Introduction
Aerobic organisms are exposed to reactive oxygen
species (ROS) due to aerobic metabolism. ROS, such as superoxide
anion (O2•−), hydrogen peroxide
(H2O2) and hydroxyl radicals
(OH•), have coalescent chemical properties that confer
reactivity to a number of biological targets (1). ROS are often associated with
oxidative stress, a condition characterized by the imbalance
between the production of free radicals and the endogenous
antioxidant mechanisms in the favor of the first (1). ROS are capable of reversibly or
irreversibly damaging biological macromolecules, such as nucleic
acids, proteins, lipids and carbohydrates (1). Thus, oxidative stress may lead to
the development of a number of pathological conditions, such as
cancer, diabetes, obesity, and neurodegenerative and autoimmune
diseases (2). However, ROS also
serve as signaling molecules which regulate biological and
physiological processes, such as the regulation of signaling
pathways, gene expression and apoptosis (1). In living organisms, there is a wide
range of endogenous defensive mechanisms against free radicals,
including enzymatic and non-enzymatic antioxidants (2). Aside from the endogenous mechanisms,
an organism may also acquire antioxidant components through diet
(3,4). Polyphenols, products of secondary
metabolism in plants, are some of the most important antioxidants
(5,6).
The entire vascular system is comprised of a
monolayer of endothelial cells. Endothelial cell integrity is
necessary to the maintenance of the vessel wall and circulatory
function (7,8). Moreover, the endothelium regulates
the homeostasis, immune and inflammatory responses (8). Oxidative stress may cause damage to
the vascular endothelium due to the loss of its integrity, leading
to senescence and its detachment into the circulation (9). Thus, it consists one of the most
important factors of pathological conditions of vessels, including
atherosclerosis and thrombosis (8). In addition, oxidative stress may
cause damage to the endothelium through leukocyte adhesion
(10,11). Moreover, the interplay between ROS
and nitric oxide sets off a vicious circle which leads to further
endothelial activation and inflammation (8).
Oxidative stress also occurs in muscle tissue.
Specifically, during intense exercise, a high rate of O2
consumption takes place in skeletal muscle that can cause
incomplete O2 reduction and electron leakage from the
electron transfer chain, leading to ROS generation and oxidative
stress. Moreover, exercise induces oxidative stress through
xanthine oxidase mechanisms (12). Oxidative stress, in turn, results
in muscle fatigue, cell damage and apoptosis (13,14).
The medicinal properties of Olea europaea
(olive tree) and its main product, olive oil (OO), has been known
since ancient times (15). OO is
the most important component of the Mediterranean diet (16), characterized by the intake of
plant foods, such as fruits, vegetables, nuts and seeds (17). Epidemiological evidence indicates
an inverse association with proper adherence to this diet and death
due to coronary artery disease and various types of cancer, such as
urinary bladder cancer, suggesting a potential protective role of
regularly consumed OO and its hydrophilic fraction physiologically
excreted through urine (18–20). The results of in vitro
studies further support this evidence by revealing a protective
effect of OO against various types of cancer (21–24), including urogenital neoplasms
(25–27), paving the way towards research for
the development of novel anticancer strategies. It has been shown
in human prostate cell cultures for example, that exposure to OO
induces an antioxidant effect on benign prostatic hyperplasia-1
cells and a pro-oxidant effect on malignant cells, suggesting that
OO may potentially be used for prostate cancer prevention (25). Furthermore, it has been reported
that increased OO consumption raises the concentration of phenol
glucuronide conjugates in a dose-dependent manner in human urine
(28) and that extra virgin OO
(EVOO) extract suppresses the migration and invasion of T24 human
bladder cancer cells by significantly inhibiting
proliferation/motility in a dose-dependent manner (26). These promising results have been
replicated in another study showing that EVOO extract significantly
inhibited the proliferation and clonogenic ability of T24 and 5637
human bladder cancer cells in a dose-dependent manner by blocking
the cell cycle, and that EVOO modulated the chemotherapeutic
toxicity in bladder cancer cells (27).
Over the past 25 years, the consumption of OO
worldwide has increased by >30% due to its two key
characteristics, namely its nutritional and organoleptic properties
(16,29). The nutritional properties of OO
are provided by its fatty acid (FA) profile and high
monounsaturated/saturated FA ratio (MUFA/SFA), as well as by its
rich antioxidant content, particularly that of biophenols which are
believed to play a role in the prevention of diseases (16,30). OO biophenols act as oxidation
chain-breakers, reacting with free radicals and forming inactive
radicals. Some of the most important biophenols found in OO with
marked biological activities, are oleocanthal (OLEO), oleacein
(OLEA), elenoic acid, oleuropein and its derivatives, tyrosol (T)
and hydroxytyrosol (HT) (31).
These compounds have the ability to scavenge free radicals by
donating them an electron or hydrogen atom or by chelating metals
(16). Among the OO biophenols,
HT has attracted distinct attention due to its potent antioxidant
activity attributed mainly to its orthodiphenolic structure
(32). It is mainly found in
olive tree leaves, olive pulp and OO (33). It is worth mentioning that HT can
also be synthesized endogenously in the human body, as a product of
dopamine oxidative metabolism, known as 3,4-dihydroxyphenyl ethanol
(DOPET) (34). HT has been shown
to exert preventive effects in several pathological conditions,
such as metabolic syndrome, neurodegenerative diseases and cancer
(34).
The aim of the present study was to examine the
antioxidant properties of a EVOO total polyphenolic fraction (TPF)
rich in biophenols, from a Greek endemic variety of Olea
europea, grown on Mount Athos, as well as that of pure HT. It
is worth noting, that to date, at least to the best of our
knowledge, there are no studies available investigating the
antioxidant properties of an OO TPF in cell cultures. The
antioxidants effects were assessed at a cellular level,
particularly in EA.hy926 endothelial cells and C2C12 myoblasts. The
obtained results will provide important information on the possible
use of the TPF as an antioxidant food supplement.
Materials and methods
Reagents and materials
n-Hexane, ethyl acetate, ethanol (etOH) and
acetonitrile were purchased from Carlo Erba Reactifs SDS (Val de
Reuil, France). Methanol (MeOH), dichloromethane and sulfuric acid
(H2SO4 >95%) were obtained from Fisher
Scientific UK (Leicestershire, UK). All solvents were of analytical
grade. Deionized water was used to prepare all aqueous solutions.
Vanillin standard were purchased from Sigma-Aldrich (Poole, UK)
with purity of >95%.
Extraction of TPF from EVOO
EVOO was procured from Northern Greece (Mount Athos)
which was freshly produced in January 2015). The extraction of TPF
was achieved following a previously described procedure (35). Liquid-liquid extraction was
carried out on a laboratory-scales Fast Centrifugal Partition
Extractor FCPE300® (Rousselet-Robatel Kromaton, Anonay,
France; column capacity of 300 ml) connected to a LabAlliance
preparative pump. The extraction process consisted of several
'Extraction-Recovery' cycles (multi-dual-mode method) using as
'mobile phase' a mixure of n-hexane/OO in proportion of 3:2
(v/v) and as 'stationary phase' etOH/water in proportion of 3:2
(v/v). The procedure starts by filling the CPE column with 0.3
liters of the stationary phase in ascending mode and at 200 rpm.
The rotation speed was increased up to 1,000 rpm and the mobile
phase (feed oil phase) was pumped at 60 ml/min in ascending mode.
After passing 2.5 liters of mobile phase (extraction step) the
pumping mode was switched to 'descending' and a volume of 0.3
liters of aqueous extraction phase was pumped (at the same flow
rate and rotation speed) in order to replace the concentrated in
biophenols stationary phase with fresh one (recovery step). This
cycle of extraction-recovery was repeated 5 more times extracting
totally 15 liters of feed oil phase corresponding to 6 liters of
OO. The 6 collected stationary phases were evaporated under a
vacuum and the obtained viscous extract was defatted using the
biphasic system n-hexane/acetonitrile (1/1 v/v) and stored at 4°C
for further use. Finally, the extraction of 6 liters of OO resulted
in the recovery of 6.35 g of OO biophenol extract.
Purification of HT from TPF
High purity HT was recovered from the treatment of
TPF in a two steps separation process. Initially, the extract was
fractionated by gradient-elution centrifugal partition
chromatography (CPC) and the enriched in the HT fraction was
further analyzed by using Sephadex LH-20 (Sigma-Aldrich) column
chromatography. The CPC experiment was performed on a
FCPC1000® apparatus (Rousselet-Robatel Kromaton)
equipped with a preparative column (1,000 ml total column capacity)
and connected to a LabAlliance Preparative pump. The system was
coupled to a SPECTRA SYSTEM UV 2000 detector set at 254, 280 and
366 nm (Thermo Fisher Scientific, Inc., Waltham, MA, USA), while
fractions were collected using a Büchi B-684 fraction collector
(Büchi Labortechnik AG, Flawil, Switzerland).
The TPF was fractionated in a step-gradient elution
mode following a previously described method (35). For this purpose, a series of 4
biphasic systems consisting of the solvents, n-hexane, ethyl
acetate, etOH, and water in proportions 4/1/2/3 (S1), 3/2/2/3 (S2),
2/3/2/3 (S3) and 1/4/2/3 v/v/v/v (S4) were used. The solvents were
thoroughly mixed in a separating funnel at room temperature prior
to use, and the 2 phases of each system were separated after
equilibration of the mixture. The column was initially filled with
the lower phase of the first biphasic system (stationary phase) in
ascending mode at a flow rate of 30 ml/min and 200 rpm. The
rotation speed was increased to 1,000 rpm and the mobile phase I
(upper phase of S1) was pumped at a flow rate of 15 ml/min. When
the hydrodynamic equilibrium of the two liquid phases inside the
CPC column was established (Sf=67%), 5 g of sample (diluted in 20
ml of lower and 10 ml of upper phase of S1) was injected into the
column via a 30 ml sample loop. A sequential pumping of the upper
mobile phases of S1, S2, S3 and S4 was then performed in volumes of
500, 1,100, 1,400 and 1,000 ml, respectively (gradient elution
step). The experiment completed by pumping the stationary phase in
ascending mode (at the same flow rate and rotation speed) and
collection of 30 more fractions (extrusion step). Fraction
collector was set to collect 25 ml fractions during the all
experiment.
All collected fractions were evaluated using thin
layer chromatography (TLC). The plates were coated (Merck
Millipore, Billerica, MA, USA) with silica gel 60 F254 and
developed in dichloromethane/MeOH in different proportions. After
detection at UV254 and UV366, the plates were sprayed with
vanillin-sulfuric acid and heated. Based on the TLC qualitative
results, the fractions containing HT (195 fractions of 25 ml) were
combined and evaporated to dryness resulting to 95 mg of an
enriched HT fraction (86% purity). For further purification,
Sephadex LH-20 column chromatography was incorporated.
Specifically, 90 mg of the sample (diluted in 1 ml
EtOH) were subjected to column chromatography (Sephadex®
LH-20, 10×285 mm) and eluted with EtOH. All obtained fractions were
initially analyzed by TLC and those of similar chemical content
were combined giving finally three main fractions (Fractions A-C).
Fraction B contained 71.3 mg HT in high purity (>97%). The
purity was determined by quantitative high-performance liquid
chromatography (HPLC) analysis.
HPLC-diode-array detection (DAD)
qualitative and quantitative analysis
The qualification analysis of TPF and quantification
of HT, T, OLEO and OLEA were performed on a Thermo Finnigan HPLC
instrument (Ontario, Canada) equipped with a SpectraSystem P4000
pump, a SpectraSystem 1000 degasser, a SpectraSystem AS3000
automated injector, and a UV SpectraSystem UV6000LP detector
monitored at 235, 280 and 365 nm. The samples were analyzed in a
Discovery HS C18 (15cm x 4.6mm, particle size 5 µm)
analytical column (Supelco, Bellefonte, PA, USA) using two
different methods. The first one (method A) was used for the
qualification analysis of the stable TPF components and
quantification of HT and T, and was based on a previously published
method with minor modifications (36). Water acidified with 0.2% acetic
acid (solvent A) and acetonitrile (solvent B) was used as a solvent
in the following gradient system: 0–40 min 2–30% B, 40–45 min 30%
B, 45–50 min 30–2% B. The flow rate was set at 1 ml/min, the
injection volume was 20 µl (from a solution of 1 mg TPF/ml
MeOH) and the total run duration was 50 min. The qualification
analysis of the labile TPF components and quantification analysis
of OLEA and OLEO was performed using the second method (method B).
The mobile phase consisted of water (solvent A) and acetonitrile
(solvent B), while the gradient elution began with 80% of solvent A
decreasing to 70% in 20 min, remaining stable for 15 min and
increasing again to 80% in the next 5 min (total duration, 40 min).
The flow rate was set at 1 ml/min, while the injection volume was
20 µl (from a solution of 1 mg TPF/ml MeOH), as previously
described (37,38).
The quantification analysis of HT and T was
performed using method A. The calibration curves were created using
the concentrations 2, 3, 20, 50 and 100 µg/ml and was used
to calculate the percentage of the target compounds in EVOO and
TPF, as well as to examine the purity of HT both in CPC fraction
and in the final pure form. The peak area values (measured at 280
nm) constitute the average of 3 measurements. Linear regression
analysis with the use of syringaldehyde (98% HPLC; ExtraSynthese,
Genay Cedex, France) as an internal standard (ISTD) was
performed.
The quantification of OLEO and OLEA was performed
using method B. The calibration curves were constructed using 10
concentrations levels (50,100, 200, 300, 400, 500, 600, 700, 800,
900 and 1,000 µg/ml). ISTD was prepared in a mixture of
MeOH/water 1:1 (v/v) at a concentration of 1 mg/ml and stored at
−4°C. Both compounds are eluted, as double peaks and thus the
quantification was based on the total area of both peaks (measured
at 235 nm) as previously described by Impellizzeri and Lin
(37). In the case of HT and T,
the quantification was based on the ratio between the peak area of
the analyte and the peak area of ISTD. The equations and the
coefficient of determination (R2) of the standard curves
were as follows: y=45130x+22444, R2=0.995 for OLEO,
y=43653x-1E+06, R2=0.997 for OLEA, y=0.041x-0.011,
R2=0.999 for HT and y=0.025x-0,014, R2=0.999
for T.
TLC and NMR analysis
The TLC analysis of TPF and CPC fractions was
performed on Merck 60 F254pre-coated silica gel plates and
developed with DCM/MeOH 95:5 (v/v). The TLC chromatograms were
firstly revelation by using UV light at 254 and 365 nm and the TLC
plates were then sprayed by a vanillin (5% w/v in
ethanol)-H2SO4 (50% v/v in methanol) solution
and heated at 100–120°C for 2–3 min. Structure confirmation of the
isolated HT was achieved by NMR analysis. The 1H-NMR
experiments were performed on a 600 MHz on a Bruker AvanceAVIII-600
spectrometer (Karlsruhe, Germany) equipped with a TXI cryoprobe
(Wissembourg, France) in CD3OD.
Assessment of the total polyphenolic
content (TPC) of the extracts
The TPC of the OO extract was determined using the
Folin-Ciocalteu reagent, as previously described (39). A total of 20 µl of extract
were added to a tube containing 1 ml distilled water, followed by
the addition of 100 µl of Folin-Ciocalteu reagent and
incubation for 3 min at room temperature. Subsequently, 280
µl of 25% w/v sodium carbonate solution along with 600
µl of distilled water were added to the mixture. Finally,
following 1 h incubation at room temperature in the dark, the
absorbance was measured at 765 nm versus a blank lacking the
extract. The measurement was carried out on a Hitachi U-1900 radio
beam spectrophotometer (serial no. 2023–029; Hitachi, Ltd., Tokyo,
Japan). The optical density of the sample without the
Folin-Ciocalteu reagent at 765 nm was also measured. The TPC was
determined using a gallic acid standard curve (50–1,500
µg/ml). The TPC is presented as µg of gallic acid
equivalents per mg of extract.
2,2-diphenyl-1-picrylhydrazyl (DPPH)
radical scavenging assay
The free-radical scavenging capacity (RSC) of the
TPF and HT extracts was evaluated using the DPPH radical assay
(40). Briefly, a 1 ml freshly
prepared methanolic solution of DPPH radical (100 µM) was
mixed with the tested extract solution at various concentrations
(7.8–250 µg/ml TPF and 0.7–100 µg/ml HT). The mixture
was vortexed and incubated at room temperature in the dark for 20
min, followed by absorbance measurement at 517 nm on a Hitachi
U-1900 radio beam spectrophotometer (serial no. 2023–029; Hitachi,
Ltd.). MeOH was used as a blank and DPPH alone in MeOH was used as
the control.
The percentage RSC of the tested extracts was
calculated using the following equation: RSC (%) =
[(ODcontrol − ODsample)/ODcontrol]
×100, where ODcontrol and ODsample are the
optical density (OD) values of the control and the test sample,
respectively. Moreover, the IC50 value indicating the
polyphenolic amount that caused 50% scavenging of the DPPH radical
was calculated. In order to compare the radical scavenging
efficiency of the extracts, the specific activity was determined.
The specific activity was evaluated by dividing the IC50
value obtained from the DPPH and
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
(ABTS•+) assays by the amount of polyphenols contained
in each mg of the TPF and HT. All experiments were carried out in
triplicate and on at least 3 separate occasions.
ABTS•+ radical scavenging
assay
The ABTS•+ RSC of the extract was
determined as previously described by Cano et al (41) with minor modifications. Briefly, 1
ml reactions were prepared in distilled water containing
ABTS•+ (1 mM), H2O2 (30 µM)
and horseradish peroxidase (6 µM) in 50 mM
phosphate-buffered saline (PBS; pH 7.5). The solution was vortexed,
followed by incubation for 45 min at room temperature in the dark.
Subsequently, 10 µl of the tested extracts, at various
concentrations, were added and the absorbance at 730 nm was read on
a Hitachi U-1900 radio beam spectrophotometer (serial no. 2023–029;
Hitachi, Ltd.). In each experiment, a blank lacking the peroxidase
was used, while the ABTS•+ radical solution without the
extract was used as the control. The RSC percentage and the
specific activity values were determined as described above for the
DPPH method. All experiments were carried out in triplicate and on
at least 3 separate occasions.
Cell culture conditions
The C2C12 murine myoblasts were cultured in
Dulbecco's modified Eagle's medium (DMEM), containing 10% (v/v)
fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml of penicillin
and 100 U/ml of streptomycin (all from Gibco, Paisley, UK) in
plastic disposable tissue culture flasks at 37°C in 5%
CO2 (42). The C2C12
myoblasts were donated by from Professor Koutsilieris (National and
Kapodistrian University of Athens, Athens, Greece).
EA.hy926 endothelial cells were cultured as
described previously in tissue culture flasks at 37°C in 5%
CO2 (34,42). The medium used was DMEM,
containing 10% (v/v) FBS, 25 mM HEPES, 2 mM L-glutamine, 100 U/ml
of penicillin and 100 U/ml of streptomycin (Gibco) (42). The EA.hy926 cells were donated by
Professor Koukoulis (University of Thessaly, Larissa, Greece).
XTT assay
Cell viability was assessed using the XTT assay kit
(Roche, Mannheim, Germany) as described previously (42). Briefly, the C2C12 and EA.hy926
cells were cultured in a 96-well plate in a density of
1×104 cells per well in DMEM. Following 24 h of
incubation, various concentrations of the TPF and HT extracts in
serum-free DMEM were administered for 24 h. Afterwards, 50
µl of XTT test solution were added to each well. Following 4
h of incubation, absorbance was measured at 450 nm and also at 630
nm as a reference wavelength in a BioTek ELx800 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). Serum-free DMEM was
used as a negative control. In addition, the absorbance of the TPF
and HT alone in serum-free DMEM and XTT test solution was measured
at 450 nm. The absorbance values of the TPF and HT alone were
subtracted from those derived from cell treatment with the tested
compounds. Data were calculated as the percentage of viability
using the following formula: Viab ility (%) =
[(ODcontrol − ODsample)/ODcontrol]
×100, where ODcontrol and ODsample indicate
the OD of the negative control and the tested compounds,
respectively. All experiments were carried out in triplicate and on
3 separate occasions.
Assessment of GSH and ROS levels in cells
by flow cytometry
The intracellular GSH and ROS levels were assessed
using the fluorescent dyes, mercury orange and
2,7-dichlorofluorescein diacetate (DCF-DA), respectively (43). Mercury orange binds directly to
GSH, while DCF-DA is deacetylated within cells by esterases and is
further converted to fluorescent DCF by the oxidative action of
ROS. A 400 µM stock solution of mercury orange was prepared
in acetone and stored at 4°C, and a fresh 400 µM stock
solution of DCF-DA was prepared in MeOH. To measure the GSH and ROS
levels the cells were first trypsinized and centrifuged at 300 × g
for 5 min at 4°C. The supernatant was discarded and the cell pellet
was resuspended in PBS at 1×106 cells/ml and incubated
in the presence of mercury orange (40 µM) or DCF-DA (10
µM) in the dark at 37°C for 30 min. The cells were then
washed, resuspended in PBS, and subjected to flow cytometric
analysis using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) with excitation and emission wavelengths
at 488 and 530 nm for ROS and at 488 and 580 nm for GSH,
respectively. In addition, forward-angle and right-angle light
scattering showing the cell size and cell internal complexity,
respectively, were measured. The cells were analyzed at a flow rate
of 1,000 events/sec. Analyses were performed on 10,000 cells per
sample, and the fluorescence intensities were measured on a
logarithmic scale. Data were analyzed using BD Cell Quest software
(BD Biosciences). Each experiment was repeated at least 6
times.
Statistical analysis
All results are expressed as the means ± standard
error of the mean. A Spearman's correlation analysis for examining
the results from the TPC, DPPH and ABTS•+ assays was
performed. A value of P<0.05 was considered to indicate a
statistically significant difference. In addition, one-way ANOVA
was applied, followed by Dunnett's tests for multiple pair wise
comparisons using SPSS software (SPSS, Inc., Chicago, IL, USA).
Results and Discussion
Preliminary analysis by our research group on
different EVOOs demonstrated that EVOO originating from the olive
tree variety grown in Mount Athos is rich in biophenols (data not
shown). Thus, this EVOO was used for the isolation of the TPF
extract, as well as that of pure HT. TPF extract was also
characterized chemically by determining the amount of HT, T, OLEO
and OLEA biophenols. Moreover, both TPF and HT were examined for
their antioxidant activity in vitro and at a cellular level
in endothelial cells and myoblasts.
The recovery of the TPF was performed by applying a
recently developed CPE-based extraction process which included the
use of the biphasic system n-hexane/EVOO/etOH/water-3/2/3/2
v/v/v/v and a multiple dual-mode CPE method for the treatment of OO
(27,35). Compared to other purposed OO
extraction methods (44,45), this green process allows the
treatment of large volumes of OO in a continuous extraction
procedure and with extremely low solvent consumption. Thus, 6
extraction-recovery cycles were performed by pumping per each cycle
2.5 liters of feed oil phase (at 60 ml/min in ascending mode) and
replacing the concentrated in biophenols stationary phase with 300
ml of fresh aqueous extraction phase (see experimental part). It is
important to note that the treatment by this process of 6 liters of
OO (corresponding to 5,478 g) resulted the recovery of 6.35 g of
TPF in only 5 h. However, due to the high complexity of TPF the
first step of the separation process included a step-gradient CPC
fractionation with a series of four biphasic systems consisting of
the solvents n-hexane, ethyl acetate, etOH and water in
proportions 4/1/2/3 (S1), 3/2/2/3 (S2), 2/3/2/3 (S3) and 1/4/2/3
v/v/v/v (S4) (27,35). Even if this method afforded
approximately HT in 87% purity, an additional purification step was
required and therefore size exclusion column chromatography was
used. The combination of these two chromatographic techniques has
the advantage of using different criteria of selectivity between
two purification steps and has been successfully applied for the
recovery of pure compounds from complex natural extracts (46). The result of this two-step
purification procedure was the effective recovery of high purity
(>97%) HT. The purity of the recovered compounds was firstly
examined by proton nuclear magnetic resonance (1H-NMR)
and was then evaluated by HPLC-DAD analysis.
In addition, since the compounds under investigation
behave differently in the presence or absence of acid two different
elution systems have been used. Specifically, for the analysis of
the TPF constituents, such as the phenyl alcohols (HT and T), the
mobile phase of the used HPLC method (method A, see Materials and
methods) contained acidified solvent (water acidified with 0.2%
acetic acid) in order to increase the quality of the analysis. On
the other hand, OLEA and OLEO are labile components and they are
not stable in acidic conditions. Thus, the mobile phase of the HPLC
method (method B, see Materials and methods) used for the analysis
of those compounds was composed of water (solvent A) and
acetonitrile (solvent B) without acidification. The profiling of
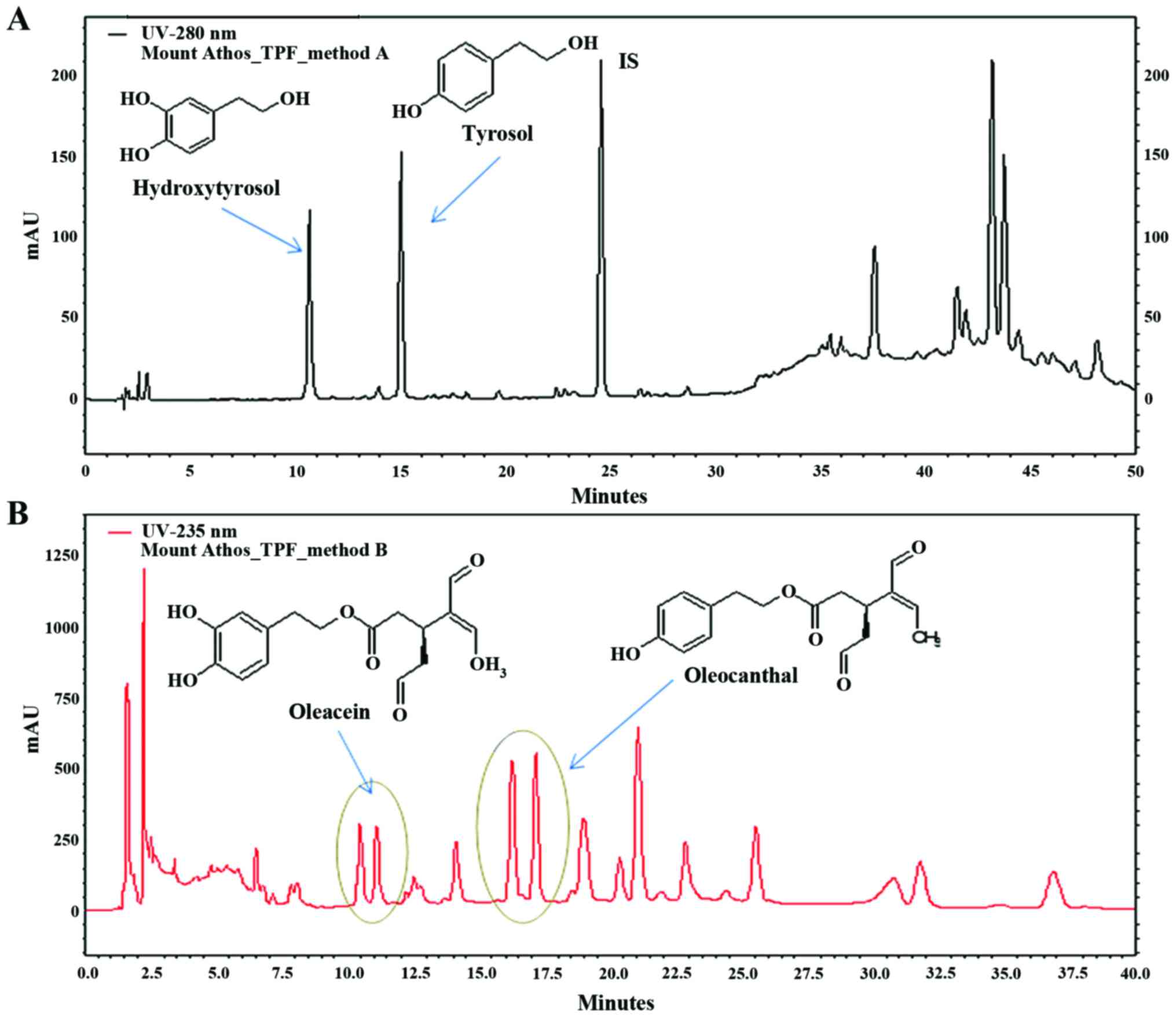
TPF as resulting from HPLC-DAD chromatograms is depicted in
Fig. 1. The results of the
quantitative analysis are expressed in mg/g TPF. Specifically, TPF
extract contained 19.4 mg/g HT, 24.4 mg/g T, 56.3 mg/g OLEA and
135.5 mg/ml OLEO.
As indicated by the quantitative analysis, the TPF
was rich in biophenols. Specifically, the TPC as measured by
Folin-Ciocalteau assay was 484 µg of biophenols per mg of
TPF extract. According to a comparative study in which 221 EVOOs
were extracted from 4 olive mono-cultivars (Koroneiki, Tsounati,
Adramitini, and Throubolia) originating from 4 divisions of Greece
(47), the range of TPC was
between 30–351 mg/kg EVOOs. Of note, according to a health claim on
OO polyphenols approved by the European Food Safety Authority
(EFSA; Commission Regulation EU 432/2012), OOs are considered to
protect from oxidative stress-induced lipid peroxidation in blood,
when they contain about 5 mg of HT and its derivatives (e.g.,
oleuropein complex and T) per 20 g of OO. Based on the content of
TPF in biophenols, OO in the present study, contain 4.15 mg of HT
and its derivatives per 20 g.
The chemical analysis of TPF indicated that the T
levels were high (28.28 mg/kg TPF) and close to those of HT (22.48
mg/kg TPF), according to a comparative study, where the T levels
fluctuated between 1.97–16.2 mg/kg OO (47). Additionally, OLEO exhibited the
highest concentration which was >2-fold higher than that of
OLEA.
Following the determination of the biophenolic
content, the antioxidant activities of TPF and HT were evaluated by
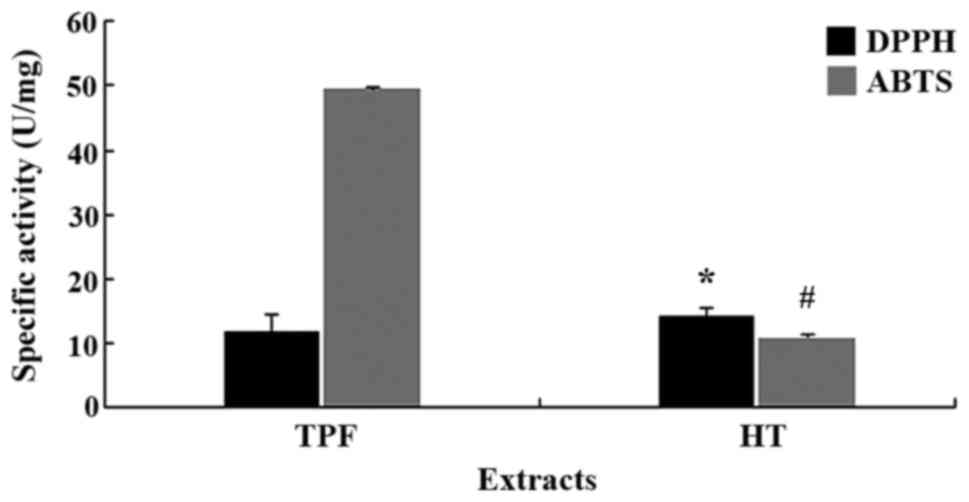
DPPH and ABTS•+ assays (Fig. 2). In order to examine the
antioxidant potency of the biophenols contained in the tested
compounds, the specific activity was determined. Specific activity
is a unit that compares extracts with varying composition. Since HT
was a pure polyphenol, for the calculation of the specific
activity, its polyphenolic content was considered as 100%.
According to DPPH assay, HT exhibited a significantly (P<0.05)
higher antioxidant activity compared with TPF. by contrast, in
ABTS•+ assay, the antioxidant activity of HT was
significantly (P<0.05) lower than that of TPF. These differences
between the DPPH and ABTS•+ assays may be ascribed to
the different solvents used in each assay (48). In particular, in DPPH assay the
solvent is MeOH while in ABTS•+ assay the solvent is
water (49). Namely, the
bioactive compounds of TPF may be more polar and so they were
dissolved more in water and less in MeOH than HT.
The antioxidant activity of the tested compounds was
also examined in C2C12 myoblasts and EA.hy926 endothelial cells. In
order to use non-cytotoxic concentrations of the tested compounds
for these experiments, their cytotoxicity was assessed by XTT
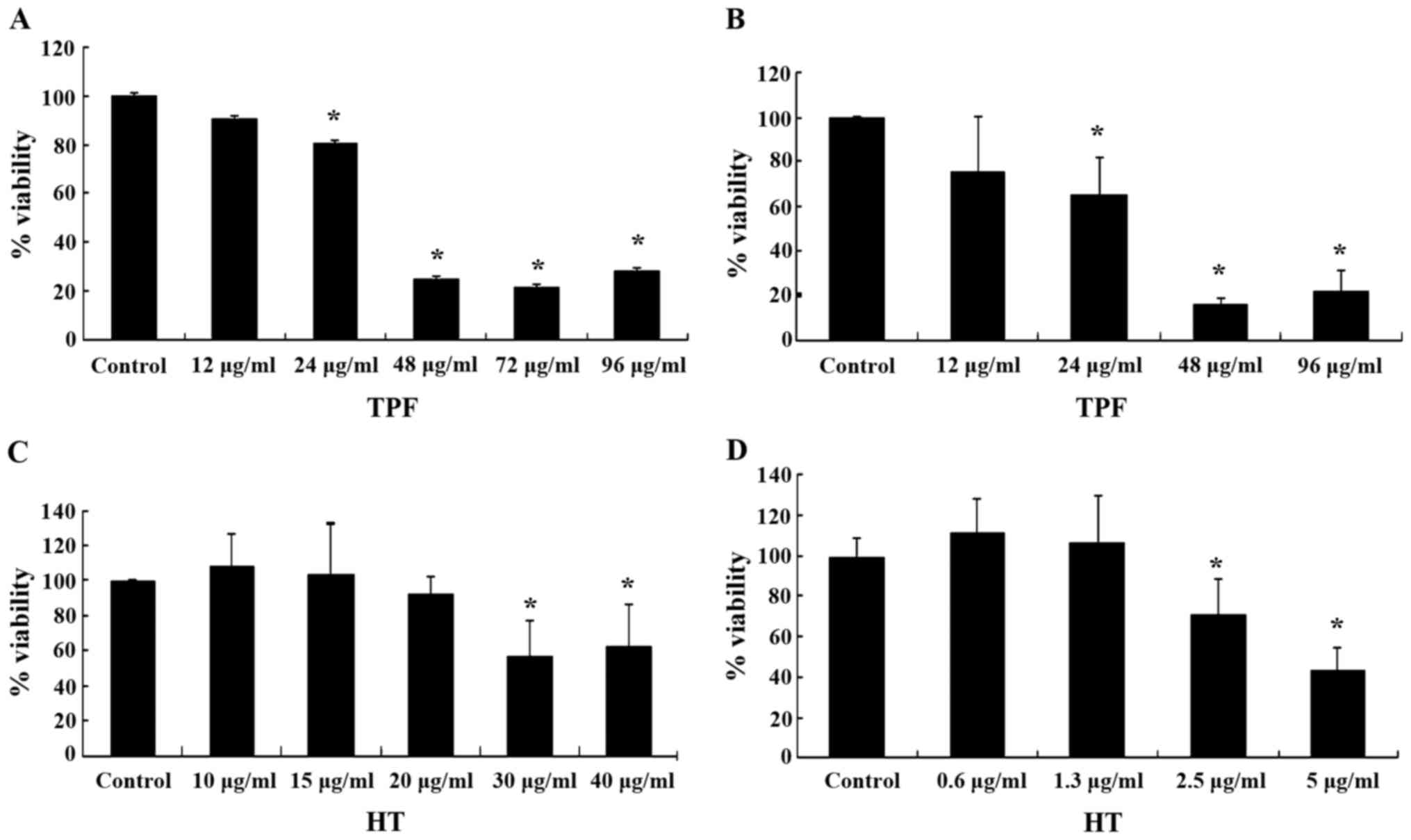
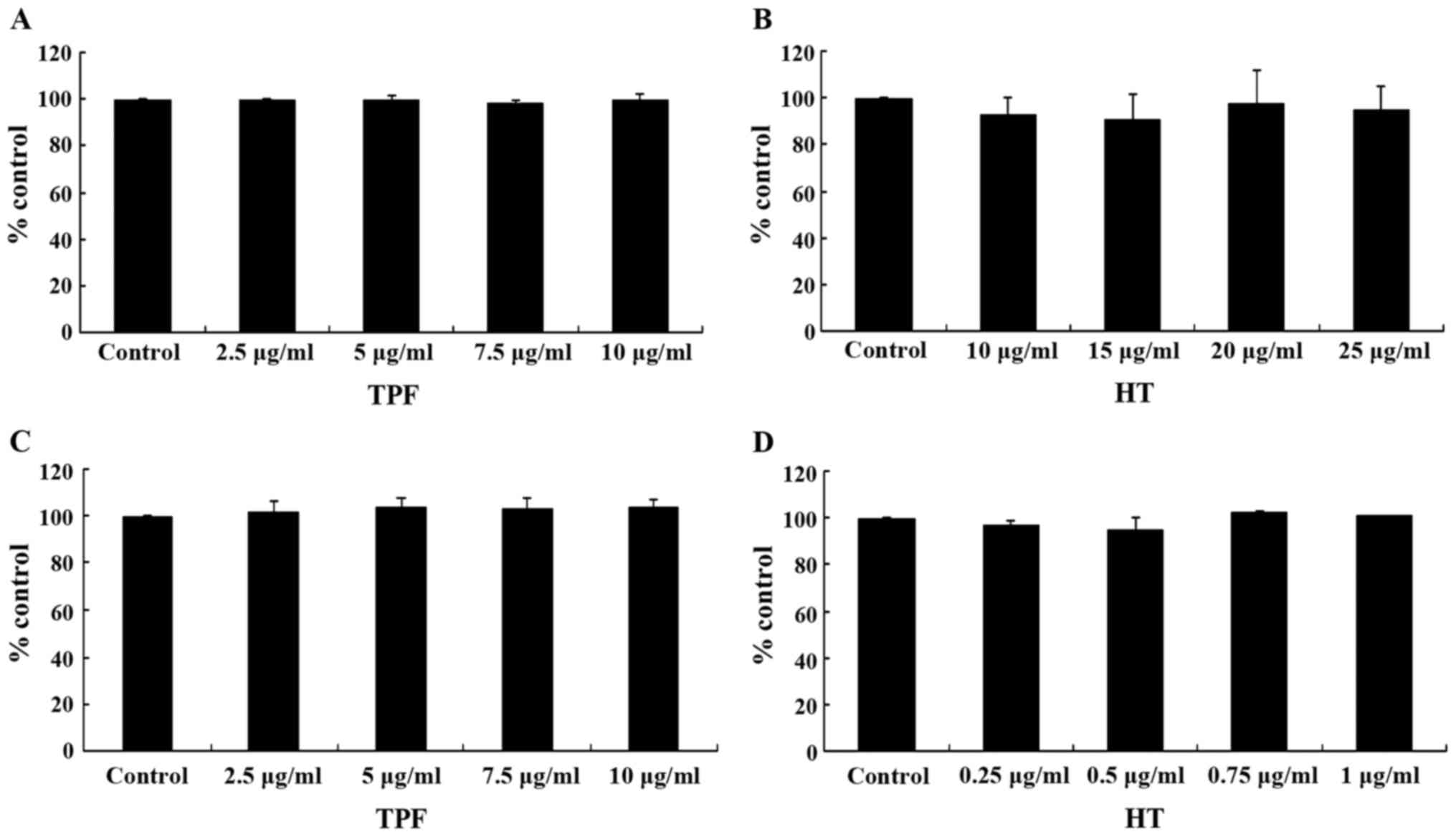
assay. The results from XTT assay revealed that TPF exhibited
cytotoxicity at concentrations >24.0 µg/ml in both the
C2C12 and EA.hy926 cells (Fig. 3A and
B). HT exerted cytotoxic effects at concentrations >30.0
µg/ml in the C2C12 cells and >2.5 µg/ml in the
EA.hy926 cells (Fig. 3C and D).
Thus, the range of concentrations used for assessing the
antioxidant activity of the tested compounds were 2.5–10.0
µg/ml for TPF in both the C2C12 and EA.hy926 cells, and
10–25 and 0.25–1.0 µg/ml for HT in the C2C12 and EA.hy926
cells, respectively.
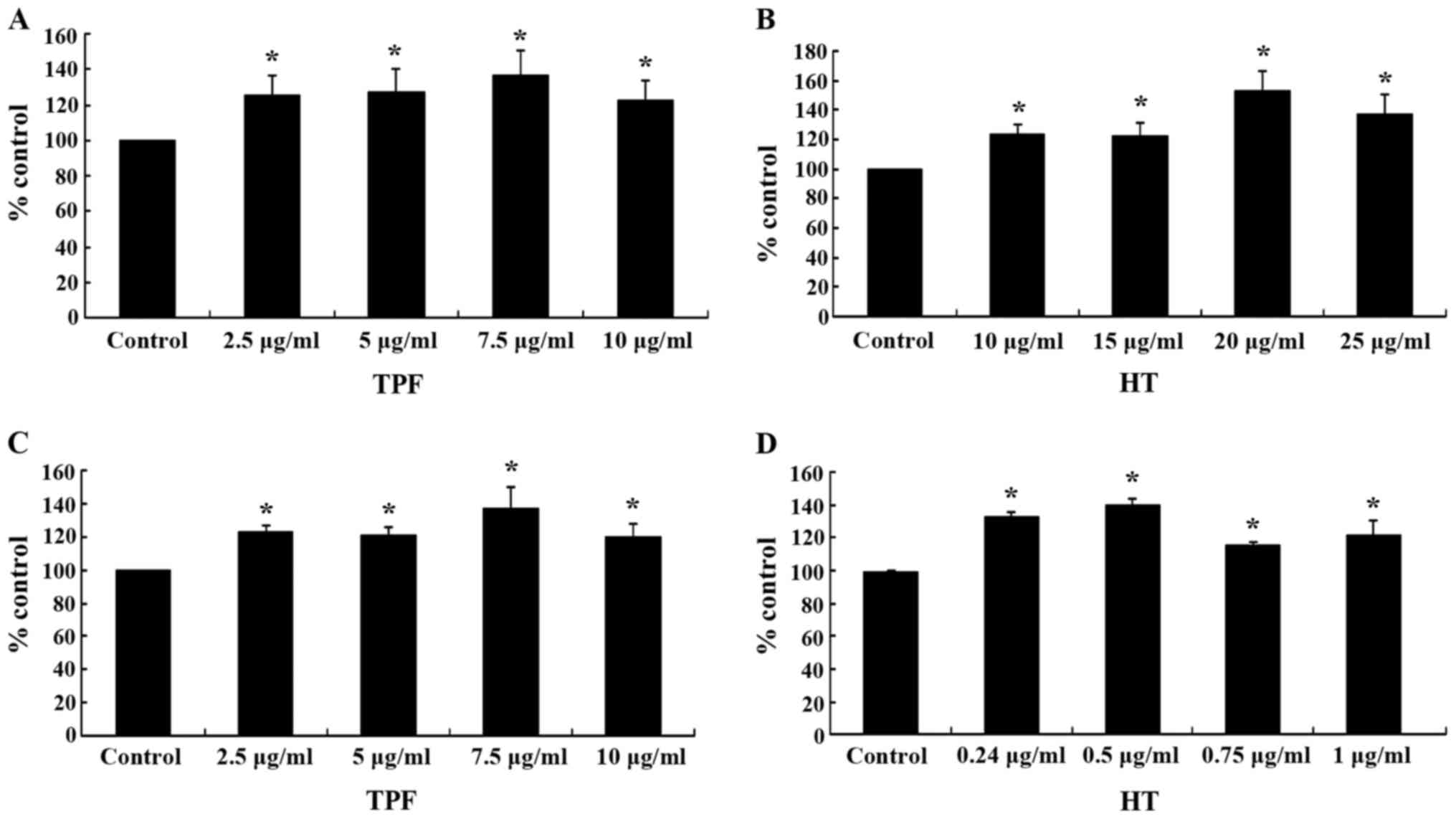
The results from flow cytometric analysis revealed
that treatment of the C2C12 cells with TPF significantly increased
the GSH levels by 26, 28, 37 and 23% at 2.5, 5, 7.5 and 10
µg/ml, respectively, compared with control (Fig. 4A). Treatment of the C2C12 cells
with HT also significantly increased the GSH levels by 24, 23, 54
and 38% at 10, 15, 20 and 25 µg/ml, respectively (Fig. 4B). Moreover, treatment of the
EA.hy926 cells with TPF and HT significantly increased the GSH
levels compared to the controls (Fig.
4C and D). Thus, the effects of TPF and HT on the GSH levels
seemed to be cell type-independent. Particularly, TPF increased the
GSH levels in the EA.hy926 cells by 23, 22, 38 and 20% at 2.5, 5,
7.5 and 10 µg/ml, respectively, while HT by 34, 40, 15 and
22% at 0.25, 0.5, 0.75 and 1.0 µg/ml respectively, compared
to controls (Fig. 4C and D). The
HT-induced increase in GSH levels suggested that this polyphenol
was one of the bioactive compounds in TPF accounting for its
effects on the GSH levels.
HT has been shown to increase GSH synthesis in human
retinal pigment epithelial cells by the activation of the nuclear
factor (erythroid-derived-2)-like 2 (Nrf2), a transcription factor
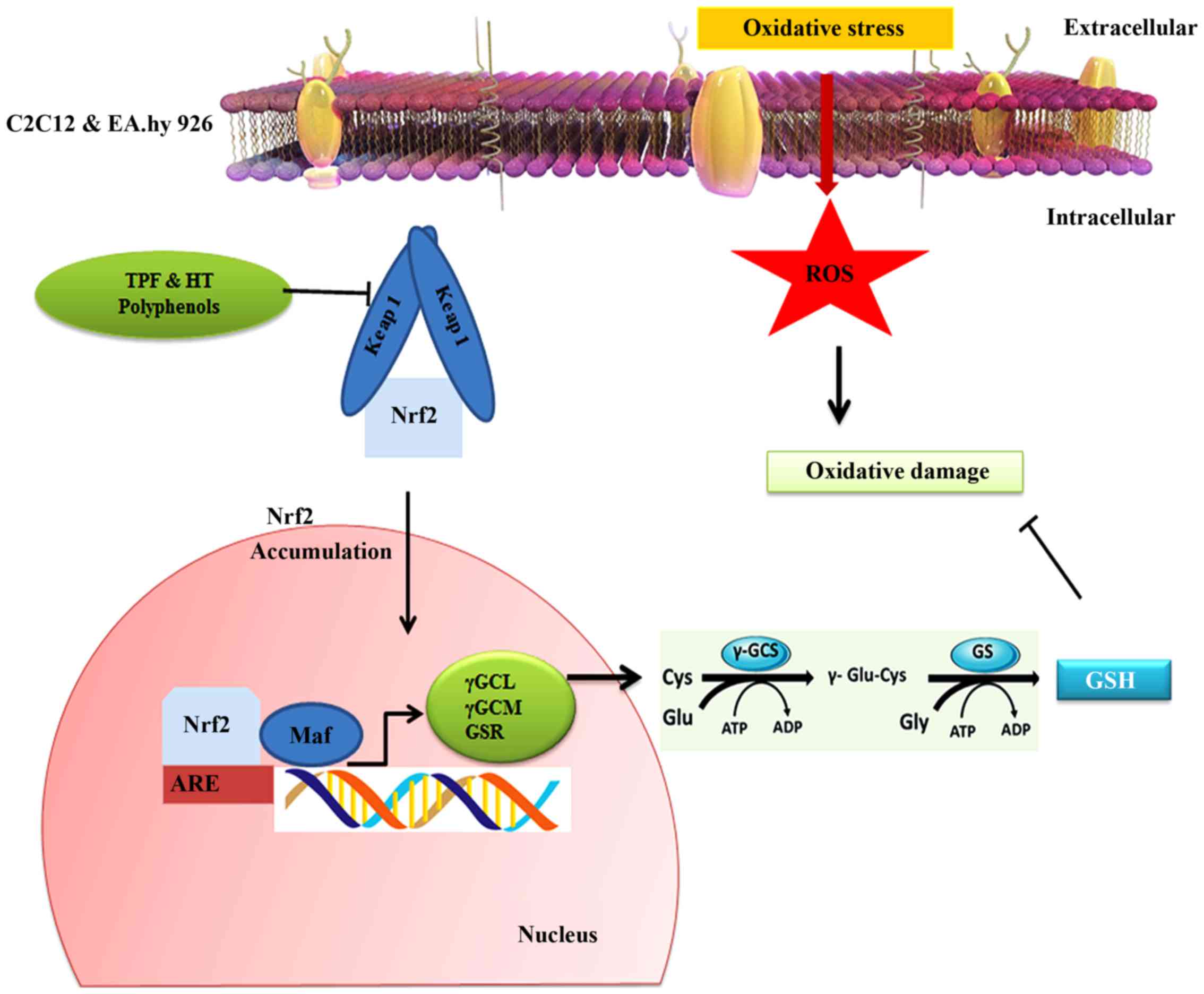
and a key regulator of the antioxidant defense system (Fig. 6) (50–53). Nrf2 induces the expression of
gamma-glutamylcysteine ligase (GCL), which has the ability to
regulate the synthesis of GSH (54). Moreover, HT seems to activate the
NK-p62/SQSTM1 pathway, required for Nrf2-induced GSH synthesis
(42,50). Manzanares et al (55) indicated that after a high EVOO
diet, the activation of Nrf2 was observed in rat livers and mammary
glands.
Moreover, OO biophenols, from olive mill waste water
have been shown to increase GSH concentrations in human blood
(48,56). This increase in GSH levels may be
due to the upregulation of GCL and GSH synthetase, mediated by the
antioxidant response element (ARE; a target of Nrf2) (Fig. 6) (56). Covas et al indicated that
an EVOO rich in biophenols (366 mg/kg) beneficially modulated the
balance between GSH and oxidized glutathione (GSSG). Thus, EVOO
biophenols exert their antioxidant effects as a part of the
induction of Nrf2 (57).
It is also worth mentioning that at the highest
concentration, both TPF and HT exhibited less potency for
increasing the GSH levels than at lower concentrations. This
observed decrease in the potency of TPF and HT at the highest
concentrations may be explained by a potential pro-oxidant activity
of polyphenols after reaching a 'crucial' concentration (43,58–61). A potential pro-oxidant activity
was also supported by the cytotoxicity exerted by TPF and HT after
these high concentrations.
Oleuropein and HT have been reported to exert
pro-oxidant effects, due to their iron- and copper-reducing
activities. These reduced metals, in turn, can catalyze the
production of OH• radicals by the Fenton reaction. The
ability of dietary polyphenols to act as antioxidants/prooxidants
under in vitro and in vivo systems is dependent on a
number of factors such their concentration and structure (62).
The observed TPF- and HT-induced increase in GSH
levels in C2C12 myoblasts and EA.hy926 endothelial cells is of
importance. GSH is a conserved molecule among many species,
reflecting its crucial biological role. In particular, it has been
established that the thiol moiety of GSH is important for the
direct scavenging of radical species (63). Thus, a decrease at GSH levels
contributes to oxidative stress associated with aging and many
pathological conditions, such as neurodegenerative diseases,
inflammation, and infections (63).
Unlike GSH, the ROS levels were not significantly
affected by the two test compounds compared to the controls in
neither cell line (Fig. 5). It
should be mentioned that the measured ROS levels corresponded to
the naturally occurring levels in these cells; that is, there was
not any treatment of cells with oxidizing agents before the
addition of TPF and HT. Previous studies have also reported that
changes in oxidative stress levels or antioxidant mechanisms are
not always accompanied by changes in ROS levels (42,43,64).
In conclusion, EVOO from a Greek endemic olive tree
variety, Olea europea, was used for the isolation of TPF, as
well as that of pure HT. For the extraction process, it was used a
recently developed green CPE-based method that allows the treatment
of large volumes of OO in a continuous extraction procedure and
with extremely low solvent consumption. For the isolation of pure
HT, a combination of two chromatographic techniques was used, which
has the advantage of using different criteria of selectivity
between two purification steps and thus it is effective for the
recovery of pure compounds from complex natural extracts. As
indicated from the quantitative analysis, the TPF extract was rich
in biophenols, such as T, HT, OLEO and OLEA. Finally, TPF and HT
exhibited in vitro potent free radical scavenging activity,
while they increased (at low concentrations), the levels of GSH,
one of the most important antioxidant molecules, in both
endothelial cells and myoblasts.
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
OOs
|
olive oils
|
|
H2O2
|
hydrogen peroxide
|
|
FA
|
fatty acid
|
|
MUFA/SFA
|
monounsaturated/saturated fatty acid
ratio
|
|
GSH
|
glutathione
|
|
HT
|
hydroxytyrosol
|
|
EVOO
|
extra virgin olive oil
|
|
TPF
|
total polyphenolic fraction
|
|
T
|
tyrosol
|
|
OLEO
|
oleocanthal
|
|
OLEA
|
oleacein
|
|
DMEM
|
Dulbecco's modified Eagle's
medium
|
|
FBS
|
fetal bovine serum
|
|
TLC
|
thin layer chromatography
|
|
CPC
|
centrifugal partition
chromatography
|
|
etOH
|
ethanol
|
|
MeOH
|
methanol
|
|
ISTD
|
internal standard
|
|
RSC
|
radical scavenging capacity
|
|
1H-NMR
|
proton nuclear magnetic resonance
|
|
OD
|
optical density
|
|
Nrf2
|
nuclear factor
(erythroid-derived-2)-like 2
|
Acknowledgments
The present study was funded in part by a grant
(Demetrios Kouretas) from the 'Tsantali Wineries'.
References
|
1
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
3
|
Landete JM: Dietary intake of natural
antioxidants: Vitamins and polyphenols. Crit Rev Food Sci Nutr.
53:706–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang YZ, Yang S and Wu G: Free radicals,
antioxidants, and nutrition. Nutrition. 18:872–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Landete JM: Updated knowledge about
polyphenols: functions, bioavailability, metabolism, and health.
Crit Rev Food Sci Nutr. 52:936–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HY, Yang M, Li Z and Meng Z: Curcumin
inhibits angio-tensin II-induced inflammation and proliferation of
rat vascular smooth muscle cells by elevating PPAR-γ activity and
reducing oxidative stress. Int J Mol Med. 39:1307–1316.
2017.PubMed/NCBI
|
|
7
|
Kerasioti E, Stagos D, Georgatzi V, Bregou
E, Priftis A, Kafantaris I and Kouretas D: Antioxidant effects of
sheep whey protein on endothelial cells. Oxid Med Cell Longev.
2016:65857372016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deanfield JE, Halcox JP and Rabelink TJ:
Endothelial function and dysfunction: Testing and clinical
relevance. Circulation. 115:1285–1295. 2007.PubMed/NCBI
|
|
9
|
Woywodt A, Bahlmann FH, De Groot K, Haller
H and Haubitz M: Circulating endothelial cells: Life, death,
detachment and repair of the endothelial cell layer. Nephrol Dial
Transplant. 17:1728–1730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kokura S, Wolf RE, Yoshikawa T, Granger DN
and Aw TY: Molecular mechanisms of neutrophil-endothelial cell
adhesion induced by redox imbalance. Circ Res. 84:516–524. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou Y, Yoon S, Jung KJ, Kim CH, Son TG,
Kim MS, Kim YJ, Lee J, Yu BP and Chung HY: Upregulation of aortic
adhesion molecules during aging. J Gerontol A Biol Sci Med Sci.
61:232–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spanou C, Veskoukis AS, Kerasioti T,
Kontou M, Angelis A, Aligiannis N, Skaltsounis AL and Kouretas D:
Flavonoid glycosides isolated from unique legume plant extracts as
novel inhibitors of xanthine oxidase. PLoS One. 7:e322142012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goutzourelas N, Stagos D, Spanidis Y,
Liosi M, Apostolou A, Priftis A, Haroutounian S, Spandidos DA,
Tsatsakis AM and Kouretas D: Polyphenolic composition of grape stem
extracts affects antioxidant activity in endothelial and muscle
cells. Mol Med Rep. 12:5846–5856. 2015.PubMed/NCBI
|
|
14
|
Reid MB: Free radicals and muscle fatigue:
Of ROS, canaries, and the IOC. Free Radic Biol Med. 44:169–179.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rafehi H, Smith AJ, Balcerczyk A, Ziemann
M, Ooi J, Loveridge SJ, Baker EK, El-Osta A and Karagiannis TC:
Investigation into the biological properties of the olive
poly-phenol, hydroxytyrosol: Mechanistic insights by genome-wide
mRNA-Seq analysis. Genes Nutr. 7:343–355. 2012. View Article : Google Scholar
|
|
16
|
Montaño A, Hernández M, Garrido I, Llerena
JL and Espinosa F: Fatty acid and phenolic compound concentrations
in eight different monovarietal virgin olive oils from extremadura
and the relationship with oxidative stability. Int J Mol Sci.
17:172016. View Article : Google Scholar
|
|
17
|
Local Food-Nutraceuticals Consortium:
Understanding local Mediterranean diets: a multidisciplinary
pharmacological and ethnobotanical approach. Pharmacol Res.
52:353–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trichopoulou A, Costacou T, Bamia C and
Trichopoulos D: Adherence to a Mediterranean diet and survival in a
Greek population. N Engl J Med. 348:2599–2608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trichopoulou A, Lagiou P, Kuper H and
Trichopoulos D: Cancer and Mediterranean dietary traditions. Cancer
Epidemiol Biomarkers Prev. 9:869–873. 2000.PubMed/NCBI
|
|
20
|
Brinkman MT, Buntinx F, Kellen E, Van
Dongen MC, Dagnelie PC, Muls E and Zeegers MP: Consumption of
animal products, olive oil and dietary fat and results from the
Belgian case-control study on bladder cancer risk. Eur J Cancer.
47:436–442. 2011. View Article : Google Scholar
|
|
21
|
Oliveras-Ferraros C, Fernández-Arroyo S,
Vazquez-Martin A, Lozano-Sánchez J, Cufí S, Joven J, Micol V,
Fernández-Gutiérrez A, Segura-Carretero A and Menendez JA: Crude
phenolic extracts from extra virgin olive oil circumvent de novo
breast cancer resistance to HER1/HER2-targeting drugs by inducing
GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of
Histone H3. Int J Oncol. 38:1533–1547. 2011.PubMed/NCBI
|
|
22
|
Tunca B, Tezcan G, Cecener G, Egeli U, Ak
S, Malyer H, Tumen G and Bilir A: Olea europaea leaf extract alters
microRNA expression in human glioblastoma cells. J Cancer Res Clin
Oncol. 138:1831–1844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouallagui Z, Han J, Isoda H and Sayadi S:
Hydroxytyrosol rich extract from olive leaves modulates cell cycle
progression in MCF-7 human breast cancer cells. Food Chem Toxicol.
49:179–184. 2011. View Article : Google Scholar
|
|
24
|
Cárdeno A, Sánchez-Hidalgo M,
Cortes-Delgado A and Alarcón de la Lastra C: Mechanisms involved in
the antiproliferative and proapoptotic effects of unsaponifiable
fraction of extra virgin olive oil on HT-29 cancer cells. Nutr
Cancer. 65:908–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Acquaviva R, Di Giacomo C, Sorrenti V,
Galvano F, Santangelo R, Cardile V, Gangia S, D'Orazio N, Abraham
NG and Vanella L: Antiproliferative effect of oleuropein in
prostate cell lines. Int J Oncol. 41:31–38. 2012.PubMed/NCBI
|
|
26
|
Coccia A, Bastianelli D, Mosca L,
Monticolo R, Panuccio I, Carbone A, Calogero A and Lendaro E: Extra
virgin olive oil phenols suppress migration and invasion of T24
human bladder cancer cells through modulation of matrix
metalloproteinase-2. Nutr Cancer. 66:946–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coccia A, Mosca L, Puca R, Mangino G,
Rossi A and Lendaro E: Extra-virgin olive oil phenols block cell
cycle progression and modulate chemotherapeutic toxicity in bladder
cancer cells. Oncol Rep. 36:3095–3104. 2016.PubMed/NCBI
|
|
28
|
Miró-Casas E, Covas MI, Fitó M,
Farré-Albadalejo M, Marrugat J and de la Torre R: Tyrosol and
hydroxytyrosol are absorbed from moderate and sustained doses of
virgin olive oil in humans. Eur J Clin Nutr. 57:186–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keys A, Menotti A, Karvonen MJ, Aravanis
C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys
MH, et al: The diet and 15-year death rate in the seven countries
study. Am J Epidemiol. 124:903–915. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Obied HK, Prenzler PD, Omar SH, Ismael R,
Servili M, Esposto S, Taticchi A, Selvaggini R and Urbani S:
Pharmacology of Olive Biophenols. Advances in Molecular Toxicology.
Fishbein JC and Heilman JM: 6. Elsevier; Amsterdam: pp. 195–242.
2012, View Article : Google Scholar
|
|
31
|
Lewandowska U, Szewczyk K, Hrabec E,
Janecka A and Gorlach S: Overview of metabolism and bioavailability
enhancement of polyphenols. J Agric Food Chem. 61:12183–12199.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaffer S, Podstawa M, Visioli F, Bogani
P, Müller WE and Eckert GP: Hydroxytyrosol-rich olive mill
wastewater extract protects brain cells in vitro and ex vivo. J
Agric Food Chem. 55:5043–5049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rietjens SJ, Bast A and Haenen GR: New
insights into controversies on the antioxidant potential of the
olive oil antioxidant hydroxytyrosol. J Agric Food Chem.
55:7609–7614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodríguez-Morató J, Boronat A, Kotronoulas
A, Pujadas M, Pastor A, Olesti E, Pérez-Mañá C, Khymenets O, Fitó
M, Farré M, et al: Metabolic disposition and biological
significance of simple phenols of dietary origin: Hydroxytyrosol
and tyrosol. Drug Metab Rev. 48:218–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Angelis A, Hamzaoui M, Aligiannis N, Nikou
T, Michailidis D, Gerolimatos P, Termentzi A, Hubert J, Halabalaki
M, Renault JH, et al: An integrated process for the recovery of
high added-value compounds from olive oil using solid support free
liquid-liquid extraction and chromatography techniques. J
Chromatogr A. 1491:126–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mastralexi A, Nenadis N and Tsimidou MZ:
Addressing analytical requirements to support health claims on
'olive oil polyphenols' (EC Regulation 432/2012). J Agric Food
Chem. 62:2459–2461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Impellizzeri J and Lin J: A simple
high-performance liquid chromatography method for the determination
of throat-burning oleocanthal with probated antiinflammatory
activity in extra virgin olive oils. J Agric Food Chem.
54:3204–3208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vougogiannopoulou K, Lemus C, Halabalaki
M, Pergola C, Werz O, Smith AB III, Michel S, Skaltsounis L and
Deguin B: One-step semisynthesis of oleacein and the determination
as a 5-lipoxygenase inhibitor. J Nat Prod. 77:441–445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singleton VL, Orthofer R and
Lamuela-Raventos RM: Analysis of total phenols and other oxidation
substrates and antioxidants by means of Folin-Ciocalteu reagent.
Methods Enzymol. 299:152–178. 1998. View Article : Google Scholar
|
|
40
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
Lebensm Wiss Technol. 28:25–30. 1995. View Article : Google Scholar
|
|
41
|
Cano A, Hernandez-Ruiz J, García-Cánovas
F, Acosta M and Arnao MB: An end-point method for estimation of the
total antioxidant activity in plant material. Phytochem Anal.
9:196–202. 1998. View Article : Google Scholar
|
|
42
|
Goutzourelas N, Stagos D, Demertzis N,
Mavridou P, Karterolioti H, Georgadakis S, Kerasioti E, Aligiannis
N, Skaltsounis L, Statiri A, et al: Effects of polyphenolic grape
extract on the oxidative status of muscle and endothelial cells.
Hum Exp Toxicol. 33:1099–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Priftis A, Stagos D, Konstantinopoulos K,
Tsitsimpikou C, Spandidos DA, Tsatsakis AM, Tzatzarakis MN and
Kouretas D: Comparison of antioxidant activity between green and
roasted coffee beans using molecular methods. Mol Med Rep.
12:7293–7302. 2015.PubMed/NCBI
|
|
44
|
Vulcano I, Halabalaki M, Skaltsounis L and
Ganzera M: Quantitative analysis of pungent and anti-inflammatory
phenolic compounds in olive oil by capillary electrophoresis. Food
Chem. 169:381–386. 2015. View Article : Google Scholar
|
|
45
|
Keiler AM, Zierau O, Bernhardt R,
Scharnweber D, Lemonakis N, Termetzi A, Skaltsounis L, Vollmer G
and Halabalaki M: Impact of a functionalized olive oil extract on
the uterus and the bone in a model of postmenopausal osteoporosis.
Eur J Nutr. 53:1073–1081. 2014. View Article : Google Scholar
|
|
46
|
Angelis A, Hubert J, Aligiannis N,
Michalea R, Abedini A, Nuzillard JM, Gangloff SC, Skaltsounis AL
and Renault JH: Bio-guided isolation of methanol-soluble
metabolites of common spruce (Picea abies) bark by-products and
investigation of their dermo-cosmetic properties. Molecules.
21:212016. View Article : Google Scholar
|
|
47
|
Agiomyrgianaki A, Petrakis PV and Dais P:
Influence of harvest year, cultivar and geographical origin on
Greek extra virgin olive oils composition: A study by NMR
spectroscopy and biometric analysis. Food Chem. 135:2561–2568.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pliszka B, Huszcza-Ciołkowska G and
Wierzbicka E: Effects of solvents and extraction methods on the
content and antiradical activity of polyphenols from fruits
Actinidia arguta, Crataegus monogyna, Gaultheria procumbens and
Schisandra chinensis. Acta Sci Pol Technol Aliment. 15:57–63. 2016.
View Article : Google Scholar
|
|
49
|
Schlesier K, Harwat M, Böhm V and Bitsch
R: Assessment of antioxidant activity by using different in vitro
methods. Free Radic Res. 36:177–187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zou X, Feng Z, Li Y, Wang Y, Wertz K,
Weber P, Fu Y and Liu J: Stimulation of GSH synthesis to prevent
oxidative stress-induced apoptosis by hydroxytyrosol in human
retinal pigment epithelial cells: Activation of Nrf2 and
JNK-62/SQSTM1 pathways. J Nutr Biochem. 23:994–1006. 2012.
View Article : Google Scholar
|
|
51
|
Moi P, Chan K, Asunis I, Cao A and Kan YW:
Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic
leucine zipper transcriptional activator that binds to the tandem
NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl
Acad Sci USA. 91:9926–9930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar :
|
|
53
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kobayashi M and Yamamoto M: Nrf2-Keap1
regulation of cellular defense mechanisms against electrophiles and
reactive oxygen species. Adv Enzyme Regul. 46:113–140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Manzanares MA, Solanas M, Moral R, Escrich
R, Vela E, Costa I and Escrich E: Dietary extra-virgin olive oil
and corn oil differentially modulate the mRNA expression of
xeno-biotic-metabolizing enzymes in the liver and in the mammary
gland in a rat chemically induced breast cancer model. Eur J Cancer
Prev. 24:215–222. 2015. View Article : Google Scholar
|
|
56
|
Visioli F, Wolfram R, Richard D, Abdullah
MI and Crea R: Olive phenolics increase glutathione levels in
healthy volunteers. J Agric Food Chem. 57:1793–1796. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Covas MI, Nyyssönen K, Poulsen HE,
Kaikkonen J, Zunft HJ, Kiesewetter H, Gaddi A, de la Torre R, Mursu
J, Bäumler H, et al EUROLIVE Study Group: The effect of polyphenols
in olive oil on heart disease risk factors: A randomized trial. Ann
Intern Med. 145:333–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Procházková D, Boušová I and Wilhelmová N:
Antioxidant and prooxidant properties of flavonoids. Fitoterapia.
82:513–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lambert JD and Elias RJ: The antioxidant
and pro-oxidant activities of green tea polyphenols: A role in
cancer prevention. Arch Biochem Biophys. 501:65–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fukumoto LR and Mazza G: Assessing
antioxidant and prooxidant activities of phenolic compounds. J
Agric Food Chem. 48:3597–3604. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sakihama Y, Cohen MF, Grace SC and
Yamasaki H: Plant phenolic antioxidant and prooxidant activities:
Phenolics-induced oxidative damage mediated by metals in plants.
Toxicology. 177:67–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Maurya DK and Devasagayam TP: Antioxidant
and prooxidant nature of hydroxycinnamic acid derivatives ferulic
and caffeic acids. Food Chem Toxicol. 48:3369–3373. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aquilano K, Baldelli S and Ciriolo MR:
Glutathione: New roles in redox signaling for an old antioxidant.
Front Pharmacol. 5:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kerasioti E, Stagos D, Priftis A,
Aivazidis S, Tsatsakis AM, Hayes AW and Kouretas D: Antioxidant
effects of whey protein on muscle C2C12 cells. Food Chem.
155:271–278. 2014. View Article : Google Scholar : PubMed/NCBI
|