasion in
glioma cells, Yan et al presumed that the secretary nature
of OPN splice variants may be induced by the absence of exon 5 or
exon 4. As a consequence, OPN-b without out exon 5 may aggregate
within the cytoplasm and exert a significant anti-apoptotic effect,
while OPN-c without exon 4 may be easily secreted to culture
supernatants and has a remarkable effect on cellular invasion
(74).
Therefore, it would be necessary to delineate which
isoform of OPN is responsible for pathophysiological events, and
furthermore, sOPN and iOPN should be independently targeted in any
potential therapies.
Tumour cells and their microenvironment mutually
influence tumour formation, progression and metastasis. The tumour
microenvironment includes a variety of non-tumour cell types, such
as fibroblasts, immune cells, vascular and smooth muscle cells. The
host's reaction to neoplastic cells and the ability of
environmental modification by tumour cells themselves are both
involved in tumour formation. A number of studies have demonstrated
that OPN can be synthesised by tumour and other cells types in the
tumour microenvironment, such as macrophages and stromal cells
(87,88). However, whether tumour-derived OPN
differs from host-derived OPN, either structurally or functionally,
is largely unknown. There is evidence indicating that the role of
OPN in metastasis is dependent on the site of production. For
example, historically, stroma-derived OPN has been considered to be
intrinsically involved in the defensive mechanism against tumour
development by acting as a macrophage chemoattractant, yet
stroma-derived OPN can effectively regulate melanoma growth,
angiogenesis and metastasis with the host OPN-tumour interaction
(89–91). In addition, tumour-derived OPN may
promote the metastatic process by inhibiting macrophage
cytotoxicity against tumours (92). By contrast, some evidence suggests
that at least in some instances, the presence of OPN in macrophages
promote the development and activity of type I natural killer (NK)
T cells (NK T cells) and that OPN may inhibit tumour activity via
these NK cells (93).
Thus, host-derived OPN and tumour-derived OPN may
mutually affect each other and the interaction between the tumour
and tumour microenvironment may determine whether the overall
effect of OPN will be tumour promoting or tumour inhibiting.
The similar heterogeneity of OPN splice variants
exists in cancers. the differential effects of the three splice
variants may be due to the distinctive functions of the spliced
exons that individual OPN isoforms contain. For example, the
sequences encoded by exon 5, absent in OPN-b, contain one of
several clusters of phosphorylated serine/threonine residues. Exon
4 however encodes two glutamine residues essential for
transglutaminase cross-linking. As exon 4 is absent in OPN-c, but
present in OPN-a and OPN-b, OPN-c has no specific functions exerted
by exon 4 (94). In breast cancer
cells, it has been reported that OPN-a, not OPN-c may have a
tendency to aggregate in response to physical concentrations of
calcium, and thus have a greater capacity to enhance cell adhesion
(95). By contrast, OPN-c may be
more soluble and able to support anchorage-independent growth of
breast cancer cells (96,97). OPN-c possesses a more exposed
Arg-Ser-Lys (RSK) motif compared to OPN-a (98). Using the RSK motif as a cleavage
site, systemically circulating thrombin can cleave OPN-c easily
into two fragments, the N-terminal and C-terminal, of approximately
equivalent size (98). The
N-terminal GRGDS-containing fragment produced by thrombin cleavage
has the potential to enhance tumour cell migration (98). The thrombin-cleaved C-terminal
fragment of OPN has also been reported to influence breast cancer
cell migration and invasion in vitro (99). The absence of the transglutaminase
acting site in OPN-c may explain why OPN-c cannot be cross-linked
with the extracellular matrix (94).
On the other hand, some investigations suggest that
the possible reasons for OPN isoforms cell-type specific patterns
and their roles are closely related to these differing PTMs of the
three OPN splice variants. Gimba et al demonstrated that the
potential of OPN-a, OPN-b and OPN-c for specific phosphorylation
patterns, and the deletion of exon 4 or 5 altered the pattern of
PTMs, ultimately resulting in functional modifications (46).
OPN was initially identified in bone and later
characterised based on its splice variants and their structures and
functions. It is now well established that the isoforms of OPN are
differentially expressed in many tumour types and play critical
roles at different stages of cancer development and progression.
OPN may be a useful biomarker to monitor cancer progression and one
of the significant predictors of poor prognosis and survival rates,
in certain cancers for example in breast cancer and lung cancer.
Thus, OPN may have several potential clinical implications:
firstly, as a convenient tool for clinical test. As a secreted
protein, OPN can easily detected in body fluids, such as blood,
urine and body cavity fluids (namely pleural and peritoneal
ascites). This gives rise to convenient sampling, taking advantages
of sample quantity, safe detection, procedure speed and minimal
invasiveness, collectively providing a convenient approach for
clinical testing. Secondly, the power of combining OPN with other
traditional biomarkers, such as VEGF, MMPs and E-cadherin, for
example, provide more accurate predictions for prognosis and
potential response to therapies (43,50,100).
Yet, changes remain, especially given the complexity
of the distribution patterns and presence of the variant forms for
OPN. Methods of OPN detection are currently very limited.
Traditional protein detection methods such as ELISA have shown
their limit due to a lack of sensitivity and the nature of samples
required for the testings. Several studies have focused on the
cost-effectiveness and ease of implementing immunosensors for OPN
detection demonstrating better sensitivity than ELISA assays, and
providing greater potential to develop simple test kits for OPN
combined with other protein biomarkers (101–104).
Due to the pre- and post-translational regulation,
the expression and function patterns of OPN isoforms usually
exhibit tissue specificity. It has been suggested that this
provides the possibility to develop cancer therapy strategies to
target those OPN isoforms which are specific to tumour cells or
play a key role in tumour progression. But the pre- and
post-translational regulation are complex and ubiquitous,
developing drugs that target only cancer cells with minimal impact
on healthy cells is extremely difficult. Thus the expression
patterns and activities of tumour-specific OPN isoforms should be
further defined with multidisciplinary and large scale clinical
study. The development of specific OPN-based approaches for
individual cancer types is equally important as well.
Collectively, OPN and its variants have been shown
to play an important role in regulating the aggressive nature of
cancer cells and promote the growth of tumours. Although there is
more to learn with regards to the mechanisms of action of the
specific OPN variants, it is clear that there is clinical
prospect(s) for this protein and its variants. OPN appears to have
good clinical value in evaluating disease progression and cancer
patient outcome. There is a good prospect for developing a OPN
based clinical test for cancer patients in order to evaluate their
prognosis and response to therapies. Wht is most exciting is the
prospect of developing tools to target the protein and its variants
in novel ways. It is anticipated that in the coming years we will
see some significant progress along these fronts.
The authors wish to thank Cancer Research Wales,
Cardiff University China Medical Scholarship, Life Science Research
Network for Wales (LSRNW) and the Albert Hung Foundation for
supporting their study.
|
1
|
Giachelli CM, Liaw L, Murry CE, Schwartz
SM and Almeida M: Osteopontin expression in cardiovascular
diseases. Ann NY Acad Sci. 760:109–126. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber GF and Cantor H: The immunology of
Eta-1/osteopontin. Cytokine Growth Factor Rev. 7:241–248. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Denhardt DT and Noda M: Osteopontin
expression and function: role in bone remodeling. J Cell Biochem
Suppl. 30–31(S30–31): 92–102. 1998. View Article : Google Scholar
|
|
4
|
Sodek J, Ganss B and McKee MD:
Osteopontin. Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christensen B, Kazanecki CC, Petersen TE,
Rittling SR, Denhardt DT and Sørensen ES: Cell type-specific
post-translational modifications of mouse osteopontin are
associated with different adhesive properties. J Biol Chem.
282:19463–19472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christensen B, Nielsen MS, Haselmann KF,
Petersen TE and Sørensen ES: Post-translationally modified residues
of native human osteopontin are located in clusters: identification
of 36 phosphorylation and five O-glycosylation sites and their
biological implications. Biochem J. 390:285–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christensen B, Petersen TE and Sørensen
ES: Post-translational modification and proteolytic processing of
urinary osteopontin. Biochem J. 411:53–61. 2008. View Article : Google Scholar
|
|
8
|
Denhardt DT and Guo X: Osteopontin: a
protein with diverse functions. FASEB J. 7:1475–1482.
1993.PubMed/NCBI
|
|
9
|
Cho HJ, Cho HJ and Kim HS: Osteopontin: a
multifunctional protein at the crossroads of inflammation,
atherosclerosis, and vascular calcification. Curr Atheroscler Rep.
11:206–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coppola D, Szabo M, Boulware D, Muraca P,
Alsarraj M, Chambers AF and Yeatman TJ: Correlation of osteopontin
protein expression and pathological stage across a wide variety of
tumor histologies. Clin Cancer Res. 10:184–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Tanani MK, Campbell FC, Kurisetty V,
Jin D, McCann M and Rudland PS: The regulation and role of
osteopontin in malignant transformation and cancer. Cytokine Growth
Factor Rev. 17:463–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellahcène A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith LL, Cheung HK, Ling LE, Chen J,
Sheppard D, Pytela R and Giachelli CM: Osteopontin N-terminal
domain contains a cryptic adhesive sequence recognized by
alpha9beta1 integrin. J Biol Chem. 271:28485–28491. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yokosaki Y, Matsuura N, Sasaki T, Murakami
I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y and
Sheppard D: The integrin alpha(9)beta(1) binds to a novel
recognition sequence (SVVYGLR) in the thrombin-cleaved
amino-terminal fragment of osteopontin. J Biol Chem.
274:36328–36334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scatena M, Liaw L and Giachelli CM:
Osteopontin: a multifunctional molecule regulating chronic
inflammation and vascular disease. Arterioscler Thromb Vasc Biol.
27:2302–2309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun BS, Li Y, Zhang ZF, You J and Wang CL:
Osteopontin combined with CD44v6, a novel prognostic biomarker in
non-small cell lung cancer undergoing curative resection. Ann
Thorac Surg. 96:1943–1951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao YL, Xing LQ, Ren TJ, Hou JF, Xue Q,
Liu C and Han YM: The expression of osteopontin in breast cancer
tissue and its relationship with 21ras and CD44V6 expression. Eur J
Gynaecol Oncol. 37:41–47. 2016.
|
|
19
|
Yang L, Shang X, Zhao X, Lin Y and Liu J:
Correlation study between OPN, CD44v6, MMP-9 and distant metastasis
in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi. 26:989–992. 2012.In Chinese.
|
|
20
|
Kim JS, Bashir MM and Werth VP: Gottron's
papules exhibit dermal accumulation of CD44 variant 7 (CD44v7) and
its binding partner osteopontin: a unique molecular signature. J
Invest Dermatol. 132:1825–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allan AL, George R, Vantyghem SA, Lee MW,
Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka
CO, et al: Role of the integrin-binding protein osteopontin in
lymphatic metastasis of breast cancer. Am J Pathol. 169:233–246.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Courter D, Cao H, Kwok S, Kong C, Banh A,
Kuo P, Bouley DM, Vice C, Brustugun OT and Denko NC: The RGD domain
of human osteopontin promotes tumor growth and metastasis through
activation of survival pathways. PLoS One. 5:e96332010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui R, Takahashi F, Ohashi R, Gu T,
Yoshioka M, Nishio K, Ohe Y, Tominaga S, Takagi Y, Sasaki S, et al:
Abrogation of the interaction between osteopontin and alphavbeta3
integrin reduces tumor growth of human lung cancer cells in mice.
Lung Cancer. 57:302–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu CM, Chen PC, Li TM, Fong YC and Tang
CH: Si-Wu-tang extract stimulates bone formation through
I3K/Akt/NF-κB signaling pathways in osteoblasts. BMC Complement
Altern Med. 13:2772013. View Article : Google Scholar
|
|
25
|
Wang Y, Yan W, Lu X, Qian C, Zhang J, Li
P, Shi L, Zhao P, Fu Z, Pu P, et al: Overexpression of osteopontin
induces angiogenesis of endothelial progenitor cells via the
avβ3/I3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell
Biol. 90:642–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogata T, Ueyama T, Nomura T, Asada S,
Tagawa M, Nakamura T, Takahashi T, Matsubara H and Oh H:
Osteopontin is a myosphere-derived secretory molecule that promotes
angiogenic progenitor cell proliferation through the
phosphoinositide 3-kinase/Akt pathway. Biochem Biophys Res Commun.
359:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen RX, Xia YH, Xue TC, Zhang H and Ye
SL: Down-regulation of osteopontin inhibits metastasis of
hepatocellular carcinoma cells via a mechanism involving MMP-2 and
uPA. Oncol Rep. 25:803–808. 2011.
|
|
28
|
Mi Z, Guo H, Wai PY, Gao C and Kuo PC:
Integrin-linked kinase regulates osteopontin-dependent MMP-2 and
uPA expression to convey metastatic function in murine mammary
epithelial cancer cells. Carcinogenesis. 27:1134–1145. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tuck AB, Hota C and Chambers AF:
Osteopontin(OPN)-induced increase in human mammary epithelial cell
invasiveness is urokinase (uPA)-dependent. Breast Cancer Res Treat.
70:197–204. 2001. View Article : Google Scholar
|
|
30
|
Kerenidi T, Kazakou AP, Lada M, Tsilioni
I, Daniil Z and Gourgoulianis KI: Clinical significance of
circulating osteopontin levels in patients with lung cancer and
correlation with VEGF and MMP-9. Cancer Invest. 34:385–392. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Babarović E, Valković T, Budisavljević I,
Balen I, Štifter S, Duletić-Načinović A, Lučin K and Jonjić N: The
expression of osteopontin and vascular endothelial growth factor in
correlation with angiogenesis in monoclonal gammopathy of
undetermined significance and multiple myeloma. Pathol Res Pract.
212:509–516. 2016. View Article : Google Scholar
|
|
32
|
Lin Q, Guo L, Lin G, Chen Z, Chen T, Lin
J, Zhang B and Gu X: Clinical and prognostic significance of OPN
and VEGF expression in patients with non-small-cell lung cancer.
Cancer Epidemiol. 39:539–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Terpos E, Kiagia M, Karapanagiotou EM,
Charpidou A, Dilana KD, Nasothimiou E, Harrington KJ, Polyzos A and
Syrigos KN: The clinical significance of serum markers of bone
turnover in NSCLC patients: surveillance, management and prognostic
implications. Anticancer Res. 29:1651–1657. 2009.PubMed/NCBI
|
|
34
|
Hsieh IS, Huang WH, Liou HC, Chuang WJ,
Yang RS and Fu WM: Upregulation of drug transporter expression by
osteopontin in prostate cancer cells. Mol Pharmacol. 83:968–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anborgh PH, Caria LB, Chambers AF, Tuck
AB, Stitt LW and Brackstone M: Role of plasma osteopontin as a
biomarker in locally advanced breast cancer. Am J Transl Res.
7:723–732. 2015.PubMed/NCBI
|
|
36
|
Friedmann-Morvinski D, Bhargava V, Gupta
S, Verma IM and Subramaniam S: Identification of therapeutic
targets for glioblastoma by network analysis. Oncogene. 35:608–620.
2016. View Article : Google Scholar
|
|
37
|
Güttler A, Giebler M, Cuno P, Wichmann H,
Keßler J, Ostheimer C, Söling A, Strauss C, Illert J, Kappler M, et
al: Osteopontin and splice variant expression level in human
malignant glioma: radiobiologic effects and prognosis after
radiotherapy. Radiother Oncol. 108:535–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Etiz D, Ataizi FC, Bayman E, Akcay M,
Acikalin MF, Colak E and Ciftci E: Prognostic value of osteopontin
in patients treated with primary radiotherapy for head and neck
cancer. Asian Pac J Cancer Prev. 14:5175–5178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imano M, Satou T, Itoh T, Sakai K,
Ishimaru E, Yasuda A, Peng YF, Shinkai M, Akai F, Yasuda T, et al:
Immunohistochemical expression of osteopontin in gastric cancer. J
Gastrointest Surg. 13:1577–1582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Higashiyama M, Ito T, Tanaka E and Shimada
Y: Prognostic significance of osteopontin expression in human
gastric carcinoma. Ann Surg Oncol. 14:3419–3427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ng L, Wan TM, Lam CS, Chow AK, Wong SK,
Man JH, Li HS, Cheng NS, Pak RC, Cheung AH, et al: Post-operative
plasma osteopontin predicts distant metastasis in human colorectal
cancer. PLoS One. 10:e01262192015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ng L, Wan T, Chow A, Iyer D, Man J, Chen
G, Yau TC, Lo O, Foo CC, Poon JT, et al: Osteopontin overexpression
induced tumor progression and chemoresistance to oxaliplatin
through induction of stem-like properties in human colorectal
cancer. Stem Cells Int. 2015:2478922015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poruk KE, Firpo MA, Scaife CL, Adler DG,
Emerson LL, Boucher KM and Mulvihill SJ: Serum osteopontin and
tissue inhibitor of metalloproteinase 1 as diagnostic and
prognostic biomarkers for pancreatic adenocarcinoma. Pancreas.
42:193–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koopmann J, Fedarko NS, Jain A, Maitra A,
Iacobuzio-Donahue C, Rahman A, Hruban RH, Yeo CJ and Goggins M:
Evaluation of osteopontin as biomarker for pancreatic
adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 13:487–491.
2004.PubMed/NCBI
|
|
45
|
Liu G, Fan X, Tang M, Chen R, Wang H, Jia
R, Zhou X, Jing W, Wang H, Yang Y, et al: Osteopontin induces
autophagy to promote chemo-resistance in human hepatocellular
carcinoma cells. Cancer Lett. 383:171–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gimba ER and Tilli TM: Human osteopontin
splicing isoforms: known roles, potential clinical applications and
activated signaling pathways. Cancer Lett. 331:11–17. 2013.
View Article : Google Scholar
|
|
47
|
Salem M, Atti SA, Raziky ME, Darweesh SK
and Sharkawy ME: Clinical significance of plasma osteopontin level
as a biomarker of hepatocellular carcinoma. Gastroenterology Res.
6:191–199. 2013.PubMed/NCBI
|
|
48
|
Xu ST, Guo C, Ding X, Fan WJ, Zhang FH, Xu
WL and Ma YC: Role of osteopontin in the regulation of human
bladder cancer proliferation and migration in T24 cells. Mol Med
Rep. 11:3701–3707. 2015.PubMed/NCBI
|
|
49
|
Moszynski R, Szubert S, Szpurek D,
Michalak S and Sajdak S: Role of osteopontin in differential
diagnosis of ovarian tumors. J Obstet Gynaecol Res. 39:1518–1525.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakae M, Iwamoto I, Fujino T, Maehata Y,
Togami S, Yoshinaga M and Douchi T: Preoperative plasma osteopontin
level as a biomarker complementary to carbohydrate antigen 125 in
predicting ovarian cancer. J Obstet Gynaecol Res. 32:309–314. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Subramani VN, Narasimhan M, Thiyagarajan
M, Munuswamy BD and Jayamani L: Expression of osteopontin in oral
squamous cell carcinoma and its surgical margins-an
immunohistochemical study. J Clin Diagn Res. 9:ZC66–ZC69.
2015.PubMed/NCBI
|
|
52
|
Kiss T, Ecsedi S, Vizkeleti L, Koroknai V,
Emri G, Kovács N, Adany R and Balazs M: The role of osteopontin
expression in melanoma progression. Tumour Biol. 36:7841–7847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Blasberg JD, Pass HI, Goparaju CM, Flores
RM, Lee S and Donington JS: Reduction of elevated plasma
osteopontin levels with resection of non-small-cell lung cancer. J
Clin Oncol. 28:936–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zduniak K, Ziolkowski P, Ahlin C, Agrawal
A, Agrawal S, Blomqvist C, Fjällskog ML and Weber GF: Nuclear
osteopontin-c is a prognostic breast cancer marker. Br J Cancer.
112:729–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ortiz-Martínez F, Perez-Balaguer A,
Ciprián D, Andrés L, Ponce J, Adrover E, Sánchez-Payá J, Aranda FI,
Lerma E and Peiró G: Association of increased osteopontin and
splice variant-c mRNA expression with HER2 and
triple-negative/basal-like breast carcinomas subtypes and
recurrence. Hum Pathol. 45:504–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun J, Feng A, Chen S, Zhang Y, Xie Q,
Yang M, Shao Q, Liu J, Yang Q, Kong B, et al: Osteopontin splice
variants expressed by breast tumors regulate monocyte activation
via MCP-1 and TGF-β1. Cell Mol Immunol. 10:176–182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu
N, Lan F, Liu Y, Yan S, Dong X, et al: Prognostic values of
osteopontin-c, E-cadherin and β-catenin in breast cancer. Cancer
Epidemiol. 37:985–992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Patani N, Jouhra F, Jiang W and Mokbel K:
Osteopontin expression profiles predict pathological and clinical
outcome in breast cancer. Anticancer Res. 28:4105–4110. 2008.
|
|
59
|
Sun SJ, Wu CC, Sheu GT, Chang HY, Chen MY,
Lin YY, Chuang CY, Hsu SL and Chang JT: Integrin β3 and CD44 levels
determine the effects of the OPN-a splicing variant on lung cancer
cell growth. Oncotarget. 7:55572–55584. 2016.PubMed/NCBI
|
|
60
|
Zhao B, Sun T, Meng F, Qu A, Li C, Shen H,
Jin Y and Li W: Osteopontin as a potential biomarker of
proliferation and invasiveness for lung cancer. J Cancer Res Clin
Oncol. 137:1061–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Blasberg JD, Goparaju CM, Pass HI and
Donington JS: Lung cancer osteopontin isoforms exhibit angiogenic
functional heterogeneity. J Thorac Cardiovasc Surg. 139:1587–1593.
2010. View Article : Google Scholar :
|
|
62
|
Nakamura KD, Tilli TM, Wanderley JL,
Palumbo A Jr, Mattos RM, Ferreira AC, Klumb CE4, Nasciutti LE and
Gimba ER: Osteopontin splice variants expression is involved on
docetaxel resistance in PC3 prostate cancer cells. Tumour Biol.
37:2655–2663. 2016. View Article : Google Scholar
|
|
63
|
Tilli TM, Bellahcène A, Castronovo V and
Gimba ER: Changes in the transcriptional profile in response to
overexpression of the osteopontin-c splice isoform in ovarian
(OvCar-3) and prostate (PC-3) cancer cell lines. BMC Cancer.
14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tilli TM, Mello KD, Ferreira LB, Matos AR,
Accioly MT, Faria PA, Bellahcène A, Castronovo V and Gimba ER: Both
osteopontin-c and osteopontin-b splicing isoforms exert
pro-tumorigenic roles in prostate cancer cells. Prostate.
72:1688–1699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin J, Myers AL, Wang Z, Nancarrow DJ,
Ferrer-Torres D, Handlogten A, Leverenz K, Bao J, Thomas DG, Wang
TD, et al: Osteopontin (OPN/SPP1) isoforms collectively enhance
tumor cell invasion and dissemination in esophageal adenocarcinoma.
Oncotarget. 6:22239–22257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang MX, Xu YJ, Zhu MC and Yan F:
Overexpressed ostepontin-c as a potential biomarker for esophageal
squamous cell carcinoma. Asian Pac J Cancer Prev. 14:7315–7319.
2013. View Article : Google Scholar
|
|
67
|
Chae S, Jun HO, Lee EG, Yang SJ, Lee DC,
Jung JK, Park KC, Yeom YI and Kim KW: Osteopontin splice variants
differentially modulate the migratory activity of hepatocellular
carcinoma cell lines. Int J Oncol. 35:1409–1416. 2009.PubMed/NCBI
|
|
68
|
Takafuji V, Forgues M, Unsworth E,
Goldsmith P and Wang XW: An osteopontin fragment is essential for
tumor cell invasion in hepatocellular carcinoma. Oncogene.
26:6361–6371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tang X, Li J, Yu B, Su L, Yu Y, Yan M, Liu
B and Zhu Z: Osteopontin splice variants differentially exert
clinicopathological features and biological functions in gastric
cancer. Int J Biol Sci. 9:55–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Siddiqui AA, Jones E, Andrade D, Shah A,
Kowalski TE, Loren DE, Chipitsyna G and Arafat HA: Osteopontin
splice variant as a potential marker for metastatic disease in
pancreatic adenocarcinoma. J Gastroenterol Hepatol. 29:1321–1327.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sarosiek K, Jones E, Chipitsyna G,
Al-Zoubi M, Kang C, Saxena S, Gandhi AV, Sendiky J, Yeo CJ and
Arafat HA: Osteopontin (OPN) isoforms, diabetes, obesity, and
cancer; what is one got to do with the other? A new role for OPN. J
Gastrointest Surg. 19:639–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ferreira LB, Eloy C, Pestana A, Lyra J,
Moura M, Prazeres H, Tavares C, Sobrinho-Simões M, Gimba E and
Soares P: Osteopontin expression is correlated with differentiation
and good prognosis in medullary thyroid carcinoma. Eur J
Endocrinol. 174:551–561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ferreira LB, Tavares C, Pestana A, Pereira
CL, Eloy C, Pinto MT, Castro P, Batista R, Rios E, Sobrinho-Simões
M, et al: Osteopontin-a splice variant is overexpressed in
papillary thyroid carcinoma and modulates invasive behavior.
Oncotarget. 7:52003–52016. 2016.PubMed/NCBI
|
|
74
|
Yan W, Qian C, Zhao P, Zhang J, Shi L,
Qian J, Liu N, Fu Z, Kang C, Pu P, et al: Expression pattern of
osteopontin splice variants and its functions on cell apoptosis and
invasion in glioma cells. Neuro-oncol. 12:765–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hahnel A, Wichmann H, Greither T, Kappler
M, Würl P, Kotzsch M, Taubert H, Vordermark D and Bache M:
Prognostic impact of mRNA levels of osteopontin splice variants in
soft tissue sarcoma patients. BMC Cancer. 12:1312012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Inoue M and Shinohara ML: Intracellular
osteopontin (iOPN) and immunity. Immunol Res. 49:160–172. 2011.
View Article : Google Scholar
|
|
77
|
Shinohara ML, Kim HJ, Kim JH, Garcia VA
and Cantor H: Alternative translation of osteopontin generates
intracellular and secreted isoforms that mediate distinct
biological activities in dendritic cells. Proc Natl Acad Sci USA.
105:7235–7239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yushi Q, Li Z, Von Roemeling CA, Doeppler
H, Marlow LA, Kim BY, Radisky DC, Storz P, Copland JA and Tun HW:
Osteopontin is a multi-faceted pro-tumorigenic driver for central
nervous system lymphoma. Oncotarget. 7:32156–32171. 2016.PubMed/NCBI
|
|
79
|
Zohar R, Lee W, Arora P, Cheifetz S,
McCulloch C and Sodek J: Single cell analysis of intracellular
osteopontin in osteogenic cultures of fetal rat calvarial cells. J
Cell Physiol. 170:88–100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhao W, Wang L, Zhang L, Yuan C, Kuo PC
and Gao C: Differential expression of intracellular and secreted
osteopontin isoforms by murine macrophages in response to toll-like
receptor agonists. J Biol Chem. 285:20452–20461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Junaid A, Moon MC, Harding GE and Zahradka
P: Osteopontin localizes to the nucleus of 293 cells and associates
with polo-like kinase-1. Am J Physiol Cell Physiol. 292:C919–C926.
2007. View Article : Google Scholar
|
|
82
|
Zohar R, Suzuki N, Suzuki K, Arora P,
Glogauer M, McCulloch CA and Sodek J: Intracellular osteopontin is
an integral component of the CD44-ERM complex involved in cell
migration. J Cell Physiol. 184:118–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shinohara ML, Lu L, Bu J, Werneck MB,
Kobayashi KS, Glimcher LH and Cantor H: Osteopontin expression is
essential for interferon-alpha production by plasmacytoid dendritic
cells. Nat Immunol. 7:498–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen R, Crispin DA, Pan S, Hawley S,
McIntosh MW, May D, Anton-Culver H, Ziogas A, Bronner MP and
Brentnall TA: Pilot study of blood biomarker candidates for
detection of pancreatic cancer. Pancreas. 39:981–988. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fedarko NS, Jain A, Karadag A, Van Eman MR
and Fisher LW: Elevated serum bone sialoprotein and osteopontin in
colon, breast, prostate, and lung cancer. Clin Cancer Res.
7:4060–4066. 2001.PubMed/NCBI
|
|
86
|
Collins AL, Rock J, Malhotra L, Frankel WL
and Bloomston M: Osteopontin expression is associated with improved
survival in patients with pancreatic adenocarcinoma. Ann Surg
Oncol. 19:2673–2678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ashkar S, Weber GF, Panoutsakopoulou V,
Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT,
Glimcher MJ and Cantor H: Eta-1 (osteopontin): an early component
of type-1 (cell-mediated) immunity. Science. 287:860–864. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chan SC, Tekari A, Benneker LM, Heini PF
and Gantenbein B: Osteogenic differentiation of bone marrow stromal
cells is hindered by the presence of intervertebral disc cells.
Arthritis Res Ther. 18:292015. View Article : Google Scholar
|
|
89
|
Kumar S, Sharma P, Kumar D, Chakraborty G,
Gorain M and Kundu GC: Functional characterization of stromal
osteopontin in melanoma progression and metastasis. PLoS One.
8:e691162013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY,
Shun CT, Tsai MF, Chen CH and Yang PC: Tumor-associated
macrophages: the double-edged sword in cancer progression. J Clin
Oncol. 23:953–964. 2005. View Article : Google Scholar
|
|
91
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wai PY, Guo L, Gao C, Mi Z, Guo H and Kuo
PC: Osteopontin inhibits macrophage nitric oxide synthesis to
enhance tumor proliferation. Surgery. 140:132–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sato K, Iwai A, Nakayama Y, Morimoto J,
Takada A, Maruyama M, Kida H, Uede T and Miyazaki T: Osteopontin is
critical to determine symptom severity of influenza through the
regulation of NK cell population. Biochem Biophys Res Commun.
417:274–279. 2012. View Article : Google Scholar
|
|
94
|
Collighan RJ and Griffin M:
Transglutaminase 2 cross-linking of matrix proteins: biological
significance and medical applications. Amino Acids. 36:659–670.
2009. View Article : Google Scholar
|
|
95
|
He B, Mirza M and Weber GF: An osteopontin
splice variant induces anchorage independence in human breast
cancer cells. Oncogene. 25:2192–2202. 2006. View Article : Google Scholar
|
|
96
|
Shen H and Weber GF: The osteopontin-c
splice junction is important for anchorage-independent growth. Mol
Carcinog. 53:480–487. 2014. View Article : Google Scholar
|
|
97
|
Shi Z, Wang B, Chihanga T, Kennedy MA and
Weber GF: Energy metabolism during anchorage-independence.
Induction by osteopontin-c. PLoS One. 9:e1056752014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sivakumar S and Niranjali Devaraj S:
Tertiary structure prediction and identification of druggable
pocket in the cancer biomarker - osteopontin-c. J Diabetes Metab
Disord. 13:132014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mi Z, Oliver T, Guo H, Gao C and Kuo PC:
Thrombin-cleaved COOH(−) terminal osteopontin peptide binds with
cyclophilin C to CD147 in murine breast cancer cells. Cancer Res.
67:4088–4097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chimparlee N, Chuaypen N, Khlaiphuengsin
A, Pinjaroen N, Payungporn S, Poovorawan Y and Tangkijvanich P:
Diagnostic and prognostic roles of serum osteopontin and
osteopontin promoter polymorphisms in hepatitis B-related
hepatocellular carcinoma. Asian Pac J Cancer Prev. 16:7211–7217.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sharma A, Hong S, Singh R and Jang J:
Single-walled carbon nanotube based transparent immunosensor for
detection of a prostate cancer biomarker osteopontin. Anal Chim
Acta. 869:68–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Faria M, Halquist MS, Yuan M, Mylott W Jr,
Jenkins RG and Karnes HT: Comparison of a stable isotope labeled
(SIL) peptide and an extended SIL peptide as internal standards to
track digestion variability of an unstable signature peptide during
quantification of a cancer biomarker, human osteopontin, from
plasma using capillary microflow LC-MS/MS. J Chromatogr B Analyt
Technol Biomed Life Sci. 1001:156–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Meirinho SG, Dias LG, Peres AM and
Rodrigues LR: Development of an electrochemical RNA-aptasensor to
detect human osteopontin. Biosens Bioelectron. 71:332–341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen H, Mei Q, Jia S, Koh K, Wang K and
Liu X: High specific detection of osteopontin using a
three-dimensional copolymer layer support based on electrochemical
impedance spectroscopy. Analyst (Lond). 139:4476–4481. 2014.
View Article : Google Scholar
|