Introduction
Cartilages are avascular, tough, flexible, fibrous
connective tissues that have multiple roles. Transient cartilages
are a major component of embryonic skeletons and offer a model for
bone formation, while permanent cartilages exhibit various
biomechanical characteristics, including elastic cartilage, hyaline
cartilage and fibrocartilage (1).
The present study focused on articular cartilage, a sort of hyaline
cartilage, which serves crucial roles in supporting and allotting
forces that emerge during joint loading, and supplying a
frictionless lubricating surface to protect the joint from wear or
degradation (2). On account of
the deficiency of nerves, blood vessels, and the inherently poor
differentiating ability of chondrocytes, damaged articular
cartilage has an extremely limited capacity to self-heal (3,4).
Finally, these cartilage injuries result in premature joint
degeneration and posttraumatic arthritis (5). Despite the development of numerous
methods for the restoration of cartilage lesions, some shortcomings
remain (6). Therefore, the search
for novel therapies with low cost, stable activity and security for
cartilage injuries is required.
Herb Epimedium (HEP), a traditional Chinese
medicine, has been widely used for arthritis treatment in China,
Korea and Japan (7,8). Icariin is the major effective
constituent of HEP (9,10). Research has indicated that icariin
promoted osteoblast differentiation through inducing BMP-2, BMP-4
and SMAD4 expression (11).
Additionally, in bone tissue engineering, a study by Zhao et
al (12) demonstrated
efficient osteoinductivity of icariin that was able to enhance
in vivo bone formation. In a murine model of dexamethasone
(DXM)-induced osteoporosis, icariin exerted protective effects
against bone deteriorations and promoted bone remodeling, with
significant decreases in bone resorption markers C-terminal
telopeptide of type II collagen (CTX-II) and tartrate-resistant
acid phosphatase (TRAP)-5b being observed (13). Icariin has been demonstrated to be
a safe and powerful chondrocyte anabolic agent to stimulate
chondrocyte proliferation and attenuate extracellular matrix (ECM)
degradation (14,15). ECM, in response to the properties
of cartilage, is synthesized by chondrocytes (16), thus icariin may be a potential
catalyst for chondrogenesis in cartilage tissue engineering. In the
present study, the effects of icariin were assessed in a rat model
of DXM-induced cartilage injury.
Materials and methods
Animal experiments and drug
administration
A total of 90 6-week-old male Wistar rats, weighing
160–230 g, were obtained from the Centre of Laboratory Animals of
Harbin Medical University (Harbin, China). The rats were given free
access to food and water and were caged individually in a
controlled temperature (21–22°C) in 50–60% humidity, with an
artificial light cycle (12-h light/dark). All rats were acclimated
for 5 days and randomized into the following three groups
(n=10/group): vehicle (control), DXM, and icariin (DXM group
treated with icariin). The vehicle group received normal feed for
12 weeks; the DXM group were injected intramuscularly with 5 mg/kg
body weight DXM (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
three times a week for 12 weeks; rats in the icariin group were
dosed orally for 12 weeks with icariin (100 mg/kg/day;
Sigma-Aldrich; Merck KGaA) combined with DXM for 12 weeks. The
animal protocol was approved by the Committee on the Ethics of
Animal Experiments of Harbin Medical University.
Cell culture
Articular chondrocytes were isolated from the knee
joints of rats in the vehicle group as previously described
(17), with some alterations.
Articular cartilage tissues were cut into small pieces (<1
mm3) and digested with 0.2% trypsin and 0.2% type II
collagenase for 30 min and 2 h, respectively. The released cells
were maintained in Dulbecco's modified Eagle's medium/F12
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA), 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C in a humidified atmosphere of CO2 in air. When the
cells reached ~90% confluence, they were treated with various
concentrations of DXM (0, 10, 50 and 100 µM) for 48 h at
37°C. For miR-206 inhibitor treatment, cells were transfected with
anti-miR-206 inhibitor (200 nM; Riboxx GmbH, Radebeul, Germany)
using GeneCellin transfection reagent (BioCellChallenge, Toulon,
France), according to manufacturer's instructions. A total of 48 h
after transfection, cells were harvested and used for further
experiments. The sequence of the miR-206 inhibitor was
5′-CCACACACUUCCUUACAUUCCA-3′.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously reported
(18), with some changes.
Briefly, the cartilage tissues were crushed under liquid nitrogen
conditions and total RNA extraction was performed with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to manufacturer's instructions. Samples (1
µg RNA) were reverse-transcribed using a Reverse
Transcription System for real-time PCR (Takara Biotechnology Co.,
Ltd., Dalian, China), according to the manufacturer's instructions.
Synthesized cDNA was used in qPCR experiments using SsoFast™
EvaGreen Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
qPCR was performed with the following primers: alkaline phosphatase
(ALP) sense, 5′-GCCCTCTCCAAGACATATA-3′ and antisense,
5′-CCATGATCACGTCGATATCC-3′; TRAP sense, 5′-AGATCTCCAAGCGCTGGAAC-3′
and antisense, 5′-AGGTAGCCGTTGGGGACCTT-3′; osteocalcin sense,
5′-ATGAGAGCCCTCACACTCCTC-3′ and antisense,
5′-CTAGACCGGGCCGTAGAAGCG-3′; cathepsin K sense,
5′-GGGAGACATGACCAGCGAAG-3′ and antisense,
5′-CTGAAAGCCCAACAGGAACC-3′; collagen type I (Col-1) sense,
5′-TCCTGCCGATGTCGCTATC-3′ and antisense,
5′-CCATGTAGGCTACGCTGTTCTTG-3′; matrix metalloproteinase (MMP)-13
sense, 5′-CCCTGGAGCCCTGATGTTT-3′ and antisense,
5′-CTCTGGTGTTTTGGGGTGCT-3′; transforming growth factor (TGF)-β1
sense, 5′-CCAAGGAGACGGAATACAGG-3′ and antisense,
5′-GTGTTGGTTGTAGAGGGCAAG-3′; miR-612 sense,
5′-CAGGGCTTCTGAGCTCCTTA-3′ and antisense,
5′-TGAGAGTCCTGTCCTGGCTG-3′; miR-206 sense,
5′-GATTCGCCAAAGGAAATAGC-3′ and antisense,
5′-GTTACAAGGTCATCCAAGAC-3′; miR-28-5p sense,
5′-GTGCACTGTCACGGGTTTTC-3′ and antisense, 5′-CCTCTGCAGCCTGGTGAC-3′;
miR-714 sense, 5′-CTGCAAGGGTGGAGGTGTAG-3′ and antisense,
5′-AGGCAGTGGTCTAAACTCGC-3′; miR-7a sense,
5′-TGTTGGCCTAGTTCTGTGTGG-3′ and antisense,
5′-GGCAGACTGTGATTTGTTGTCG-3′; miR-365 sense,
5′-AAATGAGGGACTTTCAGGGGC-3′ and antisense,
5′-AACAATAAGGATTTTTAGGGGCATT-3′; and GAPDH sense, 5′-GTCGGTGTGAAC
GGATTTG-3′ and antisense, 5′-CTTGCGTGGGTAGAGTCAT-3′. qPCR
amplification was performed with an initial denaturation at 95°C
for 5 min, followed by 27 cycles of 95°C for 1 min, 65°C for 1 min
and 72°C for 1 min, with a final extension step of 72°C for 10 min.
Results were analyzed with Opticon Monitor software 3.1 (Bio-Rad
Laboratories, Inc.). Specificity was determined by 1% agarose gel
electrophoretic analysis of the reaction products. GAPDH was used
as an internal standard. Data were analyzed using the
2−ΔΔCq method, as previously described (19).
Western blotting
Western blotting was performed as previously
reported (20). Briefly, proteins
were extracted by lysing cells in buffer (50 mM Tris pH 7.4, 150 mM
NaCl, 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 1
mM phenyl methylsulfonyl fluoride, 25 mg/ml leupeptin and 25 mg/ml
aprotinin). Protein concentration was determined using a
bicinchoninic acid protein assay (Bio-Rad Laboratories, Inc.).
Subsequently, proteins (20 µg) were separated by 10%
SDS-PAGE mini-gel and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA) for 60 min at 100 V.
Following incubation in blocking buffer (Tris-buffered saline
containing 150 mM NaCl, 50 nM Tris and 0.05% Tween-20; pH 7.5) for
1 h at room temperature, the membrane was hybridized in blocking
buffer with specific primary antibodies against cathepsin K
(sc-48353; 1:100), B-cell lymphoma 2 (Bcl-2; sc-56015; 1:50),
Bcl-2-associated X protein (Bax; sc-70407; 1:200), caspase-3
(sc-271759; 1:200), caspase-9 (sc-8355; 1:200) and β-actin
(sc-70319; 1:200) overnight at 4°C. Subsequently, the membrane was
incubated with secondary antibodies labeled with horseradish
peroxidase (sc-516102; 1:200) for 1 h at room temperature, followed
by detection with an enhanced chemiluminescence system (GE
Healthcare, Chicago, IL, USA). All antibodies were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). β-actin was used
as the protein loading control. Band intensities were quantified by
immunoblot densitometry using Image-Pro Plus 4.5 (Media
Cybernetics, Inc., Rockville, MD, USA).
Serum biochemical markers of cartilage
metabolism
Anesthetized rats were placed in a euthanasia
chamber. Directly after aspiration, blood was transferred to plain
tubes. Serum was centrifuged at 2,000 × g for 1 min at 4°C, and
stored at −80°C until analysis. Serum levels of CTX-II and
deoxypyridinoline (DPD) were measured using a rat ELISA assay
(60-2700; Immutopics, Inc., San Clemente, CA, USA), as previously
described (21).
Histomorphological analysis
Cartilage tissues were dissected along the axial
plane into pieces of 10×5×7 mm with a thin layer of subchondral
bone and were fixed in 4% formaldehyde for over 24 h at room
temperature. Following decalcification in 10% EDTA solution for
over 2 weeks, the samples were embedded in paraffin. The specimens
were cut into 4-µm sections and stained with safranin-O for
5 min at room temperature. To evaluate the volume of cartilage
formation, the area occupied by chondrocytes and cartilage matrix
stained with safranin-O was quantified using an image analysis
system (ImageJ version 1.43u; National Institutes of Health,
Bethesda, MD, USA).
Luciferase activity assay
The potential binding sites between cathepsin K and
miR-206 were predicted using TargetScan (targetscan.org/mamm_31/). A luciferase assay was used
to validate cathepsin K as a target of miR-206. Articular
chondrocytes (~7.5×104 cells/well) were seeded in
12-well plates, then transfected with the wild-type, mutant
cathepsin K 3′ untranslated region (3′UTR) or the vector alone
(co-transfected using pGL3-control) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), in accordance with
the manufacturer's instructions. After 48 h, cells were lysed, then
the firefly and Renilla luciferase activities were measured
using the dual luciferase reporter assay kit (Promega, Madison, WI,
USA) on a luminometer (Orion II Microplate Luminometer; Berthold
Detection Systems GmbH, Pforzheim, Germany). To check the
specificity of the miR-206 effect, chondrocytes were co-transfected
with wild-type cathepsin K 3′UTR or miR-206 before luciferase
activities were measured. Control cells were transfected with the
scramble sequence. Renilla reporter luciferase activity was
normalized to the firefly luciferase activity.
Cell viability and apoptosis assays
Cell viability was assessed by LIVE/DEAD assay as
previously reported (22).
Briefly, cells were stained using a LIVE/DEAD stain kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. This kit contains two fluorescent dyes, calcein-AM to
stain living cells green and ethidium homodimer-1 (Ethd-1) to stain
dead cells red. Following staining, samples were observed through
an epifluorescent microscope (Carl Zeiss AG, Oberkochen, Germany)
at a magnification of ×200. For each well, at least five different
fields were examined, and a minimum of 1,000 cells were counted to
determine the fraction of calcein-AM-positive cells vs.
Ethd-1-positive cells.
In addition, to evaluate apoptotic activity of
chondrocytes, terminal deoxynucleotidyl-transferase-mediated dUTP
nick end labelling (TUNEL) staining was performed, as previously
described (23). Frozen cartilage
sections were fixed with 4% methanol-free formaldehyde solution in
PBS for 10 min at room temperature, then washed with PBS three
times. The DNA fragments were labeled with fluorescein-12-dUTP in
terminal deoxynucleotidyl transferase incubation buffer (Promega)
in a humidified chamber (37°C for 60 min) to avoid exposure to
light. The reactions were stopped by transferring the slides to SSC
buffer (0.3 M NaCl, 0.03 M sodium citrate, pH 7.0) for 15 min and
washing with PBS to remove unincorporated fluorescein-12-dUTP. The
slides were then counterstained with 1 μg/ml
4′,6-diamidino-2-phenylindole (Vector Laboratories, Inc.,
Burlingame, CA, USA) for 5 min at room temperature to provide a
blue background. The green fluorescence of apoptotic cells
(fluorescein-12-dUTP) may be detected using a fluorescence
microscope at 520 nm.
Caspase-3 activity
Caspase-3 activity was evaluated as previously
reported (24). Assays were
performed in 96-well microtiter plates by incubating 20 µg
cell lysate in 100 µl reaction buffer (1% NP-40, 20 mM
Tris-HCl pH 7.5, 137 mM NaCl and 10% glycerol) containing 5
µM caspase-3 substrate (Ac-DEVD-pNA; Sigma-Aldrich; Merck
KGaA). Lysates were incubated at 37°C for 2 h. Subsequently, the
absorbance was measured at 405 nm with a spectrophotometer.
Statistical analysis
All experiments were conducted at least three times.
Data were presented as the mean ± standard error of the mean.
Statistical analyses were performed using PRISM version 4.0
(GraphPad Software, Inc., La Jolla, CA, USA). The differences among
groups were analyzed by one-way analysis of variance, followed by
Tukey's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of icariin on biomarkers of bone
metabolism in experimental rats
The expression of biochemical markers of bone
turnover in the three experimental groups, including TRAP as a bone
resorption marker, and ALP and osteocalcin as bone formation
markers, were first determined using RT-qPCR. Compared with the
vehicle control group, the DXM group demonstrated significantly
increased expression of ALP and TRAP; however, icariin treatment
significantly eliminated the enhancing effect of DXM on the
expression of ALP and TRAP compared with the DXM group (Fig. 1A and B). In contrast, icariin
significantly stimulated osteocalcin expression following DXM
treatment compared with the levels observed in the DXM treatment
group (Fig. 1C). Additionally, it
was also determined that icariin abolished the promotion effect of
DXM on cathepsin K expression, an enzyme expressed by osteoclasts
in response to bone resorption, at the mRNA and protein expression
levels (Fig. 1D and E).
 | Figure 1mRNA expression of (A) ALP, (B) TRAP,
(C) osteocalcin and (D) cathepsin K in the three experimental
groups measured by reverse transcription-quantitative polymerase
chain reaction. Compared with the vehicle control group, the DXM
group demonstrated significantly increased expression levels of
ALP, TRAP and cathepsin K, and decreased osteocalcin expression.
However, icariin treatment significantly eliminated the enhancing
effect of DXM on the expression of ALP, TRAP and cathepsin K, and
stimulated osteocalcin expression. (E) Protein expression of
cathepsin K in the three experimental groups measured by western
blotting. Icariin significantly abolished the promotion effect of
DXM on cathepsin K. Values are expressed as the mean ± standard
error of the mean (n=10/group). ***P<0.001 vs.
vehicle group; ##P<0.01 and ###P<0.001
vs. DXM group. ALP, alkaline phosphatase; TRAP, tartrate-resistant
acid phosphatase; DXM, dexamethasone. |
Effects of icariin on cartilage
metabolism in experimental rats
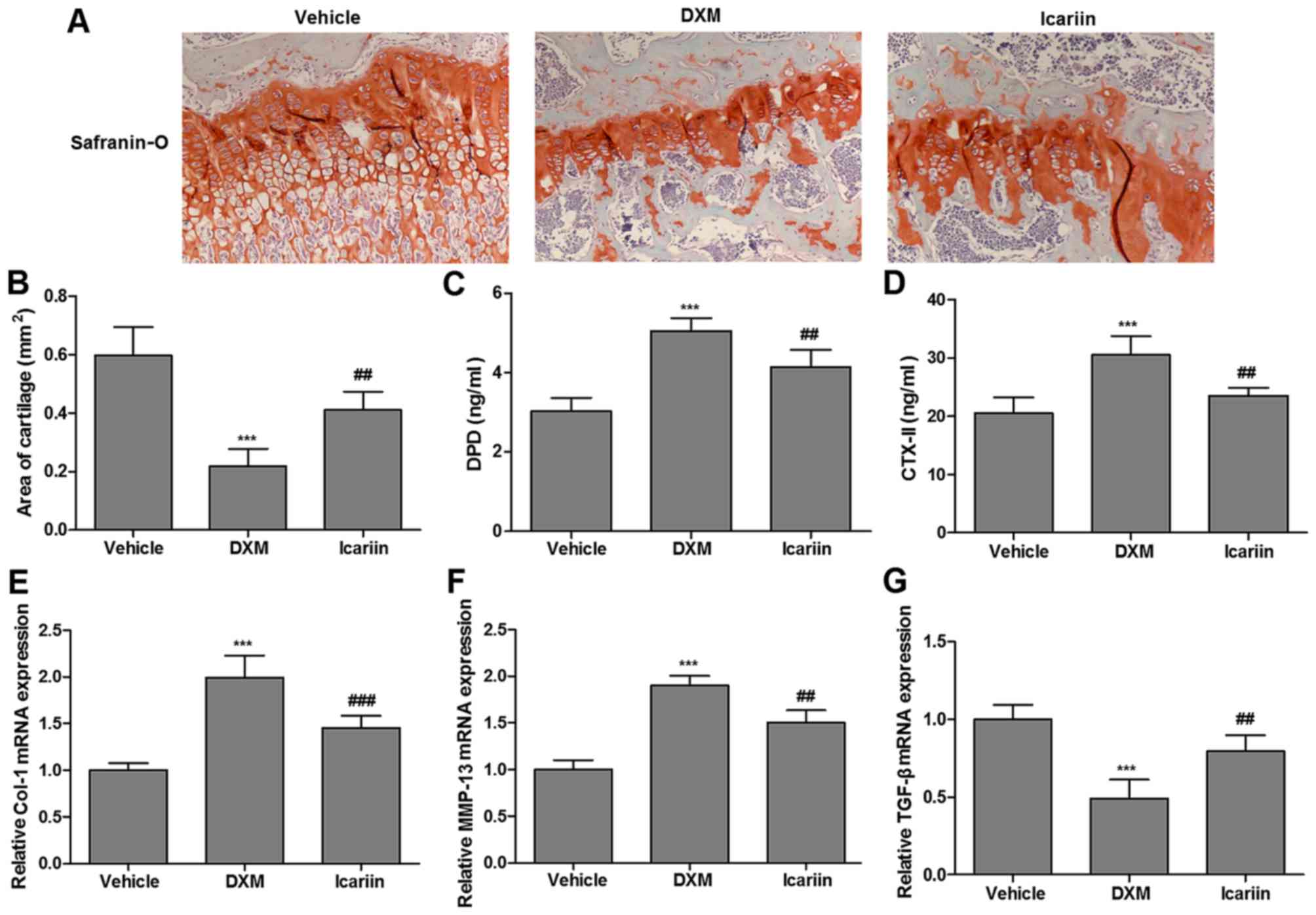
The present study assessed the effects of icariin on
articular cartilage in the three groups. As demonstrated in
Fig. 2A, a small volume of
cartilage formation was observed in the DXM group by safranin-O
staining, relative to the control group; however, continuous
administration of icariin enhanced the volume of cartilage
formation. Similarly, administration of icariin to DXM-treated rats
significantly elevated the reduced cartilage area (occupied by
chondrocytes and cartilage matrix dyed with safranin-O; Fig. 2B). More importantly, icariin
significantly reversed the DXM-induced increase in serum
concentrations of DPD and CTX-II, the commonly used serum markers
of cartilage metabolism, in experimental rats (Fig. 2C and D). Furthermore, the
expression of Col-1, MMP-13 and TGF-β in the three experimental
groups, which are cartilage metabolism-related genes, were
determined. Icariin significantly reduced the elevated expression
of Col-1 and MMP-13 induced by DXM, but promoted TGF-β expression
(Fig. 2E–G).
Validation of dysregulated microRNA
(miRNA) in articular cartilage of experimental rats
The results in Fig.
2 demonstrated the beneficial effect of icariin on cartilage
metabolism in DXM-treated rats, thus it was speculated whether
icariin affected articular cartilage of experimental rats at the
miRNA level. The relative expression of the miRNA in articular
cartilage of all experimental rats were presented as a heatmap
(Fig. 3A). The deeper the color,
the higher the expression. A total of five miRNA were further
determined to be significantly upregulated in the icariin group,
compared with the reduced levels in the DXM-treated group,
including miR-612, -206, -28-5p, -7a and -365 (Fig. 3B-G). In view of the role of
miR-206 as a key regulator during osteoblast differentiation
(25), miR-206 was selected as
the candidate for the following experiments.
 | Figure 3(A) Heatmap of all miR related to
articular cartilage in experimental rats. Red indicates high
relative expression and green indicates low relative expression.
mRNA expression of (B) miR-612, (C) miR-206, (D) miR-28-5p, (E)
miR-714, (F) miR-7a and (G) miR-365 in the three experimental
groups were determined by reverse transcription-quantitative
polymerase chain reaction. DXM significantly reduced miR-612,
miR-206, miR-28-5p, miR-7a, miR-714 and miR-365 expression;
however, icariin significantly rescued the expression of these miR,
except for the expression of miR-714. Values are expressed as the
mean ± standard error of the mean. ***P<0.001 vs.
vehicle group; #P<0.05 and ###P<0.001
vs. DXM group. miR, microRNA; DXM, dexamethasone. |
miR-206 targets cathepsin K
To determine whether miR-206 regulated cathepsin K
through the predicted binding sites in its 3′UTR (Fig. 4A), two luciferase constructs were
employed by incorporating wild-type or mutant 3′UTR of cathepsin K,
which expressed luciferase unless repressed by the incorporated
3′UTR. As demonstrated in Fig.
4B, co-transfection with the pMIR-REPORT construct containing
mutant cathepsin K 3′UTR and PLemiR-206 did not exhibit a
significant difference compared with the control; however,
co-transfection with the luciferase construct containing wild-type
cathepsin K 3′UTR and PLemiR-206 resulted in an ~70% decline in
luciferase activity compared with the control. As demonstrated in
Fig. 4C and D, compared with the
control group, miR-206 overexpression significantly decreased the
expression of cathepsin K; however, the inhibition of miR-206
elevated the level of cathepsin K, both at the mRNA and protein
expression levels.
 | Figure 4Luciferase activity assay and the
expression of Cstk in chondrocytes. (A) Schematic representation of
the putative miR-206 binding site in the Cstk 3′UTR in TargetScan.
(B) Luciferase activity assay of Cstk. Co-transfection with the
luciferase construct containing wild-type Cstk K 3′UTR and
PLemiR-206 resulted in a significant decline in luciferase activity
compared with the control. miR-206 overexpression significantly
decreased the expression of Cstk; however, inhibiting miR-206
elevated the level of Cstk, both at the (C) mRNA and (D) protein
levels. mRNA expression of Cstk was measured by reverse
transcription-quantitative polymerase chain reaction, and protein
expression was measured by western blotting. Values are expressed
as the mean ± standard error of the mean. *P<0.05 and
**P<0.01 vs. con or NC group;
&&&P<0.001 vs. scramble group. Cstk,
cathepsin K; UTR, untranslated region; Con, control; NC, normal
control; miR, microRNA; wild, wild-type; Mut, mutant. |
Effects of icariin on chondrocytes in
experimental rats and the implicated molecular mechanism
Finally, the present study assessed the effects of
icariin on chondrocytes in the presence of DXM at the indicated
concentrations (Fig. 5). As
demonstrated in Fig. 5A, the
addition of DXM (100 µM) to chondrocytes resulted in a
maximum reduction in cell viability compared with the control
cells; however, this effect was reversed significantly following
treatment with icariin (100 µM). As demonstrated in Fig. 5B and C, icariin (100 µM)
significantly suppressed DXM-induced elevation of chondrocyte
apoptosis, accompanied by an increase in the level of Bcl-2, and
decreases in the levels of Bax, caspase-3 and caspase-9 (Fig. 5D). RT-qPCR further indicated that
icariin (100 µM) abolished the maximal inhibition effect of
DXM (100 µM) on miR-206 expression in chondrocytes (Fig. 5E). Additionally, Fig. 5F and G demonstrated that the
dose-dependent addition of DXM significantly elevated the
expression of cathepsin K, which peaked at a dose of 100 µM.
This level was significantly decreased following icariin
stimulation (100 µM).
Discussion
In bone tissue engineering, the effect of icariin on
bone metabolism is of interest, including the stimulation of bone
formation and osteoblast differentiation (26), as well as the inhibition of bone
resorption and osteoclast differentiation (27). However, little is known about the
effect of icariin on cartilage tissue and how icariin acts on
cartilage tissue engineering, although icariin has been
demonstrated to stimulate chondrocyte proliferation and reduce ECM
degradation (15). The present
data provided available information about the effect of icariin in
rats with cartilage disease, as well as the implicated mechanism.
Changes in bone markers not only simply predict the response in
bone metabolism (28), but may
also be detected earlier than the alterations in bone mineral
density (29). The present study
first identified that the administration of icariin to DXM-treated
rats significantly decreased bone resorption (reduced expression of
TRAP and cathepsin K), as well as significantly increased the level
of bone formation (elevated expression of osteocalcin), which were
consistent with the results observed in mice with DXM-induced
osteoporosis (13), suggesting a
beneficial effect of icariin on bone generation.
There is increasing evidence supporting the
important role of icariin in cartilage tissue engineering. An in
vivo study of the effect of icariin on cartilage tissue
engineering revealed that icariin stimulated ECM secretion and the
expression of cartilage-related genes of chondrocytes, implying the
possibility of icariin as a promoter in cartilage tissue
engineering (30). A study by Sun
et al (31) reported that
icariin suppressed bone and cartilage deteriorations in mice with
collagen-induced arthritis, suggesting that icariin holds promise
as a treatment for patients with joint diseases. A study by Yuan
et al (32) reported that
integrating icariin into hydrogel scaffolds facilitated the
synthesis of cartilage matrix and improved the quality of newly
formed cartilage. Furthermore, cytokines, particularly TGF-β, have
been demonstrated to be strong regulators of chondrocyte
differentiation and to modulate ECM formation (33,34). A recent study has identified that
icariin stimulated cartilage repair through the activation of
hypoxia inducible factor-1α in chondrocytes (35). It should be noted that DPD and
CTX-II, commonly used serum markers of cartilage metabolism, are
important for cartilage turnover. In the present study, as
expected, it was observed that icariin markedly enhanced cartilage
formation (increased cartilage area) and improved cartilage
metabolism (reduced serum concentrations of DPD and CTX-II, reduced
expression of Col-1 and MMP-13, and elevated expression of TGF-β)
in DXM-treated rats.
More importantly, the present study determined that
miR-206 was significantly upregulated following continuous
administration of icariin to DXM-treated rats, and cathepsin K was
further validated as the target RNA of miR-206. miRNA are small
non-coding RNA that act as key post-transcriptional gene regulators
and are implicated in various biological processes, including
proliferation, development and disease occurrence (36–38). miR-206 has been demonstrated to be
a key regulator during osteoblast differentiation (25). Various studies have explored the
role of miRNA in cartilage development and diseases. A study by
Sumiyoshi et al (39)
discovered a novel role of miR-181a in cartilage metabolism. A
study by Mirzamohammadi et al (40) reported that overexpression of
hsa-miR-148a stimulated cartilage formation by inhibiting
hypertrophic differentiation and inducing type II collagen
production of osteoarthritis chondrocytes. Guérit et al
(41) reported that miR-29a was
greatly downregulated during chondrogenesis and that overexpression
of miR-29a observably inhibited chondrocyte-specific gene
expression during chondrogenic differentiation of mesenchymal stem
cells. The present study also revealed that the addition of icariin
(100 µM) to DXM-treated chondrocytes resulted in a
significant increase in cell viability, as well as a significant
reduction in cell apoptosis, which were extremely similar to the
findings in rabbit chondrocytes (42). Additionally, the activation of
miR-206 targeting to cathepsin K following the addition of icariin
(100 µM) to DXM-treated chondrocytes further suggested a
novel miR-206-dependent mechanism that may be responsible for the
chondroprotective efficacy of icariin in DXM-induced cartilage
injuries in rats.
In conclusion, the present results identified
icariin as a potential accelerator exerting protective effects
against cartilage degradation and promoting cartilage regeneration
in a rat model of DXM-induced cartilage injury. The present study
revealed that the activation of miR-206 targeting to cathepsin K,
at least partly, was involved in this mechanism.
Acknowledgments
The present study was supported by the Natural
Science Foundation for Young Scholars of Heilongjiang, China (grant
no. QC20160117).
References
|
1
|
Dang AC and Kuo AC: Cartilage biomechanics
and implications for treatment of cartilage injuries. Oper Tech
Orthop. 24:288–292. 2014. View Article : Google Scholar
|
|
2
|
Mow VC, Ratcliffe A and Poole AR:
Cartilage and diarthrodial joints as paradigms for hierarchical
materials and structures. Biomaterials. 13:67–97. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frenkel SR, Clancy RM, Ricci JL, Di Cesare
PE, Rediske JJ and Abramson SB: Effects of nitric oxide on
chondrocyte migration, adhesion, and cytoskeletal assembly.
Arthritis Rheum. 39:1905–1912. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein TJ, Rizzi SC, Reichert JC, Georgi N,
Malda J, Schuurman W, Crawford RW and Hutmacher DW: Strategies for
zonal cartilage repair using hydrogels. Macromol Biosci.
9:1049–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furman BD, Strand J, Hembree WC, Ward BD,
Guilak F and Olson SA: Joint degeneration following closed
intraarticular fracture in the mouse knee: a model of posttraumatic
arthritis. J Orthop Res. 25:578–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seyedin MS and Matava M: Cartilage repair
methods. US Patent. 20070128155 A1. Filed December 7, 2006; issued
June 7, 2007. https://www.google.ms/patents/US20070128155.
|
|
7
|
Yu S, Chen K, Li S and Zhang K: In vitro
and in vivo studies of the effect of a Chinese herb medicine on
osteoclastic bone resorption. Chin J Dent Res. 2:7–11.
1999.PubMed/NCBI
|
|
8
|
Zhang DW, Cheng Y, Wang NL, Zhang JC, Yang
MS and Yao XS: Effects of total flavonoids and flavonol glycosides
from Epimedium koreanum Nakai on the proliferation and
differentiation of primary osteoblasts. Phytomedicine. 15:55–61.
2008. View Article : Google Scholar
|
|
9
|
Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY,
Yao XS, Leung PC and Wong MS: The osteoprotective effect of Herba
epimedii (HEP) extract in vivo and in vitro. Evid Based Complement
Alternat Med. 2:353–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian G, Zhang X, Lu L, Wu X, Li S and Meng
J: Regulation of Cbfa1 expression by total flavonoids of Herba
epimedii. Endocr J. 53:87–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010. View Article : Google Scholar
|
|
12
|
Zhao J, Ohba S, Komiyama Y, Shinkai M,
Chung UI and Nagamune T: Icariin: a potential osteoinductive
compound for bone tissue engineering. Tissue Eng Part A.
16:233–243. 2010. View Article : Google Scholar
|
|
13
|
Zhang J, Song J and Shao J: Icariin
attenuates glucocorticoid-induced bone deteriorations, hypocalcemia
and hypercalciuria in mice. Int J Clin Exp Med. 8:7306–7314.
2015.PubMed/NCBI
|
|
14
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MH, Sun JS, Tsai SW, Sheu SY and Chen
MH: Icariin protects murine chondrocytes from
lipopolysaccharide-induced inflammatory responses and extracellular
matrix degradation. Nutr Res. 30:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi WN and Scully SP: Type II collagen
modulates the composition of extracellular matrix synthesized by
articular chondrocytes. J Orthop Res. 21:282–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsubara J, Yamada Y, Nakajima TE, Kato
K, Hamaguchi T, Shirao K, Shimada Y and Shimoda T: Clinical
significance of insulin-like growth factor type 1 receptor and
epidermal growth factor receptor in patients with advanced gastric
cancer. Oncology. 74:76–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Kim JE, Lee MH, Nam DH, Song HK, Kang YS,
Lee JE, Kim HW, Cha JJ, Hyun YY, Han SY, et al: Celastrol, an NF-κB
inhibitor, improves insulin resistance and attenuates renal injury
in db/db mice. PLoS One. 8:e62068. 2013. View Article : Google Scholar
|
|
21
|
DeLaurier A, Jackson B, Pfeiffer D, Ingham
K, Horton MA and Price JS: A comparison of methods for measuring
serum and urinary markers of bone metabolism in cats. Res Vet Sci.
77:29–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tu Y, Xue H, Francis W, Davies AP,
Pallister I, Kanamarlapudi V and Xia Z: Lactoferrin inhibits
dexamethasone-induced chondrocyte impairment from osteoarthritic
cartilage through up-regulation of extracellular signal-regulated
kinase 1/2 and suppression of FASL, FAS, and caspase 3. Biochem
Biophys Res Commun. 441:249–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou RR, Jia SF, Zhou Z, Wang Y, Bucana CD
and Kleinerman ES: Adenovirus-E1A gene therapy enhances the in vivo
sensitivity of Ewing's sarcoma to VP-16. Cancer Gene Ther.
9:407–413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JW, Choi YJ, Suh SI, Baek WK, Suh MH,
Jin IN, Min DS, Woo JH, Chang JS, Passaniti A, et al: Bcl-2
overexpression attenuates resveratrol-induced apoptosis in U937
cells by inhibition of caspase-3 activity. Carcinogenesis.
22:1633–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inose H, Ochi H, Kimura A, Fujita K, Xu R,
Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:20794–20799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XF, Xu H, Zhao YJ, Tang DZ, Xu GH, Holz
J, Wang J, Cheng SD, Shi Q and Wang YJ: Icariin augments bone
formation and reverses the phenotypes of osteoprotegerin-deficient
mice through the activation of Wnt/β-catenin-BMP signaling. Evid
Based Complement Alternat Med. 2013:6523172013. View Article : Google Scholar
|
|
27
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011. View Article : Google Scholar
|
|
28
|
Mehl B, Delling G, Schlindwein I, Heilmann
P, Voia C, Ziegler R, Nawroth P and Kasperk C: Do markers of bone
metabolism reflect the presence of a high- or low-turnover state of
bone metabolism? Med Klin. 97:588–594. 2002. View Article : Google Scholar
|
|
29
|
Terzi T, Terzi M, Tander B, Cantürk F and
Onar M: Changes in bone mineral density and bone metabolism markers
in premenopausal women with multiple sclerosis and the relationship
to clinical variables. J Clin Neurosci. 17:1260–1264. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Y, Wu X and Zhang X, Li D, Xiao Y, Fan
Y and Zhang X: The in vivo study of the effect of icariin on ECM
secretion and gene expression of chondrocytes. Pharmacol Clin Chin
Mater Med. 2012.
|
|
31
|
Sun P, Liu Y, Deng X, Yu C, Dai N, Yuan X,
Chen L, Yu S, Si W, Wang X, et al: An inhibitor of cathepsin K,
icariin suppresses cartilage and bone degradation in mice of
collagen-induced arthritis. Phytomedicine. 20:975–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan T, He L, Yang J, Zhang L, Xiao Y, Fan
Y and Zhang X: Conjugated icariin promotes tissue-engineered
cartilage formation in hyaluronic acid/collagen hydrogel. Process
Biochem. 50:2242–2250. 2015. View Article : Google Scholar
|
|
33
|
Blanco FJ, Geng Y and Lotz M:
Differentiation-dependent effects of IL-1 and TGF-beta on human
articular chondrocyte proliferation are related to inducible nitric
oxide synthase expression. J Immunol. 154:4018–4026.
1995.PubMed/NCBI
|
|
34
|
Roberts AB, Flanders KC, Heine UI,
Jakowlew S, Kondaiah P, Kim SJ and Sporn MB: Transforming growth
factor-beta: multifunctional regulator of differentiation and
development. Philos Trans R Soc Lond B Biol Sci. 327:145–154. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang P, Zhang F, He Q, Wang J, Shiu HT,
Shu Y, Tsang WP, Liang S, Zhao K and Wan C: Flavonoid compound
icariin activates hypoxia inducible factor-1α in chondrocytes and
promotes articular cartilage repair. PLoS One. 11:e01483722016.
View Article : Google Scholar
|
|
36
|
Shantikumar S, Caporali A and Emanueli C:
Role of microRNAs in diabetes and its cardiovascular complications.
Cardiovasc Res. 93:583–593. 2012. View Article : Google Scholar :
|
|
37
|
Schoolmeesters A, Eklund T, Leake D,
Vermeulen A, Smith Q, Force Aldred S and Fedorov Y: Functional
profiling reveals critical role for miRNA in differentiation of
human mesenchymal stem cells. PLoS One. 4:e56052009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bueno MJ, Pérez de Castro I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sumiyoshi K, Kubota S, Ohgawara T, Kawata
K, Abd El Kader T, Nishida T, Ikeda N, Shimo T, Yamashiro T and
Takigawa M: Novel role of miR-181a in cartilage metabolism. J Cell
Biochem. 114:2094–2100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mirzamohammadi F, Papaioannou G and
Kobayashi T: MicroRNAs in cartilage development, homeostasis, and
disease. Curr Osteoporos Rep. 12:410–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guérit D, Brondello JM, Chuchana P,
Philipot D, Toupet K, Bony C, Jorgensen C and Noël D: FOXO3A
regulation by miRNA-29a controls chondrogenic differentiation of
mesenchymal stem cells and cartilage formation. Stem Cells Dev.
23:1195–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L, Zhang X, Li KF, Li DX, Xiao YM,
Fan YJ and Zhang XD: Icariin promotes extracellular matrix
synthesis and gene expression of chondrocytes in vitro. Phytother
Res. 26:1385–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|