Introduction
Angle class II malocclusion is a common condition
that presents most commonly as mandibular retrusion (1). Treatment of this condition involves
the use of functional appliances to stimulate mandibular growth by
forward posturing of the mandible. During this process, a series of
morphological and histological changes are observed in the region
of the temporomandibular joint (TMJ), which manifest as
reconstruction of the condyle and glenoid fossa (2). Studies have demonstrated that
mandibular advancement may induce endochondral bone formation in
the condyle (3-5). The present study established a mouse
model of stepwise mandibular advancement to study bone homeostasis
associated with TMJ modification in the absence and presence of
platelet-rich plasma (PRP).
The coordination between bone matrix formation by
osteoblasts and bone resorption by osteoclasts maintains bone
homeostasis (6). Shifting of the
balance either towards bone-resorbing osteoclasts or boneforming
osteoblasts is implicated in the causation of several bone
disorders. Osteoclasts are multinucleated cells of hematopoietic
origin responsible for bone resorption and have a critical role in
maintaining bone remodeling and mineral homeostasis (7,8).
Two cytokines, the macrophage colony-stimulating factor (M-CSF) and
the receptor activator of nuclear factor-κB ligand (RANKL) are
known to regulate osteoclast differentiation (9).
PRP refers to plasma with an enhanced concentration
of platelets. As a natural source of a variety of growth factors,
PRP has an important role in bone repair, cell proliferation and
differentiation during tissue regeneration (10). PRP has been demonstrated to
stimulate bone regeneration and healing by inducing proliferation
and differentiation of osteoblasts (11,12). However, its effect on
RANKL-induced osteoclast differentiation has remained to be fully
elucidated.
The aim of the present study was to evaluate the
effect of PRP on osteoclast differentiation during mandibular
condyle remodeling, and to determine the underlying molecular
mechanisms involved in this biological process.
Materials and methods
Animals and experimental design
A total of 100, 3 week-old female BALB/c mice were
maintained in a temperature-controlled room (24±1°C) with relative
humidity (50±10%) at the animal center of Nanjing Medical
University (Nanjing, China) with free access to mouse chow and
water and underwent a 12 h light/dark cycle. All animal
experimentation was performed according to protocols approved by
the Experimental Animal Care and Use Committee of Nanjing Medical
University (Nanjing, China). Mice were provided by the Model Animal
Research Center of Nanjing University (Nanjing, China).
Induction of mandibular forward movement was
performed as described previously (13). In brief, in 4-week-old mice, both
sides of the lower incisors were trimmed by 1 mm at the incisal
third every 3 days until they were sacrificed. This was performed
to induce a mandibular protrusion movement that occurs when mice
are feeding.
Four-week-old female mice were divided into two
groups (n=5 in each): The negative control group (received normal
saline) and the PRP group (received PRP treatment). Normal saline
or PRP were directly injected into the articular cavity of the TMJ
by a micro syringe once a week. After injection, the animals were
allowed to recover and were sacrificed at 7, 14 and 28 days after
mandibular forward movement.
Preparation of PRP
Whole blood collected from age-matched female BALB/c
mice was transferred to sterile tubes and mixed with sodium citrate
(3.8%) at a ratio of 9:1 to prevent coagulation. PRP was enriched
by a two-step centrifugation method as described previously
(14). The tubes were centrifuged
at 600 × g for 10 min at room temperature and the whole blood was
separated into three phases: platelet-poor plasma (top), buffy coat
(middle) and erythrocytes (bottom). The top and middle liquid
phases were transferred to a fresh tube and centrifuged again at
1,200 × g for 15 min. The upper half of the supernatant plasma was
discarded and the lower half was blended thoroughly to yield PRP.
PRP was activated with 10% calcium chloride solution and 5,000
units of bovine thrombin to induce the formation of a gel, which
was then stored at −20°C.
Micro-computed tomography (micro-CT)
analysis
To assess differences in the density of subchondral
bone and the degree of bone resorption, the condylar bone was
cleared of marrow and soft tissue and then fixed overnight in 70%
ethanol (15). The specimens were
then scanned at a slice thickness of 18 µm using a Skyscan
1176 micro-CT device (Skyscan, Kontich, Belgium) at 50 kV and 456
µA. Images were reconstructed and analyzed using NRecon
version 1.6 and CTAn version 1.31.8.1 software (Bruker, Billerica,
MA, USA). The area of condylar cartilage and subchondral bone in
the sample was defined as the region of interest (ROI). A total of
five consecutive images from the ROI were used for
three-dimensional reconstruction and analysis.
The parameters analyzed included the trabecular bone
volume per total volume (BV/TV), trabecular number (Tb.N),
trabecular separation (Tb.Sp) and trabecular thickness (Tb.Th). The
operator who performed the scanning analysis was blinded to the
group identity of each sample.
Histological analysis
Mandibular condyles from the control and
experimental animals were resected and fixed in 10% buffered
formalin for 24 h, decalcified in 10% EDTA (pH 7.4) for 21 days,
embedded in paraffin and cut into 4-µm thick sections. The
sections were stained for total collagen, as well as with
tartrate-resistant acid phosphatase (TRAP) and Goldner's trichrome
as described previously (16,17). The histomorphometric parameters
determined included the percentage of collagen area, the number of
osteoclasts per bone perimeter (OcN/BP) and the number of
osteoblasts per bone perimeter (ObN/BP) (18). Two examiners blinded to the group
identity analyzed the slides.
Mouse osteoclast culture
Bone marrow cells and hematopoietic cells were
extracted from the tibias and femurs of three-week-old BALB/c
female mice under general anesthesia, as described previously
(19). Cells were cultured in
α-minimum essential medium (α-MEM) with 15% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin-streptomycin (PS) at 37°C and 5%
CO2 for 3 h to remove adherent cells. Non-adherent cells
were seeded onto new plates and cultured in α-MEM with M-CSF (20
ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 3 days,
which resulted in the growth of bone marrow-derived macrophages
(BMMs).
For osteoclast differentiation and TRAP activity
staining, BMMs were seeded in 6-well plates and cultured in the
presence of M-CSF (20 ng/ml) and RANKL (20 ng/ml; Sigma-Aldrich;
Merck KGaA) with or without 1% PRP for 3 days.
TRAP staining
The protocol used for cell TRAP staining was in
accordance with that of a previous study (20). In brief, cells were fixed in 4%
paraformaldehyde and stained for TRAP activity with 0.1 M acetate
solution (pH 5.0) containing 6.76 mM sodium tartrate, 0.1 mg/ml
naphthol AS-MX phosphate and 0.5 mg/ml Fast Red Violet at 37°C for
30 min. Multinucleated cells expressing TRAP and with >3 nuclei
were identified as osteoclasts.
Alizarin Red S (ARS) staining
Bone marrow stromal cells (BMSCs) flushed from the
femurs and tibias were cultured in α-MEM containing 10% FBS and 1%
PS at 37°C and 5% CO2 for 3 days, followed by removal of
non-adherent cells by replacing the medium. For osteogenic
differentiation, BMSCs were seeded at a density of 1×105
cells/well in 12-well plates in a mineralized solution containing
α-MEM, 10% FBS, 10 mM β-glycerolphosphate, 10 nM dexamethasone and
50 mg/ml ascorbate phosphate, with and without addition 1% PRP.
After 12 days of ossification induction, mineral deposition was
assessed by ARS staining. A microplate reader was used to capture
micrographs of mineralized nodules. ARS staining was performed as
per the manufacturer's instructions (Beyotime Institute of
Biotechnology, Haimen, China).
Microarray and gene expression
analysis
Total RNA was extracted from condyle and glenoid
fossa samples of test and control mice using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 14 days. A NanoDrop
ND-1000 was used for RNA quantification and quality assessment,
while RNA integrity was assessed using standard denaturing agarose
gel electrophoresis. The Whole Mouse Genome Oligo Microarray
(4×44K; Agilent Technologies, Santa Clara, CA, USA) was used to
investigate the transcriptional profiles of the samples; the array
represented >41,000 transcripts.
RNA labeling and array hybridization were performed
according to the Agilent One-Color Microarray-Based Gene Expression
Analysis protocol (Agilent Technologies). Data extracted using
Agilent Feature Extraction software (version 11.0.1.1) were
normalized and analyzed using the GeneSpring GX v12.1 software
package (Agilent Technologies). Differentially expressed genes were
identified by fold-change screening.
Gene ontology (GO) analysis and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway enrichment analysis was
performed to systematically identify the terms of differentially
expressed genes and to identify the pathways associated with
osteoclastic differentiation. The microarray data were deposited in
the NCBI Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/; accession no. GSE67644)
(21). The procedure was
performed by KangChen Bio-Tech (Shanghai, China). P<0.005 was
used as a cutoff threshold to identify significantly enriched GO
terms and pathways.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for RNA analysis
Total RNA was isolated from osteoclasts which were
treated with or without 1% PRP for 3 days using an RNA extraction
kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. The RNA was converted to complementary DNA using a Takara
PrimeScript RT reagent kit (Takara Bio, Inc.). The gene expression
level was assessed by qPCR using the ABI-7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The qPCR
mixture contained 2 µl of cDNA, 0.5 µl of each primer
and 10 µl of SYBR-Green in a final volume of 20 µl
(95°C for 30 sec; 40 cycles of 95°C for 5 sec, 60°C for 31 sec,
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec).
The following primers were used: Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′;
nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1)
forward, 5′-CGAGTTCACATCCCACAG-3′ and reverse,
5′-GACAGCACCATCTTCTTCC-3′; c-fos forward, 5′-CACTCTGGTCTCCTCCGT-3′
and reverse, 5′-ATTCTCCGTTTCTCTTCCTC-3′; TRAP forward,
5′-CAGCAGCCAAGGAGGACTAC-3′ and reverse, 5′-ACATAGCCCACACCGTTCTC-3′;
cathepsin k (Ctsk) forward, 5′-CCCATCTCTGTGTCCATC-3′ and reverse,
5′-AGTGCTTGCTTCCCTTCT-3′; carbonic anhydrase 2 (CAR2) forward,
5′-ATCCTTGCTCCCTTCTTC-3′ and reverse, 5′-ATCCAGGTCACACATTCC-3′;
matrix metalloproteinase 9 (MMP9) forward,
5′-TCACTTTCCCTTCACCTTC-3′ and reverse, 5′-ATTTGCCGTCCTTATCGT-3′;
Dickkopf-related protein 1 (Dkk1) forward, 5′-ATT
CCAGCGCTGTTACTGTG-3′ and reverse, 5′-GAATTGCTGGTTTGATGGTG-3′;
β-catenin forward, 5′-TTCCTGAGCTGACCAAACTG-3′ and reverse,
5′-GCACTATGGCAGACACCATC-3′; cyclin D1 forward,
5′-CGGATGAGAACAAGCAGA-3′ and reverse, 5′-CGGTAGCAGGAGAGGAAG-3′. The
2−ΔΔCq method was used for quantification (22).
Western blot analysis
Western blot analysis was performed according to a
previously described method (23). After osteoclasts were treated with
or without 1% PRP for 3 days and 5 days, total cellular protein was
extracted using a TPER Protein Extraction reagent kit (Thermo
Fisher Scientific, Inc.) according to manufacturer's instructions.
The protein concentrations were determined using a bicinchoninic
acid protein assay kit (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (50 µg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
transferred by electro-blotting onto a polyvinylidene difluoride
membrane (EMD Millipore Corp., Billerica, MA, USA) and the membrane
was incubated overnight at 4°C with the following primary
antibodies: Mouse Dkk1 antibody (1:1,000 dilution; cat. no. AF1765;
R&D Systems, Minneapolis, MN, USA), rabbit β-catenin antibody
(1:1,000 dilution; cat. no. 8480; Cell Signaling Technology,
Boston, MA, USA), rabbit anti-GAPDH (1:5,000 dilution; cat. no.
AP0063; Bioworld, Irving, TX, USA), followed by incubation with
horseradish peroxidase-conjugated secondary antibodies (cat. no.
ZB-2301; Origene Technologies, Beijing, China) at room temperature
for 1 h. The protein bands were visualized with ECL substrate
solution (GE Healthcare Life Sciences, Little Chalfont, UK) for 1
min, and images were captured using a MicroChemiluminescence system
4.2 (DNR, Jerusalem, Israel). Semi-quantitative analyses were
performed using ImageJ v.1.45 software (National Institutes of
Health, Bethesda, NJ, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Experiments were conducted separately at least 3 times.
SPSS 19.0 (IBM Corp., Armonk, NY, USA was used for statistical
analysis. Student's t-test was used to assess inter-group
differences. P<0.05 was considered indicative of a statistically
significant difference.
Results
Injection of PRP is associated with bone
reconstruction
To investigate the effect of PRP on bone mass,
micro-CT was used to characterize the bone phenotype and to
quantitatively analyze the relevant parameters of condylar
subchondral bone (Fig. 1).
Micro-CT analysis of the mandibular condyle from 4-week-old mice
indicated significant differences between the two groups (Fig. 1A). Mice in the PRP group exhibited
a higher BV/TV (P<0.01), and increased Tb.N (P<0.01) and a
reduced Tb.Sp (P<0.01). However, no significant difference in
Tb.Th was observed between the two groups (Fig. 1C). Together, these results
suggested a significant effect of PRP on bone formation in
vivo under this specific physiological conditions of bone
remodeling. This was also supported by the results of total
collagen staining of TMJ sections at 7, 14 and 28 days (Fig. 1B and D–F).
 | Figure 1PRP alleviates bone loss in a mouse
model of mandibular advancement. (A) Four-week-old mice were
sacrificed on day 28 after the first mandibular forward movement,
and radiographs of the transverse sections of the mandibular
condyles were obtained using a micro-CT scanner. Scale bar, 100
µm. (B) Representative photomicrographs of sections
subjected to histochemical staining for total collagen. Scale bar,
100 µm. (C) BV/TV, Tb.N and Tb.Sp of the mandibular condyles
were determined by analysis of micro-CT data with Xelis software.
No significant difference was observed with respect to trabecular
thickness between the control and experimental groups (P>0.05).
(D-F) The proportion of red-stained collagenous area within the
total area in the entire section, on (D) day 7, (E) day 14 and (F)
day 28 in the PRP group was significantly greater than that in the
control group. Values are expressed as the mean ± standard
deviation (n=5). **P<0.01. PRP, platelet-rich plasma;
RANKL, receptor activator of nuclear factor-κB ligand; BV/TV, bone
volume per total volume; Tb.Sp, trabecular separation; Tb.Th,
trabecular thickness; CT, computed tomography; Con, control. |
PRP facilitates osteoblast
differentiation but inhibits osteoclast formation in condyle
subchondral bone
To examine the effects of PRP on bone homeostasis
and osteoclastic differentiation, differences in histological
staining were compared between the experimental and control
samples. TRAP staining of TMJ sections from control and PRP mice at
7 days (Fig. 2A), 14 days
(Fig. 2B) and 28 days (Fig. 2C) indicated that the number of
mature osteoclasts in the control group was higher than that in the
PRP group. This indicated a significant attenuation of osteoclast
activity through treatment with PRP. OcN/BP was determined by TRAP
staining to quantify reduction in osteoclasts. Goldner's trichrome
staining indicated an obvious reduction in osteoclasts and an
increase in osteoblasts quantified as the OcN/BP and ObN/BP
(Fig. 2). These results indicated
that PRP inhibits osteoclast formation and promotes osteoblast
formation in the TMJ of mice with mandibular protrusion.
 | Figure 2Effects of PRP on bone homeostasis and
osteoclastic differentiation. The dissected mandibular condyles of
day 7 (A), day 14 (B) and day 28 (C) were fixed, decalcified,
embedded and sectioned (eft). The sections were stained for TRAP
(top) and subjected to Goldner's trichrome staining (bottom). Scale
bar, 100 µm. The OcN/BP and ObN/BP were determined by
histomorphometric analysis (right). Values are expressed as the
mean ± standard deviation (n=5). *P<0.05 and
**P<0.01. TRAP, tartrate resistant acid phosphatase;
OcN, number of osteoclasts; ObN, number of osteoblasts; BP, bone
perimeter; PRP, platelet-rich plasma; Con, control. |
PRP suppresses RANKL-induced osteoclast
differentiation but enhances osteogenesis
To further investigate the role of PRP in osteoclast
differentiation, BMMs were cultured with M-CSF (20 ng/ml) and RANKL
(20 ng/ml), in the presence or absence of 1% PRP. Four days later,
cells were fixed and subjected to TRAP staining. As expected, the
number of TRAP-positive multinu cleated cells per field was
significantly lower in the PRP group as compared with that in the
control group. Alizarin Red S staining of BMSCs cultured with PRP
for 12 days indicated a higher number of mineralization nodules
compared with that in the control group (Fig. 3A). These results indicated that
PRP inhibited RANKL-induced osteoclast differentiation and
accelerated osteoblastic activity, which induced a positive effect
on bone regeneration.
 | Figure 3PRP suppresses the early stage of
RANKL-induced osteoclastogenesis but enhances osteoblast
differentiation. (A) Bone marrow-derived macrophages were cultured
with macrophage colony stimulating factor (20 ng/ml) and RANKL (20
ng/ml) in the presence or absence of 1% PRP for 3 days. The cells
were fixed, permeabilized and stained for TRAP. Images were
captured under a light microscope (top left) and the number of
TRAP-positive osteoclasts (>3 nuclei) per field was determined
(top right). Scale bar, 200 µm. Bone marrow stromal cells
were stained with Alizarin red and examined under a microscope
(magnification, x100; bottom left) and the wells of 12-well plates
were observed under image scanner (bottom right). (B and C) Effect
of PRP on NFATc1, c-fos, TRAP, Ctsk, CAR2 and MMP9 mRNA expression
in osteoclasts. All values are expressed as the mean ± standard
deviation (n=3). *P<0.05 and **P<0.01.
PRP, platelet-rich plasma; RANKL, receptor activator of nuclear
factor-κB ligand; TRAP, tartrate resistant acid phosphatase; Oc,
osteoclasts; NFATc1, nuclear factor of activated T-cells,
cytoplasmic 1; Ctsk, cathepsin k; CAR2, carbonic anhydrase 2; MMP9,
matrix metalloproteinase 9. |
PRP inhibits bone resorption by
downregulation of osteoclast-associated genes
The expression levels of RANKL-induced
differentiation marker genes NFATc1, TRAP and c-fos, as well as
those of the resorptive activity marker genes Ctsk, CAR2 and MMP9
were assessed by RT-qPCR in osteoclasts after stimulation with PRP
for 3 days. At day 3 of PRP treatment, the expression of NFATc1,
TRAP and c-fos was significantly lower than that in the control
group (Fig. 3B). Furthermore,
downregulation of the expression of Ctsk, CAR2 and MMP9 was
observed in the PRP group compared with that in the control group
(Fig. 3C). These results
indicated that PRP inhibited osteoclast differentiation at the gene
expression level.
Gene chip analysis
Based on the assumption that PRP may influence gene
expression, gene expression profile chip analysis was used to
compare the differences in gene expression between the control and
PRP groups. Thousands of differentially expressed transcripts were
identified, including 18 genes that exhibited a 100-fold
upregulation and 21 genes that had been previously reported to be
associated with osteoclasts exhibiting a downregulation of
>2-fold (Table I).
Furthermore, GO analysis provided terms of enriched biological
process, cellular component and molecular function of the
differentially expressed genes, the top 9 of which are presented in
Fig. 4B. These were associated
with cell adhesion molecules (CAMs), basal cell carcinoma,
spliceo-some, the Hedgehog signaling pathway, the Notch signaling
pathway, pathways in cancer, tight junction, the Wnt signaling
pathway and transcriptional misregulation in cancer. The Wnt
signaling pathway, which is considered important for osteoblast
differentiation, ranked eighth. The activation of Wnt signaling
indicates that osteoblast differentiation is enhanced by PRP.
Results of Alizarin Red S staining were also consistent with this
hypothesis. The effect of PRP on osteoblasts is well documented in
the literature (12,24). Dkk1 is a known inhibitor of Wnt
signaling, which was demonstrated to impair osteoblast activity and
potentially stimulate osteoclastogenesis (25). Therefore, among the downregulated
genes, the present study focused on the Dkk1 gene, which exhibited
a 9.972-fold change in expression. The Wnt pathway was selected for
further research.
 | Table IDownregulated genes according to the
gene chip analysis. |
Table I
Downregulated genes according to the
gene chip analysis.
| Gene | P-value | Probe name | Fold change |
|---|
| Wnt3a | 0.02624592 | A_52_P361534 | 3.4888073 |
| Dkk1a | 0.03172296 | A_51_P379069 | 9.9720192 |
| Fzd1a | 0.04910161 | A_52_P597634 | 2.0385147 |
| Fzd2a | 0.02590137 | A_51_P404077 | 7.5377999 |
| Dvl2 | 0.00029402 | A_55_P2055869 | 3.1892181 |
| Ctbp2 | 0.01244548 | A_52_P15212 | 3.1172611 |
| Wnt8ba | 0.00420996 | A_55_P2140913 | 2.0766898 |
| Wnt3aa | 0.02285019 | A_55_P2067649 | 2.9224466 |
| Wnt10aa | 0.03544225 | A_51_P171616 | 3.1686486 |
| Vangl2a | 0.03769394 | A_55_P2117984 | 2.0264866 |
| Csnk2a2a | 0.03733953 | A_52_P207614 | 5.0116083 |
| Adm | 0.04438596 | A_51_P265571 | 8.1618855 |
| Shh | 0.03644892 | A_52_P49014 | 3.8205879 |
| Ucp1 | 5.9393×10-5 | A_51_P426353 | 16.0701171 |
| Hnrnpr | 0.01911658 | A_55_P1969625 | 6.9950514 |
| Rest | 0.0265632 | A_55_P2143306 | 6.1341825 |
| Hmga2 | 0.00308199 | A_52_P300730 | 5.2488875 |
| Cd44 | 0.03947195 | A_55_P2166501 | 5.1884358 |
| Areg | 0.00801028 | A_52_P482897 | 4.3739025 |
| Vcam1 | 0.03508028 | A_52_P520495 | 4.4398901 |
| Sema3a | 0.03332904 | A_55_P2054013 | 4.4616508 |
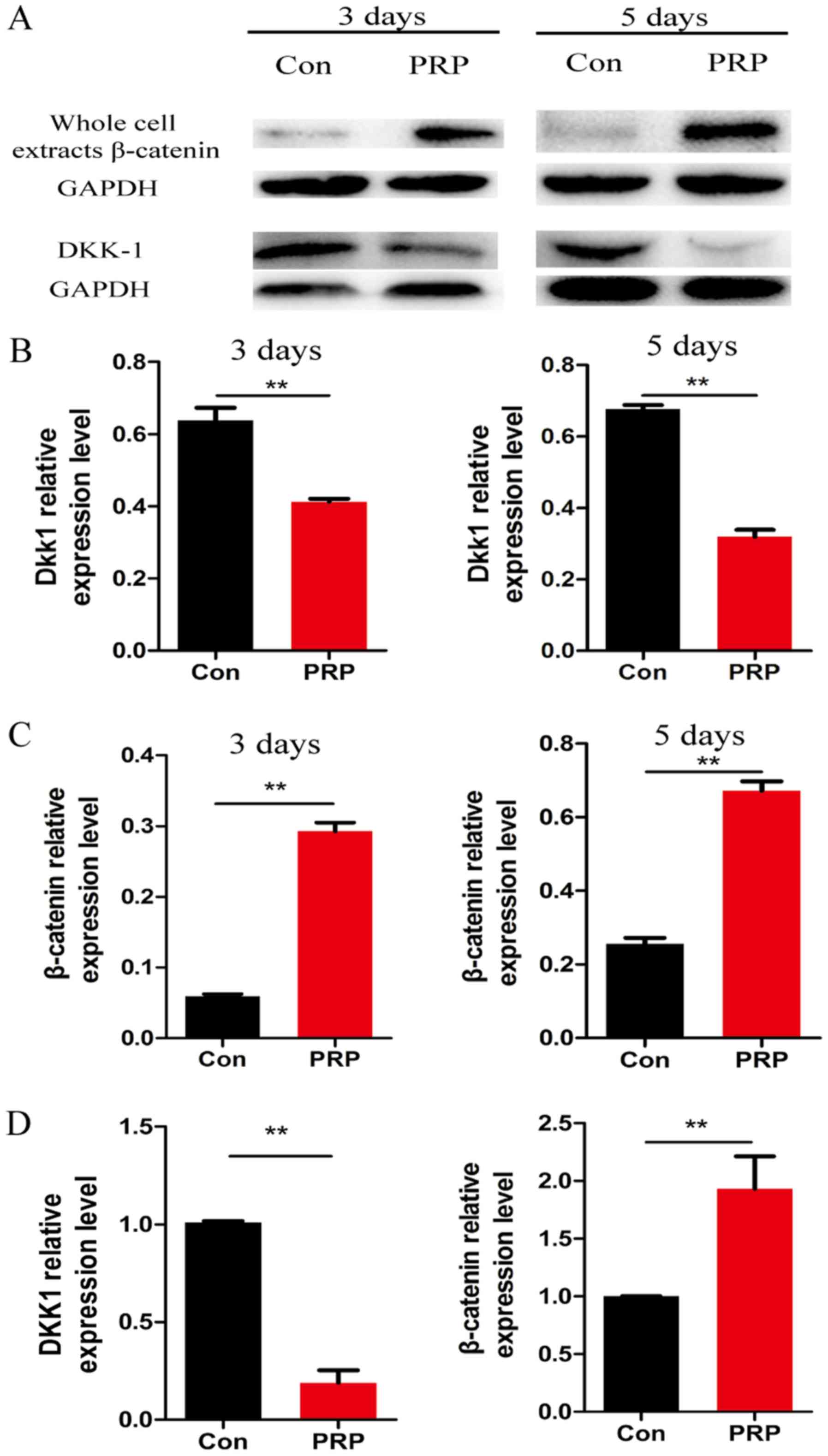
Wnt signaling pathway is activated during
osteoclast differentiation
To further explore the molecular mechanisms of the
anti-osteoclastic effects of PRP, RNA and protein from cells
pretreated with PRP was assessed for the expression of
Wnt-associated signaling molecules by RT-qPCR and western blot
analysis, respectively. Of note, β-catenin (a component of the
canonical Wnt pathway) is known to have a critical role in bone
formation and remodeling (26).
Cyclin D1, the downstream transcription factor of β-catenin, has a
mediatory role in the canonical Wnt pathway. Dkk1 is a specific
inhibitor of the canonical Wnt pathway. PRP was demonstrated to
significantly reduce RANKL-induced osteoclast differentiation by
regulating early signaling pathways and increasing the levels of
β-catenin and cyclin D1 (27).
Downregulation of Dkk1 and upregulation of β-catenin indicated that
PRP inhibited osteoclast differentiation by activating the
β-catenin/Wnt pathway (Fig. 5).
These results indicated that PRP causes a repression of Dkk1, which
induces the Wnt signaling pathway. This results in inhibition of
phosphorylation of β-catenin, leading to the accumulation of
non-phosphorylated β-catenin in the cytoplasm and its translocation
to the nucleus. This in turn induces upregulation of the downstream
target gene cyclin D1 (Fig.
6).
Discussion
Various studies have demonstrated condylar cartilage
remodeling and increases in mandibular length after insertion of
functional appliances at an early stage of growth and development
(28,29). In treatment of class II occlusion,
functional appliances produce an impact on mandibular advancement,
which in turn alters the biophysical environment in the TMJ and
induces a series of cellular changes (30). The increase in new bone formation
in the TMJ as a result of mandibular advancement has been reported
to cause adaptive remodeling of the mandibular condyle. TMJ
reconstruction relies on the fine balance between ossification and
bone resorption.
PRP was first used to enhance bone grafts for the
repair of mandibular defects (31). Ever since, PRP has found wide
applications in medicine for bone tissue engineering, regeneration
of damaged joints, oral maxillofacial surgery and oral planting due
to its self-replicating ability, multi-directional differentiation
potential, easy availability, absence of immune rejection and
minimal ethical concerns (32).
In the present study, PRP was identified as a
critical negative regulator of osteoclastogenesis and bone
resorption in vivo and in vitro. First, micro-CT and
histological techniques were used to observe the morphology of the
TMJs of the experimental mice. The outcomes of micro-CT and
histological analyses for the two groups revealed significant
differences in parameters including bone mass, OcN and ObN. It was
thus hypothesized that PRP may affect bone formation by inhibiting
osteoclast differentiation. To confirm this hypothesis, RT-qPCR was
performed to assess the expression of osteoclast marker genes
(NFATc1, c-fos, TRAP, Ctsk, CAR2 and MMP9). Downregulation of c-fos
and NFATc1 mRNA is indicative of the inhibition of osteoclast
differentiation (33). As
expected, PRP repressed the expression of RANKL-induced
differentiation marker genes. In addition, gene chip analysis
identified several differentially expressed genes, among which Dkk1
was of particular interest.
The results of the present study provide insight
into the link between PRP and osteoclast differentiation. The Wnt
signaling pathway was identified to be involved in PRP-induced
inhibition of osteoclast differentiation. To further validate this
result, RANKL-induced osteoclasts treated with PRP were examined
for the expression of Dkk1 and β-catenin using RT-qPCR and western
blot analysis. The results demonstrated that PRP affected Dkk1 and
β-catenin expression during osteoclast differentiation, which
confirmed the involvement of the Wnt pathway in PRP-mediated
inhibition of osteoclast differentiation. Several studies have
investigated the role of PRP in inducing bone formation via
promotion of osteoblastic differentiation (34). Several lignin-like compounds have
been reported to repress RANKL-induced osteoclastogenesis and
inhibit osteoclast-mediated bone resorption activity (35). The present study established
extensive functions of PRP in mouse TMJ remodeling, and it was
indicated that local injection of PRP in the damaged domain may be
effective in inhibiting TMJ remodeling, or may at least provide
symptom relief.
There are several limitations to the present study.
Firstly, no sham group was included for comparison. Secondly,
histologic results in the control and PRP groups were not
time-dependent (days 7, 14 and 28), which indicates that there may
be a complicated mechanism at work, which requires further
research.
PRP has important effects not only on osteoblastic
differentiation but also on osteoclast differentiation during bone
formation. However, the present study did not examine the
interaction between osteoblasts and osteoclasts in bone formation.
Further study is therefore required to identify additional details
regarding this potential association. To date, no uniform methods
and standards for the extraction of PRP have been established,
which makes it difficult to obtain and apply it at production level
quantities (36). Further
research will be performed to identify the precise elements
involved in the influence of PRP on bone formation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81371179), the Natural
Science Foundation of Jiangsu Province (grant no. BK20150048) and a
Project Funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions (grant no. 2014-037).
References
|
1
|
Cozza P, Baccetti T, Franchi L, De Toffol
L and McNamara JA Jr: Mandibular changes produced by functional
appliances in Class II malocclusion: A systematic review. Am J
Orthod Dentofacial Ortho. 129:599.e1–599.e12. 2006. View Article : Google Scholar
|
|
2
|
Owtad P, Potres Z, Shen G, Petocz P and
Darendeliler MA: A histochemical study on condylar cartilage and
glenoid fossa during mandibular advancement. Angle Orthod.
81:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung FY, Rabie AB and Hägg U:
Neovascularization and bone formation in the condyle during
stepwise mandibular advancement. Eur J Orthod. 26:137–141. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feres MF, Alhadlaq A and El-Bialy T:
Adjunctive techniques for enhancing mandibular growth in class II
malocclusion. Med Hypotheses. 84:301–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruf S and Pancherz H: Temporomandibular
joint remodeling in adolescents and young adults during Herbst
treatment: A prospective longitudinal magnetic resonance imaging
and cephalometric radiographic investigation. Am J Orthod
Dentofacial Orthop. 115:607–618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rho J, Takami M and Choi Y:
Osteoimmunology: Interactions of the immune and skeletal systems.
Mol Cells. 17:1–9. 2004.PubMed/NCBI
|
|
7
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteopro-tegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar
|
|
10
|
Foster TE, Puskas BL, Mandelbaum BR,
Gerhardt MB and Rodeo SA: Platelet-rich plasma: From basic science
to clinical applications. Am J Sports Med. 37:2259–2272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomoyasu A, Higashio K, Kanomata K, Goto
M, Kodaira K, Serizawa H, Suda T, Nakamura A, Nojima J, Fukuda T,
et al: Platelet-rich plasma stimulates osteoblastic differentiation
in the presence of BMPs. Biochem Biophys Res Commun. 361:62–67.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanno T, Takahashi T, Tsujisawa T,
Ariyoshi W and Nishihara T: Platelet-rich plasma enhances human
osteoblast-like cell proliferation and differentiation. J Oral
Maxillofac Surg. 63:362–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tagliaro ML, Rassi Guimarães ML, Pereira
Padilha DM, Callegari-Jacques SM and Jeckel-Neto EA: Mandibular
advancement and morphological changes in the mandibles of female
mice of different ages. Exp Gerontol. 41:1157–1164. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ba R, Wei J, Li M, Cheng X, Zhao Y and Wu
W: Cell-bricks based injectable niche guided persistent ectopic
chondrogenesis of bone marrow-derived mesenchymal stem cells and
enabled nasal augmentation. Stem Cell Res Ther. 6:162015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rafferty KL, Liu ZJ, Ye W, Navarrete AL,
Nguyen TT, Salamati A and Herring SW: Botulinum toxin in
masticatory muscles: Short-and long-term effects on muscle, bone,
and craniofacial function in adult rabbits. Bone. 50:651–662. 2012.
View Article : Google Scholar
|
|
16
|
Chen Ma J, Zhang W, Tucker L, Zhu B,
Sasaki G, Hao H, Wang L, Ci L, Jiang HH, et al: RNA
interference-mediated silencing of Atp6i prevents both periapical
bone erosion and inflammation in the mouse model of endodontic
disease. Infect Immun. 81:1021–1030. 2013. View Article : Google Scholar :
|
|
17
|
Chen W, Ma J, Zhu G, Jules J, Wu M,
McConnell M, Tian F, Paulson C, Zhou X, Wang L, et al: Cbfβ
deletion in mice recapitulates cleidocranial dysplasia and reveals
multiple functions of Cbfβ required for skeletal development. Proc
Natl Acad Sci USA. 111:8482–8487. 2014. View Article : Google Scholar
|
|
18
|
Bendre MS, Gaddy-Kurten D, Mon-Foote T,
Akel NS, Skinner RA, Nicholas RW and Suva LJ: Expression of
interleukin 8 and not parathyroid hormone-related protein by human
breast cancer cells correlates with bone metastasis in vivo. Cancer
Res. 62:5571–5579. 2002.PubMed/NCBI
|
|
19
|
Yamachika E, Tsujigiwa H, Matsubara M,
Hirata Y, Kita K, Takabatake K, Mizukawa N, Kaneda Y, Nagatsuka H
and Iida S: Basic fibroblast growth factor supports expansion of
mouse compact bone-derived mesenchymal stem cells (MSCs) and
regeneration of bone from MSC in vivo. J Mol Histol. 43:223–233.
2012. View Article : Google Scholar
|
|
20
|
Lee YD, Yoon SH, Park CK, Lee J, Lee ZH
and Kim HH: Caveolin-1 regulates osteoclastogenesis and bone
metabolism in a sex-dependent manner. J Biol Chem. 290:6522–6530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar :
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Lee HY, Lee SY, Kim SD, Shim JW, Kim HJ,
Jung YS, Kwon JY, Baek SH, Chung J and Bae YS:
Sphingosylphosphorylcholine stimulates CCL2 production from human
umbilical vein endothelial cells. J Immunol. 186:4347–4353. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Sun S and Liu H, Chen H, Rong X, Lou
J, Yang Y, Yang Y and Liu H: Use of a biological reactor and
platelet-rich plasma for the construction of tissue-engineered bone
to repair articular cartilage defects. Exp Ther Med. 12:711–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rachner TD, Göbel A, Benad-Mehner P,
Hofbauer LC and Rauner M: Dickkopf-1 as a mediator and novel target
in malignant bone disease. Cancer Lett. 346:172–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Willert K and Nusse R: Beta-catenin: A key
mediator of Wnt signaling. Curr Opin Genet Dev. 8:95–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei W, Zeve D, Suh JM, Wang X, Du Y,
Zerwekh JE, Dechow PC, Graff JM and Wan Y: Biphasic and
dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol
Cell Biol. 31:4706–4719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saikoski LZ, Cançado RH, Valarelli FP and
de Freitas KM: Dentoskeletal effects of Class II malocclusion
treatment with the Twin Block appliance in a Brazilian sample: A
prospective study. Dental Press J Orthod. 19:36–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baysal A and Uysal T: Dentoskeletal
effects of Twin Block and Herbst appliances in patients with Class
II division 1 mandibular retrognathy. Eur J Orthod. 36:164–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rabie AB, Zhao Z, Shen G, Hägg EU, Dr O
and Robinson W: Osteogenesis in the glenoid fossa in response to
mandibular advancement. Am J Orthod Dentofacial Orthop.
119:390–400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nathani DB, Sequeira J and Rao BH:
Comparison of platelet rich plasma and synthetic graft material for
bone regeneration after third molar extraction. Ann Maxillofac
Surg. 5:213–218. 2015. View Article : Google Scholar
|
|
33
|
Xu X, Liu N, Wang Y, Pan LC, Wu D, Peng Q,
Zhang M, Wang HB and Sun WC: Tatarinan O, a lignin-like compound
from the roots of Acorus tatarinowii Schott inhibits osteoclast
differentiation through suppressing the expression of c-Fos and
NFATc1. Int Immunopharmacol. 34:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goto H, Matsuyama T, Miyamoto M, Yonamine
Y and Izumi Y: Platelet-rich plasma/osteoblasts complex induces
bone formation via osteoblastic differentiation following
subcutaneous transplantation. J Periodontal Res. 41:455–462. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ito S, Ohmi A, Sakamiya A, Yano T, Okumura
K, Nishimura N and Kagontani K: Ginger hexane extract suppresses
RANKL-induced osteoclast differentiation. Biosci Biotechnol
Biochem. 80:779–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anitua E, Sánchez M, Orive G and Andía I:
The potential impact of the preparation rich in growth factors
(PRGF) in different medical fields. Biomaterials. 28:4551–4560.
2007. View Article : Google Scholar : PubMed/NCBI
|