Introduction
Inflammatory bowel diseases (IBDs), consisting
mainly of ulcerative colitis (UC) and Crohn's disease (CD), are
chronic inflammatory disorders involving the gastrointestinal tract
(1). To date, mucosal healing has
been indicated as the therapeutic goal for IBD, as healing is
associated with sustained clinical remission, reduced rates of
hospitalization and surgery, and a lower incidence of colorectal
cancer (2–4). To optimize the outcomes of patients
with IBD, frequent monitoring is required to evaluate disease
activity and treatment efficacy. Endoscopy is considered the gold
standard for evaluating intestinal inflammation and mucosal
healing. However, frequent endoscopic procedures are invasive,
unpleasant, time-consuming and costly for patients, and they
require a skilled operator and bowel preparation. Therefore, a
reliable surrogate marker capable of mirroring intestinal
inflammation, which can be used as a substitute for endoscopy, is
required.
The fecal stream is in close contact with the
intestinal mucosa and can, therefore, take up molecules serving as
markers of intestinal inflammation. There are several studies
concerning fecal markers for intestinal inflammation (5–8).
Of these, calprotectin is the most widely used marker for
evaluating the disease activity of IBD (9). Calprotectin is an abundant
heterodimeric calcium-binding protein belonging to the S100 family
(S100A8 and S100A9), which inhibits metalloproteinases, and has
antimicrobial and pro-apoptotic activities (10,11). Calprotectin is present in
neutrophils, monocytes/macrophages and potentially epithelial
cells, and it comprises up to 60% of the total cytosolic protein
content of neutrophils. Calprotectin has several clinical
advantages, including a high stability at room temperature,
resistance to degradation and a homogenous distribution in stools,
all of which have been described as prerequisites for
biomarkers.
The use of enzyme-linked immunosorbent assay (ELISA)
analyses for calprotectin has been thoroughly validated. However,
the ELISA protocol is time-consuming to perform, and this can lead
to delayed reporting of the results. As the assay also requires
specialized laboratory equipment, it is predominantly used in large
laboratories. To overcome these limitations, an
immunochromatographic point-of-care test (POCT) for calprotectin
has been developed (12,13).
In the present study, the fecal calprotectin levels
were measured using ELISA as the gold standard, and the
correlations between these levels, endoscopic and clinical disease
activities and fecal hemoglobin (Hb) were examined. In addition,
the fecal calprotectin level as measured using ELISA was compared
with that measured using the POCT and the serum calprotectin level,
in addition to examining the immunohistochemical localization of
calprotectin in the diseased intestine.
Patients and methods
Ethical consideration
The Human Ethics Committee of Kurume University
School of Medicine approved the protocol in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
each of the subjects or their parents prior to enrollment in the
study.
Patients
Between January, 2014 and December, 2015,
colonoscopies were performed and stool samples were collected from
113 patients with UC and 42 with CD. The diagnoses were based on
characteristic clinical, endoscopic, radiological and histological
features. The patient characteristics are presented in Table I. Among the patients with UC,
there were 51 men and 62 women, with a median age of 41.5 years and
median disease duration of 83 months. In terms of disease
distribution, 59 patients had pancolitis, 30 had left colon
involvement and 24 had disease limited to the rectum. Among the
patients with CD, there were 29 men and 13 women, with a median age
of 31 years and median disease duration of 54 months. The disease
affected the ileum and the colon in 29 patients, the colon alone in
nine patients, and the ileum alone in four patients. These patients
had received adequate medical therapy. In addition, 96 healthy,
age-matched subjects served as normal controls.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
|
Characteristics | Ulcerative
colitis
(n=113) | Crohn's
disease
(n=42) |
|---|
| Sex,
male/female | 51/62 | 29/13 |
| Age, years (median,
IQR) | 41.5 (30–57) | 31 (22–40.75) |
| Area involved | Total
colitis/left-side colitis/proctitis 59/30/24 |
Ileitis/ileocolitis/colitis 4/29/9 |
| Disease duration,
months (median, IQR) | 83 (38–173.5) | 54 (15.5–166) |
| Treatments | | |
| 5-Aminosalicylic
acid (%) | 100 (87.7) | 33 (78.6) |
| Prednisolone
(%) | 30 (26.3) | 3 (7.1) |
| Immunomodulator
(%) | 16 (14.0) | 9 (21.4) |
| Leukocytapheresis
(%) | 2 (1.8) | 1 (2.4) |
| Nutrition therapy
(%) | 3 (2.6) | 18 (42.9) |
| Antitumor necrosis
factor (%) | 2 (1.8) | 17 (40.5) |
| Surgery (%) | 1 (0.9) | 5 (11.9) |
Endoscopic and clinical evaluations
For the evaluation of disease activity in the
patients with UC, the endoscopic mucosal status was graded
according to the severity of inflammation using the Rachmilewitz
score (14), and clinical
activity was graded using the Mayo score (15). For the patients with CD, the
endoscopic findings were graded according to the severity of
inflammation using the Simple Endoscopic Score for CD (SES-CD)
(16), and clinical activity was
graded using the CD Activity Index (CDAI) (17).
Colonoscopy
Bowel preparation was performed using a polyethylene
glycol-based or magnesium citrate-based electrolyte solution
according to the standard protocol used in Kurume University
Hospital in Japan. Following the clearing of colonic lavage fluid,
the patients underwent a colonoscopy. Patients were excluded from
the study if the colonoscopic examination was incomplete due to
problems with the bowel preparation or if it was not possible to
insert the colonoscope into the cecum.
Collection of stool samples
The stool samples were collected on the day of
colonoscopy, or a few days prior to colonoscopy and stored in a
refrigerator until the day of the colonoscopy. Upon receipt in the
laboratory, all stool samples were registered and stored at −20°C.
Following thawing, the fecal samples were prepared and analyzed
according to the test manufacturer's protocol (Bühlmann
Laboratories AG, Schönenbuch, Switzerland). Between 40 and 120 mg
of stool was collected into the hollow cavity of the extraction
tool (Smart-Prep; Bühlmann Laboratories AG), and extraction buffer
in a weight/volume ratio of 1:50 was then added to the extraction
tube. The sample tubes were mixed for 1 min using a vortex mixer
until no large particles were present. Subsequently, 1 ml of the
homogenate was transferred to an Eppendorf tube and centrifuged for
5 min at 3,000 × g at room temperature. The supernatant (0.5 ml)
was transferred to a new tube and stored at −20°C until the
assay.
Measurement of fecal calprotectin
Fecal calprotectin was measured using a quantitative
enzyme-linked immunosorbent assay (fCAL™ ELISA; Bühlmann
Laboratories AG) according to the manufacturer's protocol (12,18). The fecal calprotectin level was
also measured using a quantitative rapid point-of-care test (POCT;
Quantum Blue® Calprotectin; Bühlmann Laboratories AG),
which uses immunochromatographic technology in a lateral flow assay
system, including an easy-to-use Quantum Blue® reader.
The test was performed in accordance with the manufacturer's
protocol.
Measurement of fecal Hb
Fecal Hb was determined using a quantitative fecal
immunochemical test, as described previously (19–21). Briefly, the stool sample was
collected using an OC-Hemodia sampling probe and was immediately
processed and examined using OC-SENSOR neo (both from Eiken
Chemical, Tokyo, Japan), which can accurately measure fecal Hb
levels between 50 and 1,000 ng/ml.
Measurement of serum calprotectin
The serum calprotectin levels were quantified using
an ELISA (sCAL™ ELISA; Bühlmann Laboratories AG). In brief, a blood
sample (0.5 ml) for serum calprotectin measurements was collected
in blood collection tubes containing ethylenediaminotetraacetic
acid. The sample was centrifuged for 10 min at 10,000 rpm and the
extracted serum was collected and frozen at −20°C for subsequent
measurement. The serum was diluted 1:50, and 100 µl of each
sample was added to the wells of a plate and incubated at room
temperature for 45 min. The plate was then washed three times with
diluted washing solution, and 100 µl of monoclonal
anti-calprotectin antibody conjugated with horseradish peroxidase
were added and incubated for 45 min at room temperature. A second
washing procedure was performed, 100 µl of enzyme substrate
solution was added to each well, and optical density was read at
450 nm. The serum calprotectin concentration was calculated from
the standards and expressed as µg/ml.
Determination of laboratory
parameters
A blood sample was also obtained from each patient
and was used to measure various laboratory parameters. The total
leukocyte count, platelet count, serum levels of Hb, albumin and
C-reactive protein and erythrocyte sedimentation rate were
determined by routine laboratory analysis within three days prior
to or following collection of the stool sample used for
calprotectin measurement.
Immunohistochemistry
To examine the expression of calprotectin using
immunocytochemistry, a primary monoclonal antibody targeting
calprotectin (clone MAC387; cat. no. M0747; Dako, Glostrup,
Denmark) was used at a dilution of 1:1,600 (incubation time, 50
min) and a secondary antibody (One-step polymer-HRP, cat. no.
HK595-50K; BioGenex, Fremont, CA, USA) was used at a dilution of
1:600 (incubation time, 15 min). Double staining was performed
using monoclonal anti-neutrophil elastase (diluted 1:400; clone
NP57, cat. no. M0752), monoclonal anti-CD68 (diluted 1:100; clone
KP1, cat. no. M0814) (both from Dako) and monoclonal
anti-calprotectin (incubation time, 50 min). The sections were
developed using 3,3′-diaminobenzidine for anti-calprotectin and
alkaline phosphatase for anti-neutrophil elastase and anti-CD68. We
used a Nikon Optiphot microscope (Nikon Corp., Tokyo, Japan) for
visualization of staining.
Statistical analysis
The results are presented as the median and range.
As calprotectin measurements were highly skewed, log-transformed
values were used in data analyses. All statistical analyses were
performed using the Statistical Package for the Social Sciences for
Windows software 14.0 (SPSS, Inc., Chicago, IL, USA). The
statistical analyses were performed using nonparametric
Mann-Whitney and Kruskal-Wallis tests. Correlations between
variables were estimated using the two-tailed Spearman's rank order
correlation coefficient. The mean differences between the assays
were calculated based on the methods described previously (22). P<0.05 was considered to
indicate a statistically significant difference.
Results
Fecal calprotectin
The individual fecal calprotectin levels of the
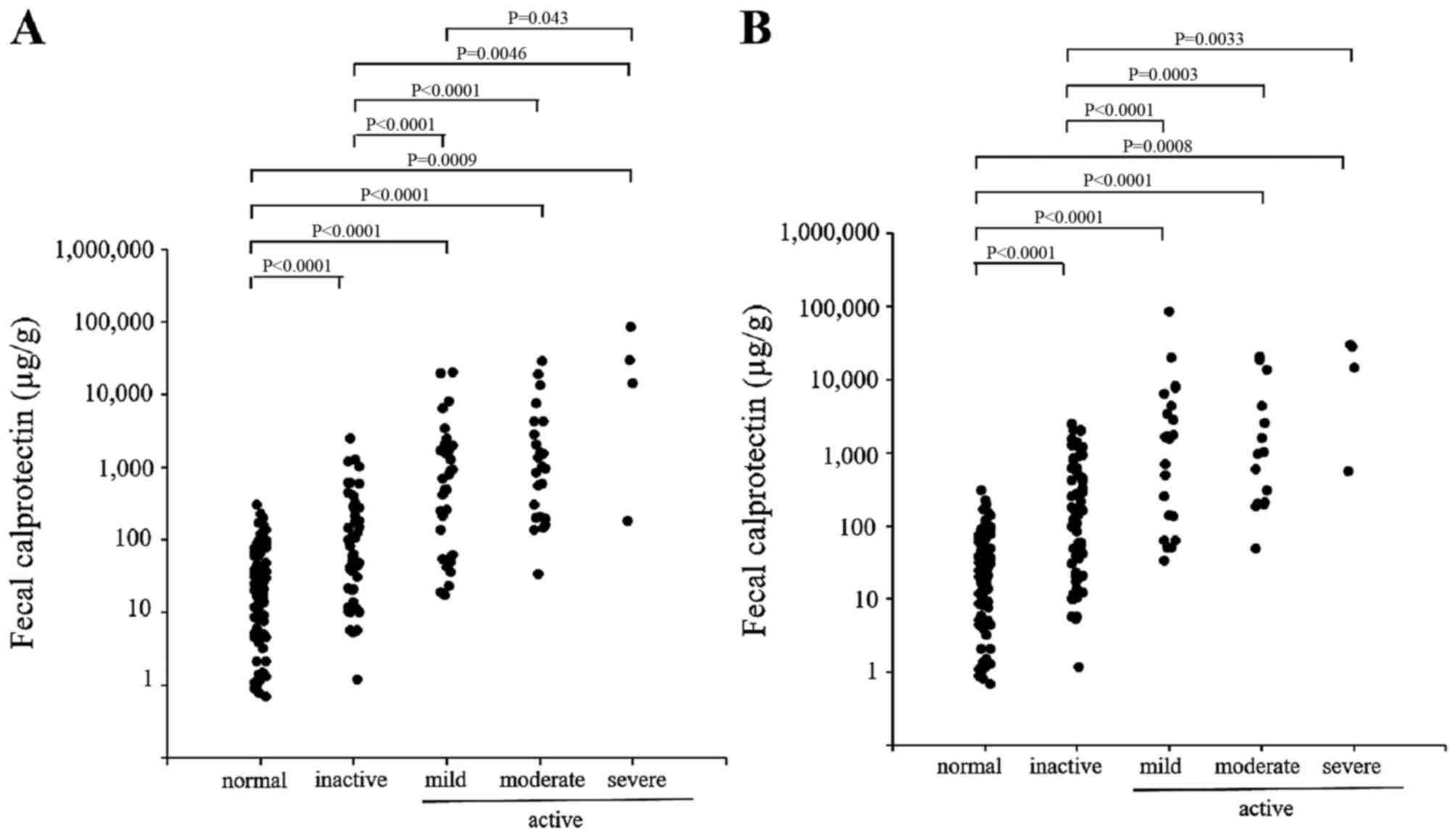
patients with UC are shown in Fig.
1. The endoscopic disease activity was graded according to the
Rachmilewitz score as either inactive, mild, moderate or severe
(Fig. 1A). The fecal calprotectin
levels were significantly higher among the patients with UC,
compared with those among the normal controls. The fecal
calprotectin level was closely correlated with the endoscopic
grade, and the concentrations differed significantly among the
groups. The clinical disease activity (Fig. 1B) was grouped according to the
Mayo score into inactive, mild, moderate or severe. A close
correlation between the fecal calprotectin level and the clinical
disease activity was also observed.
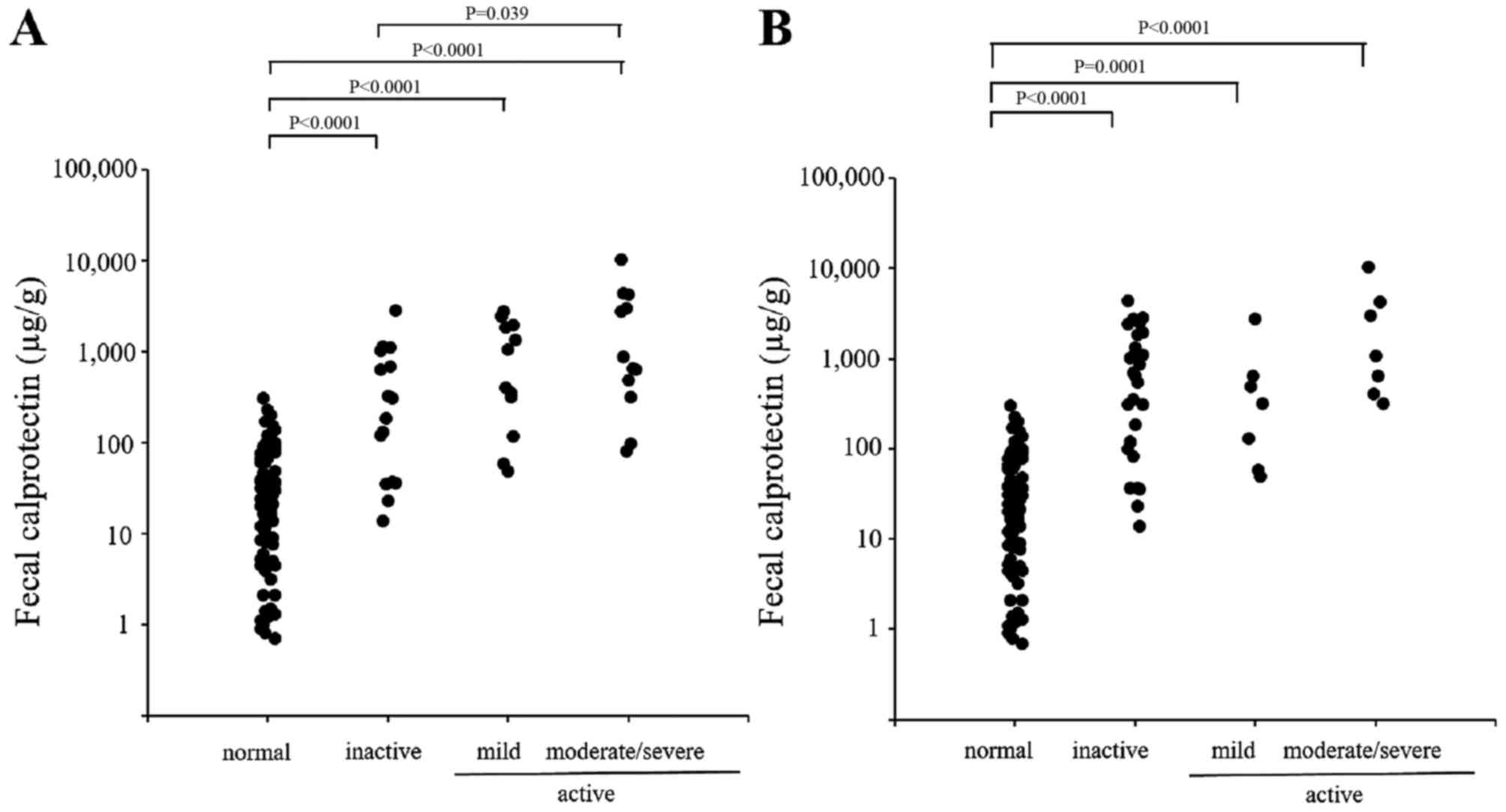
The individual fecal calprotectin levels in patients
with CD are shown in Fig. 2. The
endoscopic disease activity was graded according to the SES-CD into
inactive, mild, moderate or severe disease (Fig. 2A), and the clinical disease
activity was classified according to the CDAI into inactive, mild,
moderate or severe disease(Fig.
2B). In contrast to the patients with UC, only a weak
correlation was observed between the fecal calprotectin level and
the endoscopic and clinical disease activities in patients with
CD.
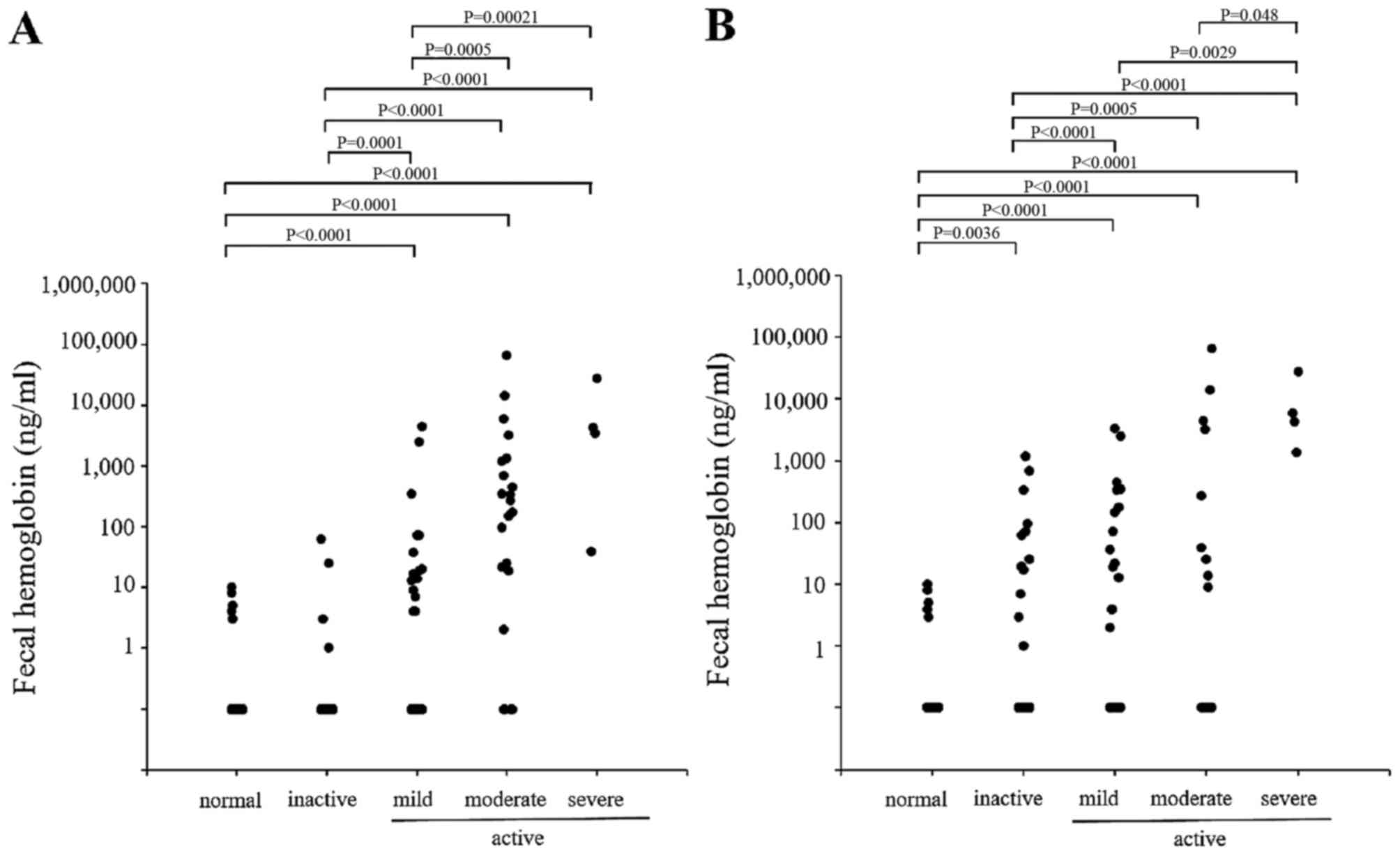
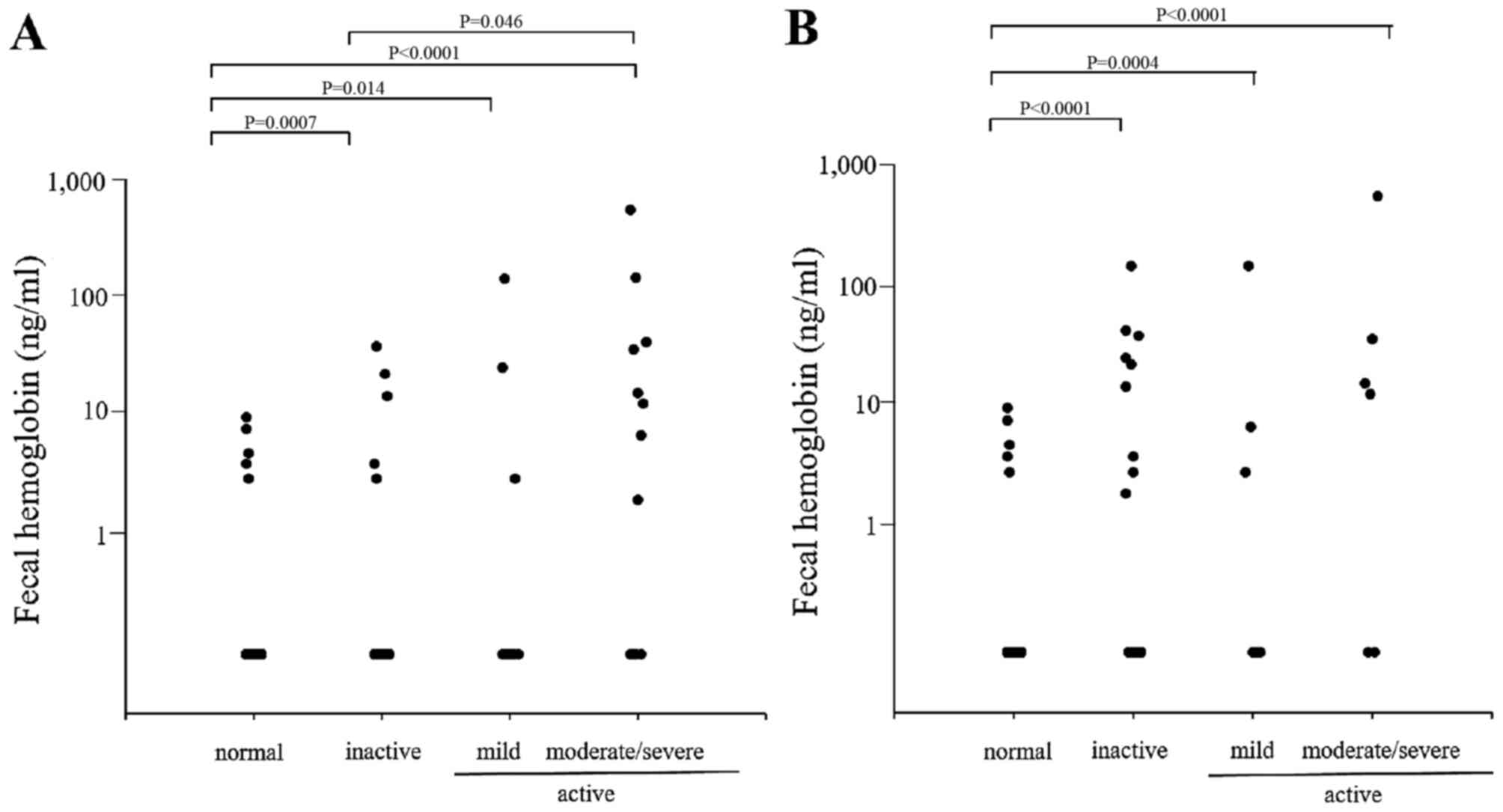
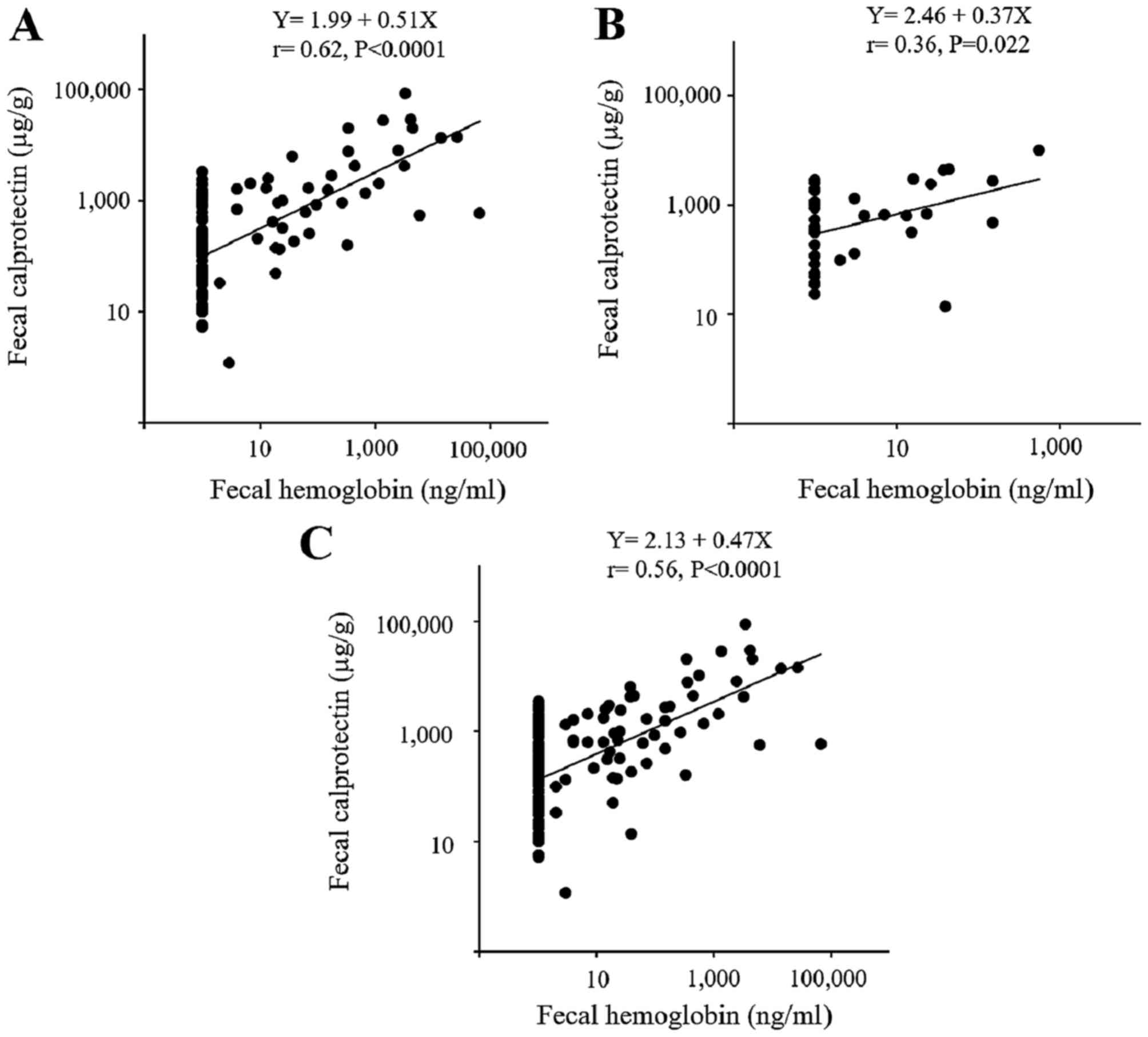
Fecal Hb
The fecal Hb levels were also measured in patients
with UC (Fig. 3) and in those
with CD (Fig. 4). Similar to the
calprotectin results, a close association was observed between the
fecal Hb level and the endoscopic and clinical disease activity in
the patients with UC, however, only a partial correlation was
observed in the patients with CD. A significant correlation was
observed between the level of fecal calprotectin and the level of
fecal Hb in the patients with UC and those with CD (Fig. 5).
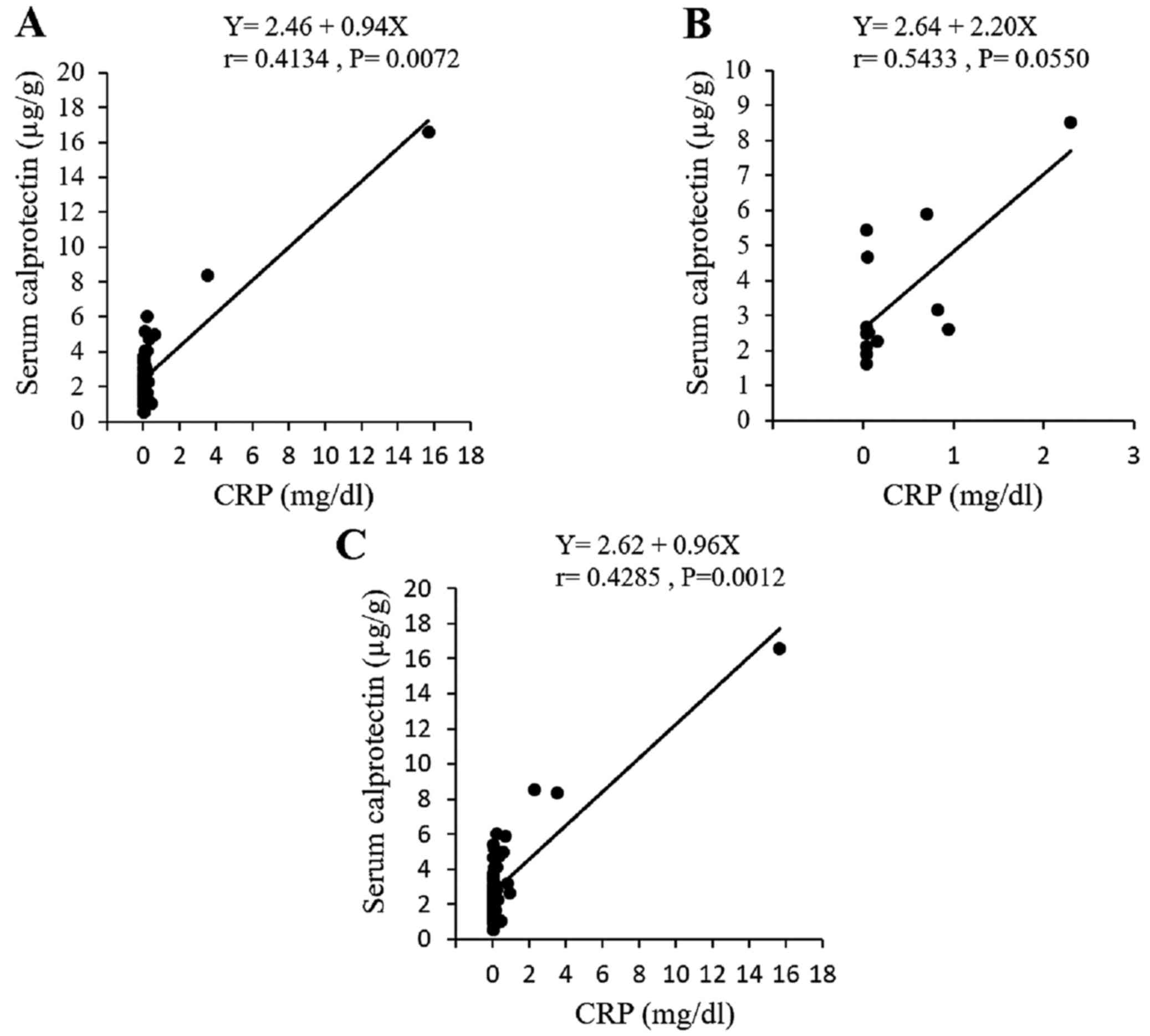
Correlation with laboratory
parameters
The correlation coefficients and significance values
between the fecal calprotectin or fecal Hb levels and the indicated
laboratory parameters are summarized in Table II. Fecal calprotectin was
significantly correlated with the leukocyte count, platelet count,
albumin and CRP levels and ESR in the patients with UC, and with
the serum albumin level, CRP level and ESR in the patients CD.
Similarly, the fecal Hb level was significantly correlated with the
leukocyte count, albumin level, CRP level and ESR in the patients
with UC, and with the platelet count, serum albumin level, CRP
level and ESR in the patients with CD.
 | Table IICorrelation coefficients and
significance of differences between fecal markers and laboratory
parameters in patients with UC or CD. |
Table II
Correlation coefficients and
significance of differences between fecal markers and laboratory
parameters in patients with UC or CD.
| Fecal calprotectin
| Fecal hemoglobin
|
|---|
UC (n=113)
| CD (n=42)
| UC (n=113)
| CD (n=42)
|
|---|
| R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value |
|---|
| Leukocyte
(µl) | 0.27 | 0.004 | −0.01 | 0.937 | 0.24 | 0.011 | −0.09 | 0.562 |
| Platelet
(×104/µl) | 0.25 | 0.007 | 0.17 | 0.291 | 0.05 | 0.575 | 0.41 | 0.008 |
| Hemoglobin
(g/dl) | −0.13 | 0.180 | −0.16 | 0.311 | −0.18 | 0.059 | −0.27 | 0.088 |
| Albumin (g/dl) | −0.45 | <.001 | −0.27 | 0.082 | −0.56 | <.001 | −0.44 | 0.004 |
| CRP (mg/dl) | 0.25 | 0.001 | 0.41 | 0.007 | 0.42 | <.001 | 0.46 | 0.002 |
| ESR 1 h (/mm) | 0.21 | 0.032 | 0.32 | 0.038 | 0.26 | 0.007 | 0.42 | 0.006 |
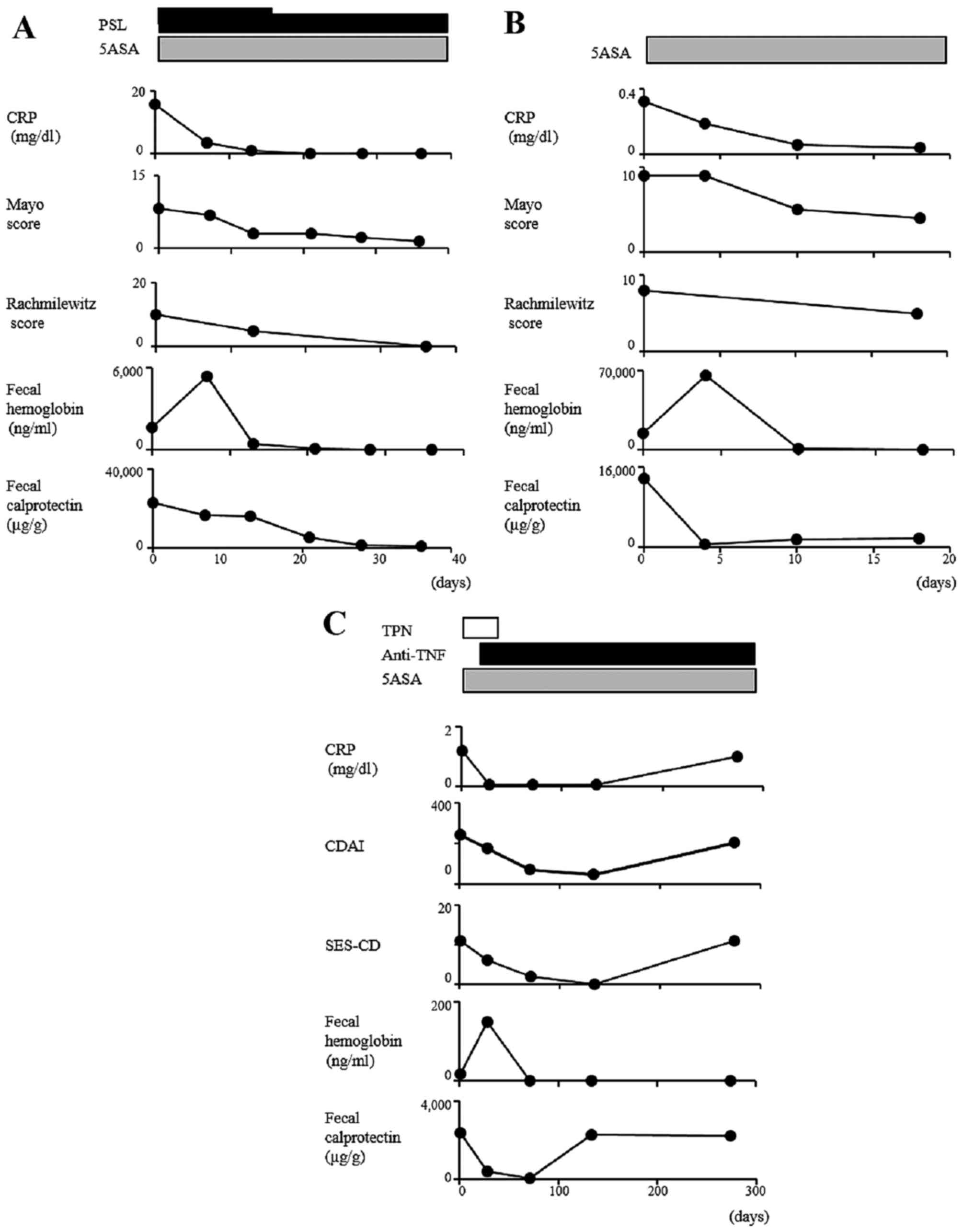
Serial measurements of fecal
calprotectin
The serial changes in fecal calprotectin and other
parameters within the same individual are shown in Fig. 6. Of note, decreasing
concentrations of fecal calprotectin were consistently found as the
disease began to abate, regardless of the treatment modality, and
these concentrations remained at lower levels when stable remission
had been achieved.
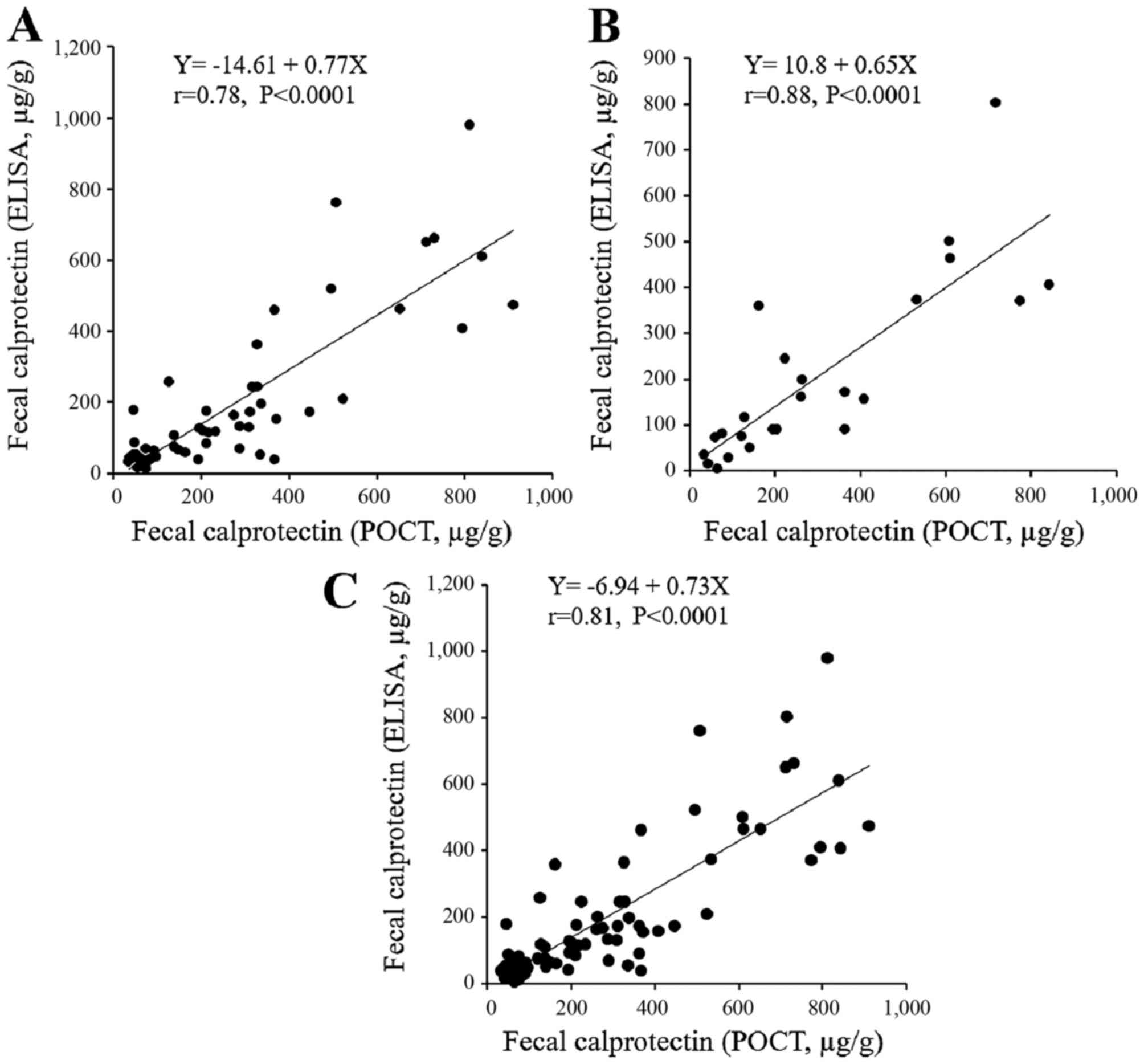
POCT
The ELISA results were then compared with those of a
quantitative, rapid POCT performed simultaneously to measure the
fecal calprotectin levels (Fig.
7). Stool samples were available from 56 patients with UC and
from 24 patients with CD. Of note, the correlation between the
results of the two methods was high for the patients with UC and
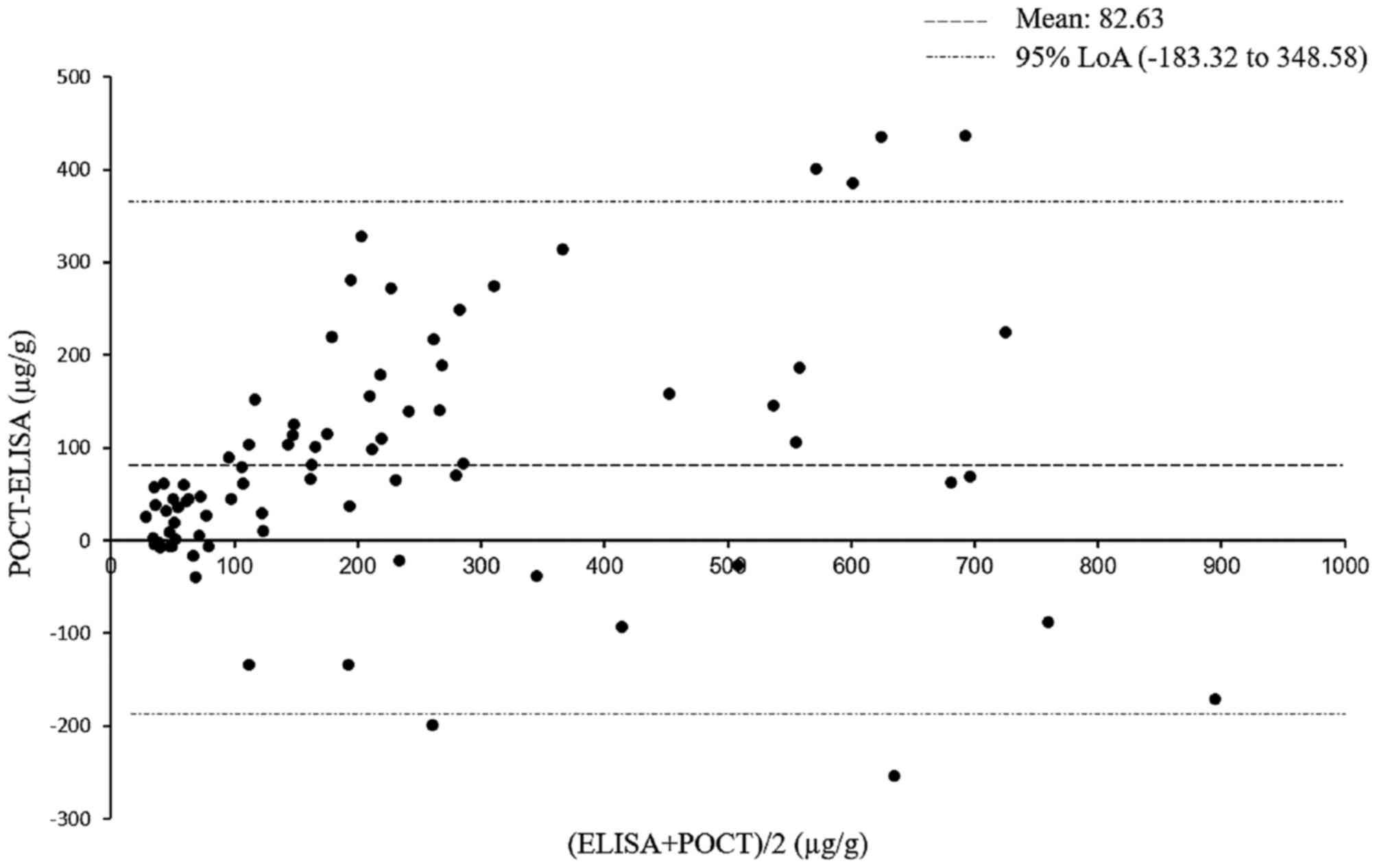
those with CD. The agreement between the two methods was asessed
using the Bland-Altman method (22). As only six of 80 paired data
(7.5%) were outside the limit of agreement, the measurements
obtained from the rapid POCT were considered to show a clinically
acceptable level of agreement with ELISA (Fig. 8).
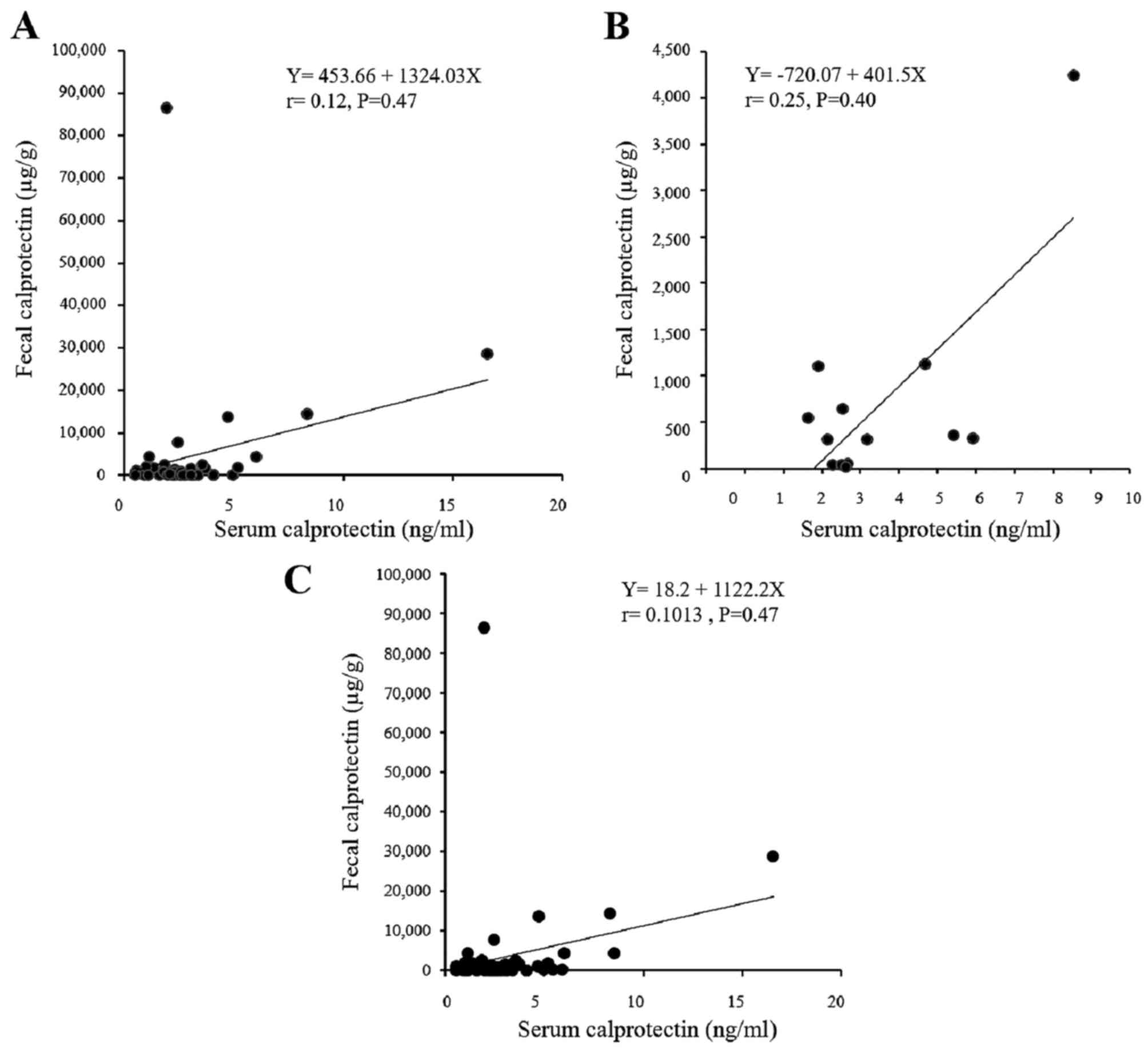
Association between serum and fecal
calprotectin
The correlation between the fecal and serum
calprotectin levels were also analyzed using an ELISA (Fig. 9). The samples were analyzed in 41
patients with UC and in 13 patients with CD. No statistical
correlation was observed between the serum and fecal calprotectin
levels for either the UC or CD groups of patients. Of note, there
was a mild but statistically significant correlation between the
serum calprotectin and CRP levels in the patients with UC, but not
in the patients with CD (Fig.
10).
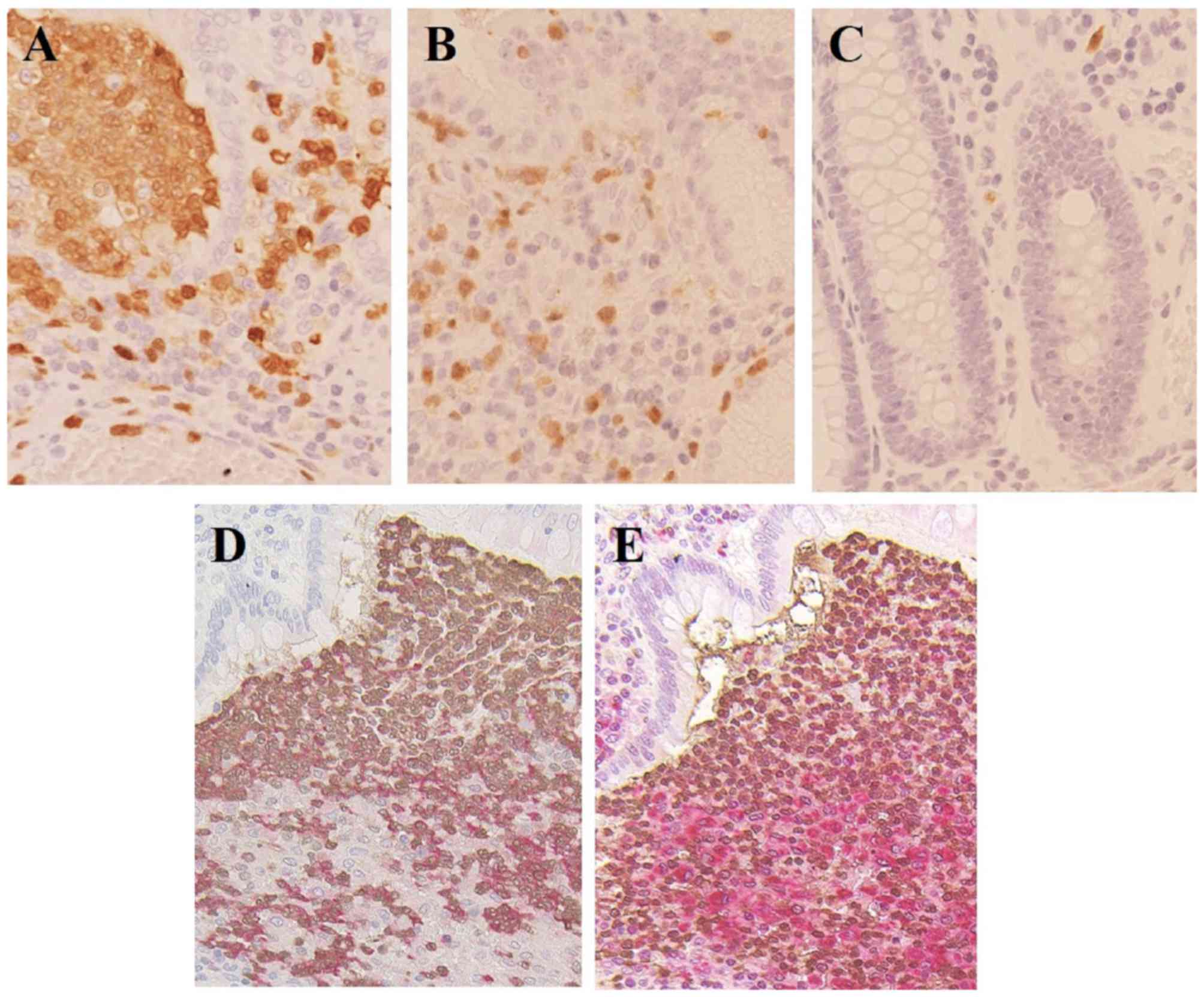
Immunocytochemistry
Immunohistochemical staining was performed using
colon tissue specimens from the patients with UC and CD, and
increased numbers of calprotectin-positive cells, including
inflammatory leukocytes, were found in the colonic mucosa, compared
with the tissues from the normal controls (Fig. 11A–C). In addition, to define the
types of leukocytes responsible for expressing calprotectin, double
immunohistochemistry for calprotectin and neutrophil
elastase-positive neutrophils (Fig.
11D) or CD68-positive macrophages (Fig. 11E) were performed in sequential
sections from the same sample of an UC specimen. The results showed
that the colonic mucosa specimens from the patients with UC and CD
had higher levels of neutrophil and monocyte calprotectin-positive
cells, compared with those observed in the normal control
specimens.
Discussion
Endoscopy is considered the gold standard for the
diagnosis and follow-up of patients with IBD. However, a less
invasive approach is now being sought for the management of these
patients. Fecal calprotectin is the most widely used marker for IBD
for evaluating intestinal inflammation and mucosal healing. The
present study focused on the detection of calprotectin in stool and
serum samples, and examined its immunohistochemical localization in
colon specimens.
In patients with UC, it was found that the fecal
calprotectin level increased significantly, and was closely
correlated with endoscopic and clinical disease activities and
laboratory parameters, particularly in patients with UC; these
results were consistent with previously published data (9,23–25). Although the data obtained in the
present study showed close correlations between the fecal
calprotectin level and laboratory parameters, the fecal
calprotectin level has been consistently shown to be superior to
laboratory parameters, due to its high specificity for the
assessment of intestinal inflammation. In patients with CD, the
fecal calprotectin level also increased, however, its correlation
with endoscopic and clinical disease activities and laboratory
markers was relatively weak, compared with the correlations
observed for patients with UC, although the number of patients with
CD was relatively small. This difference may be explained, in part,
by the characteristic features of CD, including the focality of the
disease and its uneven, deeper tissue involvement within the
intestinal wall. This leads to an unpredictable level of release
from the mucosa, and possibly weakens the correlation between the
fecal calprotectin level and disease activity. At present, the use
of fecal calprotectin levels for estimating CD activity is
controversial (26–29). Although a correlation between
elevated calprotectin levels and colonic inflammation has been
confirmed repeatedly, correlation for the small bowel is less well
established. The endoscopic assessment of all segments, including
the small bowel and colon, may be required for assessing the
disease activity in patients with CD. Taken together, the results
of the present study suggested that the fecal calprotectin level is
a reliable marker for assessing IBD disease activity, however, it
may be more useful for evaluating disease activity in patients with
UC than in those with CD.
Using the same samples, the present study
concurrently measured the fecal Hb levels using an immunoassay as
an alternate fecal marker for intestinal inflammation. Evidence on
the correlation between fecal Hb levels and disease activity
remains limited (30). The
present study found that the level of fecal Hb was close to that of
fecal calprotectin, particularly in patients with UC, which was in
agreement with the results of a previous study by Nakarai et
al, showing that fecal Hb and calprotectin levels were equally
capable of predicting mucosal healing (19). The most important difference
between fecal Hb and calprotectin is that fecal Hb is a marker of
intestinal bleeding, whereas calprotectin is specific to activated
mucosal leukocytes, which are critical in the underlying
pathophysiology of IBD. Although further investigations are
required, the performance of fecal Hb was comparable to that of
fecal calprotectin in patients with UC, suggesting its
applicability as a novel surrogate marker of inflammation. In
contrast to the results for the patients with UC, the fecal Hb
level appeared to be less sensitive in the patients with CD, which
was consistent with the findings of a previous study (21). Fecal Hb tests were originally
developed as a marker for colorectal cancer, rather than small
bowel disease, and the use of fecal Hb as a marker for CD requires
further investigation. At present, the reason for the positive
correlation between fecal Hb and calprotectin concentrations
observed in the present study remains speculative. However, the
increased permeability observed in chronic mucosal inflammatory
conditions (31) may be partly
responsible for this association.
One of the situations in which the measurement of
fecal calprotectin is likely to be useful is in evaluating the
response to treatment. However, few follow-up studies have examined
fecal calprotectin levels in patients with IBD, and the serial
changes in fecal calprotectin levels in the same individual remains
to be fully elucidated. Although the present study was largely
cross-sectional, follow-up examinations were performed for a small
number of patients, and a change in calprotectin paralleling that
of disease activity was observed throughout the course of the
disease, further supporting the potential use of this marker for
monitoring disease activity. A large-scale, longitudinal study,
examining patients with IBD, is required.
The present study also examined the reliability of
POCT measurements, compared with those obtained using ELISA, which
is the gold standard test for measuring fecal calprotectin levels.
ELISA is highly accurate, however, it must be performed in a
laboratory and requires the collection of multiple samples; POCT is
an easy and rapid technique, which also provides quantitative
results but is more widely available and can be performed for
individual samples (12,13). The data obtained in the present
study showed a high level of correlation between the two
techniques, suggesting that POCT may be used as a reliable
alternative to ELISA for evaluating disease activity and for the
follow-up of patients with IBD. Further comprehensive
investigations are required to compare the abilities of these
parameters to accurately reflect disease activity in patients with
IBD.
High serum calprotectin levels have been described
in patients with systemic lupus erythematosus, anti-neutrophil
cytoplasmic antibody (ANCA)-associated vasculitis, rheumatoid
arthritis, juvenile idiopathic arthritis, Kawasaki disease and
renal allograft rejection (32–38). At present, few studies have been
performed on serum calprotectin in IBD, and the value of this
parameter for estimating disease activity is controversial
(39,40). The present study is the first, to
the best of our knowledge, to measure serum and fecal calprotectin
levels concurrently. It was found that serum calprotectin levels
were correlated with the serum CRP levels, particularly in patients
with UC, but that the serum calprotectin levels were not correlated
with the fecal calprotectin levels. In ANCA-associated vasculitis,
patients have been shown to exhibit higher cell-surface
calprotectin expression levels in circulating neutrophils and
monocytes, as assessed using flow cytometry, in association with
increases in serum calprotectin levels (34). Accordingly, serum calprotectin is
considered to be derived predominantly from circulating leukocytes,
and not diseased intestine; therefore, the serum calprotectin level
may actually reflect systemic inflammation, rather than intestinal
inflammation. The clinical application of serum calprotectin
warrants further validation in large cohorts.
In the context of IBD, calprotectin may be released
at sites of intestinal inflammation, leading to an increase in the
fecal calprotectin level. To confirm the cellular source in the
intestine, immunohistochemistry was performed in the present study.
It was found that calprotectin was expressed in neutrophils and
monocytes/macrophages, and the excess calprotectin released from
these cell types in response to inflammatory stimuli may spill into
the gut lumen (6). In addition to
being an inflammatory marker, calprotectin exerts several effects,
suggesting that it may have a more direct pathogenic role in IBD.
Calprotectin-induced stimulation of monocytes/macrophages, acting
through Toll-like recepror-4, activates nuclear factor-κB and other
transcription factors, leading to the increased production of
metalloproteinases (41) and
proinflammatory cytokines (42),
in addition to stimulating interleukin-17-producing T cells
(43), which have been implicated
in IBD. Of note, a previous study using calprotectin-knockout mice
showed that calprotectin was critical for the development of
glomerulonephritis and that it promoted inflammatory
leukocyte-renal cell interactions (44). Taken together, these findings
suggest that calprotectin, which is derived predominantly from
activated neutrophils and monocytes/macrophages, may contribute to
the activation of innate immunity and be involved in the
pathophysiology of IBD.
The present study had several limitations. Firstly,
although a prospective design was used, the study was performed at
a single center and involved a limited number of patients,
particularly those with CD. Secondly, the endoscopic disease
activity was evaluated for the region with the most severe
inflammation, whereas the disease extent and location were not
considered. In patients with CD, disease activity may exist in the
upper gastrointestinal tract or in other segments of the small
bowel, which cannot be reached when performing an endoscopy. In
addition, the calprotectin concentration may depend on the sampling
position within the stool, although the distribution of
calprotectin within a single stool sample appears to be
homogeneous. Finally, although clinical studies, including the
present study, have demonstrated that fecal calprotectin is
correlated with endoscopic disease activity, whether the
normalization of fecal calprotectin levels reflects mucosal healing
in IBD, which is an important treatment target, remains a topic of
debate. No confirmed definition of mucosal healing exists at
present. In future investigations, histologic findings require
consideration in addition to endoscopic findings to enable the
precise evaluation of disease activity.
Taken together, the data obtained in the present
study suggested that fecal calprotectin, which is derived
predominantly from neutrophils and monocytes/macrophages, is a
reliable marker for the noninvasive monitoring of disease activity,
particularly in patients with UC. Fecal calprotectin for POCT
offers potential as a rapid and simple measurement in clinical
settings.
Acknowledgments
This study was supported partly by a Grant-in-Aid
from the Ministry of Science and Education (grant no. 25460964),
and by the Health and Labour Sciences Research Grants for Research
on Intractable Diseases from the Ministry of Health, Labour and
Welfare of Japan.
Abbreviations:
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
POCT
|
point-of-care test
|
|
IBDs
|
inflammatory bowel diseases
|
|
UC
|
ulcerative colitis
|
|
CD
|
Crohn's disease
|
References
|
1
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Invest.
117:514–521. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pineton de Chambrun G, Peyrin-Biroulet L,
Lémann M and Colombel JF: Clinical implications of mucosal healing
for the management of IBD. Nat Rev Gastroenterol Hepatol. 7:15–29.
2010. View Article : Google Scholar
|
|
3
|
Baert F, Moortgat L, Van Assche G,
Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts
P, Vermeire S, et al: Mucosal healing predicts sustained clinical
remission in patients with early-stage Crohn's disease.
Gastroenterology. 138:463–468. PubMed/NCBI
|
|
4
|
Frøslie KF, Jahnsen J, Moum BA, Vatn MH,
Group I and IBSEN Group: Mucosal healing in inflammatory bowel
disease: Results from a Norwegian population-based cohort.
Gastroenterology. 133:412–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sands BE: Biomarkers of inflammation in
inflammatory bowel disease. Gastroenterology. 149:1275–1285.e1272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foell D, Wittkowski H and Roth J:
Monitoring disease activity by stool analyses: From occult blood to
molecular markers of intestinal inflammation and damage. Gut.
58:859–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kopylov U, Rosenfeld G, Bressler B and
Seidman E: Clinical utility of fecal biomarkers for the diagnosis
and management of inflammatory bowel disease. Inflamm Bowel Dis.
20:742–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehmann FS, Burri E and Beglinger C: The
role and utility of faecal markers in inflammatory bowel disease.
Therap Adv Gastroenterol. 8:23–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schoepfer AM, Beglinger C, Straumann A,
Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet
V and Vavricka SR: Fecal calprotectin more accurately reflects
endoscopic activity of ulcerative colitis than the Lichtiger Index,
C-reactive protein, platelets, hemoglobin, and blood leukocytes.
Inflamm Bowel Dis. 19:332–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johne B, Fagerhol MK, Lyberg T, Prydz H,
Brandtzaeg P, Naess-Andresen CF and Dale I: Functional and clinical
aspects of the myelomonocyte protein calprotectin. Mol Pathol.
50:113–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinbakk M, Naess-Andresen CF, Lingaas E,
Dale I, Brandtzaeg P and Fagerhol MK: Antimicrobial actions of
calcium binding leucocyte L1 protein, calprotectin. Lancet.
336:763–765. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coorevits L, Baert FJ and Vanpoucke HJ:
Faecal calprotectin: Comparative study of the Quantum Blue rapid
test and an established ELISA method. Clin Chem Lab Med.
51:825–831. 2013. View Article : Google Scholar
|
|
13
|
Rogler G, Aldeguer X, Kruis W, Lasson A,
Mittmann U, Nally K, Peyrin-Biroulet L, Schoepfer A, Vatn M,
Vavricka S, et al: Concept for a rapid point-of-care calprotectin
diagnostic test for diagnosis and disease activity monitoring in
patients with inflammatory bowel disease: Expert clinical opinion.
J Crohn's Colitis. 7:670–677. 2013. View Article : Google Scholar
|
|
14
|
Rachmilewitz D: Coated mesalazine
(5-aminosalicylic acid) versus sulphasalazine in the treatment of
active ulcerative colitis: A randomised trial. BMJ. 298:82–86.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schroeder KW, Tremaine WJ and Ilstrup DM:
Coated oral 5-aminosalicylic acid therapy for mildly to moderately
active ulcerative colitis. A randomized study. N Engl J Med.
317:1625–1629. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daperno M, D'Haens G, Van Assche G, Baert
F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A and Gevers A:
Development and validation of a new, simplified endoscopic activity
score for Crohn's disease: The SES-CD. Gastrointest Endosc.
60:505–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Best WR, Becktel JM and Singleton JW:
Rederived values of the eight coefficients of the Crohn's Disease
Activity Index (CDAI). Gastroenterology. 77:843–846.
1979.PubMed/NCBI
|
|
18
|
Oyaert M, Trouvé C, Baert F, De Smet D,
Langlois M and Vanpoucke H: Comparison of two immunoassays for
measurement of faecal calprotectin in detection of inflammatory
bowel disease: (pre)-analytical and diagnostic performance
characteristics. Clin Chem Lab Med. 52:391–397. 2014. View Article : Google Scholar
|
|
19
|
Nakarai A, Kato J, Hiraoka S, Kuriyama M,
Akita M, Hirakawa T, Okada H and Yamamoto K: Evaluation of mucosal
healing of ulcerative colitis by a quantitative fecal
immunochemical test. Am J Gastroenterol. 108:83–89. 2013.
View Article : Google Scholar
|
|
20
|
Takashima S, Kato J, Hiraoka S, Nakarai A,
Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H, et
al: Evaluation of mucosal healing in ulcerative colitis by fecal
calprotectin vs. fecal immunochemical test. Am J Gastroenterol.
110:873–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inokuchi T, Kato J, Hiraoka S, Takashima
S, Nakarai A, Takei D, Sugihara Y, Takahara M, Kawano S, Harada K,
et al: Fecal immunochemical test versus fecal calprotectin for
prediction of mucosal healing in Crohn's disease. Inflamm Bowel
Dis. 22:1078–1085. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bland JM and Altman DG: Statistical
methods for assessing agreement between two methods of clinical
measurement. Lancet. 1:307–310. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langhorst J, Elsenbruch S, Koelzer J,
Rueffer A, Michalsen A and Dobos GJ: Noninvasive markers in the
assessment of intestinal inflammation in inflammatory bowel
diseases: Performance of fecal lactoferrin, calprotectin, and
PMN-elastase, CRP, and clinical indices. Am J Gastroenterol.
103:162–169. 2008. View Article : Google Scholar
|
|
24
|
Hanai H, Takeuchi K, Iida T, Kashiwagi N,
Saniabadi AR, Matsushita I, Sato Y, Kasuga N and Nakamura T:
Relationship between fecal calprotectin, intestinal inflammation,
and peripheral blood neutrophils in patients with active ulcerative
colitis. Dig Dis Sci. 49:1438–1443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawashima K, Ishihara S, Yuki T, Fukuba N,
Oshima N, Kazumori H, Sonoyama H, Yamashita N, Tada Y, Kusunoki R,
et al: Fecal calprotectin level correlated with both endoscopic
severity and disease extent in ulcerative colitis. BMC
Gastroenterol. 16:472016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ricanek P, Brackmann S, Perminow G,
Lyckander LG, Sponheim J, Holme O, Høie O and Rydning A: Evaluation
of disease activity in IBD at the time of diagnosis by the use of
clinical, biochemical, and fecal markers. Scand J Gastroenterol.
46:1081–1091. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones J, Loftus EV Jr, Panaccione R, Chen
LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG,
Harmsen SW, et al: Relationships between disease activity and serum
and fecal biomarkers in patients with Crohn's disease. Clin
Gastroenterol Hepatol. 6:1218–1224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ,
Deng FH, Nie B and Jiang B: Meta-analysis: Fecal calprotectin for
assessment of inflammatory bowel disease activity. Inflamm Bowel
Dis. 20:1407–1415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sipponen T, Kärkkäinen P, Savilahti E,
Kolho KL, Nuutinen H, Turunen U and Färkkilä M: Correlation of
faecal calprotectin and lactoferrin with an endoscopic score for
Crohn's disease and histological findings. Aliment Pharmacol Ther.
28:1221–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato J, Hiraoka S, Nakarai A, Takashima S,
Inokuchi T and Ichinose M: Fecal immunochemical test as a biomarker
for inflammatory bowel diseases: Can it rival fecal calprotectin.
Intest Res. 14:5–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmad R, Sorrell MF, Batra SK, Dhawan P
and Singh AB: Gut permeability and mucosal inflammation: bad, good
or context dependent. Mucosal Immunol. 10:307–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haga HJ, Brun JG, Berntzen HB, Cervera R,
Khamashta M and Hughes GR: Calprotectin in patients with systemic
lupus erythematosus: Relation to clinical and laboratory parameters
of disease activity. Lupus. 2:47–50. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soyfoo MS, Roth J, Vogl T, Pochet R and
Decaux G: Phagocyte-specific S100A8/A9 protein levels during
disease exacerbations and infections in systemic lupus
erythematosus. J Rheumatol. 36:2190–2194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pepper RJ, Hamour S, Chavele KM, Todd SK,
Rasmussen N, Flint S, Lyons PA, Smith KG, Pusey CD, Cook HT, et al:
Leukocyte and serum S100A8/S100A9 expression reflects disease
activity in ANCA-associated vasculitis and glomerulonephritis.
Kidney Int. 83:1150–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Rycke L, Baeten D, Foell D, Kruithof E,
Veys EM, Roth J and De Keyser F: Differential expression and
response to anti-TNFalpha treatment of infiltrating versus resident
tissue macrophage subsets in autoimmune arthritis. J Pathol.
206:17–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frosch M, Ahlmann M, Vogl T, Wittkowski H,
Wulffraat N, Foell D and Roth J: The myeloid-related proteins 8 and
14 complex, a novel ligand of toll-like receptor 4, and
interleukin-1beta form a positive feedback mechanism in
systemic-onset juvenile idiopathic arthritis. Arthritis Rheum.
60:883–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirono K, Foell D, Xing Y, Miyagawa-Tomita
S, Ye F, Ahlmann M, Vogl T, Futatani T, Rui C, Yu X, et al:
Expression of myeloid-related protein-8 and -14 in patients with
acute Kawasaki disease. J Am Coll Cardiol. 48:1257–1264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burkhardt K, Radespiel-Tröger M, Rupprecht
HD, Goppelt-Struebe M, Riess R, Renders L, Hauser IA and Kunzendorf
U: An increase in myeloid-related protein serum levels precedes
acute renal allograft rejection. J Am Soc Nephrol. 12:1947–1957.
2001.PubMed/NCBI
|
|
39
|
Leach ST, Yang Z, Messina I, Song C, Geczy
CL, Cunningham AM and Day AS: Serum and mucosal S100 proteins,
calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis
in children with inflammatory bowel disease. Scand J Gastroenterol.
42:1321–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meuwis MA, Vernier-Massouille G, Grimaud
JC, Bouhnik Y, Laharie D, Piver E, Seidel L, Colombel JF and Louis
E: GETAID (Groupe d'Étude Thérapeutique Des Affections
Inflammatoires Digestives): Serum calprotectin as a biomarker for
Crohn's disease. J Crohn's Colitis. 7:e678–e683. 2013. View Article : Google Scholar
|
|
41
|
van Lent PL, Grevers LC, Schelbergen R,
Blom A, Geurts J, Sloetjes A, Vogl T, Roth J and van den Berg WB:
S100A8 causes a shift toward expression of activatory Fcγ receptors
on macrophages via toll-like receptor 4 and regulates Fcγ receptor
expression in synovium during chronic experimental arthritis.
Arthritis Rheum. 62:3353–3364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sunahori K, Yamamura M, Yamana J, Takasugi
K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S and Makino
H: The S100A8/A9 heterodimer amplifies proinflammatory cytokine
production by macrophages via activation of nuclear factor kappa B
and p38 mitogen-activated protein kinase in rheumatoid arthritis.
Arthritis Res Ther. 8:R692006. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Loser K, Vogl T, Voskort M, Lueken A,
Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, et al:
The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the
development of autoreactive CD8+ T cells. Nat Med.
16:713–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pepper RJ, Wang HH, Rajakaruna GK,
Papakrivopoulou E, Vogl T, Pusey CD, Cook HT and Salama AD:
S100A8/A9 (calprotectin) is critical for development of
glomerulonephritis and promotes inflammatory leukocyte-renal cell
interactions. Am J Pathol. 185:1264–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|