Introduction
In the United States, the incidence of urothelial
cell carcinoma (UCC) of the bladder or transitional cell carcinoma
(TCC) is approximately 70,000 new cases annually, leading to
approximately 15,000 deaths. The risk factors for TCC are many, but
most commonly include smoking, chronic cystitis and chemical
exposure, including exposure to aniline dye. Initial presentation
most commonly appears in the form of gross hematuria, but can also
present with irritative voiding symptoms, anemia andrenal failure
due to obstruction. The most common subtype of bladder cancer is
superficial bladder cancer, namely the non-muscle invasive form
limited to the urothelium. Of all new cases of bladder cancer, the
non-muscle invasive type accounts for nearly 70%. The rate of
progression and recurrence is up to 40 and 70%, respectively
(1,2).
The gold standard initial treatment for bladder
cancer includes cystoscopy and transurethral resection of the
bladder tumor. During this time, malignant cells can float onto the
adjacent epithelium, increasing the risk of recurrence. Once
pathological evaluation confirms a non-muscle invasive disease, the
standard of care to prevent recurrence and progression is the
intravesical instillation of immunotherapeutic agents. Intravesical
immunotherapy results in a massive local immune response
characterized by the induced expression of cytokines in the urine
and bladder wall and by an influx of granulocytes, and mononuclear
and dendritic cells (3,4). Current intravesical treatments are
costly and require the special handling of these agents. Patients
are treated with intravesical therapy with bacillus Calmette-Guerin
(BCG) bacterium, or mitomycin C (MMC) following resection. Both of
these can cause severe side-effects, which are rarely
life-threatening (3).
BCG, is an attenuated live vaccine for tuberculosis
that has shown antitumor activity. The proposed mechanism of action
is T-cell activation to attack abnormal urothelial cells and this
may have a direct inhibitory effect on tumor cell invasion.
Intravesical BCG has been shown to decrease tumor recurrence and
progression. Intravesical treatment usually begins 2–4 weeks
following transurethral resection to minimize systemic absorption
and undesirable side-effects. Treatment regimens include an
induction phase that consists of weekly instillation for 6 weeks as
long as the patient can tolerate the agent. This is followed by a
maintenance regimen that can last for 1–3 years, depending on the
tumor grade and characteristics. Side-effects of the agent include
cystitis, dysuria, malaise, fatigue and systemic inflammatory
response syndrome, and can be as severe as BCG sepsis.
Contraindications include immunosuppression, traumatic
catheterization, active urinary tract infection or gross hematuria
and a personal history of BCG sepsis (3,5–8).
MMC is an alkylating agent that inhibits DNA synthesis.
Intravesical MMC has a clear impact on tumor recurrence when
immediately instilled following the transurethral resection of
bladder tumor and in the adjuvant setting. There is no clear
evidence of an impact on progression. MMC is usually administered
immediately following the resection of the bladder tumor, as long
as bladder perforation has not occurred. Systemic absorption, which
can cause myelosuppression, is rare as MMC has a high molecular
weight. Common side-effects include contact dermatitis and
irritative voiding symptoms (3,5–8).
A meta-analysis of 9 clinical trials compared its
efficacy on progression with that of BCG. Within a median follow-up
of 26 months, 7.67% of the patients in the BCG group and 9.44% of
the patients in the MMC group developed tumor progression (9). Another review found a 38% reduction
in tumor recurrence with MMC. This was not as effective as BCG, but
was considered in most studies to make MMC a viable option in light
of its lesser side-effects, particularly the low, but real risk of
sepsis (10).
We previously examined the efficacy of
epigallocatechin-3-gallate (EGCG) in comparison with MMC in the
prevention of tumor cell implantation/growth in an animal model of
superficial bladder cancer (11).
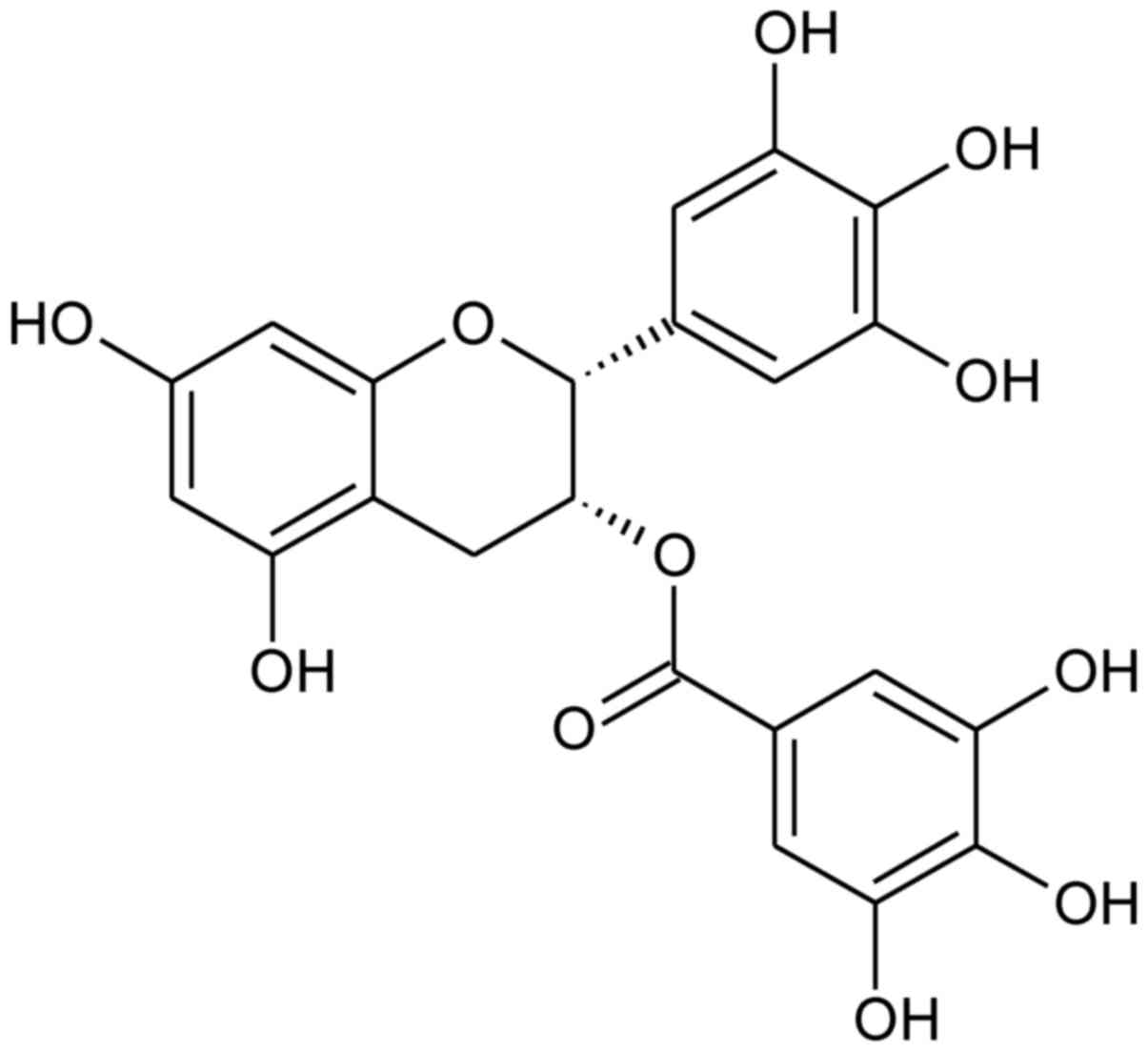
EGCG
{[(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl]
3,4,5-trihydroxybenzoate; Fig. 1}
is the most abundant catechin in green tea. EGCG and green tea
extracts are under investigation for their potential effects on
human health and disease (12–15). One of the promising uses of EGCG
and green tea extracts is the prevention and treatment of cancer
(16–19). Experiments on cells in
vitro and on animal models in vivo have shown the
anti-neoplastic activity of EGCG(17,20–24). However, the mechanism of action of
EGCG is poorly understood due to the deficiency of analytical tools
that can detect the precise behavior of such a small molecule.
Nevertheless, numerous possible targets have been suggested, such
as Janus kinase (JAK)/signal transducer and activator of
transcription (STAT), mitogen-activated protein kinase (MAPK),
phosphoinositide 3-kinase (PI3K)/AKT (25), urokinase-type plasminogen
activator (uPA) and matrix metalloproteinases (MMPs) (26–29). The mechanisms of action of EGCG
make matters more complicated, given the fact that it is labile in
aqueous solutions. It has been reported that auto-oxidation and
epimerization are the two major reactions responsible for the
instability of EGCG (30). Green
tea catechins, including EGCG bind to plasma proteins that alter
their plasma concentration, tissue delivery and biological
activity. Incubation with human serum albumin (HSA) green tea
extracts and pure EGCG results in full binding to HSA (31,32). Moreover, poor absorption results
in the low systemic bioavailability of EGCG following oral
administration and its conversion to the glucuronide renders EGCG
even less available in the circulation (33,34). EGCG is highly unstable under
sunlight (35). This polyphenol,
following exposure to irradiance, such as natural sunlight,
photo-degrades approximately 69%, after 1 h of irradiation. The
inclusion of UVB (290–320 nm) produces a photo-degradation EGCG of
61% (36). However, at least to
the best of our knowledge, there are no studies available to date
on the effects of ionizing radiation on the stability of this
polyphenol. Therefore, it seems that it is safer to use green tea
extracts or EGCG in situ for the treatment of different
ailments. There are several clinical studies on humans using
topical EGCG treatment (37–39). EGCG treatment has also been
approved by the United States Food and Drug Administration
(polyphenon E - purified extract of green tea) ointment for
external genital warts treatment (40,41).
We previously studied the efficacy of EGCG in
comparison with MMC to prevent tumor cell implantation/growth in an
animal model of superficial bladder cancer and investigated the
possible mechanisms of action. Female Fisher 344 rats were used to
study the in situ effects of EGCG and MMC for the prevention
of transitional rat cell tumor implantation (AY-27) (11). Experiments revile the slightly
better effects of EGCG than MMC in these experimental animals.
Thus, EGCG can be used as an agent to decrease tumor cell
implantation and consequent intravesical cancer growth in a
bladder. The use of EGCG is a potential novel therapeutic strategy
for use as an adjunct to endoscopic bladder tumor resection. These
data suggest that EGCG lowers proteolytic activity and decreases
the probability of cancer cell implantation rather than direct
cancer cell cytotoxicity. Molecular modeling suggests that EGCG
inhibits uPA and matrix MMP-9 (11,42,43). In preparation for clinical
studies, in this study, we wished to determine the stability of
EGCG during different sterilization processes for use in the
treatment of superficial transitional cell carcinoma of the human
bladder.
Materials and methods
Chemicals
EGCG and Polyphenon E® (highly purified
green tea extract) were generous gifts from Taiyo International
Inc., Minneapolis, MN, USA.
Sterilization of EGCG by filtration and
lyophilization
Under aseptic conditions, 1,100 mg of EGCG were
dissolved in 80.0 ml distilled water and filtrated through a 0.2
µm filter. The solution was aliquoted into 20 sterile vials
dosing accurately 4.0 g of solution per one vial. The solution of
EGCG (4 ml) in the vials was freeze-dried using a vacuum
freeze-dryer (Alpha type 2–4; Martin Christ, Harz, Germany)
immediately after preparation. No lyoprotectant was added to the
vials. The samples were frozen to a terminal temperature of −45°C
and were kept at this temperature for 5 h. Primary drying was
performed by keeping the vials for 43 h at a pressure of 1.03 mbar,
during which the temperature of the freeze-dryer shelf slowly
increased up to 0°C. Secondary drying was carried out by reducing
the pressure to 0.1 mbar and increasing the shelf temperature to
30°C. Secondary drying time was 7 h. Lyophilization was terminated
by venting the drying chamber with air.
Irradiation with e-beam
Approximately 0.5 g of EGCG was placed in 4 ml
colorless glass vials closed with a plastic stopper and irradiated
25, 100 and 200 kGy with the e-beam from a linear electron
accelerator Elektronika 10/10. The energy of electrons was set up
for 9.96 MeV and the current intensity of 6.2 µA.
Melting point determination
Melting temperature was determined using Meltter
Toledo MP70 (Toledo, OH, USA)instrument in temperature 180–230°C
increasing temperature1°C/min.
Fourier transform infrared spectroscopy
(FTIR)
Samples of EGCG (1 mg each) irradiated with 25 and
200 kGy dose, lyophilized, or the unirradiated control were mixed
with 300 mg of KBr to produce tablets (1.3 cm × 0.1 cm). FTIR
spectrums were collected on an IRAffinity-1S Fourier Transform
Infrared Spectrophotometer (Shimadzu, Kyoto, Japan) instrument in
range of 400–4,000 cm−1, with a resolution of 4.0
cm−1 and 40 scans.
Ultraviolet (UV)-visible
spectrophotometry
Samples of EGCG (1 mg each) irradiated with 25 and
200 kGy dose, lyophilized, or the unirradiated control were
dissolved in 0.9% NaCl and diluted 1:25 in the same solution.
Samples were analyzed using a PerkinElmer spectrophotometer
(PerkinElmer, Inc., Waltham, MA, USA) in a 200–400 nm range.
High-performance liquid chromatography
(HPLC)
EGCG, irradiated EGCG and the lyophilized samples
were analyzed by the HPLC method for the quantitative determination
of the content. The HPLC equipment includes a Merck Hitachi L-7100
HPLC pump, a L-7450 photo diode array detector, a L-7200
autosampler, a D-700 interphase module and a column oven (Merck,
Hitachi, England). The analytical column was reverse phase C18
(LiChrospher 100, endcapped 5 µm) 250×4 mm. The following
solvents were used: 1% acetic acid – acetonitryle 75:25 (v/v), flow
0.8 ml/min. All solvents were filtered through a 0.45 µm
filter and degassed by ultrasonication. Separation was performed at
25°C and the analytical wavelength was set up for 280 nm.
Electron paramagnetic resonance (EPR)
spectroscopy
Continuous-wave X-band (9.4 GHz) electron
paramagnetic resonance measurements were performed on a Bruker
ELEXSYS E500 spectrometer with a ER49X SuperX microwave bridge
equipped with super high sensitivity probehead (Bruker BioSpin
GmbH, Rheinstetten, Germany). Magnetic field measurements were
achieved by an NMT-Teslameter ER 036™ (Bruker BioSpin GmbH). All
EPR experiments were performed at room temperature. Spin
concentration was obtained after double integration of EPR spectra
according to the procedure described elsewhere (44). The number of free radicals was
calculated from EPR spectra recorded at low microwave power (0.5
mW) to avoid saturation effects.
Microbiological assay
The following reference strains were used:
Pseudomonas aeruginosa ATCC 9027, Staphylococcus
aureus ATCC 6538, Bacillus subtilis ATCC 6633,
Clostridium sporogenes ATCC 19404, Candida albicans
ATCC 10231, and Aspergillus brasiliensis ATCC 16404. The
strains were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Culture media and solutions were as
follows: soybean-casein digest medium and fluid thioglycollate
medium (both from Merck Millipore, VWR International SAS,
Fontenay-sous-Bois, France). Sterile sodium chloride, 9 g/l
solution was from Polpharma (Starogard, Poland). Growth promotion
test of aerobes, anaerobes and fungi was done under aseptic
condition. Portion of fluid thioglycollate medium was inoculated
with 100 CFU of the separate species of micro-organism:
Clostridium sporogenes, Pseudomonas aeruginosa and
Staphylococcus aureus. A portion of soyabean-casein digest
medium was inoculated with Bacillus subtilis, Candida
albicans and Aspergillus brasiliensis. All media were
incubated for 3 days for bacteria and 5 days for fungi.
Cell proliferation assay
The AY-27 rat transitional cell line was a generous
gift from Dr Samuel Cohen, Department of Pathology and
Microbiology, University of Nebraska Medical Center and was grown
in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS),
penicillin and streptomycin. Cells (104 cells/well) were
treated with 100 µM of control, irradiated (25 kGy) and
lyophilized EGCG for 2 h after which fresh media replaced and cells
were grown for 48 h (42). Cell
proliferation assay was carried out using the CellTiter
96®Non-Radioactive Cell Proliferation Assay kit from
Promega (Madison, WI, USA). The assay was carried out according to
the manufacturer's instructions
Results
The research methodology and analytical methods were
selected to show changes in chemical structure and a polymorphic
form to demonstrate the presence of degradation products and free
radicals in sterilized EGCG in comparison to the control samples.
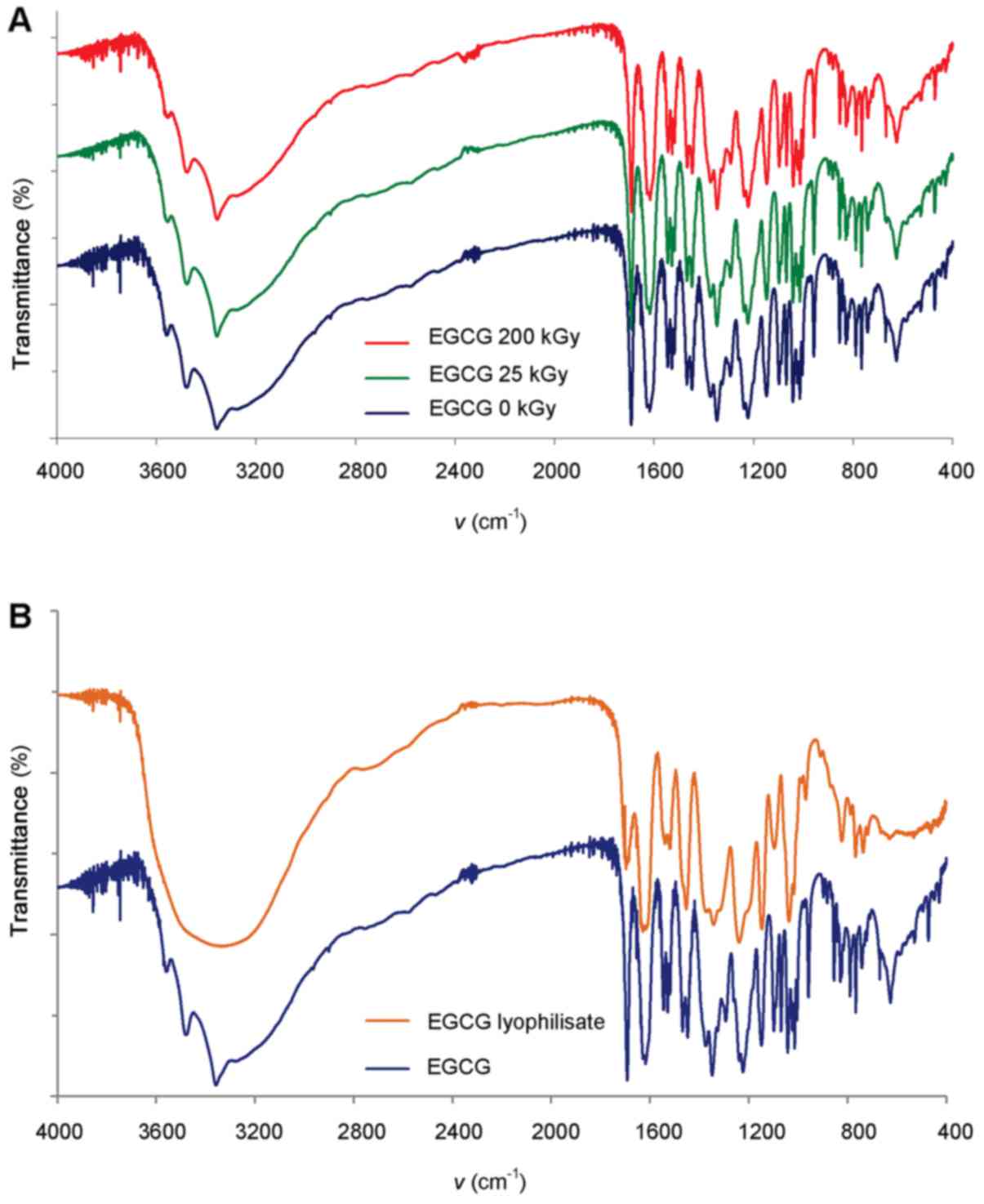
By comparing the spectra of FTIR-irradiated EGCG and control
samples, we did not detect differences in the individual absorption
bands or shifts in spectrums, showing that ionizing radiation at a
dose of both 25 and 200 kGy does not cause the production of
degradation products detectable by FTIR, or changes the form of
polymorphic forms (Fig. 2A).
Different results were obtained for EGCG sterilized by filtration
and freeze-dried. FTIR spectrums showed most dominant changes in
the range of 3,600-3,200 cm−1 among some others
(Fig. 2B), which may be the
result of hydration of compound in the fingerprint region or
changes of morphological form of this compound.
Changes in the form of EGCG can be monitored by the
determination of the temperature of the melting point using the
capillary method as well. The melting point analysis determines
that EGCG does not show the properties of a typical crystalline
compound. Samples of EGCG during heating change color from a light
pink to dark pink followed by black products thermo-degradation,
and then finally the test substance is melted and completely
degraded. The process was similar to the samples irradiated and not
radiated at a dose of 25 kGy. However, EGCG samples irradiated with
doses of 100 and 200 kGy showed little reduction in the melting
temperature (1.0–1.3°C) (Table
I). For a sample of EGCG sterilized by filtration and dried by
lyophilization, we did not observe characteristic melting, which
can be an indication of the completely amorphic form of this
compound. Regardless of some changes in the polymorphic form of
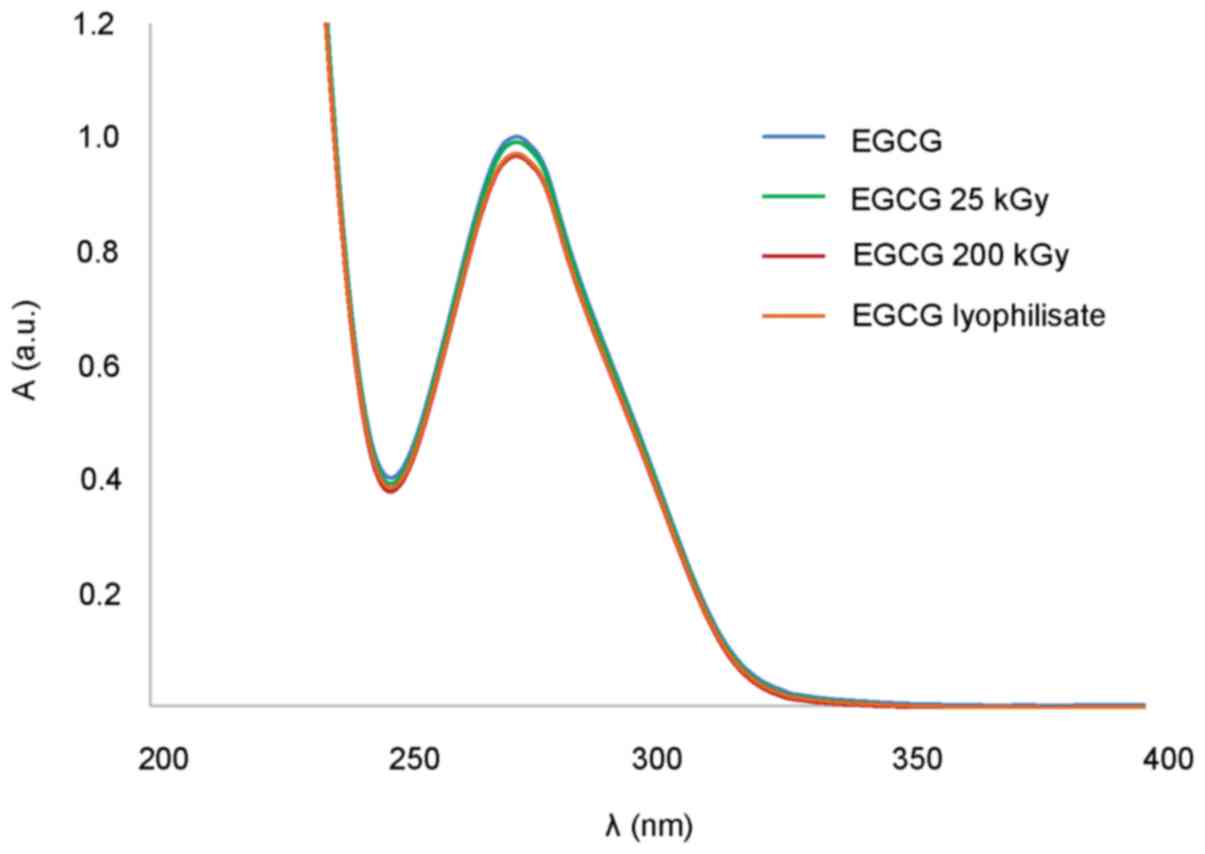
EGCG during radiation and specially the freeze drying process,
aqueous solutions exhibit very similar spectrum in the range of
200–400 nm (Fig. 3).
 | Table IParameters of EGCG melting point
before and after irradiation. |
Table I
Parameters of EGCG melting point
before and after irradiation.
| Dose (kGy) | Beginning of
melting process Tb (°C) | End of melting
process Te (°C) |
ΔTe-Tb (°C) |
|---|
| 0 | 202.2 | 206.2 | 4.0 |
| 25 | 202.3 | 206.2 | 3.9 |
| 100 | 201.2 | 204.0 | 3.8 |
| 200 | 200.9 | 203.8 | 3.8 |
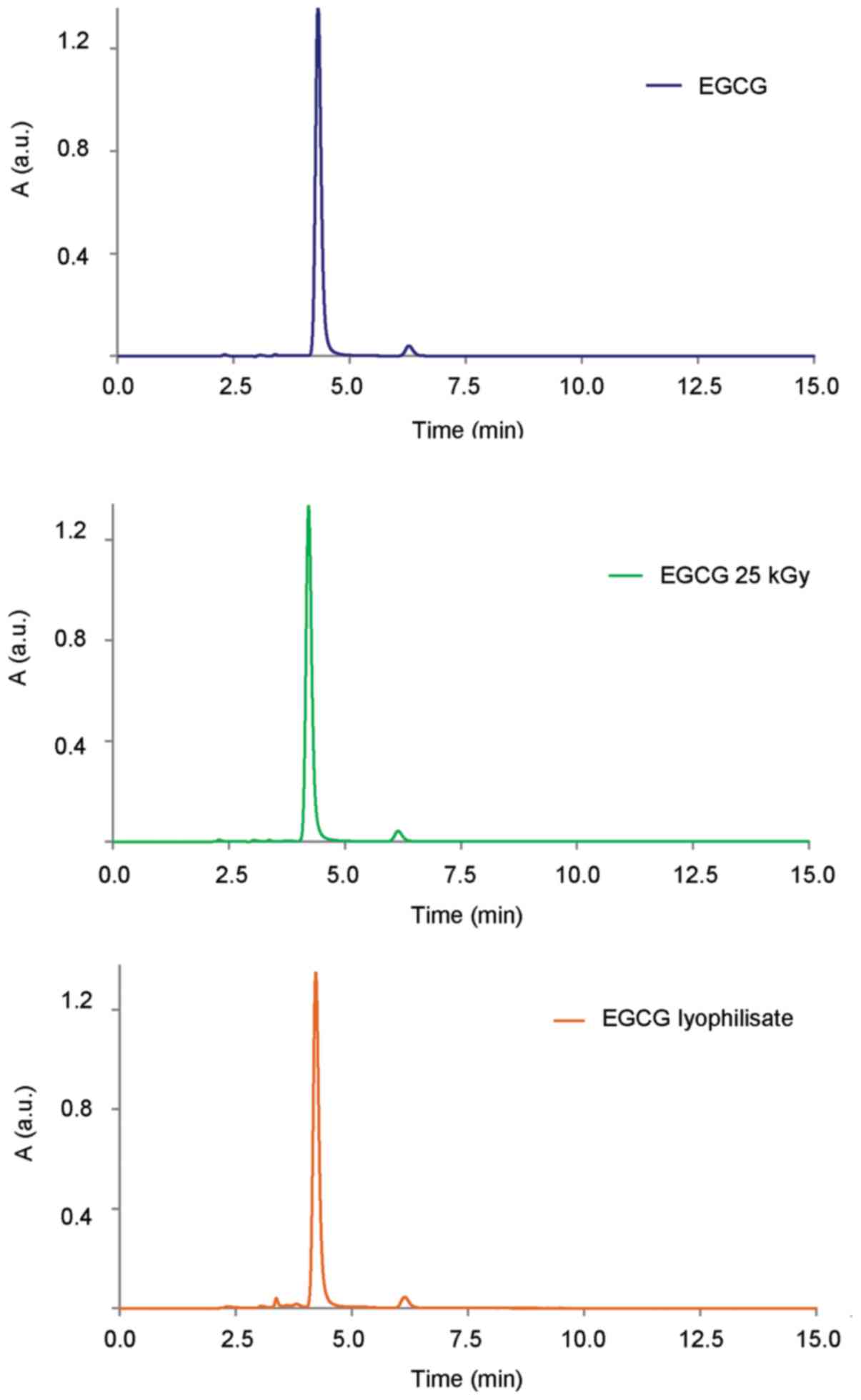
HPLC analysis revealed that the control EGCG and
EGCG irradiated with a dose of 25 kGy was not altered (Fig. 4). Quantitative analysis of the
results indicated that the content of EGCG following irradiation
with a dose of 25 kGy was changed by 0.5%. With increasing doses of
radiation, we further observed the loss of EGCG content (100 kGy of
1.4% and 200 kGy of 2.1%), which is an indication that ionizing
radiation causes some distraction of the tested compound. The
chromatogram of lyophilized EGCG had additional peaks between 3 and
4 min of retention time, which are evidence of the presence of
degradation products, which can also be a cause of changes in the
FTIR spectra. Moreover, quantitative analysis of the results
obtained indicated that the content of EGCG after the freeze-drying
process decreased by 0.6% (data not shown).
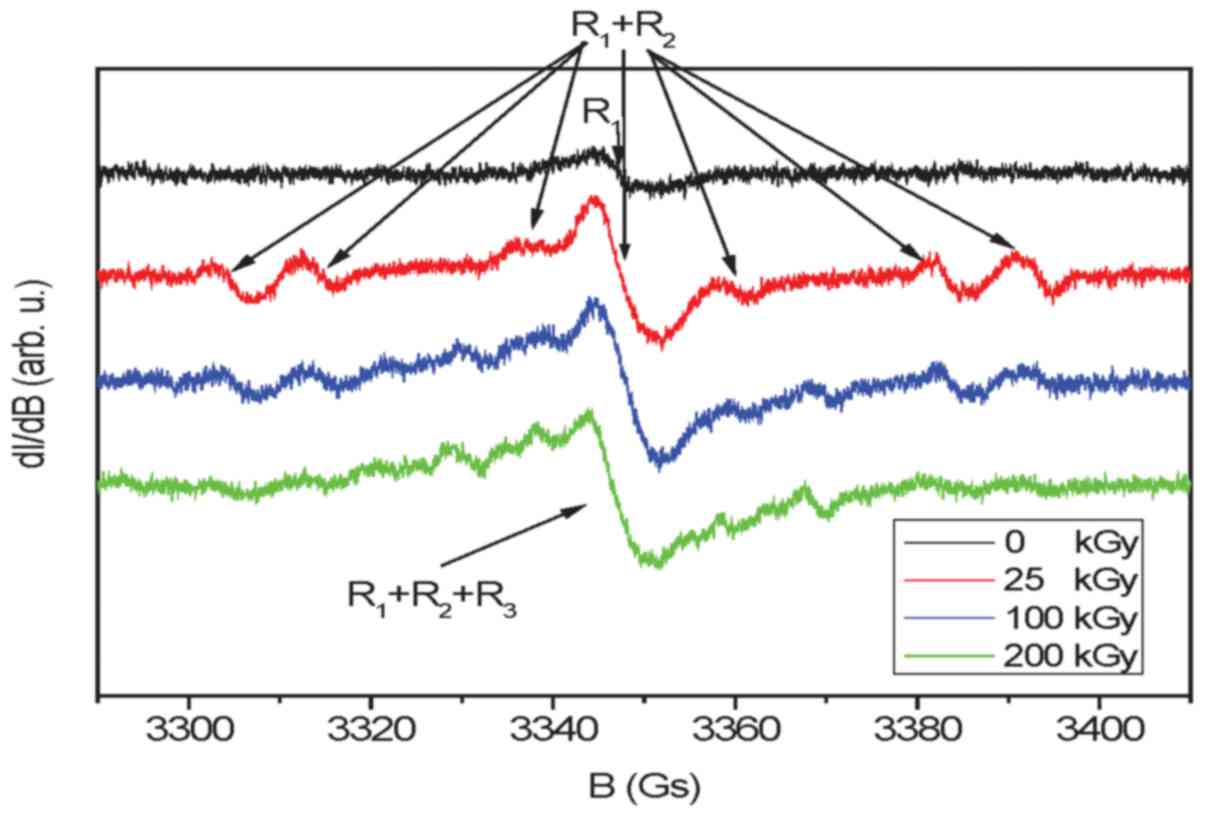
EPR spectrum of unirradiated EGCG consists of a
single free radical line R1 characterized by the
spectroscopic coefficient g = 2.0040 (±0.0005) and line width 6
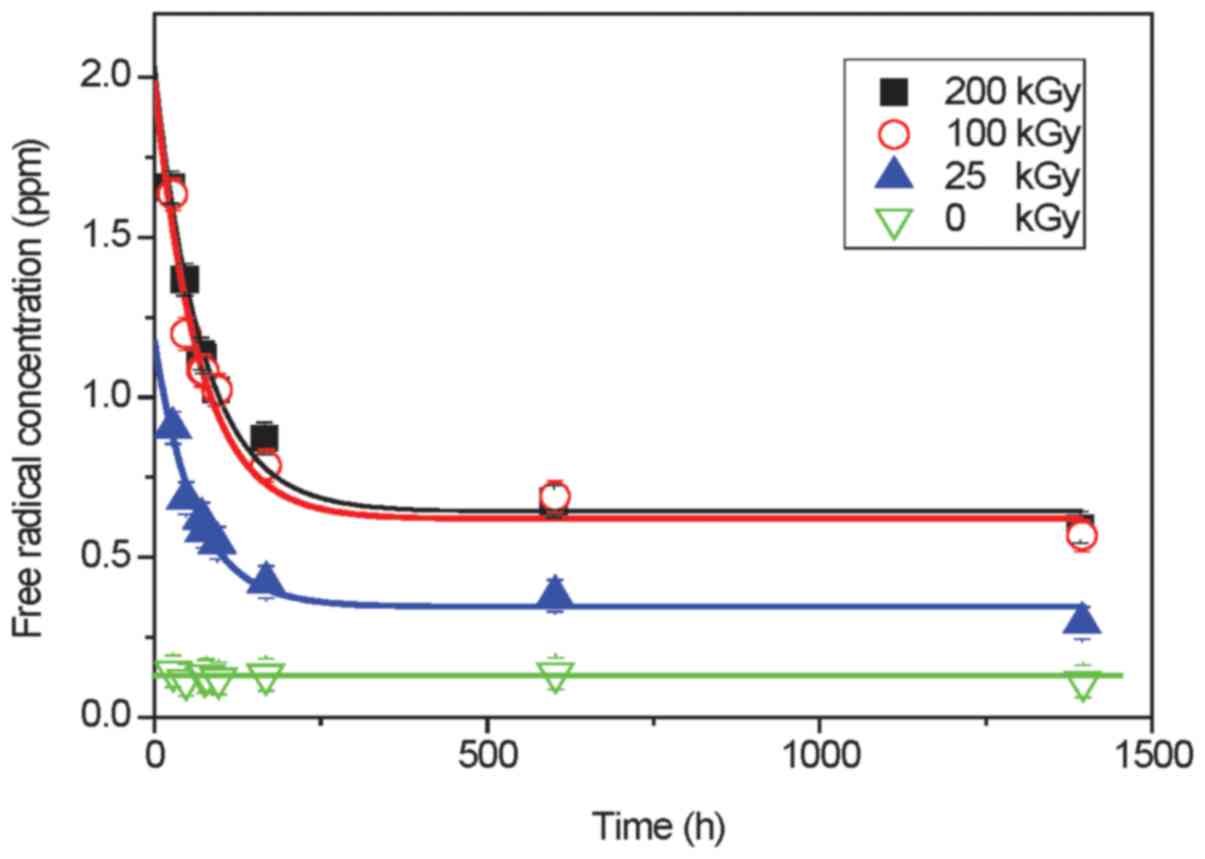
(±0.5) Gs (Fig. 5). The level of
free radicals for this sample was time-independent (Fig. 6) and reached only 0.13 ppm
(Table II). No hyperfine
structure indicates that the unpaired electron is localized mainly
on oxygen and free radicals are formed by the removal of one
hydrogen atom from the OH group. An irradiation dose of 25 kGy
creates additional free radicals of which the concentration reaches
1.18 ppm. In addition to free radical R1, one can
observe another radical R2 (the same g-factor value as
for R1) with hyperfine structure derived from the three
non-equivalent hydrogen nuclei. This radical is characterized by
hyperfine constant: AH1=44.5 (±1) Gs,
AH2=34.5 (±1) Gs, AH3=10 (±1) Gs, and most
probably is created by the removal of one hydrogen atom from the
CH2 or CH group of chromene ring. The spectra of more
irradiated EGCG (100 and 200 kGy) show additional lines
(R3) coming from radicals produced as a result of
subsequent hydrogen radiation defects. Time-dependent free radical
concentration C(t) for sterilized samples (Fig. 6) can be described by the following
equation:
 | Table IIParameters describing the
concentration of free radicals vs. time for no irradiated and
irradiated EGCG. |
Table II
Parameters describing the
concentration of free radicals vs. time for no irradiated and
irradiated EGCG.
| Samples | Initial
concentration of radicals at t=0 C(t=0) = Cf +
C0 (ppm) | Concentration of
stable radicals Cf (ppm) | Concentration of
unstable radicals at t=0 C0 (ppm) | Mean lifetime of
unstable radicals T0 (h) | Half-life of
unstable radicals T1/2 (h) |
|---|
| 0 kGy | 0.13±0.5 | 0.13±0.5 | – | – | – |
| 25 kGy | 1.18±0.11 | 0.35±0.02 | 0.83±0.09 | 61.8±10.4 | 42.8±7.2 |
| 100 kGy | 1.98±0.26 | 0.62±0.06 | 1.36±0.20 | 66.4±15.4 | 46.0±10.6 |
| 200 kGy | 2.02±0.16 | 0.64±0.04 | 1.38±0.12 | 71.6±10.0 | 49.6±6.9 |
where Cf is the concentration of stable free radicals,
C0 is the concentration of unstable free radicals and
T0 is the mean lifetime of unstable free radicals, t is
time.
Although, as shown in Table II unstable free radicals are
characterized by comparable values of the mean lifetimes for all
sterilized drugs, one can notice that increasing dose of
irradiation causes a slight increase in the mean lifetime.
Moreover, the concentration of free radicals does not increase
linear with a dose of irradiation (almost the same parameters in
Table II for 100 and 200 kGy)
suggesting additional creation of non-radical defects.
We did not observe any statistically significant
changes in the growth characteristics of the cells treated with
control EGCG, irradiated and lyophilized EGCG (data not shown).
Discussion
Local treatment of bladder cancer offers several
benefits such us: (i) precise control of the amount of medication
delivered to the bladder; (ii) high bioavailability with a low dose
used; and (iii) the short time of treatment prevents the
degradation or modification of the drug used. However, current
intra-vesical treatments are extremely costly and require the
special handling of these anti-neoplastic agents. Initially,
superficial bladder cancer is treated with transurethral resection
(TUR), during which cancer cells float freely onto the adjacent
epithelium, potentially increasing the risk of recurrence (45–50). To prevent this scenario, patients
are treated with intravesical therapy with BCG bacterium related to
tuberculosis (51,52), or MMC following resection or
fulguration (destroying the lesion by electric current) or both
agents (53). BCG, a form of
immunotherapy, can cause side-effects, such as flu-like syndrome
with fever, chills and fatigue, cystitis, or a more serious
systemic reaction which rarely leads to death (54,55). MMC is utilized similarly and
carries its own drawbacks to clinical use, including bladder
infection, irritative voiding symptoms, special handling and
preparation precautions, and bladder calcifications among others
(55–58).
We previously evaluated green tea extracts
containing EGCG as a potential novel intravesical agent for the
prevention of cell tumor implantation. Preliminary experiments were
very promising. EGCG treatment for 30–60 min in the bladder of
experimental animals was equally or slightly superior to that of
the commonly used MMC. Thus, EGCG inhibits uPA and MMP-9 enzymes,
preventing cancer cell implantation and cancer recurrence (11).
EGCG and green tea extracts have been shown to exert
many health-promoting effects, functioning through numerous
pathways such as antioxidant and anti-inflammatory pathways,
exhibiting gene expression activity, acting through growth factor
pathways. However, the results of in vitro and in
vivo experiments are frequently conflicting (59). This may be related to poor EGCG
bioavailability, its oxidation that starts during contact with air,
gastrointestinal inactivation, liver metabolism, or hard water
(27,59,60). The majority of clinical studies on
human treatment with EGCG were carried out using the oral
administration route (33,61,62).
This treatment does not require the stringent sterile requirement
of EGCG; however, bladder installation following transurethral
resection does.
The sterilization of EGCG can be challenging. For
example, canned and bottled green tea beverages are widely popular
worldwide, but preparation requires pasteurization by autoclaving
at 120°C for several minutes. During this process, considerable
amounts (approximately 50%) of EGCG undergo epimerization. The
relatively high concentration epimerized EGCG in tea beverages is
due to the thermal conversion (63,64). Therefore, heat sterilization in
the autoclave (typically temperature 121–134°C, steam, under
pressure 100 kPa, 20 min), dry heat sterilization (typically 2 h at
160 or 190°C for 6 min) have been eliminated. Chemical
sterilization due to the high reactivity of EGCG is not suitable
either.
We conclude that EGCG sterilization should be done
through the use of low temperature sterilization methods, more
precisely by ionizing radiation or the filtration method in
conjunction with freeze-drying. Both of these methods use a low
temperature process to reduce microbial contamination to an
acceptable level that is recommended for years by the American and
European Pharmacopoeias (65–67). It is estimated that approximately
90% of medications can be sterilized in the solid phase with a
standard dose of 25 kGy. However, there is a risk that ionizing
radiation in the standard sterilization dose may adversely affect
the structure of EGCG. Therefore, we began to irradiate EGCG with a
dose of 25 kGy, while increasing it to 200 kGy to capture all
changes occurring under the high-energy electron beam, and then
subjected to the analytical tests (EGCG, EGCG irradiated and
lyophilization) using a variety of analytical methods. These should
clearly answer the question of whether EGCG can be sterilized by
radiation or by filtration. The sterilization dose (25 kGy) caused
only a 0.5–0.6% decline in the content of EGCG, and ensured the
sterility of the sample, which was confirmed by microbiological
methods.
Measurement of the melting point method can be used
to rapidly monitor the changes taking place after radiation.
Literature data indicate that radiation degradation products reduce
the melting point of irradiated drugs, by a very small value of
<1°C, as observed for the ketoconazole irradiated dose of 200
kGy (68) or be a few °C. For
example, hydrocortisone following irradiation at a dose of 100 kGy
changed the melting point by 7°C (69). EGCG irradiated with different
doses led to changes in the melting point between these values and
did not answer conclusively what occurred following radiation
sterilization. In the case that the filtration and lyophilization
of EGCG melting point analysis was not conclusive, this is most
likely due to the fact that amorphous EGCG degraded before
melting.
UV spectra of EGCG solutions before and after
sterilization by different methods showed some changes in the case
of filtration, confirmed by FTIR spectra of EGCG where we observed
some differences in the range of 3,600-3,200 cm−1. In
addition, HPLC chromatograms of EGCG before and after sterilization
processes by filtration showed small additional peeks around
retention time of approximately 3 min, but were not observed in
EGCG sterilized by radiation. That can be a result of
auto-oxidation or epimerization, which are the two major reactions
responsible for the instability of EGCG in aqueous solution
(30).
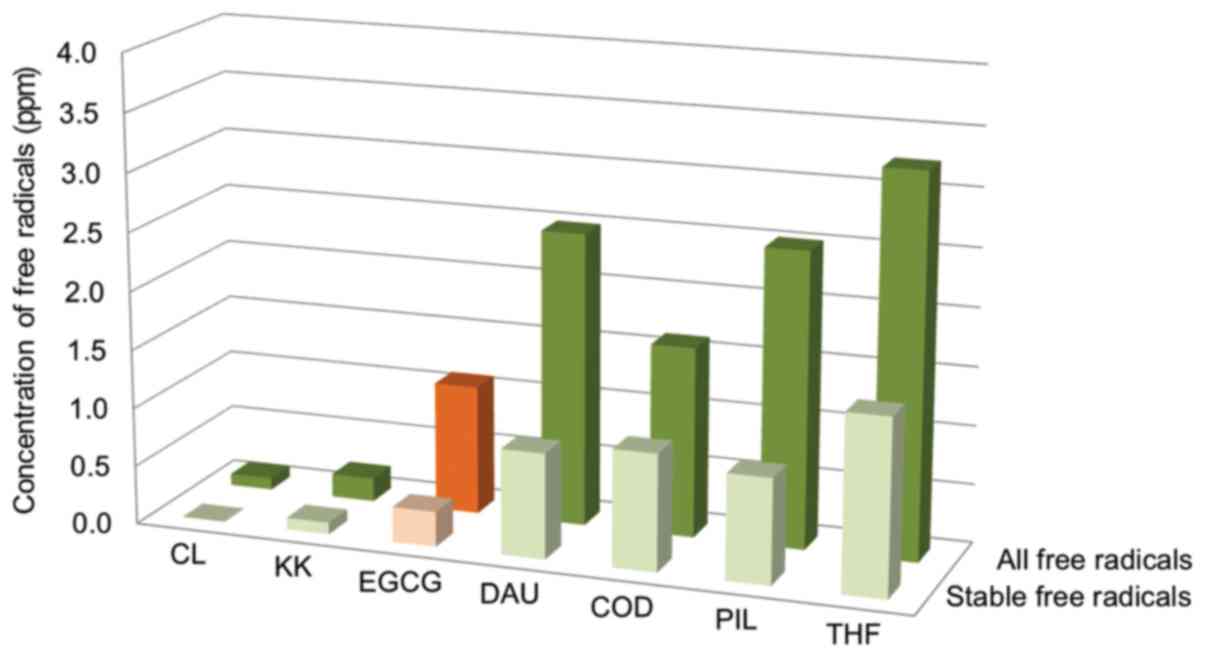
There are no pharmacopeia standards for the quantity
of free radicals in irradiated drugs. We found that the
concentration of free radicals in irradiated samples of EGCG was
very similar to the values obtained for drugs from different
chemical groups and therapeutic (70–74) with the same dose, source and
conditions of irradiation (Fig.
7). There are, however, drugs that following irradiation, show
much higher levels of free radicals such as epidoxorubicin (EPI),
4.7/18.0; doxorubicin (DOX), 26.4/60.0 ppm of stable/all free
radicals after irradiation. Nevertheless, there was no association
between the concentration of free radicals and the degree of
distribution relationship and all 3 tested antibiotics (DAU, DOX
and EPI). It has been proven that the high content of free radicals
in the sterilized subjected EPI and DOX causes the beneficial
effect-higher cytotoxic activity against cells of the lymphoblastic
leukemia line CCRF-VCR1000 (74).
This, similarly, can be beneficial for EGCG, as well.
In conclusion, the sterilization by radiation and
filtration (0.2 µm) followed by freeze-drying cause only
0.5–0.6%decline in the content of EGCG, and at the same time both
methods ensure the sterility of the sample, which was confirmed the
microbiological research. Therefore, we can conclude that both
methods are suitable to produce a sterile form of EGCG. However,
infrared and HPLC analysis show better stability of irradiated EGCG
over the filtration and lyophilization method. The concentration of
stable free radicals following irradiation sterilization was 0.35
ppm, which is similar to the other drugs sterilized by the same
method. The concentration is low and it is unlikely that the
resulting free radicals exert any damaging effects to EGCG. The
treatment of cells by EGCG sterilized by either method did not
alter cell growth pattern as compared to untreated EGCG. This
indicates that small modifications of EGCG will unlikely have any
biological effects. Therefore, we conclude that radiation is the
preferred method of EGCG sterilization, which is to be used in the
treatment of superficial bladder cancer.
References
|
1
|
Raman JD, Messer J, Sielatycki JA and
Hollenbeak CS: Incidence and survival of patients with carcinoma of
the ureter and renal pelvis in the USA, 1973–2005. BJU Int.
107:1059–1064. 2011. View Article : Google Scholar
|
|
2
|
Madeb R, Golijanin D, Knopf J and Messing
EM: Current state of screening for bladder cancer. Expert Rev
Anticancer Ther. 7:981–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen Z, Shen T, Wientjes MG, O'Donnell MA
and Au JL: Intravesical treatments of bladder cancer: review. Pharm
Res. 25:1500–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan CW, Shen ZJ and Ding GQ: The effect of
intravesical instillation of antifibrinolytic agents on bacillus
Calmette-Guerin treatment of superficial bladder cancer: a pilot
study. J Urol. 179:1307–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macleod LC, Ngo TC and Gonzalgo ML:
Complications of intra-vesical bacillus calmette-guérin. Can Urol
Assoc J. 8:E540–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Yu HS, Chen Z, Li JL, Hu ZM and
Gao JM: A novel immunotherapy for superficial bladder cancer by the
immobilization of streptavidin-tagged bioactive IL-2 on the
biotinylated mucosal surface of the bladder wall. Chin J Cancer.
29:611–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harding GE and Lawlor DK: Ruptured mycotic
abdominal aortic aneurysm secondary to Mycobacterium bovis after
intravesical treatment with bacillus Calmette-Guérin. J Vasc Surg.
46:131–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mack D, Höltl W, Bassi P, Brausi M,
Ferrari P, de Balincourt C and Sylvester R; European Organization
for Research and Treatment of Cancer Genitourinary Group: The
ablative effect of quarter dose bacillus Calmette-Guerin on a
papillary marker lesion of the bladder. J Urol. 165:401–403. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böhle A and Bock PR: Intravesical bacille
Calmette-Guérin versus mitomycin C in superficial bladder cancer:
formal meta-analysis of comparative studies on tumor progression.
Urology. 63:682–686. 2004. View Article : Google Scholar
|
|
10
|
Huncharek M and Kupelnick B:
Chemotherapeutic prophylaxis of superficial bladder tumors.
Oncology (Williston Park). 15:11062001.
|
|
11
|
Jankun J, Keck RW and Selman SH:
Epigallocatechin-3-gallate prevents tumor cell implantation/growth
in an experimental rat bladder tumor model. Int J Oncol.
44:147–152. 2014.
|
|
12
|
Suzuki Y, Miyoshi N and Isemura M:
Health-promoting effects of green tea. Proc Jpn Acad Ser B Phys
Biol Sci. 88:88–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito ST, Gosmann G, Pungartnik C and
Brendel M: Green tea extract-patents and diversity of uses. Recent
Pat Food Nutr Agric. 1:203–215. 2009. View Article : Google Scholar
|
|
14
|
Cooper R, Morré DJ and Morré DM: Medicinal
benefits of green tea: part I. Review of noncancer health benefits.
J Altern Complement Med. 11:521–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higdon JV and Frei B: Tea catechins and
polyphenols: health effects, metabolism, and antioxidant functions.
Crit Rev Food Sci Nutr. 43:89–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown MD: Green tea (Camellia sinensis)
extract and its possible role in the prevention of cancer. Altern
Med Rev. 4:360–370. 1999.PubMed/NCBI
|
|
17
|
Jung YD and Ellis LM: Inhibition of tumour
invasion and angio-genesis by epigallocatechin gallate (EGCG), a
major component of green tea. Int J Exp Pathol. 82:309–316. 2001.
View Article : Google Scholar
|
|
18
|
Shirakami Y, Shimizu M and Moriwaki H:
Cancer chemoprevention with green tea catechins: from bench to bed.
Curr Drug Targets. 13:1842–1857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Wei Y and Zhang J: Novel
mechanisms of anticancer activities of green tea component
epigallocatechin-3-gallate. Anticancer Agents Med Chem. 14:779–786.
2014. View Article : Google Scholar
|
|
20
|
Zhu K and Wang W: Green tea polyphenol
EGCG suppresses osteosarcoma cell growth through upregulating
miR-1. Tumour Biol. 37:4373–4382. 2016. View Article : Google Scholar
|
|
21
|
Tran PL, Kim SA, Choi HS, Yoon JH and Ahn
SG: Epigallocatechin-3-gallate suppresses the expression of HSP70
and HSP90 and exhibits anti-tumor activity in vitro and in vivo.
BMC Cancer. 10:2762010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thangapazham RL, Singh AK, Sharma A,
Warren J, Gaddipati JP and Maheshwari RK: Green tea polyphenols and
its constituent epigallocatechin gallate inhibits proliferation of
human breast cancer cells in vitro and in vivo. Cancer Lett.
245:232–241. 2007. View Article : Google Scholar
|
|
23
|
Ahn WS, Huh SW, Bae SM, Lee IP, Lee JM,
Namkoong SE, Kim CK and Sin JI: A major constituent of green tea,
EGCG, inhibits the growth of a human cervical cancer cell line,
CaSki cells, through apoptosis, G(1) arrest, and regulation of gene
expression. DNA Cell Biol. 22:217–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tosetti F, Ferrari N, De Flora S and
Albini A: 'Angioprevention': angiogenesis is a common and key
target for cancer chemopreventive agents. FASEB J. 16:2–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garbisa S, Sartor L, Biggin S, Salvato B,
Benelli R and Albini A: Tumor gelatinases and invasion inhibited by
the green tea flavanol epigallocatechin-3-gallate. Cancer.
91:822–832. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobalka AJ, Keck RW and Jankun J:
Synergistic anticancer activity of biologicals from green and black
tea on DU 145 human prostate cancer cells. Cent Eur J Immunol.
40:1–4. 2015.PubMed/NCBI
|
|
28
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of cell invasion and MMP
production by a nutrient mixture in malignant liposarcoma cell line
SW-872. Med Oncol. 24:394–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swiercz R, Skrzypczak-Jankun E, Merrell
MM, Selman SH and Jankun J: Angiostatic activity of synthetic
inhibitors of urokinase type plasminogen activator. Oncol Rep.
6:523–526. 1999.PubMed/NCBI
|
|
30
|
Hou Z, Sang S, You H, Lee MJ, Hong J, Chin
KV and Yang CS: Mechanism of action of
(-)-epigallocatechin-3-gallate: auto-oxidation-dependent
inactivation of epidermal growth factor receptor and direct effects
on growth inhibition in human esophageal cancer KYSE 150 cells.
Cancer Res. 65:8049–8056. 2005.PubMed/NCBI
|
|
31
|
Bae MJ, Ishii T, Minoda K, Kawada Y,
Ichikawa T, Mori T, Kamihira M and Nakayama T: Albumin stabilizes
(-)-epigallocatechin gallate in human serum: binding capacity and
antioxidant property. Mol Nutr Food Res. 53:709–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zinellu A, Sotgia S, Scanu B, Forteschi M,
Giordo R, Cossu A, Posadino AM, Carru C and Pintus G: Human serum
albumin increases the stability of green tea catechins in aqueous
physiological conditions. PLoS One. 10:e01346902015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dryden GW, Lam A, Beatty K, Qazzaz HH and
McClain CJ: A pilot study to evaluate the safety and efficacy of an
oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in
patients with mild to moderate ulcerative colitis. Inflamm Bowel
Dis. 19:1904–1912. 2013.PubMed/NCBI
|
|
34
|
Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ,
Seril DN, Sturgill MG and Yang CS: Epigallocatechin-3-gallate is
absorbed but extensively glucuronidated following oral
administration to mice. J Nutr. 133:4172–4177. 2003.PubMed/NCBI
|
|
35
|
Scalia S, Marchetti N and Bianchi A:
Comparative evaluation of different co-antioxidants on the
photochemical- and functional-stability of
epigallocatechin-3-gallate in topical creams exposed to simulated
sunlight. Molecules. 18:574–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bianchi A, Marchetti N and Scalia S:
Photodegradation of (-)-epigallocatechin-3-gallate in topical cream
formulations and its photostabilization. J Pharm Biomed Anal.
56:692–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colomer R, Sarrats A, Lupu R and Puig T:
Natural polyphenols and their synthetic analogs as emerging
anticancer agents. Curr Drug Targets. 18:147–159. 2017. View Article : Google Scholar
|
|
38
|
Joe AK, Schnoll-Sussman F, Bresalier RS,
Abrams JA, Hibshoosh H, Cheung K, Friedman RA, Yang CS, Milne GL,
Liu DD, et al: Phase Ib randomized, double-blinded,
placebo-controlled, dose escalation study of polyphenon E in
patients with Barrett's esophagus. Cancer Prev Res (Phila).
8:1131–1137. 2015. View Article : Google Scholar
|
|
39
|
Kato MT, Leite AL, Hannas AR and Buzalaf
MA: Gels containing MMP inhibitors prevent dental erosion in situ.
J Dent Res. 89:468–472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stockfleth E and Meyer T: The use of
sinecatechins (polyphenon E) ointment for treatment of external
genital warts. Expert Opin Biol Ther. 12:783–793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gross G, Meyer KG, Pres H, Thielert C,
Tawfik H and Mescheder A: A randomized, double-blind, four-arm
parallel-group, placebo-controlled phase II/III study to
investigate the clinical efficacy of two galenic formulations of
polyphenon E in the treatment of external genital warts. J Eur Acad
Dermatol Venereol. 21:1404–1412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kemberling JK, Hampton JA, Keck RW, Gomez
MA and Selman SH: Inhibition of bladder tumor growth by the green
tea derivative epigallocatechin-3-gallate. J Urol. 170:773–776.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selman SH and Keck RW: A comparative study
of the inhibiting effects of mitomycin C and polyphenolic catechins
on tumor cell implantation/growth in a rat bladder tumor model. J
Urol. 186:702–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mai VC, Bednarski W, Borowiak-Sobkowiak B,
Wilkaniec B, Samardakiewicz S and Morkunas I: Oxidative stress in
pea seedling leaves in response to Acyrthosiphon pisum infestation.
Phytochemistry. 93:49–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mossanen M and Gore JL: The burden of
bladder cancer care: direct and indirect costs. Curr Opin Urol.
24:487–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Srinivasan H, Allory Y, Sill M, Vordos D,
Alhamdani MS, Radvanyi F, Hoheisel JD and Schröder C: Prediction of
recurrence of non muscle-invasive bladder cancer by means of a
protein signature identified by antibody microarray analyses.
Proteomics. 14:1333–1342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lohi J, Kyyrönen P, Kauppinen T, Kujala V
and Pukkala E: Occupational exposure to solvents and gasoline and
risk of cancers in the urinary tract among Finnish workers. Am J
Ind Med. 51:668–672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siemiatycki J, Dewar R, Krewski D, Désy M,
Richardson L and Franco E: Are the apparent effects of cigarette
smoking on lung and bladder cancers due to uncontrolled confounding
by occupational exposures? Epidemiology. 5:57–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Isharwal S and Konety B: Non-muscle
invasive bladder cancer risk stratification. Indian J Urol.
31:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mansoor M, Ali S, Fasihuddin Q and Baloch
MU: Superficial bladder tumours: recurrence and progression. J Coll
Physicians Surg Pak. 21:157–160. 2011.PubMed/NCBI
|
|
51
|
Palou-Redorta J, Solsona E, Angulo J,
Fernández JM, Madero R, Unda M, Martínez-Piñeiro JA, Portillo J,
Chantada V and Moyano JL: Retrospective study of various
conservative treatment options with bacilli Calmette-Guérinin
bladder urothelial carcinoma T1G3: maintenance therapy. Actas Urol
Esp. 40:370–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Iida K, Naiki T, Kawai N, Etani T, Ando R,
Ikegami Y, Okamura T, Kubota H, Okada A, Kohri K, et al: Bacillus
Calmette-Guerin therapy after the second transurethral resection
significantly decreases recurrence in patients with new onset
high-grade T1 bladder cancer. BMC Urol. 16:82016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Porten SP, Leapman MS and Greene KL:
Intravesical chemotherapy in non-muscle-invasive bladder cancer.
Indian J Urol. 31:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
deKernion JB, Huang MY, Lindner A, Smith
RB and Kaufman JJ: The management of superficial bladder tumors and
carcinoma in situ with intravesical bacillus Calmette-Guerin. J
Urol. 133:598–601. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Serretta V: Management, and prevention, of
intravesical therapy complications. Urologia. 76:19–28. 2009.In
Italian. PubMed/NCBI
|
|
56
|
Chopin DK: Intravesical instillation in
the treatment of superficial tumors of the bladder. Prog Urol.
8:268–273. 1998.In French. PubMed/NCBI
|
|
57
|
Llopis M, Moreno J, Botella R and Algado
M: Incrusted cystitis after intravesical mitomycin C treatment.
Acta Urol Belg. 61:21–23. 1993.PubMed/NCBI
|
|
58
|
Punga-Maole ML, Hubert J and Mangin P:
Bladder retraction, a complication of chemoprophylaxis of
superficial bladder cancer using intravesical mitomycin C. Prog
Urol. 5:580–585. 1995.In French. PubMed/NCBI
|
|
59
|
Mereles D and Hunstein W:
Epigallocatechin-3-gallate (EGCG) for clinical trials: more
pitfalls than promises? Int J Mol Sci. 12:5592–5603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jankun J, Kondray V and Skrzypczak-Jankun
E: Analysis of the inhibition of PAI-1 by metal the aflavin
complexes and their degradation products. Int J Mol Med.
31:1153–1158. 2013.PubMed/NCBI
|
|
61
|
Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun
X, Yu J and Xing L: A phase I study of concurrent chemotherapy and
thoracic radiotherapy with oral epigallocatechin-3-gallate
protection in patients with locally advanced stage III
non-small-cell lung cancer. Radiother Oncol. 110:132–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shanafelt TD, Call TG, Zent CS, Leis JF,
LaPlant B, Bowen DA, Roos M, Laumann K, Ghosh AK, Lesnick C, et al:
Phase 2 trial of daily, oral polyphenon E in patients with
asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia.
Cancer. 119:363–370. 2013. View Article : Google Scholar
|
|
63
|
Chen Z, Zhu QY, Tsang D and Huang Y:
Degradation of green tea catechins in tea drinks. J Agric Food
Chem. 49:477–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee SM, Kim CW, Kim JK, Shin HJ and Baik
JH: GCG-rich tea catechins are effective in lowering cholesterol
and triglyceride concentrations in hyperlipidemic rats. Lipids.
43:419–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Council of Europe: European Pharmacopoeia.
8th edition. Strasbourg, France: 2014
|
|
66
|
The United States Pharmacopeial Convention
(USP): The United States Pharmacopoeia 39 - National Formulary 34
(USP 39-NF 34). USP; Rockville, MD: 2015
|
|
67
|
Marciniec B and Dettlaff K: Radiation
sterilization of drugs. Trends in Radiation Sterilization of Health
Care Products. IAEA; Vienna, Austria: pp. 187–230. 2008
|
|
68
|
Marciniec B, Kozak M, Wachowski L and
Ogrodowczyk M: Evaluation of radiostability of some steroid
derivatives. J Therm Anal Calorim. 73:473–485. 2003. View Article : Google Scholar
|
|
69
|
Marciniec B, Kozak M and Dettlaff K:
Thermal analysis in evaluation of the radiochemical stability of
some fungicidal drugs. J Therm Anal Calorim. 77:305–317. 2004.
View Article : Google Scholar
|
|
70
|
Marciniec B, Dettlaff K, Danikiewicz W,
Spólnik G, Jaroszkiewicz E and Naskrent M: Radiostability of
ketoconazole in the solid state. Curr Pharm Anal. 9:102–113.
2013.
|
|
71
|
Marciniec B, Dettlaff K and Naskrent M:
Influence of ionizing irradiation on clotrimazole in the solid
state. J Pharm Biomed Anal. 50:675–678. 2009. View Article : Google Scholar
|
|
72
|
Marciniec B, Kozak M, Naskrent M, Hofman
M, Dettlaff K and Stawny M: DSC and EPR analysis of some radiation
sterilized alkaloids. J Therm Anal. 102:261–267. 2010. View Article : Google Scholar
|
|
73
|
Marciniec B, Stawny M, Kozak M and
Naskrent M: The influence of radiation sterilization on
thiamphenicol. Spectrochim Acta A Mol Biomol Spectrosc. 69:865–870.
2008. View Article : Google Scholar
|
|
74
|
Paszel-Jaworska A, Totoń E, Dettlaff K,
Kaczmarek A, Bednarski W, Oszczapowicz I, Jelińska A and Rybczyńska
M: Increased proapoptotic activity of electron beam irradiated
doxorubicin and epirubicin in multidrug-resistant human leukemic
cells. Chem Biol Interact. 258:69–78. 2016. View Article : Google Scholar : PubMed/NCBI
|