Introduction
Cerebrovascular disease (CVD) is a serious human
health hazard with a high incidence rate, often resulting in
disability and death. Currently, it is the third leading cause of
death in humans (1–3). In China, there has been a rapid
increase in the incidence of CVD, with ~2 million people suffering
from this disease (3,4). Most survivors of CVD experience
permanent neurological damage, with 70% losing their ability to
work and 40% left with severe disability that impacts the quality
of life. Cerebral ischemia accounts for >75% of CVD patients
(1). No effective therapy is
currently available for treating cerebral ischemia (5). Evidence from clinical and in
vivo studies suggest that apoptosis of nerve cells produce
significant benefits for cerebral ischemia injury (6). A possible mechanism is thought to be
associated with endothelial dysfunction in cerebral ischemia
(7). Remote ischemic
postconditioning (RIP) in the treatment of CVD relieves
ischemia/reperfusion (I/R) injury (8–10).
However, it is not known if RIP induces neuroprotection against
cerebral ischemia and what the underlying mechanism is. In the
present study, the authors hypothesized that the protective effect
of RIP on neurological damage is mediated by exosomes derived from
endothelial cells in femoral arteries.
Exosomes are secreted from cells, and contain
proteins, DNA, mRNA and some non-protein coding RNAs. They carry
material and transducer information, is the carrier between cells
for material and information transduction (11). Exosomes play an important role in
the cellular microenvironment and are well-studied multi-functional
extracellular vesicles. In cancer cells, the exosomes of
5-FU-resistant CCL227-RH cells, are devoid of microRNA-200, and
accelerate the formation of circular chemorepellent-induced defects
in vascular endothelial cell monolayers as compared to exosomes
from naïve CCL227 cells (12).
The paracrine effects of human umbilical vein endothelial cells
(HUVECs) improve the generation of endothelial cells from cord
blood circulating endothelial progenitor cells and may include the
role of exosomes (13). A recent
study reported that exosomes extracted from adipose-derived
mesenchymal stem cells play a protective role against nerve injury
induced by glutamate (14).
Endothelial cell-derived exosomes potently increase the
proliferation, migration, secretion of matrix metalloproteinase
(MMP)-1, MMP-3 and nuclear factor (NF)-κB activity in the
mesenchymal stem cells, stimulating local trophic support (15). Mesenchymal stem cells promote
nerve growth through the support of Schwann cells, secreted
neurovascular factors and possibly trans-differentiation into
Schwann-like cells (16).
Condition medium from cells that were treated under hypoxic
conditions increased the number of differentiating neurons in
vitro (17). Exosomes
isolated from different kinds of cells all express the
characteristic proteins CD63, HSP70 and TSG101 (18).
In the present study, the authors established an
animal model of I/R injury with RIP in rats, and a cell model of
I/R injury in HUVECs and SH-SY5Y cells. The levels of protein
markers of exosomes were analyzed and measured and then exosomes
were extracted from both rats and HUVECs. The role of endothelial
cell-derived exosomes in proliferation, apoptosis, cell cycle,
migration and invasion of SH-SY5Y cells undergoing I/R was
evaluated. In addition, the authors detected the apoptosis-related
molecules caspase-3, Bax and Bcl-2. These findings help to
understand the mechanism underlying the protective role of remote
ischemia in I/R injury.
Materials and methods
Animals
A total of 30 adult Sprague-Dawley (SD) rats (15
male and 15 female) at (10 weeks old) were used, ranging in weight
from 220 to 250 g that were provided by the Laboratory Animal
Center, Nanchang University (Nanchang, China). Animals were
randomly divided into three groups that included the sham-operated
(sham) group, the middle cerebral artery occlusion and reperfusion
(MCAO/R) group and the RIP group, with 10 rats in each group. All
the rats received humane care, according to the criteria outlined
in the Guide for the Care and Use of Laboratory Animals, published
by the National Institute of Health (NIH publication 86-23 revised
1985). The animal protocol was approved by the Animal Ethics
Committee of the Second Affiliated Hospital of Nanchang University
(Nanchang, China).
Establishment of the MCAO/R model
Transient cerebral I/R (MCAO/R) was induced, as
previously described (19,20).
Rats were anesthetized with 7% chloral hydrate (0.5 ml/100 g) and
subjected to the operation. Bilateral femoral arteries were exposed
before the occlusion of middle cerebral artery. In the RIP group,
the middle cerebral artery was subject to a RIP protocol, which
comprised an occlusion for 2 h followed by clamping of the femoral
artery, repeated for 3 cycles. Rats in the sham group were
subjected to the same surgical procedure as rats in the MCAO/R
(model) group without occlusion of the artery. Rats in the RIP
group were subjected to the same surgical procedure as the rats in
the MCAO/R group without the use of remote ischemic conditioning.
Following reperfusion for 24 h, the infarct size, number of
apoptotic cells in the rat hippocampal tissue, expression of
exosome markers and release of exosomes were determined.
Cell culture
The human SH-SY5Y (ATCC, CRL-2266) nerve cells were
cultured in F12 + Dulbecco's modified Eagle's medium (DMEM; 1:1,
v/v; Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen, Thermo
Fisher Scientific, Inc.), L-glutamine and antibiotics and incubated
in a humidified atmosphere of 5% CO2 at 37°C. HUVECs
(American Type Culture Collection, Manassas, VA, USA; PCS-100-010)
were cultured in DMEM supplemented with 10% FBS (Invitrogen, Thermo
Fisher Scientific, Inc.) and 10% growth factor containing epidermal
growth factor, fibroblast growth factor-2, cAMP, heparin,
hydrocortisone, penicillin, streptomycin and amphotericin-B
(21).
Establishment of the model of I/R
injury
To test the appropriate I/R time for subsequent
experiments, oxygen glucose deprivation and reoxygenation of
SH-SY5Y and HUVEC cells was performed. Cells were placed in a
hypoxia chamber (1% O2, 5% CO2 at 37°C, cat.
no. 27310; StemCell Technologies, Vancouver, BC, Canada) at
different time-points followed by 24 or 21 h of reoxygenation,
respectively (22).
Evaluation of infarct volume and brain
injury
To evaluate the infarct in brain tissue, rat brains
were sectioned, fixed and stained by using
2,3,5-triphenyltetrazolium chloride (TTC, cat. no. G3005; Beijing
Solarbio Science & Technology, Co., Ltd., Beijing, China). The
TTC-stained brains were separated and the images scanned. The
infarct volume was analyzed by using ImageJ (version: v1.48u;
National Institutes of Health, Bethesda, MD, USA) using the
following formula: Infarct volume (%) = (contralateral hemisphere
volume − surgery side without infarct volume)/contralateral
hemisphere volume × 100% (20).
Pathological changes in brain hippocampal tissues were evaluated by
hematoxylin and eosin (H&E) staining. The apoptotic cells in
the rat hippocampal tissues were also measured using the TUNEL
assay kit (206386; Abcam, Cambridge, MA, USA) according to the
manufacturer's instructions. Five fields of each group were
observed under an optical microscope.
Exosome preparation
The release of exosomes was evaluated by detecting
markers of exosomes that included CD63, HSP70 and TSG101 in brain
tissue sections using immunostaining, and in rat plasma using
western blot analysis and flow cytometry. Exosomes were obtained
from plasma and cell culture media (FBS without exosomes). Exosomes
were prepared by standard differential centrifugation as follows:
centrifugation at 3,000 × g for 20 min at 4°C to obtain plasma,
then 10,000 × g for 20 min at 4°C to remove cells and platelets,
then twice at 100,000 × g for 70 min at 4°C with a SW-41 rotor,
followed by washes with phosphate-buffered saline (PBS). The
vehicle control consisted of PBS alone. A total of 5 µg of
exosomes were incubated with 1.25 µl aldehyde/sulfate latex
beads, 4% w/v (4 µm, A37304; Invitrogen, Thermo Fisher
Scientific, Inc.) for 15 min, and then incubated with anti-CD63 (5
µl/100 µl sample, cat. no. ab18235; Abcam) anti-Hsp70
(5 µl/100 µl sample, cat. no. ab183435; Abcam) and
anti-TSG101 (5 µl/100 µl sample, cat. no. ab207663;
Abcam), respectively. After fixing with 1% PFA, flow cytometry was
performed with a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). The results were analyzed by the FlowJo
7.0.
Electron microscopy
Electron microscopy was performed on a JEOL 1010
transmission electron microscope (JEOL, Ltd., Tokyo, Japan) at 120
kV after a standard staining procedure with 1% uranyl acetate. The
exosomes were imaged at 49,000 × g and their shapes and sizes were
determined.
Cell cycle detection
Cell cycle status was determined with a FACSCalibur
flow cytometer (BD Biosciences). Following trypsinization, cells
were adjusted to a concentration of 1×106 cells/ml and
treated using Cycletest Plus DNA reagent kit (BD Biosciences),
according to the manufacturer's instructions. The cell cycle status
was analyzed by flow cytometry using propidium iodide (PI).
Cell apoptosis detection
Cell apoptosis was determined by the Annexin V assay
using the FACScan (BD Biosciences) flow cytometer and the apoptotic
rate was calculated. After trypsinization and PBS washes, cells
were suspended in Annexin V binding buffer, and were incubated with
fluorescein isothiocyanate (FITC)-conjugated Annexin V followed by
PI (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China). In
addition, apoptosis was evaluated using Hoechst 33258 staining
(Invitrogen, Thermo Fisher Scientific, Inc.) and by measuring the
expressions of Bax, Bcl-2 and caspase-3.
Proliferation detection
Cell proliferation was determined by using a Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen,
China) and EdU staining assay (Invitrogen, Thermo Fisher
Scientific, Inc.). For the CCK-8 assay, 1×104 cells were
seeded in 96-well plates and then incubated for 24, 48 and 72 h.
Then, the absorbance of the solution was measured at 450 nm using
the microplate reader. For further confirmation of cell
proliferation of exosomes, treated SH-SY5Y cells were fixed and
stained with EdU.
Scratch wound assay
For the scratch wound assay (23,24), prior to the endothelial exosome
treatments, I/R-injured SH-SY5Y nerve cell monolayers were
scratched by a pipette tip after cells reached confluence in 6-well
plates, followed by two washes with PBS to remove cell debris.
Following treatment for the indicated times, images were taken with
an invert contrast microscope and digitized using a digital camera.
The wound areas were calculated to evaluate the cell migration
capacity by using ImageJ (National Institutes of Health).
Invasion assay
Invasion assays were performed, as previously
described (25,26). Complete medium was added to the
lower chamber. Matrigel was used to pre-coat the wells and then
1×105 cells were seeded. After 48 h, cells were fixed in
methanol and stained with crystal violet. Invaded cells were
quantified in at ×10 magnification by an optical microscope.
Results were presented as mean ± standard deviation of three
independent experiments repeated in triplicate.
Immunocytochemistry
The expression of caspase-3 was assessed by
immunocytochemistry. Cells slides were fixed in 4% formaldehyde for
10 min and pre-incubated in 0.5% Triton X-100 and 1.5% bovine serum
albumin (BSA; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 15
min at room temperature. Immunostaining was performed by incubating
overnight at 4°C with anti-caspase-3 (1:50, cat. no. 9662; Cell
Signaling Technology, Danvers, MA, USA) followed by Alexa Fluor 594
secondary antibody (1:500, cat. no. R37117; Invitrogen, Thermo
Fisher Scientific, Inc.) for 1 h at room temperature, washed twice
with PBS. DAPI (5 g/l) was used for counterstaining for 5 min, and
observed by a fluorescence microscope (Olympus Corp., Tokyo,
Japan).
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted using TRIzol reagent
(Invitrogen, Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Next, reverse transcription was performed
using a cDNA synthesis kit (Takara Bio, Inc., Shiga, Japan). qPCR
reactions were conducted using Takara SYBR Premix Ex Taq II (Tli
RNaseH Plus; Takara Bio, Inc.) on a PCR amplifier (CFX-96 Content
Real-time System; Bio-Rad Laboratories, Hercules, CA, USA). The
forward and reverse primer sequences are as follows: caspase-3
forward, 5′-AGCAAACCTCAGGGAAACATT-3′ and reverse,
5′-CTCAGAAGCACACAAACAAAACT-3′; BAX forward,
5′-CCCGAGAGGTCTTTTTCCG-3′ and reverse, 5′-GCCTTGAGCACCAGTTTGC-3′;
of Bcl-2 forward, 5′-GTGTGGAGAGCGTCAACC-3′ and reverse,
5′-CTTCAGAGACAGCCAGGAGA-3′; GAPDH forward,
5′-TGTTCGTCATGGGTGTGAAC-3′ and reverse, 5′-TGGCATGGACTGTGGTCAT-3′.
The data shown is representative of three independent
experiments.
Western blot analysis
Tissues or cells were homogenized in ice-cold RIPA
lysis buffer (cat. no. P0013B; Beyotime, Beijing, China). After
determining the concentration of protein by Bio-Rad protein assay
kit (cat. no. 5000002), 30–50 µg protein were loaded onto a
8–12% SDS-PAGE and separated by electrophoresis at 80 V for 30 min
and then 120 V for 1.5 h. Proteins were transferred to
nitrocellulose at 250 V for 1 h, blocked with 5% non-fat dry milk
for 1 h, and incubated with anti-CD63 (1:1,000, cat. no. ab134045;
Abcam), anti-HSP70 (1:1,000, cat. no. ab53496; Abcam), anti-TSG101
(1:1,000, cat. no. ab125011; Abcam), anti-cleaved caspase-3
(1:1,000, cat. no. sc-271759; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-Bax (1:1,000, cat. no. sc-4239; Santa Cruz
Biotechnology, Inc.), anti-Bcl-2 (1:1,000, cat. no. sc-7382; Santa
Cruz Biotechnology, Inc.) or anti-GAPDH (1:1,000, cat. no.
sc-47724; Santa Cruz Biotechnology, Inc.) overnight. After three
times washes, membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:10,000, cat. no. A4416
and A6154; Sigma-Aldrich) for 1 h at room temperature. Immunoblots
were detected using enhanced chemiluminescence (Beyotime Institute
of Biotechnology). The quantification of western blot analysis was
measured by the Image-Pro Plus 6.0.
Statistical analysis
Data were obtained from at least three independent
experiments and expressed as means ± standard deviations.
Statistical analysis was performed by one-way analysis of variance
(ANOVA), followed by the Student-Newman-Keuls multiple comparison
post hoc test or by the Mann-Whitney test by using the SPSS
software (SPSS 19.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
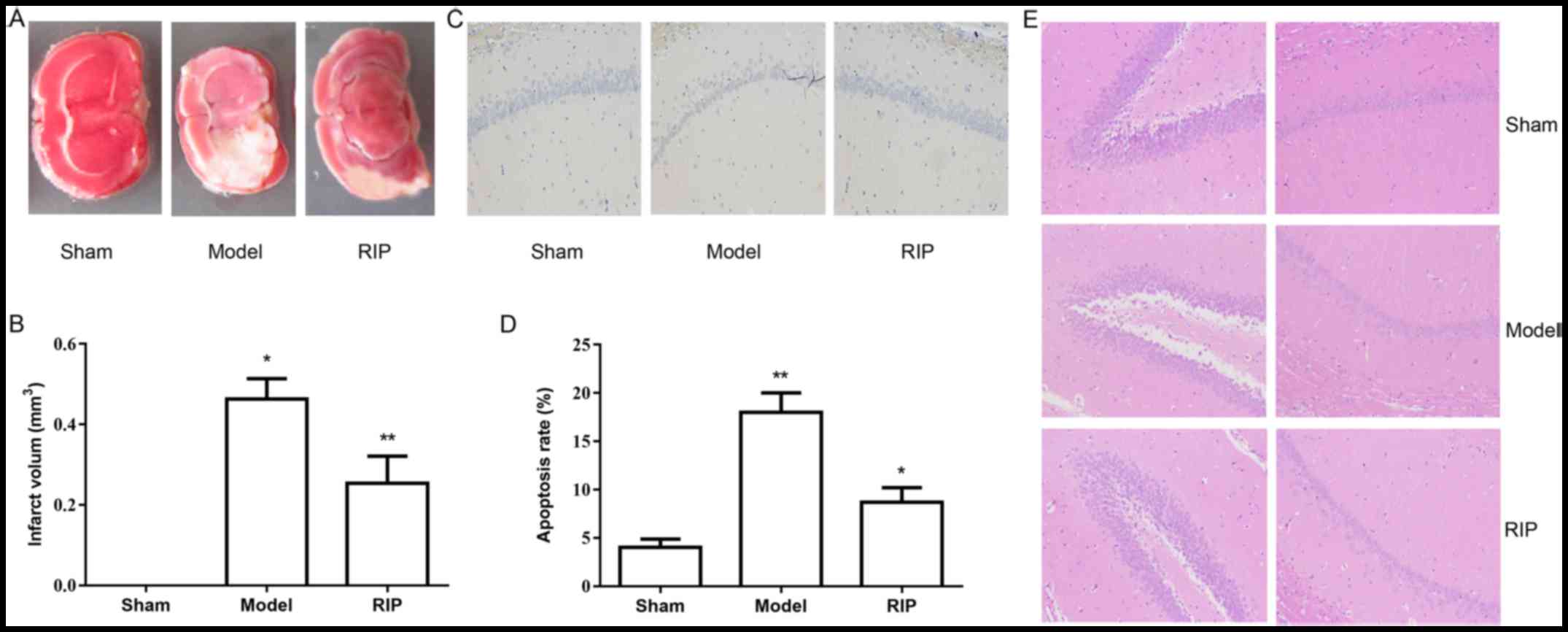
Establishment of the MCAO/R model
The changes in the MCAO/R (model) and the right
middle cerebral artery occlusion/reperfusion (RIP) groups were
assessed (Fig. 1). The authors
used TTC staining and revealed that greater infarct growth occurred
in rats in the model group compared with those in the RIP group
(Fig. 1A and B). No change in
infarct size was observed in rats in the sham group. A TUNEL assay
was used to detect apoptosis in the rat hippocampal tissues
(Fig. 1C and D). A significant
increase in the apoptotic rate was observed in the model and RIP
groups. The apoptotic rate in the RIP group was lower than that in
the model group. H&E staining demonstrated that RIP
significantly reduced the pathological changes in brain hippocampal
tissues (Fig. 1E). These results
suggested that RIP has a significant protective effect on I/R
injury.
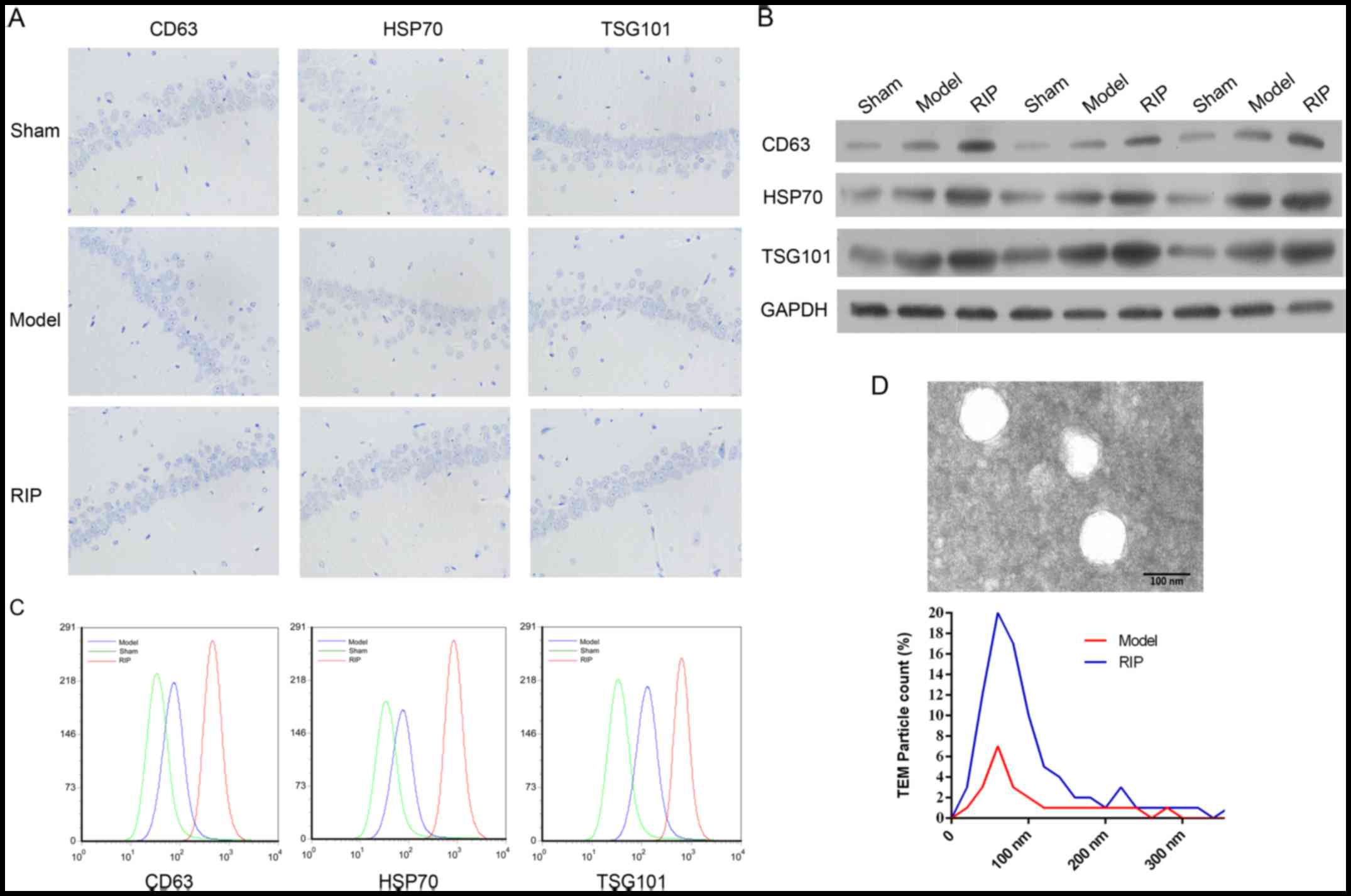
MCAO/R increases exosomes in plasma, but
not in brain hippocampal tissue
The exosome makers and exosomes in both brain
hippocampal tissue and plasma were analyzed (Fig. 2). No difference was observed in
the expressions of exosome makers CD63, HSP70 and TSG101 by
immunohistochemical staining, in brain hippocampal tissue among the
sham, model and RIP groups (Fig.
2A). However, in rat plasma, the expressions of CD63, HSP70 and
TSG101 were upregulated in the model group, and were further
increased in the RIP group by both western blot analysis (Fig. 2B) and flow cytometry (Fig. 2C). Transmission electron
microscopy revealed a significant increase in particles in the RIP
group, compared with the model group (Fig. 2D). The authors concluded that RIP
promotes the release of exosomes (40–100 nm) in rats subjected to
I/R.
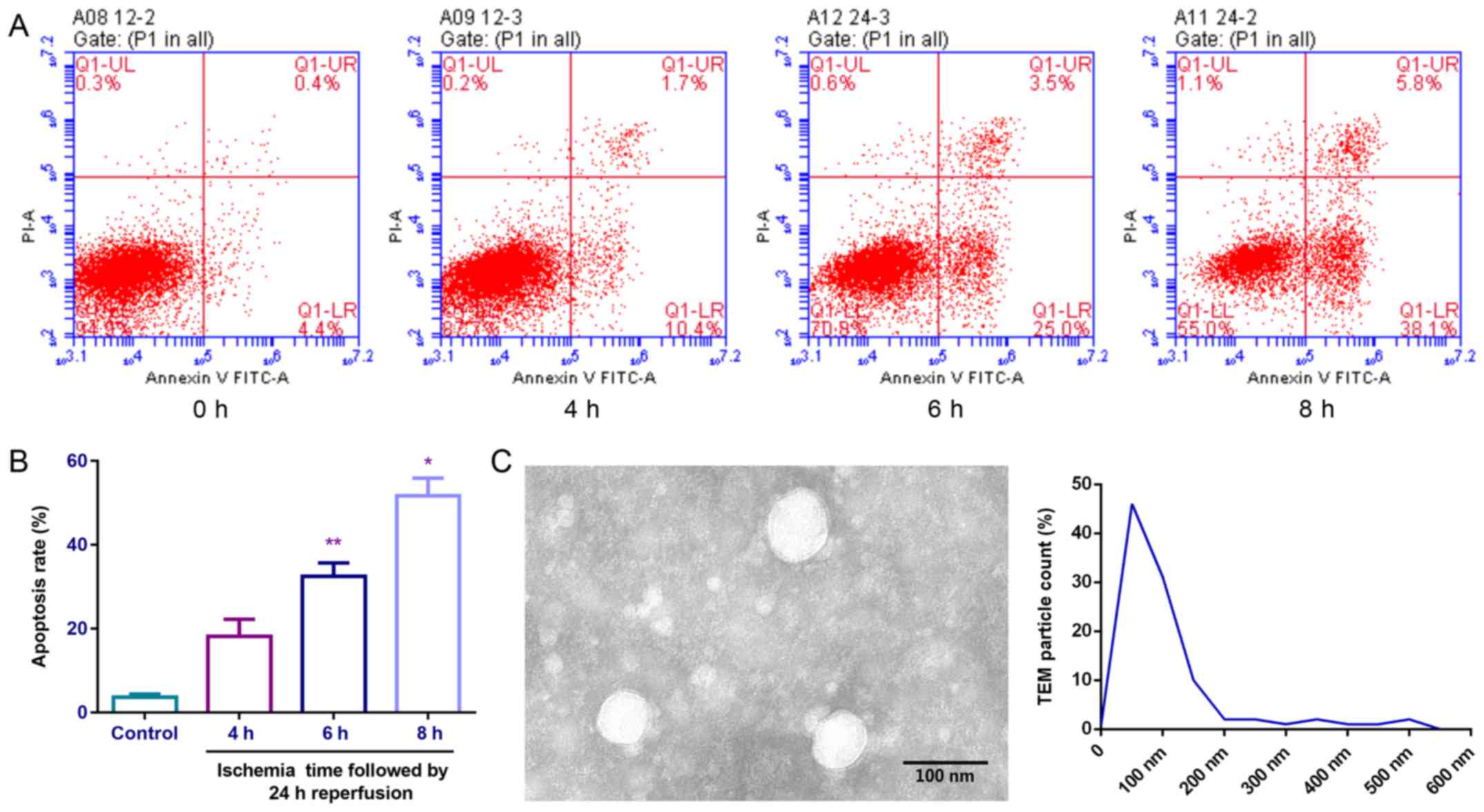
Isolation of exosomes from the
endothelial cell model of ischemia and reperfusion
An endothelial cell model of ischemia and
reperfusion was established (Fig.
3A). The authors observed a significant increase in the rate of
apoptosis with ischemia time as shown in Fig. 3A and B. Following 6 or 8 h of
ischemia followed by 24 h of reperfusion, an increase in the rate
of apoptosis was observed. The exosomes (40–100 nm) from the
endothelial cells in ischemia (6 h)-reperfusion (24 h) models in
vitro were collected and observed by transmission electron
microscope (Fig. 3C).
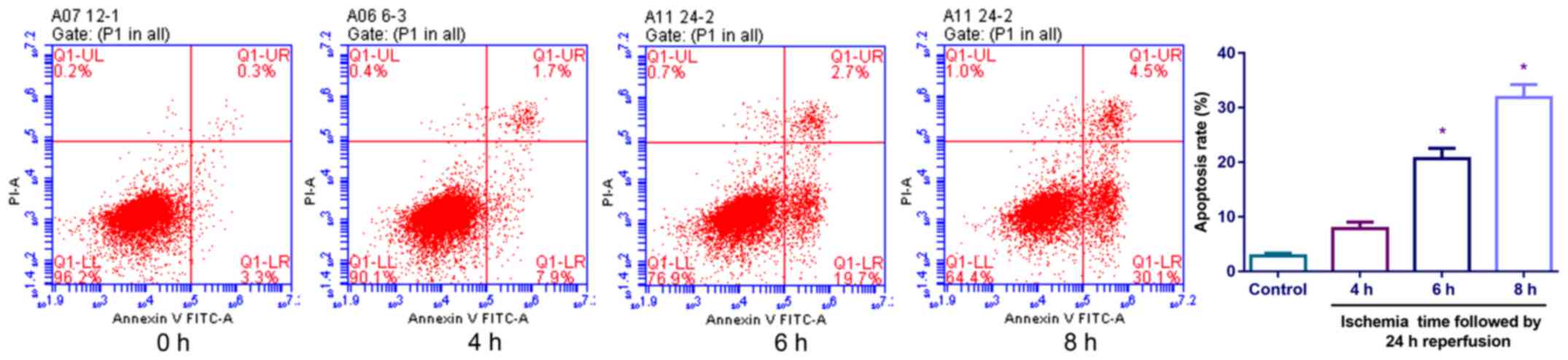
I/R-induced cell injury in SH-SY5Y nerve
cells
The authors established the SH-SY5Y nerve cell model
of ischemia and reperfusion (Fig.
4). A significant increase was observed in the rate of
apoptosis with ischemia time. Following 6 or 8 h of ischemia
followed by 24 h of reperfusion, an increase in the rate of
apoptosis was observed. Finally, the SH-SY5Y nerve cells in the
ischemia (6 h)-reperfusion (24 h) models in vitro were used
for the following tests.
Effects of exosomes extracted from
endothelial cells on cell cycle, proliferation and apoptosis of
SH-SY5Y nerve cells
The authors measured the cell cycle status,
proliferation and apoptosis of SH-SY5Y nerve cells (Fig. 5). I/R induced the G0/G1 arrest in
SH-SY5Y nerve cells, and this effect was diminished by exosomes
released from endothelial cells (Fig.
5A). Endothelial exosomes promoted the increase in SH-SY5Y
cells in S phase.
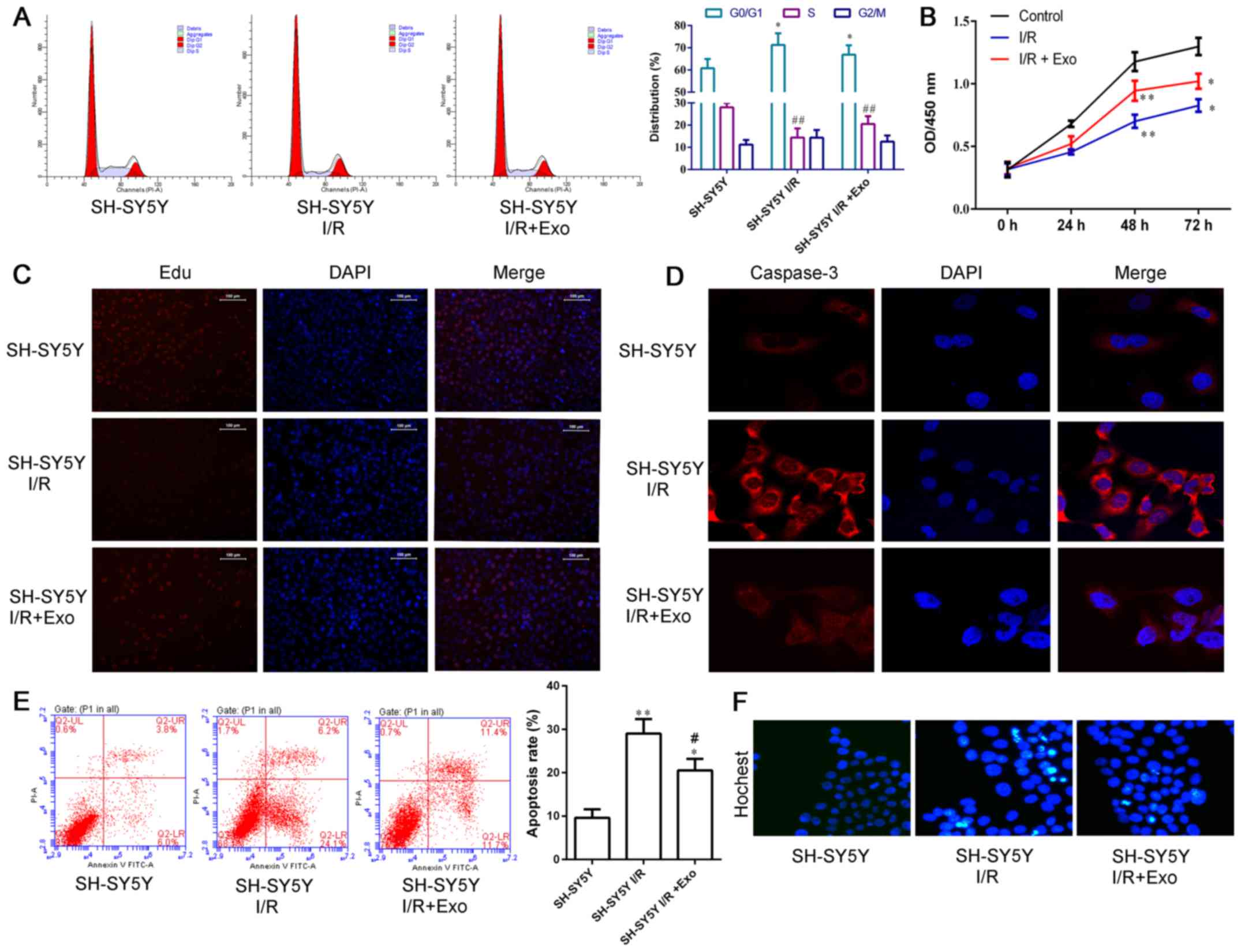 | Figure 5Exosomes from endothelial cells
diminished the I/R-induced G0/G1 arrest and apoptosis, and
inhibited proliferation in SH-SY5Y nerve cells. Effects of exosomes
derived from the endothelial cell model of ischemia and reperfusion
on cell cycle, apoptosis, and proliferation of SH-SY5Y nerve cells
were detected. (A) Cell cycle status. The percentage of G0/G1 phase
cells was higher in I/R-injured cells than control cells.
*P<0.05. Exosomes released from endothelial cells
reduced the percentage of G0/G1 phase cells significantly compared
with cells exposed to I/R, *P<0.05. The percentage of
S phase cells was lower in the I/R-injured cells than the control
cells; ##P<0.01. Exosomes released from endothelial
cells increased the percentage of S phase cells significantly
compared with cells exposed to I/R, ##P<0.01. (B)
CCK-8 assay. I/R inhibited the proliferation of SH-SY5Y nerve cells
at 48 h (control vs. I/R, **P<0.01) and 72 h (control
vs. I/R, *P<0.05). Exosomes released from endothelial
cells reversed the inhibition of cell proliferation induced by I/R
at 48 h (I/R vs. I/R+Exo, **P<0.01) and 72 h (I/R vs.
I/R+Exo, *P<0.05). (C) EdU assay. (D)
Immunofluorescence staining of caspase-3. (E) Cell apoptosis by
flow cytometry. Both I/R and I/R+Exo induced cell apoptosis
comparing with control (control vs. I/R, **P<0.01;
control vs. I/R+Exo, *P<0.05). Exosomes released from
endothelial cells reversed the induction of cell apoptosis induced
by I/R (I/R vs. I/R+Exo, #P<0.05). (F) Hoechst 33258
staining. |
Both the CCK-8 (Fig.
5B) and EdU (Fig. 5C) assays
demonstrated that I/R inhibited the cell proliferation of SH-SY5Y
nerve cells, and this inhibition decreased after exosomes were
released from endothelial cells.
I/R induced expressions of caspase-3 in SH-SY5Y
nerve cells (Fig. 5D). Exosomes
released from endothelial cells significantly inhibited I/R-induced
caspase-3. Consistent with changes in caspase-3 expression,
endothelial exosomes reduced I/R-induced apoptosis by both Annexin
V FITC-PI flow cytometry (Fig.
5E) and Hoechst 33258 assays (Fig. 5F).
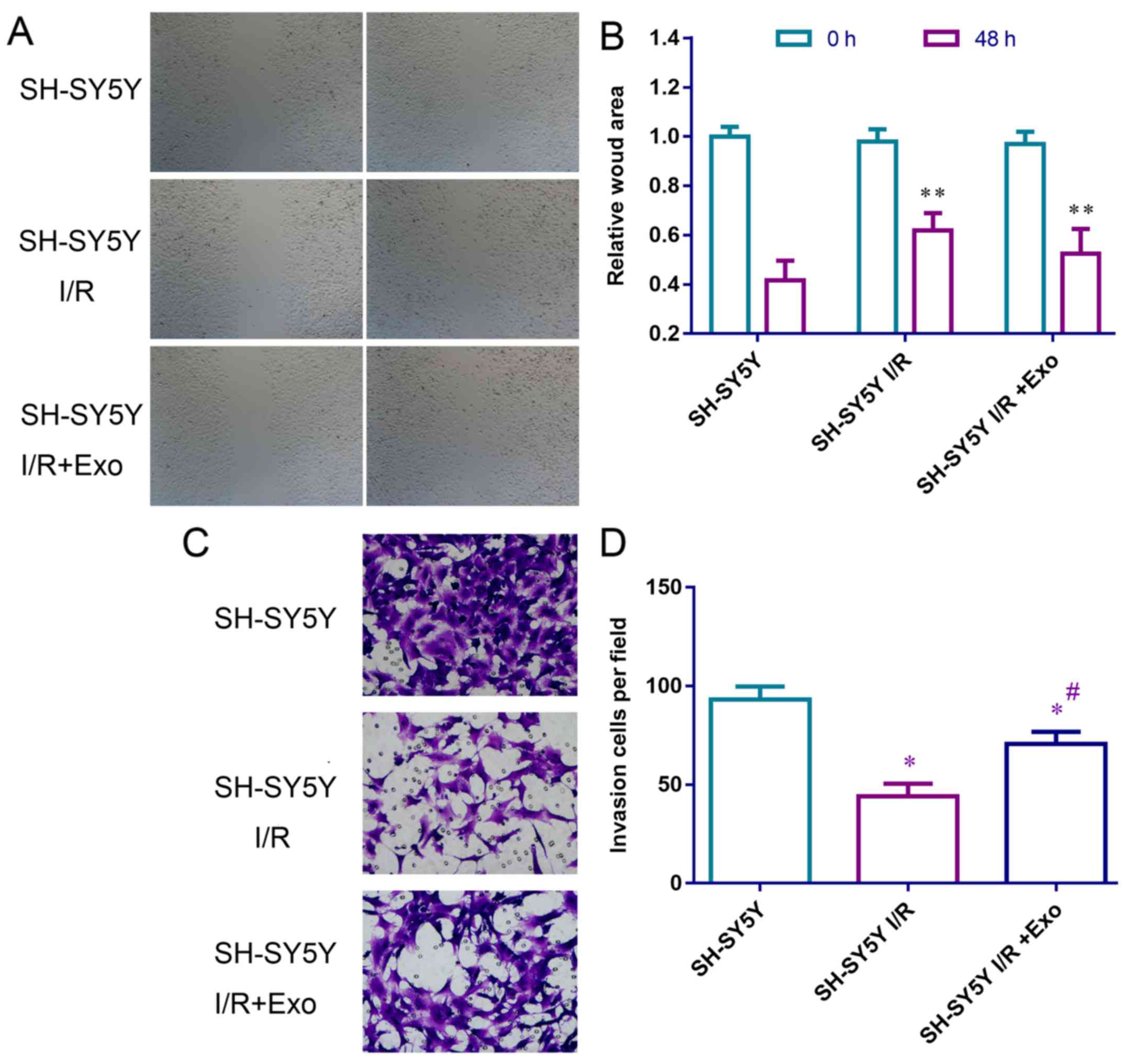
Exosomes extracted from endothelial cells
promote migration and invasion of SH-SY5Y nerve cells
I/R inhibited wound healing and invasion of SH-SY5Y
cells (Fig. 6). Following
treatment with exosomes released from endothelial cells,
I/R-inhibited cell migration (Fig.
6A) and invasion (Fig. 6B)
were partly recovered, suggesting that the exosomes from
endothelial cells promote migration and invasion of SH-SY5Y nerve
cells.
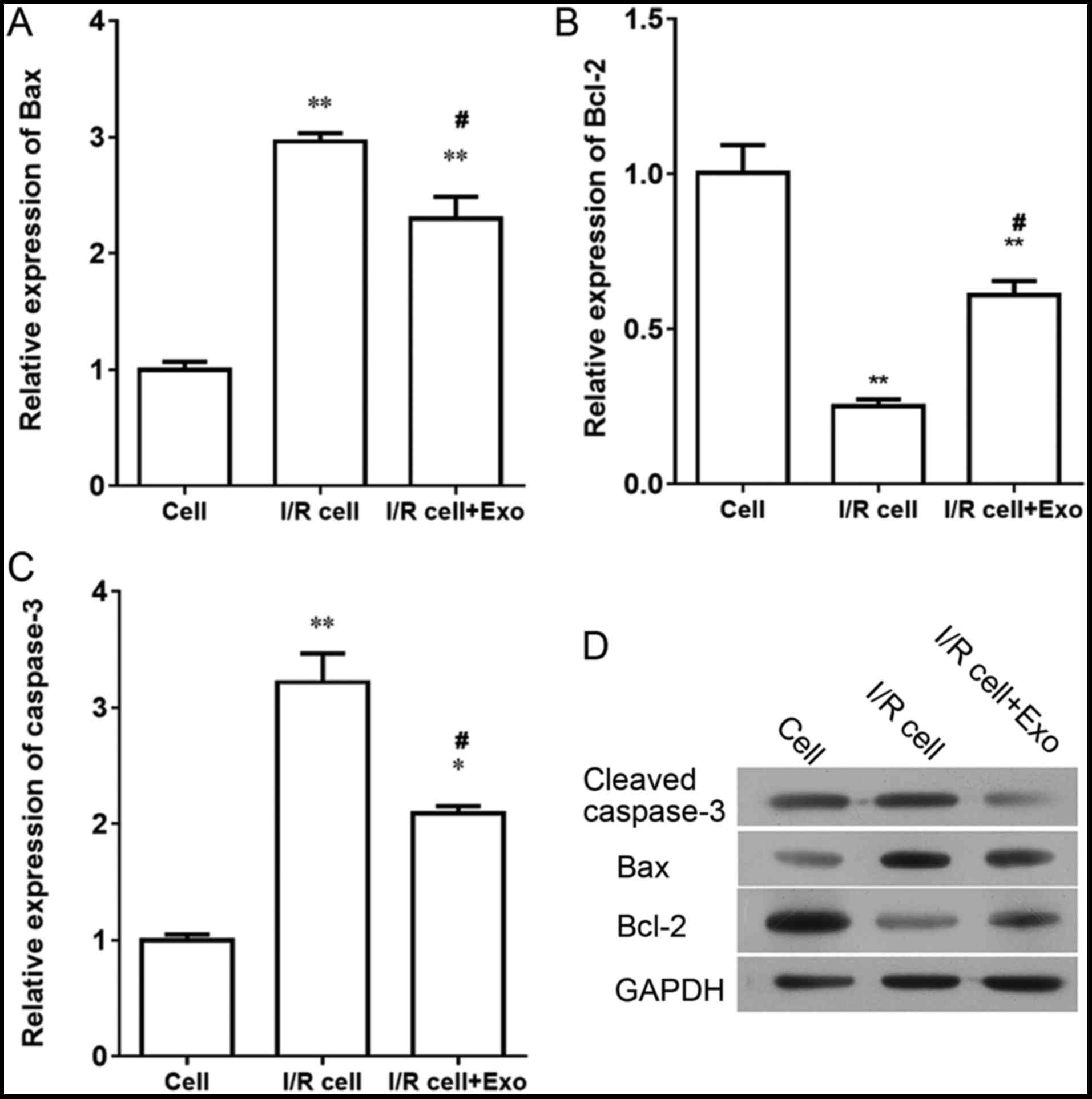
Exosomes extracted from endothelial cells
inhibit expression of cleaved caspase-3 and Bax, and upregulate
expression of Bcl-2 in SH-SY5Y nerve cells
I/R induced expression of Bax and caspase-3 and
inhibited the expression of Bcl-2 at both the mRNA and protein
levels in SH-SY5Y nerve cells (Fig.
7). After treatment with exosomes released from endothelial
cells, expression levels of Bax and caspase-3 were downregulated,
and expression levels of Bcl-2 were upregulated, suggesting that
exosomes from endothelial cells promote the expression of Bcl-2 and
inhibit the expression of Bax and caspase-3 in SH-SY5Y nerve
cells.
Discussion
In the present study, the authors presented evidence
that supports the hypothesis that exosomes released by endothelial
cells in femoral arteries mediate the protective effect of RIP on
neurological damage. MCAO/R is a widely used model for cerebral I/R
injury (27). The authors
established a right middle cerebral artery occlusion/reperfusion
rat model with RIP. In rats that were subjected to RIP, all the
infarct sizes, the rate of nerve cell apoptosis and the
pathological changes in brain hippocampal tissue were significantly
reduced. These results validated the idea that RIP serves a
protective role in I/R injury (28–30). Moreover, RIP is neuroprotective in
young male rodents after experimental stroke, and is also effective
after embolic stroke in ovariectomized female mice (31). In clinical settings, RIP protects
the aged liver against I/R injury, and is further associated with
improvement in vascular function (32). Although the mechanism involved is
not understood, here, the authors provided direct evidence that
indicates that vascular endothelial cells secrete exosomes during
cerebral I/R injury that protect neurobehavioral outcomes.
Exosomes are secreted by various cells, including
endothelial cells; the condition medium of endothelial cells is
typically rich with exosomes (33). Exosomes are microextracelluar
vesicles with a diameter of ~30–100 nm. They play important roles
in material and signaling transduction between cells in the
microenvironment (11). The
findings demonstrated that exosomes express CD63, HSP70 and TSG101.
The expressions of CD63, HSP70 and TSG101 did not increase in brain
hippocampal tissue in the sham, model and RIP groups, but increased
in plasma in the RIP group, indicating that RIP promotes the
release of exosomes. Furthermore, the authors detected exosomes
that were 40–100 nm in size using transmission electron microscopy,
in plasma of rats that underwent cerebral I/R injury. The paracrine
effects of HUVECs potentially improve endothelial cell generation
from cord blood circulating endothelial progenitor cells, and may
contain the role in the production of exosomes (13). Condition medium from cells treated
under hypoxic conditions increased the observed number of
differentiating neurons in vitro (17). The exosomes were isolated from
endothelial cells during ischemia and reperfusion injury and the
size of extracted exosomes was comparable to those observed in rats
that underwent cerebral I/R injury.
Exosomes comprise of proteins, DNA, mRNA and some
non-protein coding RNA, and have multiple functions in different
types of cells (11,34). For example, endothelial
cell-derived exosomes potently increase the proliferation,
migration, secretion of matrix metalloproteinase (MMP)-1, MMP-3 and
nuclear factor-κB activity in mesenchymal stem cells, stimulating
local trophic support (15).
Mesenchymal stem cells support nerve growth in conjunction with
Schwann cells, secreted neurovascular factors and possibly
trans-differentiation into Schwann-like cells (16). A recent study reported that
exosomes extracted from adipose-derived mesenchymal stem cells have
a protective effect against nerve injury induced by glutamate,
which may be mediated by activating the PI3/K-Akt signaling pathway
(14). Consistent with previous
studies (35,36), the current results suggested that
cell cycle arrest and apoptotic rate of SH-SY5Y nerve cells are
promoted, and proliferation, migration and invasion are inhibited
during ischemia and reperfusion injury. Treatment with I/R
endothelial cell-derived exosomes partly recovered the I/R-induced
injury to SH-SY5Y nerve cells. The role of endothelial cell-derived
exosomes on apoptosis of I/R-injured SH-SY5Y nerve cells was
further validated by observing the expression levels of caspase-3,
Bax and Bcl-2.
Taken together, the results strongly suggest that
endothelial cell-derived exosomes directly protect nerve cells
against I/R injury by promoting cell growth, migration and
invasion, as well as by inhibiting cell apoptosis. Endothelial
cell-derived exosomes could potentially be developed as a novel
treatment strategy in treating neurological damage during I/R
injury.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660420), the
Construction plan of the Superior Science and Technology Innovation
Team of Jiangxi Province (grant nos. 20152BCB24009 and
20161BCB24009), the Foreign Science and Technology Cooperation Plan
of Jiangxi Province (grant no. 20151BDH80009) and the Graduate
Innovation Foundation of Nanchang University (cx2015202).
References
|
1
|
Schreuder FH, Sato S, Klijn CJ and
Anderson CS: Medical management of intracerebral haemorrhage. J
Neurol Neurosurg Psychiatry. 88:76–84. 2017. View Article : Google Scholar
|
|
2
|
Guerram M, Zhang LY and Jiang ZZ:
G-protein coupled receptors as therapeutic targets for
neurodegenerative and cerebrovascular diseases. Neurochem Int.
101:1–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan WJ, Zheng XY, Chung KF and Zhong NS:
Impact of air pollution on the burden of chronic respiratory
diseases in China: Time for urgent action. Lancet. 388:1939–1951.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Shi YZ, Zhang N, Wang S, Ungvari
GS, Ng CH, Wang YL, Zhao XQ, Wang YJ, Wang CX, et al: The
disability rate of 5-year post-stroke and its correlation factors:
A national survey in China. PLoS One. 11:e01653412016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng Y, Liu JX, Yan ZP, Yao XH and Liu XH:
Potential microRNA biomarkers for acute ischemic stroke. Int J Mol
Med. 36:1639–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akpan N and Troy CM: Caspase inhibitors:
Prospective therapies for stroke. Neuroscientist. 19:129–136. 2013.
View Article : Google Scholar
|
|
7
|
van Ierssel SH, Conraads VM, Van
Craenenbroeck EM, Liu Y, Maas AI, Parizel PM, Hoymans VY, Vrints CJ
and Jorens PG: Endothelial dysfunction in acute brain injury and
the development of cerebral ischemia. J Neurosci Res. 93:866–872.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavi S, Abu-Romeh N, Wall S, Alemayehu M
and Lavi R: Long-term outcome following remote ischemic
postconditioning during percutaneous coronary interventions-the
RIP-PCI trial long-term follow-up. Clin Cardiol. 40:268–274. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan YY, Hu WW, Nan F and Chen Z:
Postconditioning-induced neuroprotection, mechanisms and
applications in cerebral ischemia. Neurochem Int. 107:43–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andreadou I, Iliodromitis EK, Lazou A,
Görbe A, Giricz Z, Schulz R and Ferdinandy P: Effect of
hypercholesterolaemia on myocardial function, ischaemia-reperfusion
injury and cardioprotection by preconditioning, postconditioning
and remote conditioning. Br J Pharmacol. 174:1555–1569. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vyas N and Dhawan J: Exosomes: Mobile
platforms for targeted and synergistic signaling across cell
boundaries. Cell Mol Life Sci. 74:1567–1576. 2017. View Article : Google Scholar
|
|
12
|
Holzner S, Senfter D, Stadler S,
Staribacher A, Nguyen CH, Gaggl A, Geleff S, Huttary N, Krieger S,
Jäger W, et al: Colorectal cancer cell-derived microRNA200
modulates the resistance of adjacent blood endothelial barriers in
vitro. Oncol Rep. 36:3065–3071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castelli G, Parolini I, Cerio AM, D'Angiò
A, Pasquini L, Carollo M, Sargiacomo M, Testa U and Pelosi E:
Conditioned medium from human umbilical vein endothelial cells
markedly improves the proliferation and differentiation of
circulating endothelial progenitors. Blood Cells Mol Dis. 61:58–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM,
Guan J, Bao XJ, Wu H, Han Q, Wang RZ, et al: Protection of nerve
injury with exosome extracted from mesenchymal stem cell. Zhongguo
Yi Xue Ke Xue Yuan Xue Bao. 38:33–36. 2016.PubMed/NCBI
|
|
15
|
Lozito TP and Tuan RS: Endothelial and
cancer cells interact with mesenchymal stem cells via both
microparticles and secreted factors. J Cell Mol Med. 18:2372–2384.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofer HR and Tuan RS: Secreted trophic
factors of mesenchymal stem cells support neurovascular and
musculoskeletal therapies. Stem Cell Res Ther. 7:1312016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teixeira FG, Panchalingam KM, Anjo SI,
Manadas B, Pereira R, Sousa N, Salgado AJ and Behie LA: Do
hypoxia/normoxia culturing conditions change the neuroregulatory
profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell
Res Ther. 6:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schey KL, Luther JM and Rose KL:
Proteomics characterization of exosome cargo. Methods. 87:75–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Liu Y, Zhu J, Lei S, Dong Y, Li L,
Jiang B, Tan L, Wu J, Yu S, et al: GSK-3β downregulates Nrf2 in
cultured cortical neurons and in a rat model of cerebral
ischemia-reperfusion. Sci Rep. 6:201962016. View Article : Google Scholar
|
|
20
|
Shu S, Li CM, You YL, Qian XL, Zhou S and
Ling CQ: Electroacupuncture ameliorates cerebral
ischemia-Reperfusion injury by regulation of autophagy and
apoptosis. Evid Based Complement Alternat Med. 2016:72974252016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng Y and Liu J: Role of glypican-1 in
endothelial NOS activation under various steady shear stress
magnitudes. Exp Cell Res. 348:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marutani E, Yamada M, Ida T, Tokuda K,
Ikeda K, Kai S, Shirozu K, Hayashida K, Kosugi S, Hanaoka K, et al:
Thiosulfate mediates cytoprotective effects of hydrogen sulfide
against neuronal ischemia. J Am Heart Assoc. 4:42015.
|
|
23
|
Yan Z, Liu J, Xie L, Liu X and Zeng Y:
Role of heparan sulfate in mediating CXCL8-induced endothelial cell
migration. PeerJ. 4:e16692016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng Y, Sun HR, Yu C, Lai Y, Liu XJ, Wu J,
Chen HQ and Liu XH: CXCR1 and CXCR2 are novel mechano-sensors
mediating laminar shear stress-induced endothelial cell migration.
Cytokine. 53:42–51. 2011. View Article : Google Scholar
|
|
25
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gnosa S, Capodanno A, Murthy RV, Jensen LD
and Sun XF: AEG-1 knockdown in colon cancer cell lines inhibits
radiation-enhanced migration and invasion in vitro and in a novel
in vivo zebrafish model. Oncotarget. 7:81634–81644. 2016.PubMed/NCBI
|
|
27
|
Lapi D and Colantuoni A: Remodeling of
cerebral microcirculation after ischemia-reperfusion. J Vasc Res.
52:22–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Q, Huang J, Hu J and Zhu H: Advance in
spinal cord ischemia reperfusion injury: Blood-spinal cord barrier
and remote ischemic preconditioning. Life Sci. 154:34–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Randhawa PK and Jaggi AS: Unraveling the
role of adenosine in remote ischemic preconditioning-induced
cardioprotection. Life Sci. 155:140–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heusch G and Rassaf T: Time to give up on
cardioprotection? A critical appraisal of clinical studies on
ischemic pre-, post-, and remote conditioning. Circ Res.
119:676–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoda MN, Bhatia K, Hafez SS, Johnson MH,
Siddiqui S, Ergul A, Zaidi SK, Fagan SC and Hess DC: Remote
ischemic perconditioning is effective after embolic stroke in
ovariectomized female mice. Transl Stroke Res. 5:484–490. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Limani P, Linecker M, Oberkofler CE,
Barmettler G, Kaech A, Graf R, Humar B and Clavien PA: Remote
ischemic preconditioning: A novel strategy in rescuing older livers
from ischemia-reperfusion injury in a rodent model. Ann Surg.
264:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kadota T, Fujita Y, Yoshioka Y, Araya J,
Kuwano K and Ochiya T: Extracellular vesicles in chronic
obstructive pulmonary disease. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
34
|
Huang-Doran I, Zhang CY and Vidal-Puig A:
Extracellular vesicles: Novel mediators of cell communication in
metabolic disease. Trends Endocrinol Metab. 28:3–18. 2017.
View Article : Google Scholar
|
|
35
|
Nakajima A, Tsuji M, Inagaki M, Tamura Y,
Kato M, Niiya A, Usui Y and Oguchi K: Neuroprotective effects of
propofol on ER stress-mediated apoptosis in neuroblastoma SH-SY5Y
cells. Eur J Pharmacol. 725:47–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu J, Li YH, Zhan X, Li G, Chen Z and Chen
X: The protective effect of qiancao naomaitong mixture on neuronal
damage and cerebral ischemia/reperfusion injury. Pharm Biol.
54:2304–2311. 2016. View Article : Google Scholar : PubMed/NCBI
|