Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic airway disease that leads to difficulties breathing
(1), and is characterized by
chronic inflammation of the respiratory tract with increased
numbers of inflammatory cells and molecules (2). The worldwide incidence, prevalence
and mortality of COPD are increasing (3). Cigarette smoke (CS) is a complex
mixture of chemicals generated from the burning of tobacco
(4), and is the main cause of
COPD (5). CS affects the
recruitment of inflammatory cells, including neutrophils into the
lungs and is associated with chronic inflammation of the airways
and a decline in lung function (6).
Neutrophils are the host defense inflammatory cells
that are rapidly recruited to sites of infection (7). However, neutrophilic inflammation is
the major cause of pulmonary inflammation in COPD pathophysiology
(8). Activated neutrophils
produce several cytotoxic mediators, including reactive oxygen
species (ROS) and neutrophil elastase (NE), which aggravate
pulmonary inflammation and emphysema (9). The increased production of ROS
accelerates the development of COPD through the activation of
mitogen-activated protein kinases (MAPKs) and nuclear factor-κB
(NF-κB) (10). NE activity is
increased in the lungs affected by COPD, which enhances the
destruction of alveolar structure (11). Tumor necrosis factor-α (TNF-α) is
a central inflammatory cytokine that is associated with many
immune-mediated diseases, including COPD (12). It is well known that the
constitutive overexpression of TNF-α affects the recruitment of
inflammatory cells and promotes emphysema in the lungs of animals
(13). Interleukin (IL)-6 is a
pro-inflammatory cytokine that plays a pivotal role in the
pathogenesis of COPD by modulating pulmonary function (14). Monocyte chemoattractant protein-1
(MCP-1) is one of the key chemokines that contributes to the
recruitment of inflammatory cells, such as neutrophils (15) and macrophages (16). MCP-1 levels are significantly
increased in patients with COPD compared with non-smokers (17). Inducible nitric oxide synthase
(iNOS) expression is induced by neutrophils and macrophages in
response to pro-inflammatory stimuli (18,19) and is known to have
anti-inflammatory activity (20,21). However, the continuous expression
of iNOS is associated with pulmonary inflammation and emphysema
(22). Recently, it has also been
reported that iNOS expression is higher in the lungs of patients
with COPD than non-smokers (23).
The MAPK signaling pathway promotes the inflammatory response by
enhancing inflammatory gene transcription (6,24).
NF-κB is a central transcription factor that plays an important
role in the expression of inflammatory genes, such as iNOS, TNF-α
and IL-6 (25). CS has been shown
to affect the activation of MAPKs (26) and NF-κB (27).
Neem (Azadirachta indica A. Juss.) belonging
to the family, Meliaceae is an evergreen tree, cultivated in
various parts of the Indian subcontinent (28). The neem leaf has been reported to
exhibit various pharmacological activities, including
anti-inflammatory (29),
antioxidant (30,31), antimicrobial (32) and antiviral properties (33). Active constituents of the neem
leaf include nimbin, nimbidine, isomeldenin, β-sitosterol and
quercetin (34). Quercetin
(35), β-sitoserol (36) and nimbidine (37) have been shown to exert
anti-inflammatory effects. These effects are due to the inhibition
of pro-inflammatory molecules, such as TNF-α, iNOS and NF-κB.
Recently, neem leaf extract (NLE) has been reported to protect
against endotoxemia in mice exposed to lipo-polysaccharide (LPS)
(38). However, to date, at least
to the best of our knowledge, the protective effects of NLE have
not been demonstrated in CS- and LPS-induced pulmonary
inflammation. Thus, the aim of this study was to investigate the
protective effects of NLE against cigarette smoke (CS)- and
lipopolysaccharide (LPS)-induced pulmonary inflammation.
Materials and methods
Preparation of NLE
Neem leaf was collected from ward no. 11, Hetauda,
Nepal (latitude 27°27′11.7″, longitude 85°00′11.1″ and 531 m above
sea level), and identified by Mr. M.R. Poudeyal of the
Ethnobotanical Society of Nepal (ESON). Voucher specimens recorded
as KRIB 0059759 and 760 have been deposited in the herbarium of the
Korea Research Institute of Bioscence and Biotechnolgy (KRIB).
After drying and grinding the leaves of neem, the powder (52 g) was
added to 100 liters of methanol. The extraction was carried out
using the method of repercolation at room temperature. The extract
was filtered and concentrated by a rotavapor under reduced
pressure, thereby obtaining 2.99 g of neem methanolic extract. In
the following experiment, the neem leaves were dissolved in
dimethyl sulfoxide (DMSO) at a concentration of 20 mg/ml, and then
diluted to various concentrations prior to use.
Model of CS- and LPS-induced pulmonary
inflammation
CS-and LPS-induced pulmonary inflammation was
induced using a modification of the procedure described by Lee
et al (6). Briefly, a
total of 30 C57BL/6 mice (6 weeks old; weight, 20 g; n=6/group)
were whole-body exposed to room fresh air or CS of 7 cigarettes for
50 min a day for 9 days. CS was generated by 3R4F research
cigarettes (Tobacco and Health Research Institute, University of
Kentucky, Lexington, KY, USA). LPS was instilled intrana sally on
day 8 (5 µg dissolved in 50 µl distilled water). The
mice were randomly divided into 5 groups as follows: the normal
control (NC), the CS + LPS (CS with intranasal LPS instillation)
group, the ROF (CS with intranasal LPS instillation) + roflumilast
[10 mg/kg, per os (p.o)] group, and the NLE 10 or 20 (CS
with intranasal LPS instillation) + NLE (10 or 20 mg/kg, p.o)
groups. All the animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology and performed in
compliance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals and National Animal Welfare
Law of Korea.
Measurement of inflammatory cells in
bronchoalveolar lavage fluid (BALF)
BALF collection was performed using the method of
Shin et al (5). In brief,
the mice were administered an intraperitoneal injection of a
pentobarbital (50 mg/kg; Hanlim Pharm, Co., Seoul, Korea) 24 h
after the final challenge, and a tracheostomy was performed. To
obtain the BALF, ice-cold phosphate-buffered saline (PBS) (0.7 ml)
was infused into the lung and withdrawn via tracheal cannulation
twice (total volume, 1.4 ml). To determine differential cell
counts, 100 µl of BALF were centrifuged at 1,500 rpm for 5
min and the number of neutrophils and macrophages was counted using
Diff-Quik® staining reagent according to the
manufacturer's instructions (IMEB Inc., Deerfield, IL, USA).
Measurement of ROS and NE in BALF
The effects of NLE on the production of ROS were
determined using 2′,7′-dichloroflu-orescein diacetate (DCFH-DA;
Sigma-Aldrich, St. Louis, MO, USA). Briefly, the inflammatory cells
were isolated from BALF and incubated with 20 µM DCFH-DA for
10 min at 37°C. The level of intracellular ROS was then determined
using a fluorescence microscope at 488 nm excitation and 525 nm
emission (8). The activity of NE
was examined using N-succinyl-(Ala)3-p-nitroanilide (Sigma-Aldrich)
in 37°C for 90 min, according to the protocol described by Sakuma
et al (39).
Measurement of the level of
pro-inflammatory cytokines in BALF
The levels of pro-inflammatory cytokines (TNF-α and
IL-6) in BALF were determined using ELISA according to the
manufacturer's instructions (R&D Systems, Shanghai, China). The
absorbance was measured at 450 nm using a microplate reader
(Molecular Devices, Sunnyvale, CA, USA), as previously described
(4).
Western blot analysis
Lung tissues were homogenized using a homogenizer
with a lysis buffer (Intron Biotechnology, Inc., Seoul, Korea).
Protein samples were denatured and resolved on 10%
SDS-polyacrylamide gels and transferred onto a nitrocellulose
membrane. The membrane was incubated with blocking solution for 1
h. Specific antibodies against MCP-1 (1;1,000; ab25124; Abcam,
Cambridge, MA, USA), iNOS (1;1,000; ADI-905-431; Enzo Life
Sciences, Farmingdale, NY, USA), p-ERK (1:1,000; #9101; Cell
Signaling Technology, Inc., Danvers, MA, USA), ERK (1:1,000;
sc-154; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-JNK
(1:1,000; KAP-SA011; Enzo Life Sciences), JNK (1:1,000; sc-474;
Santa Cruz Biotechnology), p-p38 (1:1,000; ADI-KAP-MA022; Enzo Life
Sciences), p-38 (1:1,000; sc-7149; Santa Cruz Biotechnology), p-p65
(1:1,000; #3033; Cell Signaling Technology, Inc.), p65 (1:1,000;
sc-372; Santa Cruz Biotechnology), p-inhibitor of NF-κB (IκB;
1:1,000; sc-371; Santa Cruz Biotechnology) and β-actin (1;2,500;
#4967; Cell Signaling Technology, Inc.) were incubated overnight at
4°C with 5% skim milk. The membranes were washed in Tris-buffered
saline with Tween 20 (TBST) and incubated with the
Peroxidase-AffiniPure goat anti-mouse IgG (H+L) (1:2,000;
115-035-003; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) and the Peroxidase-AffiniPure goat anti-rabbit IgG (H+L)
(1:2,000; 111-035-003; Jackson ImmunoResearch Laboratories, Inc.)
for 2 h at room temperature. The blots were washed 3 times with
TBST, and then developed with an enhanced chemiluminescence (ECL)
kit (Amersham Biosciences, Piscataway, NJ, USA).
Histological analysis
After the BALF samples were collected, lung tissues
were fixed in 10% (v/v) neutral-buffered formalin solution. For
histological examination, the lung tissues were embedded in
paraffin, sectioned at 4 µm thickness, and stained with a
hematoxylin and eosin (H&E) solution (Sigma-Aldrich) to
estimate the inflammatory response.
Statistical analysis
All values shown in the figures are expressed as the
means ± SD obtained from at least 3 independent experiments.
Statistical significance was carried out using a two-tailed
Student's t-test. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
NLE inhibits the infiltration of
inflammatory cells in the BALF of mice with CS- and LPS-induced
pulmonary inflammation
Given the fact that the infiltration of inflammatory
cells, such as neutrophils and macrophages is increased in the BALF
of mice with CS- and LPS-induced pulmonary inflammation (9), we investigated whether NLE inhibits
the infiltration of neutrophils and macrophages in BALF. As shown
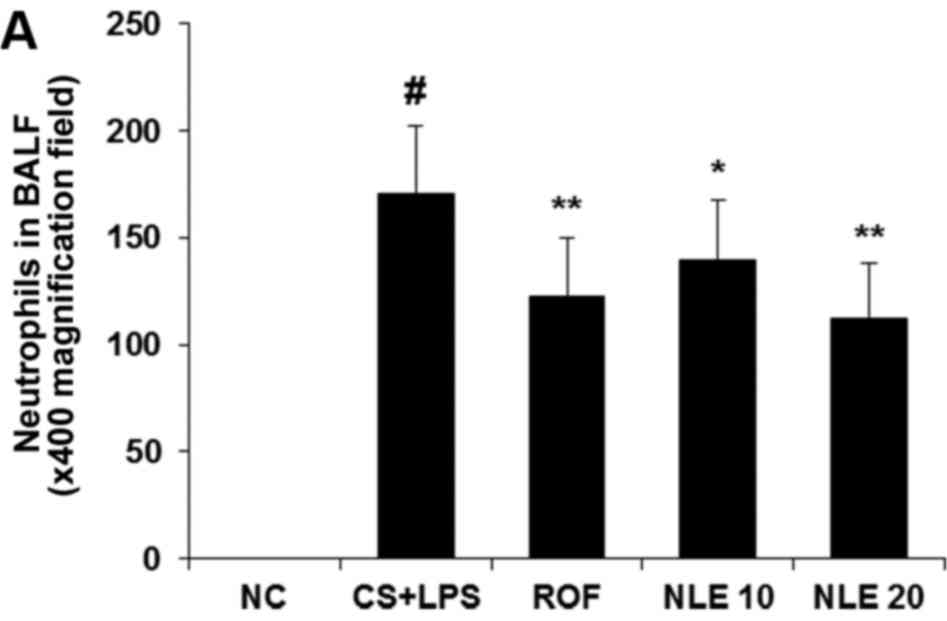
in Fig. 1, we observed that
increased numbers of neutrophils and macrophages were detected in
the BALF of mice in the CS and LPS group compared with those in the
normal control group. However, treatment with NLE significantly
attenuated the numbers of neutrophils and macrophages in BALF,
compared with the CS and LPS group in a concentration-dependent
manner (Fig. 1). The effect of 20
mg/kg NLE was similar to that of treatment with 10 mg/kg ROF.
NLE attenuates the production of ROS and
NE in BALF
It is well known that ROS production and NE activity
are increased in the BALF of mice with CS- and LPS-induced
pulmonary inflammation (5,6).
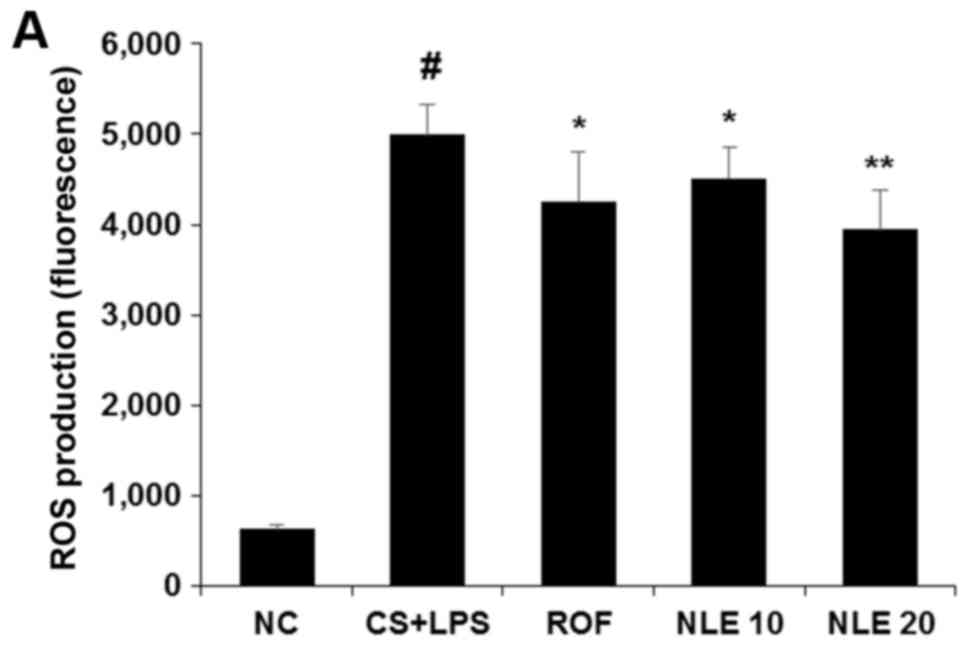
Thus, in this study, the levels of ROS and NE were examined in the
BALF of mice with CS and LPS-induced pulmonary inflammation. As
shown in Fig. 2, the levels of
ROS and NE were significantly increased in the CS and LPS group.
However, treatment with NLE significantly decreased the levels of
ROS and NE (Fig. 2). In
particular, treatment with 20 mg/kg NLE more effectively attenuated
the levels of those molecules compared with 10 mg/kg ROF.
NLE decreases the levels of TNF-α and
IL-6 in BALF
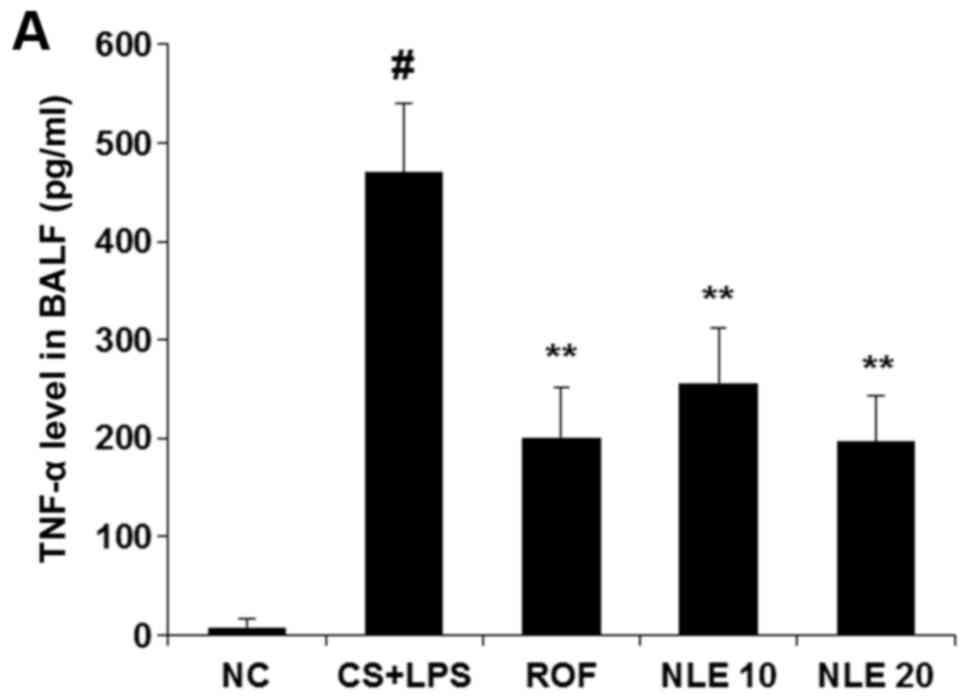
The increased release of TNF-α and IL-6 in BALF is
one of the major characteristics of COPD (5). Thus, to determine whether NLE
affects the release of pro-inflammatory cytokines in BALF, the
levels of TNF-α and IL-6 were examined by ELISA. As shown in
Fig. 3, treatment with NLE
effectively inhibited the release of these cytokines in BALF.
NLE reduces the recruitment of
inflammatory cells and the expression of MCP-1 in the lungs of mice
with CS- and LPS-induced pulmonary inflammation
To examine whether NLE affects the recruitment of
inflammatory cells and the expression of MCP-1 in the lungs of mice
with CS- and LPS-induced pulmonary inflammation, the infiltration
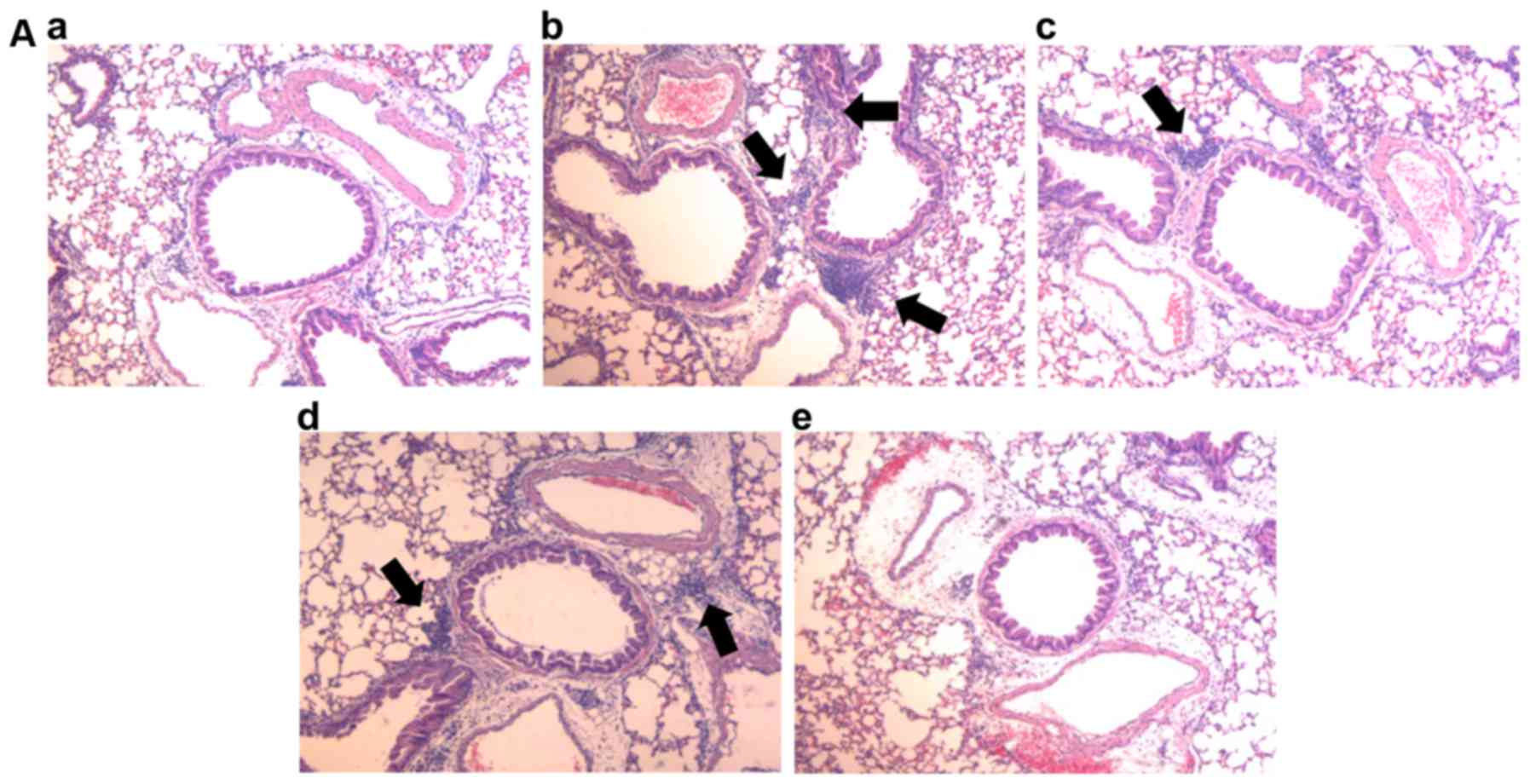
of inflammatory cells was determined by H&E staining. As shown
in Fig. 4A and B, the mice in the
CS and LPS group exhibited an increased infiltration of
inflammatory cells. However, treatment with NLE significantly
reduced the recruitment of inflammatory cells in a
concentration-dependent manner. Consistent with the decrease in
inflammatory cell recruitment, treatment with NLE also
significantly decreased the expression of MCP-1 in the lungs,
suggesting that NLE attenuated the recruitment of inflammatory
cells (Fig. 4C). Similar to the
results shown above, the effect of 20 mg/kg NLE was similar to that
of treatment with 10 mg/kg ROF.
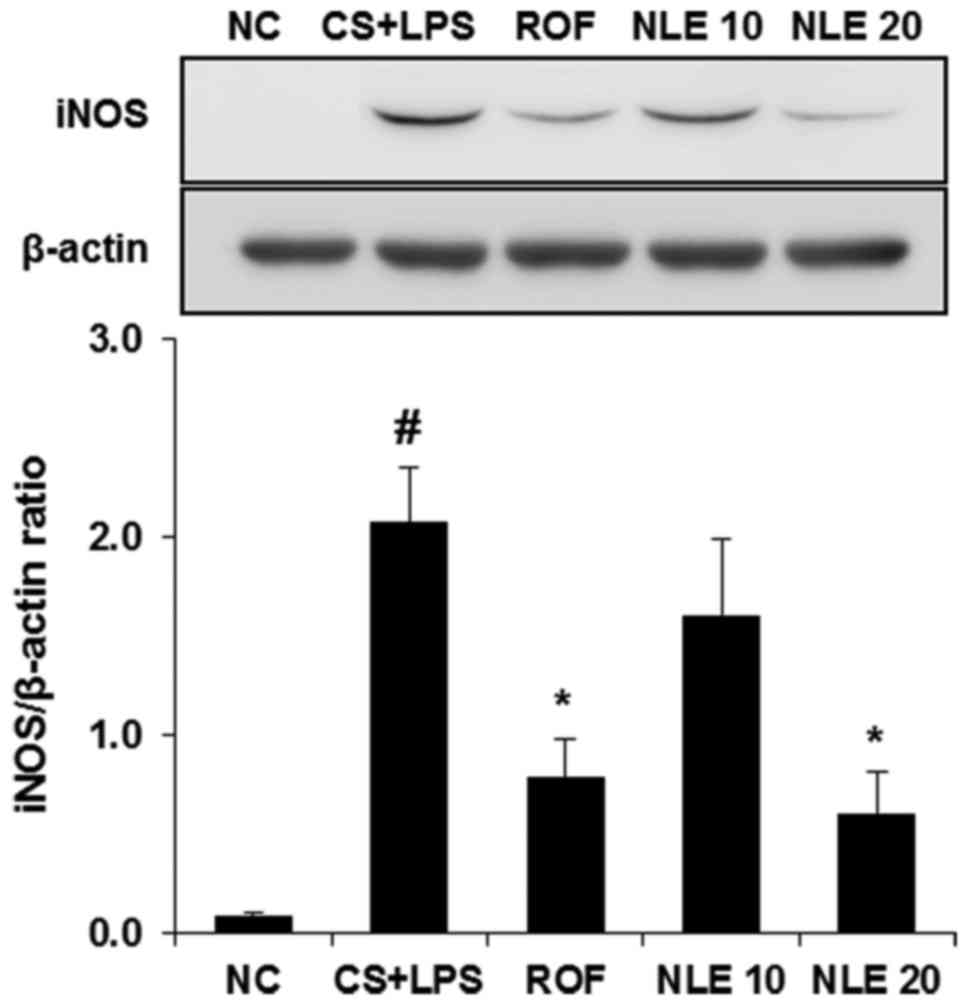
NLE inhibits the expression of iNOS in
lungs of mice with CS- and LPS-induced pulmonary inflammation
As the increased expression of iNOS induced by
neutrophils (40) and macrophages
(2) is an important in the
pathologenesis of COPD, we investigated whether NLE affects the
level of iNOS in the lungs of mice with CS- and LPS-induced
pulmonary inflammation. As shown in Fig. 5, iNOS expression was increased in
the lungs of mice in the CS and LPS group. However, treatment with
NLE effectively inhibited the expression of iNOS, compared with
normal control mice.
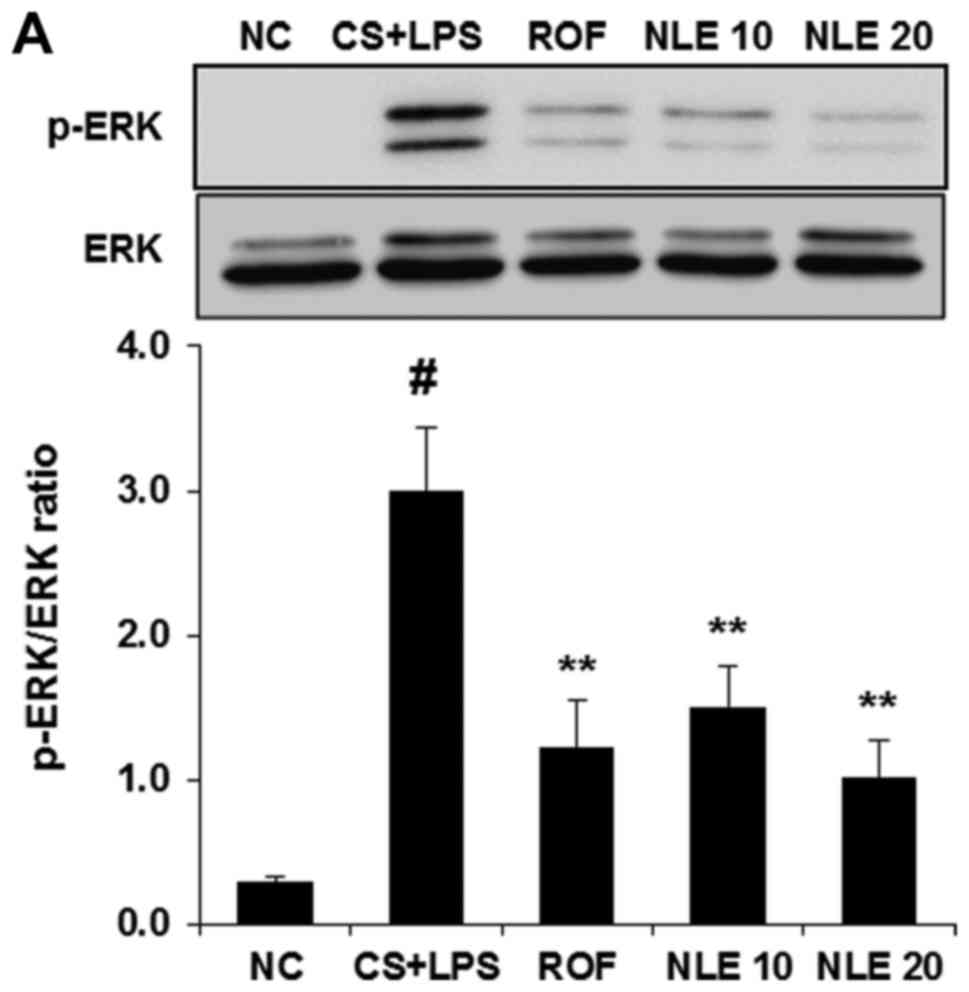
NLE attenuates the activation of ERK and
JNK in the lungs of mice with CS- and LPS-induced pulmonary
inflammation
MAPK activation plays an important role in the
inflammatory response regulating the release of pro-inflammatory
cytokines and mediators. Thus, we investigated whether NLE
treatment attenuates the activation of MAPKs in the lungs of mice
with CS- and LPS-induced pulmonary inflammation. As shown in
Fig. 6, the activation of MAPKs
(ERK, JNK and p38) was significantly increased in the lungs of mice
in the CS and LPS group. However, treatment with NLE significantly
decreased the activation of ERK and JNK in a
concentration-dependent manner (Fig.
6A and B). The inhibitory effect of 20 mg/kg NLE on ERK and JNK
activation was similar to that of treatment with 10 mg/kg ROF. No
significant attenuation of p38 activation was observed with NLE
(Fig. 6C).
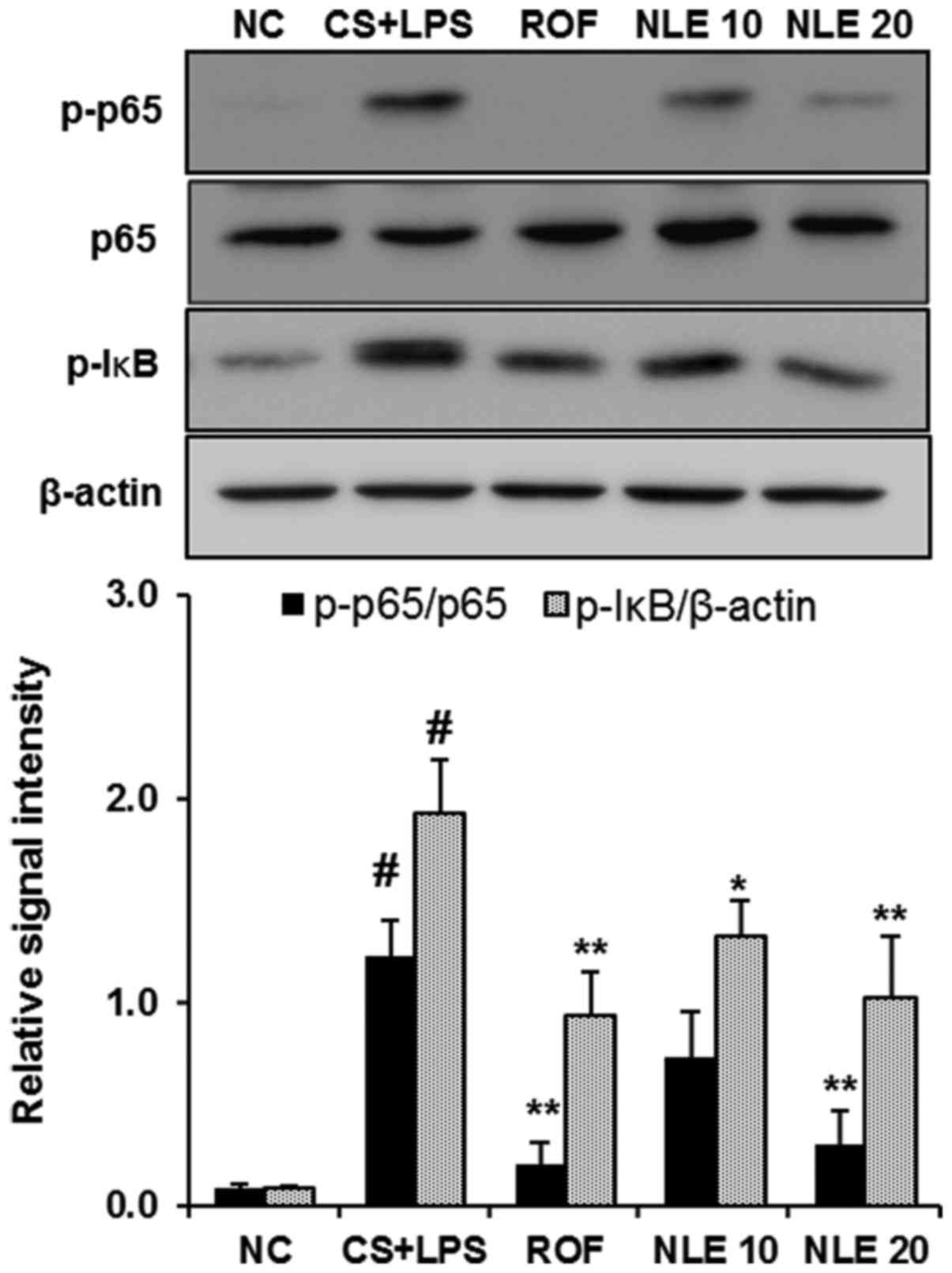
NLE decreases the phosphorylation of
NF-κB and IκB in lungs of mice with CS- and LPS-induced pulmonary
inflammation
NF-κB is activated by a number of stimuli, including
pro-inflammatory mediators and LPS. In response to these molecules,
IκB is phosphorylated, ubiquitinated and degraded, resulting in the
phosphorylation and nuclear translocation of NF-κB (20,41). In the present study, treatment
with NLE inhibited the phosphorylation of NF-κB and IκB in the
lungs of mice with CS- and LPS-induced pulmonary inflammation
(Fig. 7).
Discussion
In the present study, we examined the protective
effects of NLE against CS- and LPS-induced pulmonary inflammation.
NLE significantly inhibited the infiltration of inflammatory cells,
such as neutrophils and macrophages in BALF. NLE also reduced the
production of ROS and NE, and decreased the release of
pro-inflammatory cytokines in BALF. NLE attenuated the accumulation
of inflammatory cells and the expression of MCP-1 in the lungs of
mice with CS- and LPS-induced pulmonary inflammation. Furthermore,
NLE inhibited the expression of iNOS in the lungs of mice with CS-
and LPS-induced pulmonary inflammation. NLE also attenuated the
activation of MAPKs (ERK and JNK) and NF-κB in the lung tissue.
COPD is a global health epidemic the incidence of
which is increasing (42), and it
is associated with a high risk of morbidity and mortality (43). COPD is characterized by chronic
airway inflammation and mucus hypersecretion (44). It is also well known that CS
exposure and bacterial infection are associated with the
development of COPD (4,45,46). CS is the most important risk
factor that increases the recruitment of inflammatory cells in the
lungs and the number of goblet cells in the small airway (47). LPS is a major constituent of the
Gram-negative bacterial cell wall that stimulates the inflammatory
response (48). The recruitment
of inflammatory cells, such as neutrophils and macrophages in the
airways is a characteristic sign of COPD (6,49,50). ROS production promote the
inflammatory response in the lungs via the activation of
transcription factors, such as NF-κB and MAPK signal transduction
pathways (10). Increased ROS
production induced by neutrophils has been reported to promote the
oxidation of proteins, DNA and lipids which leads to lung damage
(10,51). A number of studies have reported
that NE levels are increased in response to CS (52,53) or CS and LPS (5,6),
which increases the inflammatory cell recruitment, emphysema and
the production of mucus in the lungs (54). CS is the most important source of
elevated levels of ROS and NE in COPD (55). In the present study, treatment
with NLE significantly inhibited inflammatory cell infiltration in
BALF and in the lungs of mice with CS- and LPS-induced pulmonary
inflammation (Figs. 1 and
4A and B). NLE also attenuated
the production of ROS and the activity of NE (Fig. 2).
Pro-inflammatory cytokines, including TNF-α and IL-6
play an important role in the pathological processes of COPD
(56). TNF-α is a central
cytokine that regulates inflammation through neutrophil recruitment
and endothelial activation (57).
IL-6 is involved in the pathogenesis of lung diseases, such as COPD
(58). It has also been reported
that exposure to CS increases macrophage accumulation that
contributes to the development of COPD by increasing the levels of
IL-6 (59). Recently, TNF-α and
IL-6 were identified to be involved in CS- and LPS-induced COPD
(4,5,60).
In the present study, NLE decreased the release of TNF-α and IL-6
in BALF (Fig. 3). MCP-1 is a
chemokine that plays a key role in the migration of neutrophils and
macrophages (15,16). Recently, the increased expression
of MCP-1 was detected in the lungs of mice exposed to CS (61). The present data demonstrated
beneficial effects of NLE against the CS- and LPS-induced
expression of MCP-1 (Fig. 4C).
iNOS has been implicated in the pathophysiology of inflammatory
diseases, including COPD (23),
and the high expression of iNOS has been reported to affect
pulmonary inflammation (62). It
has also been reported that the inhibition of iNOS exerts
protective effects in a wide variety of respiratory diseases
(63). The present study
demonstrated that NLE significantly suppressed the expression of
iNOS in the lungs of mice with CS and LPS-induced pulmonary
inflammation in a concentration-dependent manner (Fig. 5).
MAPKs have been reported to regulate
pro-inflammatory molecules (64,65) and have been widely studied in
pulmonary inflammation (4,5,66).
MAPKs (ERK, JNK and p38) mediate pro-inflammatory gene
transcription in response to cytokines and LPS (5,67).
CS leads to the activation of MAPKs (68–71). It has also been reported that MAPK
activation affects ROS production in lungs affected by COPD
(10). The present data
demonstrated that the activation of MAPKs (ERK, JNK and p38) was
induced by CS and LPS in the lungs of mice. However, NLE treatment
significantly inhibited the activation or ERK and JNK (Fig. 6A and B). No significant inhibition
of p38 activation was observed with NLE treatment (Fig. 6C).
NF-κB is a key transcription factor in the
inflammatory response, and is activated by numerous extracellular
stimuli, including pro-inflammatory cytokines, such as TNF-α and
IL-6 (10). The NF-κB-dependent
production of these cytokines affects the recruitment of
inflammatory cells, such as neutrophils and macrophages to lung
tissue, causing lung injury or emphysema (9,72,73) Therefore, NF-κB signaling is
considered to be an important therapeutic target for pulmonary
inflammation induced by CS (74).
In this study, NLE treatment significantly inhibited the elevated
phosphorylation levels of NF-κB and IκB induced by CS and LPS in
lung tissue (Fig. 7).
The neem tree (Azadirachta indica A. Juss.;
Meliaceae) is indigenous to India, and now this tree is cultivated
widely in areas of the world (75). Azadirachta indica A. Juss
has been widely used as neem and has been used in medicine for over
2,000 years (76). Various parts
of the neem tree have been used in medicines and food, as well as
as insecticides, and many bioactive constituents, including
limonoids (tetra-nortriterpenoids) have been isolated and
identified (77). NLE has been
reported to possess antibacterial activity (78–80). It has also been demonstrated that
NLE induces apoptosis in the breast cancer cells (81). Neem leaf fraction has been
reported to possess antioxidant properties (82,83). Recently, it has also been shown
that NLE protects LPS-induced endotoxemia (38). However, to date, at least to the
best of our knowledge, the protective effects of NLE have not been
investigated in CS- and LPS-induced pulmonary inflammation.
In conclusion, the present data demonstrated that
NLE significantly inhibited the infiltration of inflammatory cells,
such as neutrophils and macrophages in the lungs of mice with
CS-and LPS-induced pulmonary inflammation. NLE also attenuated the
production of inflammatory mediators, including ROS, NE, TNF-α and
IL-6 in BALF. Furthermore, NLE decreased the expression of MCP-1
and iNOS in the lungs of mice with CS-and LPS-induced pulmonary
inflammation. NLE also inhibited the activation of MAPKs (ERK and
JNK) and NF-κB in the lungs of mice. These results thus suggest
that NLE may have potential for use as a valuable therapeutic agent
in the treatment of COPD.
Acknowledgments
This study was supported by a grant from the
Ministry of Science, ICT and Future Planning (FGC 1011534),
Ministry for Health and Welfare (HI14C1277) and the KRIBB Research
Initiative Program (KGM 1221713) of the Republic of Korea.
Glossary
Abbreviations
Abbreviations:
|
COPD
|
chronic obstructive pulmonary
disease
|
|
CS
|
cigarette smoke
|
|
LPS
|
lipopolysaccharide
|
|
BALF
|
broncho-alveolar lavage fluid
|
|
ROS
|
reactive oxygen species
|
|
NE
|
neutrophil elastase
|
|
NLE
|
neem leaf extract
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin-6
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
NF-κB
|
nuclear factor-κB
|
|
IκB
|
inhibitor of NF-κB
|
References
|
1
|
Lee H, Jung KH, Lee H, Park S, Choi W and
Bae H: Casticin, an active compound isolated from Vitex Fructus,
ameliorates the cigarette smoke-induced acute lung inflammatory
response in a murine model. Int Immunopharmacol. 28:1097–1101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes PJ: Chronic obstructive pulmonary
disease * 12: new treatments for COPD. Thorax. 58:803–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park YC, Jin M, Kim SH, Kim MH, Namgung U
and Yeo Y: Effects of inhalable microparticle of flower of Lonicera
japonica in a mouse model of COPD. J Ethnopharmacol. 151:123–130.
2014. View Article : Google Scholar
|
|
4
|
Bak JH, Lee SM and Lim HB: Safety
assessment of mainstream smoke of herbal cigarette. Toxicol Res.
31:41–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin IS, Shin NR, Park JW, Jeon CM, Hong
JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, et al: Melatonin
attenuates neutrophil inflammation and mucus secretion in cigarette
smoke-induced chronic obstructive pulmonary diseases via the
suppression of Erk-Sp1 signaling. J Pineal Res. 58:50–60. 2015.
View Article : Google Scholar
|
|
6
|
Lee JW, Shin NR, Park JW, Park SY, Kwon
OK, Lee HS, Hee Kim J, Lee HJ, Lee J, Zhang ZY, et al: Callicarpa
japonica Thunb. attenuates cigarette smoke-induced neutrophil
inflammation and mucus secretion. J Ethnopharmacol. 175:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruger P, Saffarzadeh M, Weber AN, Rieber
N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J and Hartl
D: Neutrophils: between host defence, immune modulation, and tissue
injury. PLoS Pathog. 11:e10046512015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin IS, Ahn KS, Shin NR, Lee HJ, Ryu HW,
Kim JW, Sohn KY, Kim HJ, Han YH and Oh SR: Protective effect of
EC-18, a synthetic monoacetyldiglyceride on lung inflammation in a
murine model induced by cigarette smoke and lipopolysaccharide. Int
Immunopharmacol. 30:62–68. 2016. View Article : Google Scholar
|
|
9
|
Song HH, Shin IS, Woo SY, Lee SU, Sung MH,
Ryu HW, Kim DY, Ahn KS, Lee HK, Lee D, et al: Piscroside C, a novel
iridoid glycoside isolated from Pseudolysimachion rotundum var.
subinegrum suppresses airway inflammation induced by cigarette
smoke. J Ethnopharmacol. 170:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahman I and Adcock IM: Oxidative stress
and redox regulation of lung inflammation in COPD. Eur Respir J.
28:219–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shapiro SD, Goldstein NM, Houghton AM,
Kobayashi DK, Kelley D and Belaaouaj A: Neutrophil elastase
contributes to cigarette smoke-induced emphysema in mice. Am J
Pathol. 163:2329–2335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lundblad LK, Thompson-Figueroa J, Leclair
T, Sullivan MJ, Poynter ME, Irvin CG and Bates JH: Tumor necrosis
factor-alpha overexpression in lung disease: a single cause behind
a complex phenotype. Am J Respir Crit Care Med. 171:1363–1370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rincon M and Irvin CG: Role of IL-6 in
asthma and other inflammatory pulmonary diseases. Int J Biol Sci.
8:1281–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan MX, Wang Y, Liu Q, Schramm R and
Thorlacius H: CC chemokines induce P-selectin-dependent neutrophil
rolling and recruitment in vivo: intermediary role of mast cells.
Br J Pharmacol. 138:698–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): an overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Traves SL, Culpitt SV, Russell RE, Barnes
PJ and Donnelly LE: Increased levels of the chemokines GROalpha and
MCP-1 in sputum samples from patients with COPD. Thorax.
57:590–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McNeill E, Crabtree MJ, Sahgal N, Patel J,
Chuaiphichai S, Iqbal AJ, Hale AB, Greaves DR and Channon KM:
Regulation of iNOS function and cellular redox state by macrophage
Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2
activation. Free Radic Biol Med. 79:206–216. 2015. View Article : Google Scholar :
|
|
19
|
Webb JL, Polak JM and Evans TJ: Effect of
adhesion on inducible nitric oxide synthase (iNOS) production in
purified human neutrophils. Clin Exp Immunol. 123:42–48. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marinovic MP, Morandi AC and Otton R:
Green tea catechins alone or in combination alter functional
parameters of human neutrophils via suppressing the activation of
TLR-4/NFκB p65 signal pathway. Toxicol In Vitro. 29:1766–1778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roh GS, Yi CO, Cho YJ, Jeon BT,
Nizamudtinova IT, Kim HJ, Kim JH, Oh YM, Huh JW, Lee JH, et al:
Anti-inflammatory effects of celecoxib in rat lungs with
smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol.
299:L184–L191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WT, Liu XS, Xu YJ, Ni W and Chen SX:
Expression of nitric oxide synthase isoenzyme in lung tissue of
smokers with and without chronic obstructive pulmonary disease.
Chin Med J (Engl). 128:1584–1589. 2015. View Article : Google Scholar
|
|
24
|
Singh D: P38 inhibition in COPD; cautious
optimism. Thorax. 68:705–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh YC, Jeong YH, Ha JH, Cho WK and Ma JY:
Oryeongsan inhibits LPS-induced production of inflammatory
mediators via blockade of the NF-kappaB, MAPK pathways and leads to
HO-1 induction in macrophage cells. BMC Complement Altern Med.
14:2422014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Zhu M, Zhu W, Zhu J, et al: Inhibition of tobacco
smoke-induced bladder MAPK activation and epithelial-mesenchymal
transition in mice by curcumin. Int J Clin Exp Pathol. 8:4503–4513.
2015.PubMed/NCBI
|
|
27
|
Li L, Sun J, Xu C, Zhang H, Wu J, Liu B
and Dong J: Icariin ameliorates cigarette smoke induced
inflammatory responses via suppression of NF-κB and modulation of
GR in vivo and in vitro. PLoS One. 9:e1023452014. View Article : Google Scholar
|
|
28
|
Mahfuzul Hoque MD, Bari ML, Inatsu Y,
Juneja VK and Kawamoto S: Antibacterial activity of guava (Psidium
guajava L.) and Neem (Azadirachta indica A. Juss.) extracts against
foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis.
4:481–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okpanyi SN and Ezeukwu GC:
Anti-inflammatory and anti-pyretic activities of Azadirachta
indica. Planta Med. 41:34–39. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao AD, Devi KN and Thyagaraju K:
Isolation of antioxidant principle from Azadirachta seed kernels:
determination of its role on plant lipoxygenases. J Enzyme Inhib.
14:85–96. 1998. View Article : Google Scholar
|
|
31
|
Yanpallewar SU, Sen S, Tapas S, Kumar M,
Raju SS and Acharya SB: Effect of Azadirachta indica on
paracetamol-induced hepatic damage in albino rats. Phytomedicine.
10:391–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Almas K: The antimicrobial effects of
extracts of Azadi-rachta indica (Neem) and Salvadora persica (Arak)
chewing sticks. Indian J Dent Res. 10:23–26. 1999.
|
|
33
|
Badam L, Joshi SP and Bedekar SS: 'In
vitro' antiviral activity of neem (Azadirachta indica. A Juss) leaf
extract against group B coxsackieviruses. J Commun Dis. 31:79–90.
1999.
|
|
34
|
Siddiqui BS, Afshan F, Gulzar T and Hanif
M: Tetracyclic triterpenoids from the leaves of Azadirachta indica.
Phytochemistry. 65:2363–2367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang YC, Tsai MH, Sheu WH, Hsieh SC and
Chiang AN: The therapeutic potential and mechanisms of action of
quercetin in relation to lipopolysaccharide-induced sepsis in vitro
and in vivo. PLoS One. 8:e807442013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loizou S, Lekakis I, Chrousos GP and
Moutsatsou P: Beta-sitosterol exhibits anti-inflammatory activity
in human aortic endothelial cells. Mol Nutr Food Res. 54:551–558.
2010. View Article : Google Scholar
|
|
37
|
Pillai NR and Santhakumari G:
Anti-arthritic and anti-inflammatory actions of nimbidin. Planta
Med. 43:59–63. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim WH, Song HO, Jin CM, Hur JM, Lee HS,
Jin HY, Kim SY and Park H: The methanol extract of Azadirachta
indica A. Juss leaf protects mice against lethal endotoxemia and
sepsis. Biomol Ther (Seoul). 20:96–103. 2012. View Article : Google Scholar
|
|
39
|
Sakuma T, Takahashi K, Ohya N, Usuda K,
Handa M and Abe T: ONO-5046 is a potent inhibitor of neutrophil
elastase in human pleural effusion after lobectomy. Eur J
Pharmacol. 353:273–279. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rytilä P, Rehn T, Ilumets H, Rouhos A,
Sovijärvi A, Myllärniemi M and Kinnula VL: Increased oxidative
stress in asymptomatic current chronic smokers and GOLD stage 0
COPD. Respir Res. 7:692006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barnes PJ: Chronic obstructive pulmonary
disease: a growing but neglected global epidemic. PLoS Med.
4:e1122007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
44
|
Yang J, Yu HM, Zhou XD, Huang HP, Han Zh,
Kolosov VP and Perelman JM: Cigarette smoke induces mucin
hypersecretion and inflammatory response through the p66shc adaptor
protein-mediated mechanism in human bronchial epithelial cells. Mol
Immunol. 69:86–98. 2016. View Article : Google Scholar
|
|
45
|
Shao MX, Nakanaga T and Nadel JA:
Cigarette smoke induces MUC5AC mucin overproduction via tumor
necrosis factor-alpha-converting enzyme in human airway epithelial
(NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol.
287:L420–L427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chung KF: Inflammatory mediators in
chronic obstructive pulmonary disease. Curr Drug Targets Inflamm
Allergy. 4:619–625. 2005. View Article : Google Scholar
|
|
47
|
Thorley AJ and Tetley TD: Pulmonary
epithelium, cigarette smoke, and chronic obstructive pulmonary
disease. Int J Chron Obstruct Pulmon Dis. 2:409–428. 2007.
|
|
48
|
Kwak HG and Lim HB: Inhibitory effects of
Cnidium monnieri fruit extract on pulmonary inflammation in mice
induced by cigarette smoke condensate and lipopolysaccharide. Chin
J Nat Med. 12:641–647. 2014.PubMed/NCBI
|
|
49
|
Baraldo S, Turato G, Badin C, Bazzan E,
Beghé B, Zuin R, Calabrese F, Casoni G, Maestrelli P, Papi A, et
al: Neutrophilic infiltration within the airway smooth muscle in
patients with COPD. Thorax. 59:308–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li H, Yang T, Ning Q, Li F, Chen T, Yao Y
and Sun Z: Cigarette smoke extract-treated mast cells promote
alveolar macrophage infiltration and polarization in experimental
chronic obstructive pulmonary disease. Inhal Toxicol. 27:822–831.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neofytou E, Tzortzaki EG, Chatziantoniou A
and Siafakas NM: DNA damage due to oxidative stress in chronic
obstructive pulmonary disease (COPD). Int J Mol Sci.
13:16853–16864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chan KH, Chan SC, Yeung SC, Man RY, Ip MS
and Mak JC: Inhibitory effect of Chinese green tea on cigarette
smoke-induced up-regulation of airway neutrophil elastase and
matrix metal-loproteinase-12 via antioxidant activity. Free Radic
Res. 46:1123–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Iizuka T, Ishii Y, Itoh K, Kiwamoto T,
Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, et
al: Nrf2-deficient mice are highly susceptible to cigarette
smoke-induced emphysema. Genes Cells. 10:1113–1125. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bhowmik A, Chahal K, Austin G and
Chakravorty I: Improving mucociliary clearance in chronic
obstructive pulmonary disease. Respir Med. 103:496–502. 2009.
View Article : Google Scholar
|
|
55
|
van Eeden SF and Sin DD: Oxidative stress
in chronic obstructive pulmonary disease: a lung and systemic
process. Can Respir J. 20:27–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hwang JH, Lee BJ, Jung HJ, Kim KI, Choi
JY, Joo M and Jung SK: Effects of Chung-pae inhalation therapy on a
mouse model of chronic obstructive pulmonary disease. Evid Based
Complement Alternat Med. 2015:4612952015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee E, Yun N, Jang YP and Kim J: Lilium
lancifolium Thunb. extract attenuates pulmonary inflammation and
air space enlargement in a cigarette smoke-exposed mouse model. J
Ethnopharmacol. 149:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pedroza M, Schneider DJ, Karmouty-Quintana
H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D,
Gelinas R, et al: Interleukin-6 contributes to inflammation and
remodeling in a model of adenosine mediated lung injury. PLoS One.
6:e226672011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fernandez-Real JM, Broch M, Vendrell J and
Ricart W: Smoking, fat mass and activation of the tumor necrosis
factor-alpha pathway. Int J Obes Relat Metab Disord. 27:1552–1556.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu GH, Shen J, Sun P, Yang ML, Zhao PW,
Niu Y, Lu JK, Wang ZQ, Gao C, Han X, et al: Anti-inflammatory
effects of potato extract on a rat model of cigarette smoke-induced
chronic obstructive pulmonary disease. Food Nutr Res. 59:288792015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen X, Guan XJ, Peng XH, Cui ZL, Luan CY
and Guo XJ: Acetylation of lysine 9 on histone H3 is associated
with increased pro-inflammatory cytokine release in a cigarette
smoke-induced rat model through HDAC1 depression. Inflamm Res.
64:513–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao Y, Cui A, Wang F, Wang XJ, Chen X,
Jin ML and Huang KW: Characteristics of pulmonary inflammation in
combined pulmonary fibrosis and emphysema. Chin Med J (Engl).
125:3015–3021. 2012.
|
|
63
|
Hesslinger C, Strub A, Boer R, Ulrich WR,
Lehner MD and Braun C: Inhibition of inducible nitric oxide
synthase in respiratory diseases. Biochem Soc Trans. 37:886–891.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li D, Xu D, Wang T, Shen Y, Guo S, Zhang
X, Guo L, Li X, Liu L and Wen F: Silymarin attenuates airway
inflammation induced by cigarette smoke in mice. Inflammation.
38:871–878. 2015. View Article : Google Scholar
|
|
65
|
Ng DS, Liao W, Tan WS, Chan TK, Loh XY and
Wong WS: Anti-malarial drug artesunate protects against cigarette
smoke-induced lung injury in mice. Phytomedicine. 21:1638–1644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ma WJ, Sun YH, Jiang JX, Dong XW, Zhou JY
and Xie QM: Epoxyeicosatrienoic acids attenuate cigarette smoke
extract-induced interleukin-8 production in bronchial epithelial
cells. Prostaglandins Leukot Essent Fatty Acids. 94:13–19. 2015.
View Article : Google Scholar
|
|
67
|
Coskun M, Olsen J, Seidelin JB and Nielsen
OH: MAP kinases in inflammatory bowel disease. Clin Chim Acta.
412:513–520. 2011. View Article : Google Scholar
|
|
68
|
Hoshino S, Yoshida M, Inoue K, Yano Y,
Yanagita M, Mawatari H, Yamane H, Kijima T, Kumagai T, Osaki T, et
al: Cigarette smoke extract induces endothelial cell injury via JNK
pathway. Biochem Biophys Res Commun. 329:58–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu X, Balsiger R, Tyrrell J, Boyaka PN,
Tarran R and Cormet-Boyaka E: Cigarette smoke exposure reveals a
novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR.
Biochim Biophys Acta. 1850:1224–1232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marumo S, Hoshino Y, Kiyokawa H, Tanabe N,
Sato A, Ogawa E, Muro S, Hirai T and Mishima M: p38
mitogen-activated protein kinase determines the susceptibility to
cigarette smoke-induced emphysema in mice. BMC Pulm Med. 14:792014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shen N, Gong T, Wang JD, Meng FL, Qiao L,
Yang RL, Xue B, Pan FY, Zhou XJ, Chen HQ, et al: Cigarette
smoke-induced pulmonary inflammatory responses are mediated by
EGR-1/GGPPS/MAPK signaling. Am J Pathol. 178:110–118. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Metcalfe HJ, Lea S, Hughes D, Khalaf R,
Abbott-Banner K and Singh D: Effects of cigarette smoke on
toll-like receptor (TLR) activation of chronic obstructive
pulmonary disease (COPD) macrophages. Clin Exp Immunol.
176:461–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao Y, Xu Y, Li Y, Xu W, Luo F, Wang B,
Pang Y, Xiang Q, Zhou J, Wang X, et al: NF-κB-mediated inflammation
leading to EMT via miR-200c is involved in cell transformation
induced by cigarette smoke extract. Toxicol Sci. 135:265–276. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar
|
|
75
|
Akihisa T, Noto T, Takahashi A, Fujita Y,
Banno N, Tokuda H, Koike K, Suzuki T, Yasukawa K and Kimura Y:
Melanogenesis inhibitory, anti-inflammatory, and chemopreventive
effects of limonoids from the seeds of Azadirachta indicia A. Juss.
(neem). J Oleo Sci. 58:581–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Faccin-Galhardi LC, Yamamoto KA, Ray S,
Ray B, Carvalho Linhares RE and Nozawa C: The in vitro antiviral
property of Azadirachta indica polysaccharides for poliovirus. J
Ethnopharmacol. 142:86–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Akihisa T, Takahashi A, Kikuchi T, Takagi
M, Watanabe K, Fukatsu M, Fujita Y, Banno N, Tokuda H and Yasukawa
K: The melanogenesis-inhibitory, anti-inflammatory, and
chemopreventive effects of limonoids in n-hexane extract of
Azadirachta indica A. Juss. (neem) seeds. J Oleo Sci. 60:53–59.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Agyare C, Spiegler V, Sarkodie H, Asase A,
Liebau E and Hensel A: An ethnopharmacological survey and in vitro
confirmation of the ethnopharmacological use of medicinal plants as
anthelmintic remedies in the Ashanti region, in the central part of
Ghana. J Ethnopharmacol. 158:255–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Quelemes PV, Perfeito ML, Guimarães MA,
dos Santos RC, Lima DF, Nascimento C, Silva MP, Soares MJ, Ropke
CD, Eaton P, et al: Effect of neem (Azadirachta indica A. Juss)
leaf extract on resistant Staphylococcus aureus biofilm formation
and Schistosoma mansoni worms. J Ethnopharmacol. 175:287–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma J, Gairola S, Sharma YP and Gaur
RD: Ethnomedicinal plants used to treat skin diseases by Tharu
community of district Udham Singh Nagar, Uttarakhand, India. J
Ethnopharmacol. 158:140–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Othman F, Motalleb G, Lam Tsuey Peng S,
Rahmat A, Basri R and Pei Pei C: Effect of neem leaf extract
(Azadirachta indica) on c-Myc oncogene expression in 4T1 breast
cancer cells of BALB/c mice. Cell J. 14:53–60. 2012.
|
|
82
|
Manikandan P, Anandan R and Nagini S:
Evaluation of Azadirachta indica leaf fractions for in vitro
antioxidant potential and protective effects against
H2O2-induced oxidative damage to pBR322 DNA
and red blood cells. J Agric Food Chem. 57:6990–6996. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sithisarn P, Supabphol R and Gritsanapan
W: Comparison of free radical scavenging activity of Siamese neem
tree (Azadirachta indica A. Juss var. siamensis Valeton) leaf
extracts prepared by different methods of extraction. Med Princ
Pract. 15:219–222. 2006. View Article : Google Scholar : PubMed/NCBI
|