Over the past several years, intracellular signaling
targets have been intensely studied as a measure of the cellular
processes that occur following specific conditions. Extracellular
signal-regulated kinase 1/2 (ERK1/2) ligands interact with their
receptor and/or corrector in the cell and subsequently activate the
intracellular ERK1/2 signaling pathway. In vertebrates, ERK1/2
signaling begins during development and acts to regulate cell
proliferation, differentiation and fate decisions in the mature
individual. Dysfunction of ERK1/2 signaling is associated with
several human diseases, such as cancer, asthma, stroke and
Alzheimer's disease (AD) (1–4).
Due to the importance of ERK1/2 in a wide range of biological
processes in central nervous system (CNS) disease, better
understanding of the precise mechanisms of ERK1/2 signaling may
provide fundamental insight into its role in disease development as
well as help identify novel targets for therapeutic
applications.
ERK1/2, like other protein kinases, contains unique
N- and C-terminal extensions that provide signaling specificity.
Human ERK1 consist of 378 amino acid residues while ERK2 consists
of 360 amino acid residues. ERK1 and 2 differ from one anther among
various species. Gene ablation studies have provided evidence that
ERK1 and 2 are not entirely functionally identical. A study showed
that the erk1 gene is dispensable for the development of mice,
whereas ablation of the erk2 gene is embryonic lethal (5). However, ERK1 was found to play an
essential role in thymocyte development in a ERK1-knockout (KO)
mouse study (6). Whether
functions exist that are unique or preferred to ERK1 or 2 is
unknown. Maybe at one time or another during the development of an
animal, ERK1 or 2 performs functions unique to that isoform. Even
so, ERK1 and 2 have a high degree of similarity, with >95% amino
acid identity among humans, mice and rats (7). These two kinases share many
physiological and biological functions and are commonly referred to
together as ERK1/2. All known cellular stimulants of the ERK1/2
pathway lead to parallel activation of ERK1 and 2 (8). The ERK1/2 activation ratio in cells
corresponds with their expression ratio, indicating that the
isoforms are activated in parallel (9).
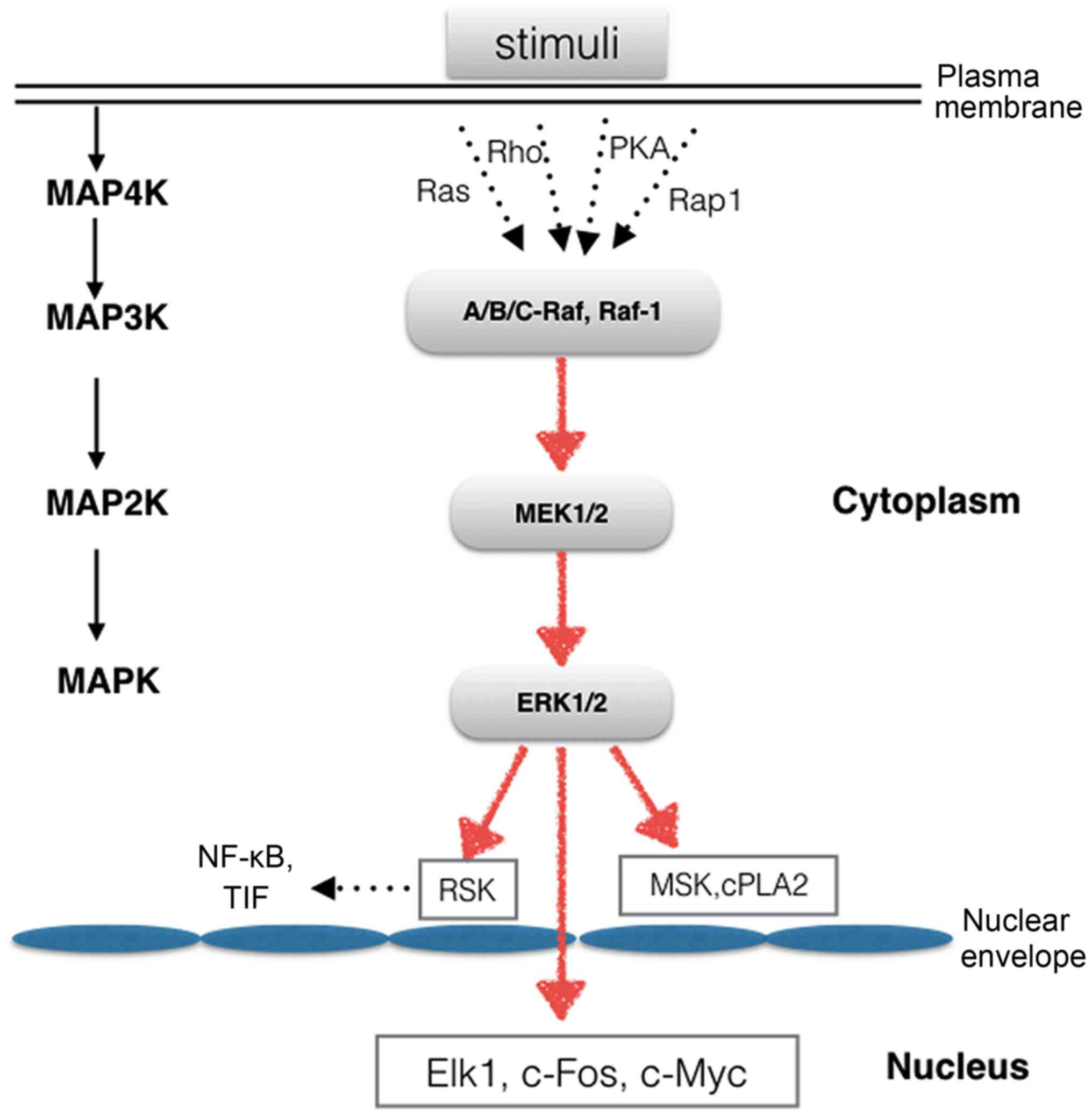
A wide variety of extracellular stimuli are capable
of activating the ERK1/2 cascade. Mitogen-activated protein
kinase/extracellular signal-regulated kinase (MEK)1 and 2 are the
immediate upstream kinases that phosphorylate and activate ERK1/2.
MEK1 and 2 are dual-specificity protein kinases that mediate the
phosphorylation of tyrosine and threonine residues. The activity of
MEK1/2 is also regulated by phosphorylation, as MEK1 and 2 are
phosphorylated by mitogen-activated protein kinase kinase kinases
(MAP3Ks). The most extensively studied MAP3Ks are the Raf proteins,
including A-Raf, B-Raf, C-Raf and Raf-1 (10). They are activated by MAP4K
proteins, such as Rap1, Ras, PKA and Rho (Fig. 1). ERK1/2 is a ubiquitously
expressed hydrophilic non-receptor protein that participates in the
Ras-Raf-MEK-ERK signal transduction cascade, which is involved in
various diseases, including cancer, cardiac hypertrophy, pain and
neuroinflam-mation (11–14). Therefore, this cascade is an
interesting target for basic and translational research, including
the development of drugs for therapeutic purposes.
Once activated, p-ERK1/2 can translocate into the
nucleus to activate a wide array of transcription factors or can
simply remain in the cytoplasm, where it regulates other
subcellular functions (Fig. 1).
ERK1 and 2 have more than 175 documented cytoplasmic and nuclear
substrates (15). ERK1/2 nuclear
targets include the ternary complex factor family of transcription
factors. These proteins mediate the expression of immediate early
genes, whose products contribute to cell survival, division and
motility (16,17). Elk1 is one of the most thoroughly
studied targets of the ERK1/2 MAPK kinase cascade, and Elk1
activation leads to increased transcriptional activity (15). Members of the ERK1/2 family of
protein kinases participate in a wide variety of cellular
processes. To date, more than 50 cytoplasmic substrates have been
identified, including the ribosomal S6 kinase (RSK) family of
protein kinases, apop-totic proteins and cytoskeletal proteins. The
RSK family consists of four human RSK isoforms (RSK1-4), mitogen-
and stress-activated kinase (MSK)1 and 2, which are directly
activated by ERK1/2 in response to stimuli. RSK1-4 are key
components downstream of the Raf-MEK-ERK signaling cascade. The RSK
family regulates transcription by mediating the phosphorylation of
various types of transcription factors, including nuclear factor-κB
(NF-κB), serum response factor (SRF) and transcription initiation
factor (TIF), in cells (18).
Scaffolds are proteins that bind to multiple
components of signaling modules. Scaffolds regulate and integrate
overall signal transduction and play a pivotal role in the spatial
and temporal regulation of the ERK1/2 signaling cascade. In
response to stimulus exposure, ERK1/2 binds to a variety of
cytoplasmic scaffold and anchor proteins, including the suppressor
of Ras (KSR1/2), MEK partner 1 (MP1), IQ motif-containing GTPase
activating protein 1 (IQGAP1) and MAP/ERK kinase kinase 1 (MEKK1)
(19,20). MP1, which is also known as MAP
kinase scaffold protein 1 and LAMTOR3, was identified as a scaffold
protein that potentiates MAPK signaling by binding to MEK1 and
ERK1. MP1 is localized to endomembrane compartments as part of
larger signaling complexes and modulates the Raf-MKK1/2-ERK1/2
pathway together with its partner, p14 (21,22). In fact, IQGAP1 is a well-known
regulator of signaling events involved in the MAPK pathway. The
interaction between IQGAP1 and ERK1/2 plays a critical role in
tumor formation, as competition for ERK1/2 binding between IQGAP1
and a peptide that encompasses the WW domain inhibits Ras and
Raf-driven tumorigenesis (23).
MEKK1, a MAP3 kinase, catalyzes the phosphorylation of MEK1 and 2,
which are components of the ERK pathway. Xu et al (24) and Karandikar et al
(25) both showed that MEKK1
binds to C-Raf, MEK1 and ERK2 of the ERK1/2 MAPK signaling module.
Recent studies have suggested that KSR1 and 2 possess catalytic
activity and that KSR2 participates in the assembly of a
MEK1/KSR2/B-Raf ternary complex that is responsible for promoting
rabbit MEK1 phosphorylation by mouse B-Raf (26,27).
ERK1/2 is abundant in the adult brain, and its
activation can play multiple roles in the activity-dependent
regulation of neuronal function. Mounting evidence indicates that
ERK1/2 signaling plays an essential role in the development of the
CNS (28). ERK1 and 2 are also
involved in neuroinflammation, neural death, learning and memory
formation and the regulation of synaptic plasticity in the adult
nervous system.
Synaptic plasticity is thought to be crucial for
information processing in the brain and to underlie many complex
behaviours. The best studied forms of synaptic plasticity in the
CNS are long-term potentiation (LTP) and long-term depression
(LTD). The regulation of protein phosphorylation has an important
role in the process of LTP and LTD.
Several recent studies have implicated the ERK1/2
pathway in the control of synaptic plasticity in the adult nervous
system (29,30). English and Sweatt (31) investigated the role of MAPKs in
regulating synaptic plasticity in adult rat neurons, with a
particular focus on the modulatory role of ERK1/2 in hippocampal
LTP. They provided the first demonstration of
N-methyl-D-aspartate (NMDA)-receptor dependent activation of
ERK2 in rat hippocampal area CA1 in response to LTP-inducing
high-frequency stimulation and suggested a crucial regulatory role
of ERK2 in synaptic plasticity. Kanterewicz et al (32) further confirmed the role of ERK1/2
in NMDA receptor-independent LTP in the hippocampus. Over the past
few years, a number of studies have demonstrated that ERK1/2
activity is required for several forms of synaptic plasticity in
the amygdala which is associated with fear-dependent learning
(33,34). Ratto and Pizzorusso (35) offered evidence, both in
vivo and in vitro, that ERK1/2 plays a crucial role in
controlling synaptic plasticity in the visual cortex. Inhibition of
ERK1/2 can prevent the induction of various forms of LTP and LTD in
the hippocampus and amygdala (33,36). These studies indicated that a
requirement for ERK1/2 activation is common to many forms of
synaptic plasticity but that the precise targets of ERK1/2 may
differ between different types of plasticity.
Evidence has shown that total ERK1/2 activity
controls the proliferation of certain late-born progenitor cells
and the differentiation of neurons and glia during fetal brain
development and that the two may compensate for each other during
this process, at least in part, due to their overlapping functions
(37,38). Samuels et al (39,40) also found that mutations that
increase ERK1/2 activity can result in macrocephaly, while
mutations that decrease ERK1/2 activity can result in microcephaly,
suggesting that the ERK1/2 pathway is involved in the expansion of
human neural progenitor cells. Furthermore, evidence indicates that
ERK1/2 also takes part in regulating the proliferation and
differentiation of astrocytes in the developing brain. Li et
al (41) found that MEK/ERK
signaling regulated the generation of glia from radial progenitors
in the developing cortex, leading to a major increase in the number
of astrocytes in the brain. This finding provides insight into the
mechanisms involved in ERK1/2-mediated regulation of normal and
abnormal astrocyte function during brain development. Recent
evidence has consistently demonstrated that the ERK1/2 pathway is
one of the dominant intracellular pathways for the regulation of
oligodendroglial development, myelination and remyelination
(38,42–44).
Although ERK1/2 activation has generally been
associated with brain cell differentiation and proliferation, a
number of studies have shown that the activation of ERK1/2 can
mediate cell death in several neuronal systems (45,46). The different effects of ERK1/2 on
brain cells may be owing to the various stimuli and cell types
involved. The activation of ERK1/2 was observed in glutamate- and
heme-induced neuronal cell death and the neuronal injury (47,48) and loss of function (49,50) were reduced when suppressing ERK1/2
activation. ERK1/2 was found to play a caspase-independent role in
promoting neuronal cell death in several other models. Okadaic acid
has been shown to induce pyramidal cell death in hippocampal area
CA3 in a manner dependent on ERK1/2 activation but not consistent
with apoptosis (51). These
findings may help us design strategies that can specifically
attenuate ERK1/2-promoted neuronal pathologies.
ERK1/2 is expressed in microglia, astrocytes and
oligodendrocytes. Microglial cells are the primary immune cells in
the CNS and promote host defense by destroying invading pathogens
(52). Intra-glial signaling,
including ERK1/2 pathway cascades, controls the regulation of
inflammatory cytokine production and iNOS expression in activated
microglia. Many in vitro experiments have demonstrated that
the ERK1/2 signaling pathway contributes to the inflammatory
response in microglia that is induced upon stimulation with
radiation, thrombin or LPS (53–55). ERK1/2 is also involved in the
inflammatory response in astrocytes (56,57). Furthermore, accumulating evidence
indicates that many of the pharmaceutical-based therapies used to
reduce neuroinflammation in stroke, neurodegenerative disorders,
intracranial infections and other diseases act by suppressing the
ERK1/2 pathway (58–61).
ERK1/2 is localized in the soma and dendritic trees
of neurons in the neocortex, hippocampus, striatum and cerebellum
(62). An increase in ERK1/2
activation, as measured as the ratio of phosphorylated to total
(phosphorylated and non-phosphorylated) ERK1/2, is necessary for
learning and the formation of memory as well as for affect and
arousal. In a seminal paper, Atkins et al (63) were the first to show that ERK1/2
is involved in memory processing in rat after fear conditioning.
Later studies showed that activation of the ERK1/2 pathway is also
required for the development of short-term memory and long-term
memory consolidation (64,65).
Treatment with ERK1/2 inhibition can impair long-term memory
retention and prevents the formation of lasting memories of an
event or association, including object recognition memory (66,67). Spatial learning and fear
conditioning are the types of long-term memory in which the
involvement of ERK1/2 has been best characterized (68). Studies of ERK1/2 KO mice
demonstrated that ERK1/2 is involved in various aspects of learning
and memory formation (69). ERK1
KO mice at first appear to be neurologically normal, whereas ERK2
KO is embryonic lethal; ERK2 KO mice die at embryonic day 6.5
(5,70,71). Selcher et al (70) showed that the ERK1 isoform is not
required for associative learning in mice; instead, they found that
the ERK2 isoform plays a predominant role in the synaptic
plasticity that underlies learning and memory. Short-term memory is
retained in ERK1-KO mice, but a marked enhancement of long-term
memory was found in a one-trial inhibitory avoidance task (72). The results of a re-consolidation
study also support the pivotal role of ERK2 in memory process
(73). These results suggest that
ERK1/2 may be a target for therapeutics to treat disorders of
learning and memory.
Consistent with its critical role in key cellular
activities, including cell proliferation, differentiation, survival
and death, the ERK1/2 signaling pathway has been implicated in the
pathogenesis of many CNS diseases, including stroke, AD, and
Parkinson's disease (PD), among others (74–78). The activation of ERK1/2 cascades
contributes to disease progression through the regulation of
neuronal apoptosis, neuroinflammation and synaptic plasticity.
ERK1/2 pathway activation is also known to play
physiological and pathological roles post-development, and a large
body of evidence suggests that ERK1/2 also contributes to the
regulation of inflammatory responses, cytokines, cell apoptosis and
death in ischemic and hemorrhagic brain injury (78–82). Several pharmacological studies
have also demonstrated that suppression of ERK1/2 activation
frequently downregulates features of apoptosis and inflammation and
reduces neurological damage after stroke (49,81–83). Madami and Edvinsson showed that
the elevated microvascular pro-inflammatory cytokine expression
observed following focal ischemia in MCAO models also involved the
ERK1/2 pathway (82). Moreover,
Shioda et al (3) found
that ERK1/2 signaling plays an important role in neurogenesis
following brain ischemia. Substantial evidence has suggested that
the ERK1/2 pathway is involved in regulating the changes in
inflammation, cyto toxicity and cerebral vasospasm that occur after
hemorrhagic stroke (76,84). Recently, Feng et al
(85) showed that Ras/Raf/ERK
signals participate in the neuronal apoptosis observed in the
hippocampus in early post-subarachnoid hemorrhage brain injury.
Taken together, these results suggest that therapies targeted at
suppression of the ERK1/2 pathway may be beneficial in stroke.
PD is the second most prevalent neurodegenerative
disease after AD and is characterized by selective dopaminergic
neuronal loss in the substantial nigra. The ERK1/2 pathway is known
to play a major regulatory role in PD-related cellular processes.
Accumulating evidence indicates that microglial cells play a
crucial role in the degeneration of dopaminergic neurons in animal
models of PD. Recent studies have shown that the oxidative stress
response plays a central role in the etiology of PD (86,87). The oxidative stress response that
occurs in microglia is mediated by the activation of the ERK1/2
signaling pathway upon stimulation with pro-inflammatory stimuli.
Furthermore, ERK1/2 has been shown to participate in L-DOPA-induced
dyskinesia through striatal synaptic plasticity (75,88). In addition, in the
dopamine-depleted striatum, ERK1/2 plays an important role in the
development of L-DOPA-induced dyskinesia in both mouse and
non-human primate models of PD (75,89). The inhibition of ERK1/2 attenuated
LID and completely inhibited all markers of angiogenesis in rat and
mouse models of LID (75,90). Therefore, the modulation of ERK1/2
in response to dopamine in PD patients may be therapeutic for motor
complications.
AD is a neurodegenerative disease that is
characterized by progressive cognitive decline and memory
dysfunction as well as the presence of neurofibrillary tangles
(NFTs) and senile plaques composed primarily of β-amyloid. ERK1/2
is one of the kinases known to phosphorylate tau and has been shown
to be associated with NFTs and senile plaques (74). Increased levels of activated
ERK1/2 have been found in AD brains, and inhibition of the pathway
can reduce β-amyloid neurotoxicity (91–93). Activated ERK1/2 is found
specifically in intracytoplasmic punctate structures and
intracellular NFTs, primarily in the subpopulation of neurons that
exhibits early AD-related protein deposition. As mentioned above,
ERK1/2 is known to play a critical role in hippocampus synaptic
plasticity and learning and memory. Abnormal ERK1/2 activation in
the hippocampus may impair hippocampal function and contribute to
memory deficits in AD patients. Therefore, improving regulation of
the ERK1/2 pathway may be a central facet for the development of
potential treatments for AD.
Drug addiction is recognized as a type of
neuroadaptive disorder. Because the ERK1/2 pathway plays an
important role in neuronal plasticity in the adult brain,
understanding of the role of this pathway is critical for overall
understanding of the molecular mechanisms underlying drug addiction
and relapse. Exposure to a variety of substances with abuse
potential, including nicotine, alcohol, amphetamine and cocaine,
acutely active ERK1/2 in the striatum and other brain areas
(94–97). Many of the enduring behavioral
effects of acute drug exposure depend on ERK1/2 signaling. Studies
have suggested that ERK1/2 is dynamically regulated following
repeated drug exposure and withdrawal and that changes in ERK1/2
activation directly affect striatal cell excitability (98,99). These effects may be responsible
for the expression of addictive behavior, and alterations of this
pathway may contribute to the drug's rewarding effects and to the
long-term maladaptation induced by drug abuse. Evidence indicates
that ERK1/2 plays a dual role in gene regulation and drug addiction
through direct activation of transcription factors, including Elk1
and cAMP response element-binding protein (CREB), and by chromatin
remodeling via MSK1 and histone H3 phosphorylation (100,101). Because ERK1/2 activation is a
key molecular process in drug self-administration, targeting it may
be a potential treatment strategy for drug addiction.
Amyotrophic lateral sclerosis (ALS) is a CNS disease
that causes the death of motor neurons and that can be either
sporadic or familial origin. Mutant SOD1 is one of the genetic
factors that contribute to the etiology of ALS, and mutant SOD1
induces motor neuron vulnerability. Phosphorylated ERK1/2 has been
shown to be increased in the hippocampus and cerebellum in SOD1
G93A transgenic models (102).
Apolloni et al (103)
showed that ERK1/2 also participates in P2X7 receptor-induced
enhancement of oxidative stress in ALS microglia, together with the
NOX2 pathway. A previous study also identified ERK1/2 as a novel
player in the pathogenesis of ALS associated with transactive
response DNA-binding protein 43 (TDP-43) (77). A recent study also showed that
depletion of TDP-43 in microglia strikingly upregulated the
production of COX-2 and PGE2 through the activation of ERK1/2
signaling (61).
Huntington's disease (HD), a devastating
neurodegenerative disease that is characterized by progressive and
severe cognitive, psychiatric and motor dysfunction, is caused by
an expanded CAG repeat in the huntingtin (Htt) gene. MAPK
signaling, and particularly the Ras-ERK cascade, is among the
pathways that have been implicated in HD. In response to mutant
huntingtin, ERK1/2 is activated and directs a protective
transcriptional response and inhibits apoptotic caspase-3 and -7
activation (104,105). Data from different model systems
indicate that ERK1/2 is involved in HD excitotoxicity at both the
intercellular and intracellular level (106–108). Pharmacological interventions
that promote ERK1/2 activation could suppress the adverse effects
of mutant Htt by activating pro-survival mechanisms and suppressing
apoptotic responses. Thus, studies in both cells and animal models
suggest that the ERK1/2 cascade may be a potential target for
therapeutic interventions for currently untreatable disorders.
ERK1/2 pathway regulated kinase is a central point
in the signaling network and is firmly established as an attractive
target for pharmacological intervention in many diseases.
Currently, inhibitors of the kinase function of Raf and MEK
represent the most studied and advanced approaches for blocking the
ERK1/2 pathway, with several inhibitors under evaluation in
clinical trials and additional inhibitors in preclinical analyses.
Morever, many neurological disease-related studies have
investigated the effects of ERK1/2 pathway inhibitors, whose main
mechanism of action is to prevent the phosphorylation of ERK1 and 2
by the upstream kinases, MEK1 and 2. A number of highly selective
MEK1/2 inhibitors have been development, and many of them have been
tested in a clinical setting. PD98059 and U0126 are
first-generation small-molecule inhibitors of MEK1/2. In
preclinical study, they feature potency and high specificity, with
no or little inhibitory effects on other kinase. Most Raf
inhibitors target mutant B-Raf and the most extensively studied
B-Raf inhibitor in neurological disease is SB386023-b. Both Raf and
MEK inhibitors have been widely applied in many experimental
studies to better understand this pathway and explore its roles in
neurological diseases (Table I).
Other selected new and emerging MEK inhibitors have not been well
studied in neurological diseases, such as PD0325901, selumetinib,
cobimetinib, refametinib and trametinib. The main results obtained
to date strongly suggest that the ERK1/2 pathway may represent a
valid therapeutic target in neurological disorder conditions.
Finally, it has also been proposed that ERK1/2 pathway may be a
significant tool through which to study stroke, neurode-generative
disease and drug addiction.
In summary, the link between the ERK1/2 signaling
pathway and a variety of neurological diseases, including stroke,
neuro-degenerative diseases and drug addiction, demonstrates the
importance of studying the ERK1/2 pathway to human health. More
detailed knowledge of the physiological and pathological functions
of ERK1/2 in the adult nervous system may not only provide insight
for the development of new therapeutic drugs for neurological
disorders but also achieve clinical benefits for patients. Over the
next several years, additional novel therapeutic strategies that
utilize ERK1/2 signaling inhibitors will likely be developed for
neurological disease clinical trials.
|
1
|
Zhu X, Castellani RJ, Takeda A, Nunomura
A, Atwood CS, Perry G and Smith MA: Differential activation of
neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the 'two hit'
hypothesis. Mech Ageing Dev. 123:39–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shioda N, Han F and Fukunaga K: Role of
Akt and ERK signaling in the neurogenesis following brain ischemia.
Int Rev Neurobiol. 85:375–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alam R and Gorska MM: Mitogen-activated
protein kinase signalling and ERK1/2 bistability in asthma. Clin
Exp Allergy. 41:149–159. 2011. View Article : Google Scholar
|
|
5
|
Yao Y, Li W, Wu J, Germann UA, Su MS,
Kuida K and Boucher DM: Extracellular signal-regulated kinase 2 is
necessary for mesoderm differentiation. Proc Natl Acad Sci USA.
100:12759–12764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pagès G, Guérin S, Grall D, Bonino F,
Smith A, Anjuere F, Auberger P and Pouysségur J: Defective
thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice.
Science. 286:1374–1377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charest DL, Mordret G, Harder KW, Jirik F
and Pelech SL: Molecular cloning, expression, and characterization
of the human mitogen-activated protein kinase p44erk1. Mol Cell
Biol. 13:4679–4690. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lefloch R, Pouysségur J and Lenormand P:
Total ERK1/2 activity regulates cell proliferation. Cell Cycle.
8:705–711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lefloch R, Pouysségur J and Lenormand P:
Single and combined silencing of ERK1 and ERK2 reveals their
positive contribution to growth signaling depending on their
expression levels. Mol Cell Biol. 28:511–527. 2008. View Article : Google Scholar :
|
|
10
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji RR, Gereau RW IV, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Brain Res Rev.
60:135–148. 2009. View Article : Google Scholar
|
|
12
|
Lorenz K, Schmitt JP, Vidal M and Lohse
MJ: Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J
Biochem Cell Biol. 41:2351–2355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui Y, Wu J, Jung SC, Park DB, Maeng YH,
Hong JY, Kim SJ, Lee SR, Kim SJ, Kim SJ, et al:
Anti-neuroinflammatory activity of nobiletin on suppression of
microglial activation. Biol Pharm Bull. 33:1814–1821. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Qi X, Chen Y, Sun B, Dai Y and Gu
Y: PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in
IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res
Clin Oncol. 137:1587–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murphy LO and Blenis J: MAPK signal
specificity: the right place at the right time. Trends Biochem Sci.
31:268–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anjum R and Blenis J: The RSK family of
kinases: emerging roles in cellular signalling. Nat Rev Mol Cell
Biol. 9:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Z and Seger R: The ERK signaling
cascade - views from different subcellular compartments.
Biofactors. 35:407–416. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lavoie H and Therrien M: Regulation of RAF
protein kinases in ERK signalling. Nat Rev Mol Cell Biol.
16:281–298. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaeffer HJ, Catling AD, Eblen ST,
Collier LS, Krauss A and Weber MJ: MP1: a MEK binding partner that
enhances enzymatic activation of the MAP kinase cascade. Science.
281:1668–1671. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brahma A and Dalby KN: Regulation of
protein phosphorylation within the MKK1-ERK2 complex by MP1 and the
MP1•P14 heterodimer. Arch Biochem Biophys. 460:85–91. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jameson KL, Mazur PK, Zehnder AM, Zhang J,
Zarnegar B, Sage J and Khavari PA: IQGAP1 scaffold-kinase
interaction blockade selectively targets RAS-MAP kinase-driven
tumors. Nat Med. 19:626–630. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu S, Robbins D, Frost J, Dang A,
Lange-Carter C and Cobb MH: MEKK1 phosphorylates MEK1 and MEK2 but
does not cause activation of mitogen-activated protein kinase. Proc
Natl Acad Sci USA. 92:6808–6812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karandikar M, Xu S and Cobb MH: MEKK1
binds raf-1 and the ERK2 cascade components. J Biol Chem.
275:40120–40127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brennan DF, Dar AC, Hertz NT, Chao WC,
Burlingame AL, Shokat KM and Barford D: A Raf-induced allosteric
transition of KSR stimulates phosphorylation of MEK. Nature.
472:366–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu J, Yu H, Kornev AP, Zhao J, Filbert EL,
Taylor SS and Shaw AS: Mutation that blocks ATP binding creates a
pseudo-kinase stabilizing the scaffolding function of kinase
suppressor of Ras, CRAF and BRAF. Proc Natl Acad Sci USA.
108:6067–6072. 2011. View Article : Google Scholar
|
|
28
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Impey S, Obrietan K and Storm DR: Making
new connections: role of ERK/MAP kinase signaling in neuronal
plasticity. Neuron. 23:11–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Cristo G, Berardi N, Cancedda L,
Pizzorusso T, Putignano E, Ratto GM and Maffei L: Requirement of
ERK activation for visual cortical plasticity. Science.
292:2337–2340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
English JD and Sweatt JD: A requirement
for the mitogen-activated protein kinase cascade in hippocampal
long term potentiation. J Biol Chem. 272:19103–19106. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanterewicz BI, Urban NN, McMahon DB,
Norman ED, Giffen LJ, Favata MF, Scherle PA, Trzskos JM,
Barrionuevo G and Klann E: The extracellular signal-regulated
kinase cascade is required for NMDA receptor-independent LTP in
area CA1 but not area CA3 of the hippocampus. J Neurosci.
20:3057–3066. 2000.PubMed/NCBI
|
|
33
|
Huang SS, He J, Zhao DM, Xu XY, Tan HP and
Li H: Effects of mutant huntingtin on mGluR5-mediated dual
signaling pathways: implications for therapeutic interventions.
Cell Mol Neurobiol. 30:1107–1115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schafe GE, Atkins CM, Swank MW, Bauer EP,
Sweatt JD and LeDoux JE: Activation of ERK/MAP kinase in the
amygdala is required for memory consolidation of pavlovian fear
conditioning. J Neurosci. 20:8177–8187. 2000.PubMed/NCBI
|
|
35
|
Ratto GM and Pizzorusso T: A kinase with a
vision: role of ERK in the synaptic plasticity of the visual
cortex. Adv Exp Med Biol. 557:122–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thiels E, Kanterewicz BI, Norman ED,
Trzaskos JM and Klann E: Long-term depression in the adult
hippocampus in vivo involves activation of extracellular
signal-regulated kinase and phosphorylation of Elk-1. J Neurosci.
22:2054–2062. 2002.PubMed/NCBI
|
|
37
|
Imamura O, Pagès G, Pouysségur J, Endo S
and Takishima K: ERK1 and ERK2 are required for radial glial
maintenance and cortical lamination. Genes Cells. 15:1072–1088.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fyffe-Maricich SL, Karlo JC, Landreth GE
and Miller RH: The ERK2 mitogen-activated protein kinase regulates
the timing of oligodendrocyte differentiation. J Neurosci.
31:843–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samuels IS, Karlo JC, Faruzzi AN,
Pickering K, Herrup K, Sweatt JD, Saitta SC and Landreth GE:
Deletion of ERK2 mitogen-activated protein kinase identifies its
key roles in cortical neurogenesis and cognitive function. J
Neurosci. 28:6983–6995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Samuels IS, Saitta SC and Landreth GE:
MAP'ing CNS development and cognition: an ERKsome process. Neuron.
61:160–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Newbern JM, Wu Y, Morgan-Smith M,
Zhong J, Charron J and Snider WD: MEK is a key regulator of
gliogenesis in the developing brain. Neuron. 75:1035–1050. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Domercq M, Alberdi E, Sánchez-Gómez MV,
Ariz U, Pérez-Samartín A and Matute C: Dual-specific phosphatase-6
(Dusp6) and ERK mediate AMPA receptor-induced oligodendrocyte
death. J Biol Chem. 286:11825–11836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Newbern JM, Li X, Shoemaker SE, Zhou J,
Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, et
al: Specific functions for ERK/MAPK signaling during PNS
development. Neuron. 69:91–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fyffe-Maricich SL, Schott A, Karl M,
Krasno J and Miller RH: Signaling through ERK1/2 controls myelin
thickness during myelin repair in the adult central nervous system.
J Neurosci. 33:18402–18408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Satoh T, Nakatsuka D, Watanabe Y, Nagata
I, Kikuchi H and Namura S: Neuroprotection by MAPK/ERK kinase
inhibition with U0126 against oxidative stress in a mouse neuronal
cell line and rat primary cultured cortical neurons. Neurosci Lett.
288:163–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Subramaniam S and Unsicker K: ERK and cell
death: ERK1/2 in neuronal death. FEBS J. 277:22–29. 2010.
View Article : Google Scholar
|
|
47
|
Jiang Q, Gu Z, Zhang G and Jing G:
Diphosphorylation and involvement of extracellular signal-regulated
kinases (ERK1/2) in glutamate-induced apoptotic-like death in
cultured rat cortical neurons. Brain Res. 857:71–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Benvenisti-Zarom L, Chen-Roetling J and
Regan RF: Inhibition of the ERK/MAP kinase pathway attenuates heme
oxygenase-1 expression and heme-mediated neuronal injury. Neurosci
Lett. 398:230–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Namura S, Iihara K, Takami S, Nagata I,
Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV and
Alessandrini A: Intravenous administration of MEK inhibitor U0126
affords brain protection against forebrain ischemia and focal
cerebral ischemia. Proc Natl Acad Sci USA. 98:11569–11574. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao
M, Xu H, Yang Y, Poon W and Fei Z: Interactions between SIRT1 and
MAPK/ERK regulate neuronal apoptosis induced by traumatic brain
injury in vitro and in vivo. Exp Neurol. 237:489–498. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rundén E, Seglen PO, Haug FM, Ottersen OP,
Wieloch T, Shamloo M and Laake JH: Regional selective neuronal
degeneration after protein phosphatase inhibition in hippocampal
slice cultures: evidence for a MAP kinase-dependent mechanism. J
Neurosci. 18:7296–7305. 1998.PubMed/NCBI
|
|
52
|
Perry VH and Teeling J: Microglia and
macrophages of the central nervous system: the contribution of
microglia priming and systemic inflammation to chronic
neurodegeneration. Semin Immunopathol. 35:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weinstein JR, Zhang M, Kutlubaev M, Lee R,
Bishop C, Andersen H, Hanisch UK and Möller T: Thrombin-induced
regulation of CD95(Fas) expression in the N9 microglial cell line:
evidence for involvement of proteinase-activated receptor(1) and
extracellular signal-regulated kinase 1/2. Neurochem Res.
34:445–452. 2009. View Article : Google Scholar
|
|
54
|
Deng Z, Sui G, Rosa PM and Zhao W:
Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling
pathway in microglial cells. PLoS One. 7:e367392012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim S, Lee MS, Lee B, Gwon WG, Joung EJ,
Yoon NY and Kim HR: Anti-inflammatory effects of
sargachromenol-rich ethanolic extract of Myagropsis myagroides on
lipopolysac-charide-stimulated BV-2 cells. BMC Complement Altern
Med. 14:2312014. View Article : Google Scholar
|
|
56
|
Park GH, Jeon SJ, Ryu JR, Choi MS, Han SH,
Yang SI, Ryu JH, Cheong JH, Shin CY and Ko KH: Essential role of
mitogen-activated protein kinase pathways in protease activated
receptor 2-mediated nitric-oxide production from rat primary
astrocytes. Nitric Oxide. 21:110–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fields J, Cisneros IE, Borgmann K and
Ghorpade A: Extracellular regulated kinase 1/2 signaling is a
critical regulator of interleukin-1β-mediated astrocyte tissue
inhibitor of metallopro-teinase-1 expression. PLoS One.
8:e568912013. View Article : Google Scholar
|
|
58
|
Wang YJ, Zheng YL, Lu J, Chen GQ, Wang XH,
Feng J, Ruan J, Sun X, Li CX and Sun QJ: Purple sweet potato color
suppresses lipopolysaccharide-induced acute inflammatory response
in mouse brain. Neurochem Int. 56:424–430. 2010. View Article : Google Scholar
|
|
59
|
Shao J, Liu T, Xie QR, Zhang T, Yu H, Wang
B, Ying W, Mruk DD, Silvestrini B, Cheng CY, et al: Adjudin
attenuates lipopolysaccharide (LPS)- and ischemia-induced
microglial activation. J Neuroimmunol. 254:83–90. 2013. View Article : Google Scholar
|
|
60
|
Zhao H, Wang SL, Qian L, Jin JL, Li H, Xu
Y and Zhu XL: Diammonium glycyrrhizinate attenuates
Aβ(1-42)-induced neuroinflammation and regulates MAPK and NF-κB
pathways in vitro and in vivo. CNS Neurosci Ther. 19:117–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H,
Chen D, Fu C, Zheng L, Zhen X, et al: Induction of COX-2-PGE2
synthesis by activation of the MAPK/ERK pathway contributes to
neuronal death triggered by TDP-43-depleted microglia. Cell Death
Dis. 6:e17022015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fiore RS, Bayer VE, Pelech SL, Posada J,
Cooper JA and Baraban JM: p42 mitogen-activated protein kinase in
brain: prominent localization in neuronal cell bodies and
dendrites. Neuroscience. 55:463–472. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Atkins CM, Selcher JC, Petraitis JJ,
Trzaskos JM and Sweatt JD: The MAPK cascade is required for
mammalian associative learning. Nat Neurosci. 1:602–609. 1998.
View Article : Google Scholar
|
|
64
|
Feld M, Dimant B, Delorenzi A, Coso O and
Romano A: Phosph-orylation of extra-nuclear ERK/MAPK is required
for long-term memory consolidation in the crab Chasmagnathus. Behav
Brain Res. 158:251–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Igaz LM, Winograd M, Cammarota M,
Izquierdo LA, Alonso M, Izquierdo I and Medina JH: Early activation
of extracellular signal-regulated kinase signaling pathway in the
hippocampus is required for short-term memory formation of a
fear-motivated learning. Cell Mol Neurobiol. 26:989–1002. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kelly A, Laroche S and Davis S: Activation
of mitogen-activated protein kinase/extracellular signal-regulated
kinase in hippo-campal circuitry is required for consolidation and
reconsolidation of recognition memory. J Neurosci. 23:5354–5360.
2003.PubMed/NCBI
|
|
67
|
Villarreal JS and Barea-Rodriguez EJ: ERK
phosphorylation is required for retention of trace fear memory.
Neurobiol Learn Mem. 85:44–57. 2006. View Article : Google Scholar
|
|
68
|
Shalin SC, Zirrgiebel U, Honsa KJ, Julien
JP, Miller FD, Kaplan DR and Sweatt JD: Neuronal MEK is important
for normal fear conditioning in mice. J Neurosci Res. 75:760–770.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Satoh Y, Endo S, Ikeda T, Yamada K, Ito M,
Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, et al:
Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show
deficits in long-term memory; ERK2 has a specific function in
learning and memory. J Neurosci. 27:10765–10776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Selcher JC, Nekrasova T, Paylor R,
Landreth GE and Sweatt JD: Mice lacking the ERK1 isoform of MAP
kinase are unimpaired in emotional learning. Learn Mem. 8:11–19.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Saba-El-Leil MK, Vella FD, Vernay B,
Voisin L, Chen L, Labrecque N, Ang SL and Meloche S: An essential
function of the mitogen-activated protein kinase Erk2 in mouse
trophoblast development. EMBO Rep. 4:964–968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mazzucchelli C, Vantaggiato C, Ciamei A,
Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pagès G,
Valverde O, et al: Knockout of ERK1 MAP kinase enhances synaptic
plasticity in the striatum and facilitates striatal-mediated
learning and memory. Neuron. 34:807–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cestari V, Costanzi M, Castellano C and
Rossi-Arnaud C: A role for ERK2 in reconsolidation of fear memories
in mice. Neurobiol Learn Mem. 86:133–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ferrer I, Blanco R, Carmona M, Ribera R,
Goutan E, Puig B, Rey MJ, Cardozo A, Viñals F and Ribalta T:
Phosphorylated map kinase (ERK1, ERK2) expression is associated
with early tau deposition in neurones and glial cells, but not with
increased nuclear DNA vulnerability and cell death, in Alzheimer
disease, Pick's disease, progressive supranuclear palsy and
corticobasal degeneration. Brain Pathol. 11:144–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Santini E, Valjent E, Usiello A, Carta M,
Borgkvist A, Girault JA, Hervé D, Greengard P and Fisone G:
Critical involvement of cAMP/DARPP-32 and extracellular
signal-regulated protein kinase signaling in L-DOPA-induced
dyskinesia. J Neurosci. 27:6995–7005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ahnstedt H, Säveland H, Nilsson O and
Edvinsson L: Human cerebrovascular contractile receptors are
upregulated via a B-Raf/MEK/ERK-sensitive signaling pathway. BMC
Neurosci. 12:52011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ayala V, Granado-Serrano AB, Cacabelos D,
Naudí A, Ilieva EV, Boada J, Caraballo-Miralles V, Lladó J, Ferrer
I, Pamplona R, et al: Cell stress induces TDP-43 pathological
changes associated with ERK1/2 dysfunction: implications in ALS.
Acta Neuropathol. 122:259–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Feld M, Krawczyk MC, Sol Fustiñana M,
Blake MG, Baratti CM, Romano A and Boccia MM: Decrease of ERK/MAPK
overac-tivation in prefrontal cortex reverses early memory deficit
in a mouse model of Alzheimer's disease. J Alzheimers Dis.
40:69–82. 2014.
|
|
79
|
Fujimoto S, Katsuki H, Ohnishi M, Takagi
M, Kume T and Akaike A: Thrombin induces striatal neurotoxicity
depending on mitogen-activated protein kinase pathways in vivo.
Neuroscience. 144:694–701. 2007. View Article : Google Scholar
|
|
80
|
Maddahi A, Ansar S, Chen Q and Edvinsson
L: Blockade of the MEK/ERK pathway with a raf inhibitor prevents
activation of pro-inflammatory mediators in cerebral arteries and
reduction in cerebral blood flow after subarachnoid hemorrhage in a
rat model. J Cereb Blood Flow Metab. 31:144–154. 2011. View Article : Google Scholar :
|
|
81
|
Ohnishi M, Katsuki H, Fujimoto S, Takagi
M, Kume T and Akaike A: Involvement of thrombin and
mitogen-activated protein kinase pathways in hemorrhagic brain
injury. Exp Neurol. 206:43–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Maddahi A and Edvinsson L: Cerebral
ischemia induces microvascular pro-inflammatory cytokine expression
via the MEK/ERK pathway. J Neuroinflammation. 7:142010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maddahi A, Povlsen GK and Edvinsson L:
Regulation of enhanced cerebrovascular expression of
proinflammatory mediators in experimental subarachnoid hemorrhage
via the mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase pathway. J Neuroinflammation. 9:2742012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ansar S, Maddahi A and Edvinsson L:
Inhibition of cerebro-vascular raf activation attenuates cerebral
blood flow and prevents upregulation of contractile receptors after
subarachnoid hemorrhage. BMC Neurosci. 12:1072011. View Article : Google Scholar
|
|
85
|
Feng D, Wang B, Ma Y, Shi W, Tao K, Zeng
W, Cai Q, Zhang Z and Qin H: The Ras/Raf/Erk pathway mediates the
subarachnoid hemorrhage-induced apoptosis of hippocampal neurons
through phosphorylation of p53. Mol Neurobiol. 53:5737–5748. 2016.
View Article : Google Scholar
|
|
86
|
Liu Y, Qin L, Li G, Zhang W, An L, Liu B
and Hong JS: Dextromethorphan protects dopaminergic neurons against
inflammation-mediated degeneration through inhibition of microglial
activation. J Pharmacol Exp Ther. 305:212–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qian L, Tan KS, Wei SJ, Wu HM, Xu Z,
Wilson B, Lu RB, Hong JS and Flood PM: Microglia-mediated
neurotoxicity is inhibited by morphine through an opioid
receptor-independent reduction of NADPH oxidase activity. J
Immunol. 179:1198–1209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Valjent E, Pascoli V, Svenningsson P, Paul
S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ,
Nairn AC, et al: Regulation of a protein phosphatase cascade allows
convergent dopamine and glutamate signals to activate ERK in the
striatum. Proc Natl Acad Sci USA. 102:491–496. 2005. View Article : Google Scholar :
|
|
89
|
Santini E, Sgambato-Faure V, Li Q, Savasta
M, Dovero S, Fisone G and Bezard E: Distinct changes in cAMP and
extracellular signal-regulated protein kinase signalling in
L-DOPA-induced dyskinesia. PLoS One. 5:e123222010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lindgren HS, Ohlin KE and Cenci MA:
Differential involvement of D1 and D2 dopamine receptors in
L-DOPA-induced angiogenic activity in a rat model of Parkinson's
disease. Neuropsychopharmacology. 34:2477–2488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pei JJ, Braak H, An WL, Winblad B, Cowburn
RF, Iqbal K and Grundke-Iqbal I: Up-regulation of mitogen-activated
protein kinases ERK1/2 and MEK1/2 is associated with the
progression of neurofibrillary degeneration in Alzheimer's disease.
Brain Res Mol Brain Res. 109:45–55. 2002. View Article : Google Scholar
|
|
92
|
Zhu X, Lee HG, Raina AK, Perry G and Smith
MA: The role of mitogen-activated protein kinase pathways in
Alzheimer's disease. Neurosignals. 11:270–281. 2002. View Article : Google Scholar
|
|
93
|
Liu F, Su Y, Li B and Ni B: Regulation of
amyloid precursor protein expression and secretion via activation
of ERK1/2 by hepatocyte growth factor in HEK293 cells transfected
with APP751. Exp Cell Res. 287:387–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lu L, Koya E, Zhai H, Hope BT and Shaham
Y: Role of ERK in cocaine addiction. Trends Neurosci. 29:695–703.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hoffmann HM, Nadal R, Vignes M and Ortiz
J: Chronic cocaine self-administration modulates ERK1/2 and CREB
responses to dopamine receptor agonists in striatal slices. Addict
Biol. 17:565–575. 2012. View Article : Google Scholar
|
|
96
|
Pascoli V, Cahill E, Bellivier F, Caboche
J and Vanhoutte P: Extracellular signal-regulated protein kinases 1
and 2 activation by addictive drugs: a signal toward pathological
adaptation. Biol Psychiatry. 76:917–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Agoglia AE, Sharko AC, Psilos KE, Holstein
SE, Reid GT and Hodge CW: Alcohol alters the activation of ERK1/2,
a functional regulator of binge alcohol drinking in adult C57BL/6J
mice. Alcohol Clin Exp Res. 39:463–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Boudreau AC, Reimers JM, Milovanovic M and
Wolf ME: Cell surface AMPA receptors in the rat nucleus accumbens
increase during cocaine withdrawal but internalize after cocaine
challenge in association with altered activation of
mitogen-activated protein kinases. J Neurosci. 27:10621–10635.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schumann J and Yaka R: Prolonged
withdrawal from repeated noncontingent cocaine exposure increases
NMDA receptor expression and ERK activity in the nucleus accumbens.
J Neurosci. 29:6955–6963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Brami-Cherrier K, Roze E, Girault JA,
Betuing S and Caboche J: Role of the ERK/MSK1 signalling pathway in
chromatin remodelling and brain responses to drugs of abuse. J
Neurochem. 108:1323–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ciccarelli A and Giustetto M: Role of ERK
signaling in activity-dependent modifications of histone proteins.
Neuropharmacology. 80:34–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chung YH, Joo KM, Lim HC, Cho MH, Kim D,
Lee WB and Cha CI: Immunohistochemical study on the distribution of
phosphorylated extracellular signal-regulated kinase (ERK) in the
central nervous system of SOD1G93A transgenic mice.
Brain Res. 1050:203–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Apolloni S, Parisi C, Pesaresi MG, Rossi
S, Carrì MT, Cozzolino M, Volonté C and D'Ambrosi N: The NADPH
oxidase pathway is dysregulated by the P2X7 receptor in
the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J
Immunol. 190:5187–5195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Apostol BL, Illes K, Pallos J, Bodai L, Wu
J, Strand A, Schweitzer ES, Olson JM, Kazantsev A, Marsh JL, et al:
Mutant huntingtin alters MAPK signaling pathways in PC12 and
striatal cells: ERK1/2 protects against mutant
huntingtin-associated toxicity. Hum Mol Genet. 15:273–285. 2006.
View Article : Google Scholar
|
|
105
|
Varma H, Cheng R, Voisine C, Hart AC and
Stockwell BR: Inhibitors of metabolism rescue cell death in
Huntington's disease models. Proc Natl Acad Sci USA.
104:14525–14530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liévens JC, Rival T, Iché M, Chneiweiss H
and Birman S: Expanded polyglutamine peptides disrupt EGF receptor
signaling and glutamate transporter expression in Drosophila. Hum
Mol Genet. 14:713–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang YY, Martin KC and Kandel ER: Both
protein kinase A and mitogen-activated protein kinase are required
in the amygdala for the macromolecular synthesis-dependent late
phase of long-term potentiation. J Neurosci. 20:6317–6325.
2000.PubMed/NCBI
|
|
108
|
Ribeiro FM, Paquet M, Ferreira LT, Cregan
T, Swan P, Cregan SP and Ferguson SS: Metabotropic glutamate
receptor-mediated cell signaling pathways are altered in a mouse
model of Hunti-ngton's disease. J Neurosci. 30:316–324. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Maddahi A, Chen Q and Edvinsson L:
Enhanced cerebrovascular expression of matrix metalloproteinase-9
and tissue inhibitor of metalloproteinase-1 via the MEK/ERK pathway
during cerebral ischemia in the rat. BMC Neurosci. 10:562009.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ahnstedt H, Mostajeran M, Blixt FW,
Warfvinge K, Ansar S, Krause DN and Edvinsson L: U0126 attenuates
cerebral vasocon-striction and improves long-term neurologic
outcome after stroke in female rats. J Cereb Blood Flow Metab.
35:454–460. 2015. View Article : Google Scholar
|
|
111
|
Vikman P, Ansar S, Henriksson M, Stenman E
and Edvinsson L: Cerebral ischemia induces transcription of
inflammatory and extracellular-matrix-related genes in rat cerebral
arteries. Exp Brain Res. 183:499–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Maddahi A, Kruse LS, Chen QW and Edvinsson
L: The role of tumor necrosis factor-α and TNF-α receptors in
cerebral arteries following cerebral ischemia in rat. J
Neuroinflammation. 8:1072011. View Article : Google Scholar
|
|
113
|
Zhang J, Xu X, Zhou D, Li H, You W, Wang Z
and Chen G: Possible role of Raf-1 kinase in the development of
cerebral vaso-spasm and early brain injury after experimental
subarachnoid hemorrhage in rats. Mol Neurobiol. 52:1527–1539. 2015.
View Article : Google Scholar
|
|
114
|
Beg SA, Hansen-Schwartz JA, Vikman PJ, Xu
CB and Edvinsson LI: ERK1/2 inhibition attenuates cerebral blood
flow reduction and abolishes ET(B) and 5-HT(1B) receptor
upregulation after subarachnoid hemorrhage in rat. J Cereb Blood
Flow Metab. 26:846–856. 2006. View Article : Google Scholar
|
|
115
|
Li J, Fan Y, Zhang YN, Sun DJ, Fu SB, Ma
L, Jiang LH, Cui C, Ding HF and Yang J: The Raf-1 inhibitor GW5074
and the ERK1/2 pathway inhibitor U0126 ameliorate PC12 cells
apoptosis induced by 6-hydroxydopamine. Pharmazie. 67:718–724.
2012.PubMed/NCBI
|
|
116
|
Bartolomé F, de Las Cuevas N, Muñoz U,
Bermejo F and Martín-Requero A: Impaired apoptosis in lymphoblasts
from Alzheimer's disease patients: cross-talk of
Ca2+/calmodulin and ERK1/2 signaling pathways. Cell Mol
Life Sci. 64:1437–1448. 2007. View Article : Google Scholar
|
|
117
|
Pei JJ, Gong CX, An WL, Winblad B, Cowburn
RF, Grundke-Iqbal I and Iqbal K: Okadaic-acid-induced inhibition of
protein phosphatase 2A produces activation of mitogen-activated
protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in
Alzheimer's disease. Am J Pathol. 163:845–858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chong YH, Shin YJ, Lee EO, Kayed R, Glabe
CG and Tenner AJ: ERK1/2 activation mediates Abeta oligomer-induced
neurotoxicity via caspase-3 activation and tau cleavage in rat
organotypic hippocampal slice cultures. J Biol Chem.
281:20315–20325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Andersen JM, Myhre O and Fonnum F:
Discussion of the role of the extracellular signal-regulated
kinase-phospholipase A2 pathway in production of reactive oxygen
species in Alzheimer's disease. Neurochem Res. 28:319–326. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pan B, Zhong P, Sun D and Liu QS:
Extracellular signal-regulated kinase signaling in the ventral
tegmental area mediates cocaine-induced synaptic plasticity and
rewarding effects. J Neurosci. 31:11244–11255. 2011. View Article : Google Scholar : PubMed/NCBI
|