Introduction
With the improvement of living standards,
nutrition-induced diabetes has become a global public health
concern, representing the 5th leading cause of mortality worldwide
(1). Diabetic cardiomyopathy
(DCM) is a major diabetic complication (2). Cardiovascular complications of
diabetes are the main cause of hospitalization and death. DCM is an
independent complication of diabetes, characterized by early-onset
diastolic dysfunction, which is largely attributed to myocardial
fibrosis. DCM is a type of cardiomyopathy independent from the
large vessels and coronary atherosclerosis, while 75% of patients
with unexplained idiopathic dilated cardiomyopathy have been found
to be diabetic (3). DCM is
characterized by impaired myocardial insulin signaling, endoplasmic
reticulum (ER) stress, mitochondrial dysfunction, activation of the
sympathetic nervous system, excessive oxidative stress, increased
inflammation, abnormal coronary microcirculation and maladaptive
immune responses. These pathophysiological changes result in
fibrosis, hypertrophy, cardiac diastolic/systolic dysfunction and,
eventually, systolic heart failure. Cardiac interstitial fibrosis
is a major characteristic of DCM (4), comprising overproduction and
deposition of myocardial interstitial collagen and resulting in
myocardial stiffness and cardiac dysfunction. A number of molecular
mechanisms have been proposed to contribute to the development of
DCM, including mechanisms involving oxidative stress, cell
apoptosis, autophagy, increased inflammation and ER stress
(5–7); however, the exact molecular
mechanisms that trigger and drive these major pathological
processes have yet to be fully elucidated.
Target organ damage in diabetes mellitus is
associated with increased inflammation and oxidative stress, which
are considered to be major factors contributing to the development
and progression of DCM. The molecular mechanisms regulating related
signal conduction remain largely unclear. Previous findings
indicate that the activation of Janus kinase/signal transducer and
activator of transcription (JAK/STAT) signaling is crucial for the
occurrence and development of myocardial fibrosis. The JAK/STAT
signaling pathway is a key point in the cytokine signal
transduction pathways, as it regulates diverse pathophysiological
processes, including proliferation, differentiation, apoptosis,
cellular immunity and inflammation (8). Hydrogen sulfide (H2S) is
a colorless, flammable gas with a characteristic odor, which, until
recently, had been known to be an endogenously produced gaseous
signaling molecule, similar to nitric oxide (NO) and carbon
monoxide, and has been implicated in the regulation of inflammatory
response, apoptosis, oxidative stress and angiogenesis (9). In addition, H2S has been
shown to exert potent cytoprotective effects against tissue injury,
including myocardial fibrosis (10). However, the specific mechanism of
cardioprotection mediated by H2S in DCM remains largely
unknown. There is little evidence on whether the JAK/STAT signaling
pathway participates in the protection of exogenous H2S
against myocardial fibrosis in diabetes mellitus. In the present
study, sodium hydrosulfide (NaHS), an exogenous donor of
H2S, was used to evaluate the antifibrotic,
anti-inflammatory and antioxidant effects of H2S in the
hearts of streptozotocin (STZ)-induced diabetic rats. The aim was
to provide insight into the molecular mechanisms underlying the
action of H2S in myocardial fibrosis associated with
diabetes mellitus, improve our understanding of the pathophysiology
of DCM, and enable the identification of new therapeutic targets
based on the modulation of H2S production.
Materials and methods
Experimental animals
The experimental protocol was approved by the Animal
Ethics Committee of the University of South China (Hengyang,
China). A total of 40 adult male Sprague Dawley rats (weight,
300±20 g), which were provided by the SJA Animal Experimental
Center of Changsha (Changsha, China), were bred in subcages in a
clean laboratory with artificial lighting (12-h light/dark cycles),
with free access to food and water.
Chemicals and reagents
NaHS was purchased from Sigma-Aldrich; Merck KGaA
(St. Louis, MO, USA). STZ was purchased from MP Biomedicals, LLC
(Santa Ana, CA, USA). Rabbit polyclonal anti-JAK-1 (cat. no.
A00330), rabbit polyclonal anti-JAK-2 (cat. no. BA3398), rabbit
polyclonal anti-collagen III (cat. no. BA0326), rabbit polyclonal
anti-transforming growth factor (TGF)-β (cat. no. BA0290), rabbit
polyclonal antitumor necrosis factor (TNF)-α (cat. no. BA14903),
rabbit polyclonal anti-nuclear factor (NF)-κB (cat. no. BM3946),
mouse anti-STAT1 (cat. no. BA0619-2), mouse anti-STAT3 (cat. no.
BA0621), mouse anti-STAT5 (cat. no. BA1411), mouse anti-STAT6 (cat.
no. BA1414), mouse anti-cystathionine-γ-lyase (CSE; cat. no.
BA3605), mouse anti-tissue inhibitor of metalloproteinase (TIMP)2
(cat. no. BA0576), mouse anti-matrix metalloproteinase (MMP)8 (cat.
no. BA2201), mouse anti-MMP14 (cat. no. BA1278) and rabbit
polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
cat. no. BM3874), were all purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). The dilution ratio of these
antibodies was 1:400. Furthermore, rabbit anti-eukaryotic
initiation factor 2α (eIF2α; cat. no. 11233-1-AP), mouse anti-Bcl-2
(cat. no. 12789-1-AP), mouse anti-caspase-3 (cat. no. 19677-1-AP)
and mouse anti-GRP94 (cat. no. 14700-1-AP) were all purchased from
Proteintech Group, Inc. (Chicago, IL, USA). The dilution ratio of
these antibodies was 1:1,000. Anti-rabbit secondary antibodies
(cat. no. SA00001-2) were also purchased from Proteintech Group,
Inc. The dilution ratio was 1:2,000. Cell lysis buffer for western
blot analysis, the ELISA kit of superoxide dismutase (SOD),
malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), glutathione (GSH),
the bicinchoninic acid (BCA) protein assay kit, the enhanced
chemiluminescence reagent kit and the SDS-PAGE gel preparation kit
were all obtained from Beyotime Institute of Biotechnology
(Shanghai, China).
Model establishment and grouping
A total of 40 experimental animals were randomly
divided into four groups (n=10) as follows: Normal (control group),
diabetes mellitus (STZ group), diabetes mellitus treated with
H2S (STZ + H2S group), and normal rats
treated with H2S (H2S group). The STZ and STZ
+ H2S groups were administered intraperitoneal (i.p.)
injections of STZ (40 mg/kg). During the same time, the rats of the
control and H2S groups were treated with saline daily
(i.p.). After 3 days of STZ injections, rat blood samples were
collected through the caudal vein to measure the blood glucose
level. Blood glucose >16.7 mmol/l suggested successful
establishment of the diabetes model. Subsequently, NaHS (100
µmol/kg, i.p.) was administered to the rats of the STZ +
H2S and H2S groups, whereas rats in the
control and STZ groups were treated with phosphate-buffered saline
daily. The experiment lasted for 8 weeks. The rats were sacrificed
following anesthesia with chloral hydrate (350 mg/kg) and all the
animals were weighed. The hearts of the rats were lavaged with
ice-cold normal saline before removing and weighing. Three rats
were selected randomly from each group. Each heart was divided into
two parts, one of which was utilized for terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) assay and the other for
biochemical analyses.
Histopathological analysis of myocardial
fibers
The removed hearts were fixed in 4%
paraformaldehyde. Each heart was dehydrated with graded alcohols,
embedded in paraffin and sliced into 5-µm sections. These
sections were stained using a hematoxylin and eosin (H&E)
staining kit and a Masson's trichrome staining kit, and observed
under a light microscope at a magnification of ×200.
TUNEL assay
The rat myocardial tissue sections were fixed in 10%
formalin, embedded in paraffin and processed for the TuNEL assay.
The slides were treated with H2O2 and
incubated with a reaction mixture containing TdT and
digoxigenin-conjugated duTP for 1 h at 37°C. Labeled DNA was
visualized with peroxidase-conjugated anti-digoxigenin antibody
using 3,3′-diaminobenzidine as the chromogen. Rat testicular tissue
was used as positive control in the TUNEL assay.
Measurement of MDA, 4-HNE, GSH and
SOD
The content of MDA, 4-HNE and GSH and the activity
of SOD in the heart were assayed by ELISA. The ELISA kits for SOD,
MDA, 4-HNE and GSH were all obtained from Beyotime Institute of
Biotechnology. The steps were conducted following the
manufacturer's instructions.
Western blot analysis
Total protein was extracted in ice-cold
radioimmunoprecipitation assay buffer containing protease
inhibitors (Beyotime Institute of Biotechnology), and quantified
using a BCA protein assay kit. Proteins were denatured, separated
by SDS-PAGE electrophoresis and transferred to a PVDF membrane by
the wet transfer method. The membranes were blocked with 5% skimmed
milk in Tris-buffered saline with Tween-20 (TBST) for 2 h at room
temperature and incubated with blocking solution containing primary
antibody (1:400, anti-collagen III; 1:400, anti-MMP8; 1:400,
anti-MMP14; 1:400, anti-TIMP2; 1:400, anti-CSE; 1:400, anti-TGF-β;
1:400, anti-TNF-α; 1:400, anti-NF-κB; 1:400, anti-STAT1/3/5/6;
1:400, anti-JAK-1/2; 1:1,000, anti-eIF2α; 1:1,000, anti-GRP94;
1:1,000, anti-caspase-3; and 1:1,000, anti-Bcl-2) overnight at 4°C.
After washing three times with TBST, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibody (1:2,000)
for 1 h at room temperature. Next, the membranes were washed in
TBST buffer three times and subjected to chemiluminescence
detection assay. The bands were analyzed with a Molecular Imager
VersaDoc MP 5000 system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical differences among the groups were assessed by one-way
analysis of variance with SPSS 18.0 software (SPSS Inc., Chicago,
IL, USA). Differences between two groups were analyzed using the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of H2S on blood
glucose concentration, body weight (BW) and heart weight (HW) in
diabetic rats
Plasma glucose concentration, BW and HW were
measured prior to sacrificing the rats. The results revealed that
BW and HW in the STZ-treated groups were significantly lower
compared with those in the control group. The concentration of
blood glucose was significantly increased in the STZ-treated group
compared with that in the control group. However, no significant
differences in BW, HW or blood glucose concentration were observed
between the H2S-treated and STZ groups (Table I).
 | Table IEffects of H2S on BG
concentration, BW and HW in diabetic rats. |
Table I
Effects of H2S on BG
concentration, BW and HW in diabetic rats.
| Parameters | Control group | STZ group | STZ +
H2S group | H2S
group |
|---|
| BW (g) | 437.71±64.75 |
269.86±20.41b |
280.14±12.06b | 471.29±24.16 |
| HW (g) | 1.46±0.17 | 1.04±0.10b | 1.07±0.13b | 1.52±0.96b |
| HW/BW (×100) | 0.34±0.02 | 0.39±0.04a | 0.38±0.05a | 0.32±0.01 |
| BG1
(mmol/l) | 5.77±0.33 | 6.12±0.83 | 6.05±0.63 | 5.83±0.77 |
| BG2
(mmol/l) | 7.26±0.58 | 28.5±2.55b | 25.2±5.54b | 8.12±2.06 |
| BG3
(mmol/l) | 6.91±0.45 | 26.90±2.42b | 25.71±2.38b | 7.32±0.91 |
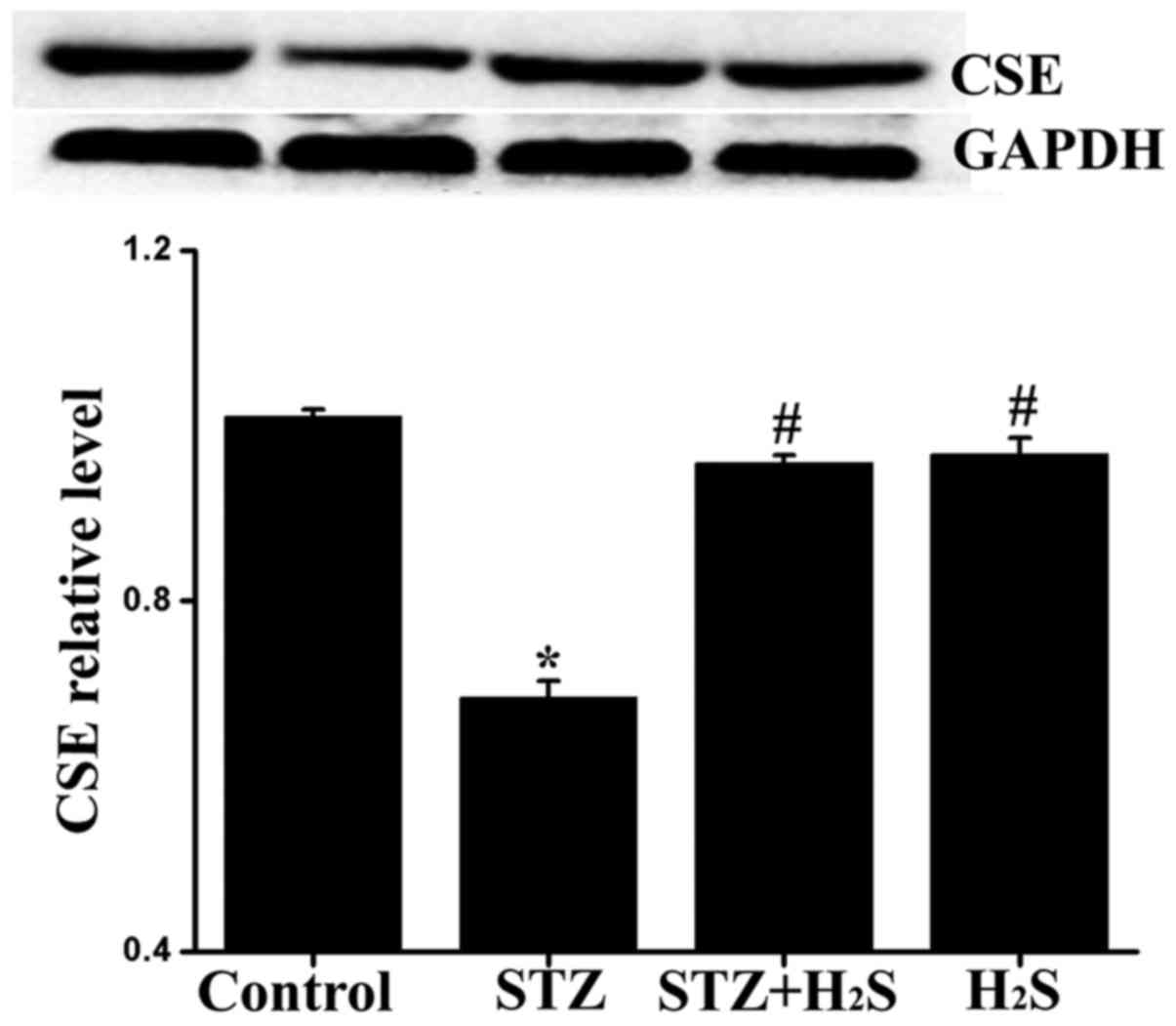
Effects of H2S on CSE
expression in diabetic rats
To determine whether diabetes-induced myocardial
damage was associated with decreased generation of endogenous
H2S, the expression level of CSE was measured by western
blot analysis. Compared with the control group, the expression
level of CSE in the STZ and STZ + H2S groups was
significantly decreased, while there was no obvious difference in
the H2S group; compared with the STZ group, the
myocardial expression of CSE was significantly increased in the STZ
+ H2S and H2S groups (Fig. 1).
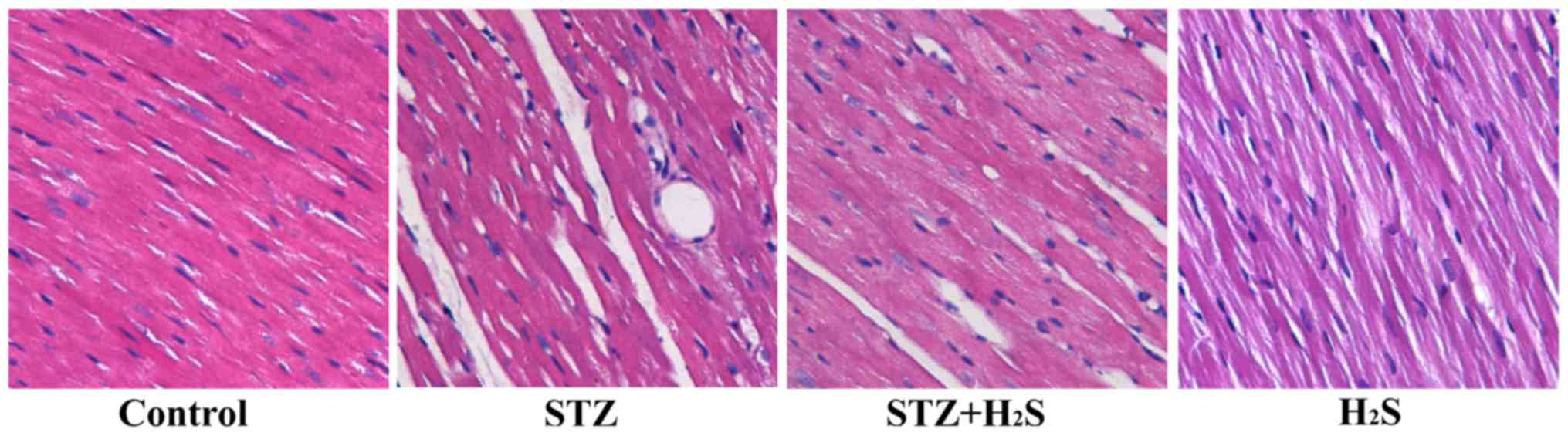
H2S improves histological
changes in rats treated with STZ
On examination under a light microscope, myocardial
cells in the control group were orderly and compactly arranged, the
intercellular space was normal, and there was less extracellular
matrix. Compared with the control group, the cardiomyocytes in the
STZ group were disordered, the intercellular spaces were broader,
and interstitial fibrosis was present. However, these changes were
markedly reversed in the STZ + H2S group. In the
H2S group, the myocardial tissue structure and
arrangement of fibers were similar to those of the control group
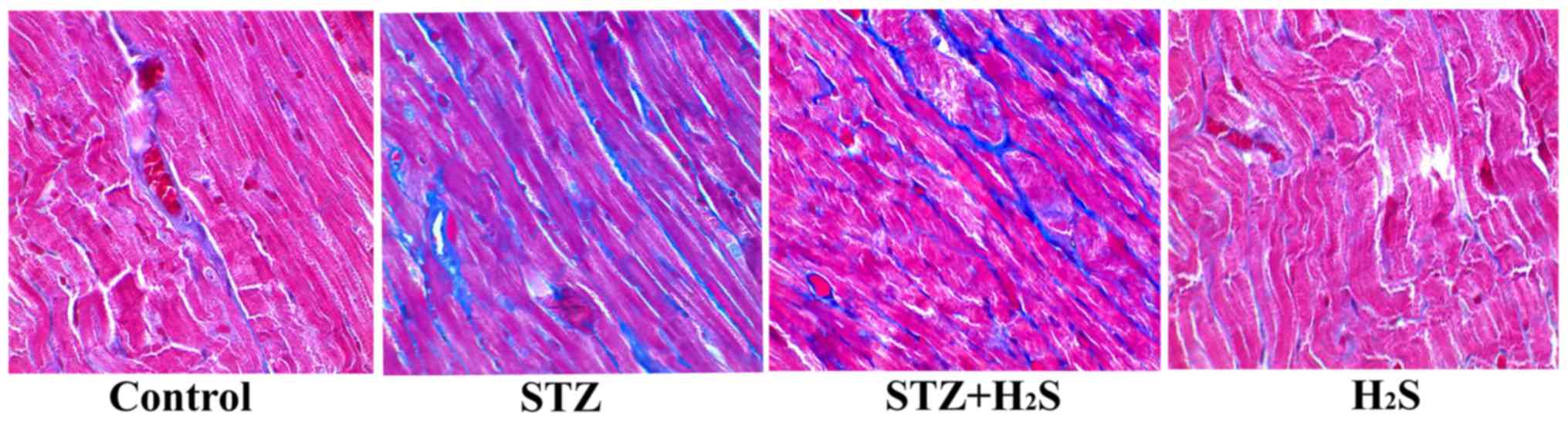
(Fig. 2). Masson's staining
revealed the deposition of collagen fibers (blue staining),
reflecting the extent of myocardial fibrosis. As demonstrated by
Masson's staining, there was little evidence of myocardial fibrosis
in the control group. However, a significantly increased degree of
fibrosis was observed in the STZ group. Compared with the STZ
group, myocardial fibrosis was markedly improved in the STZ +
H2S group; however, compared with the control group,
there were no significant histological changes in the
H2S group (Fig.
3).
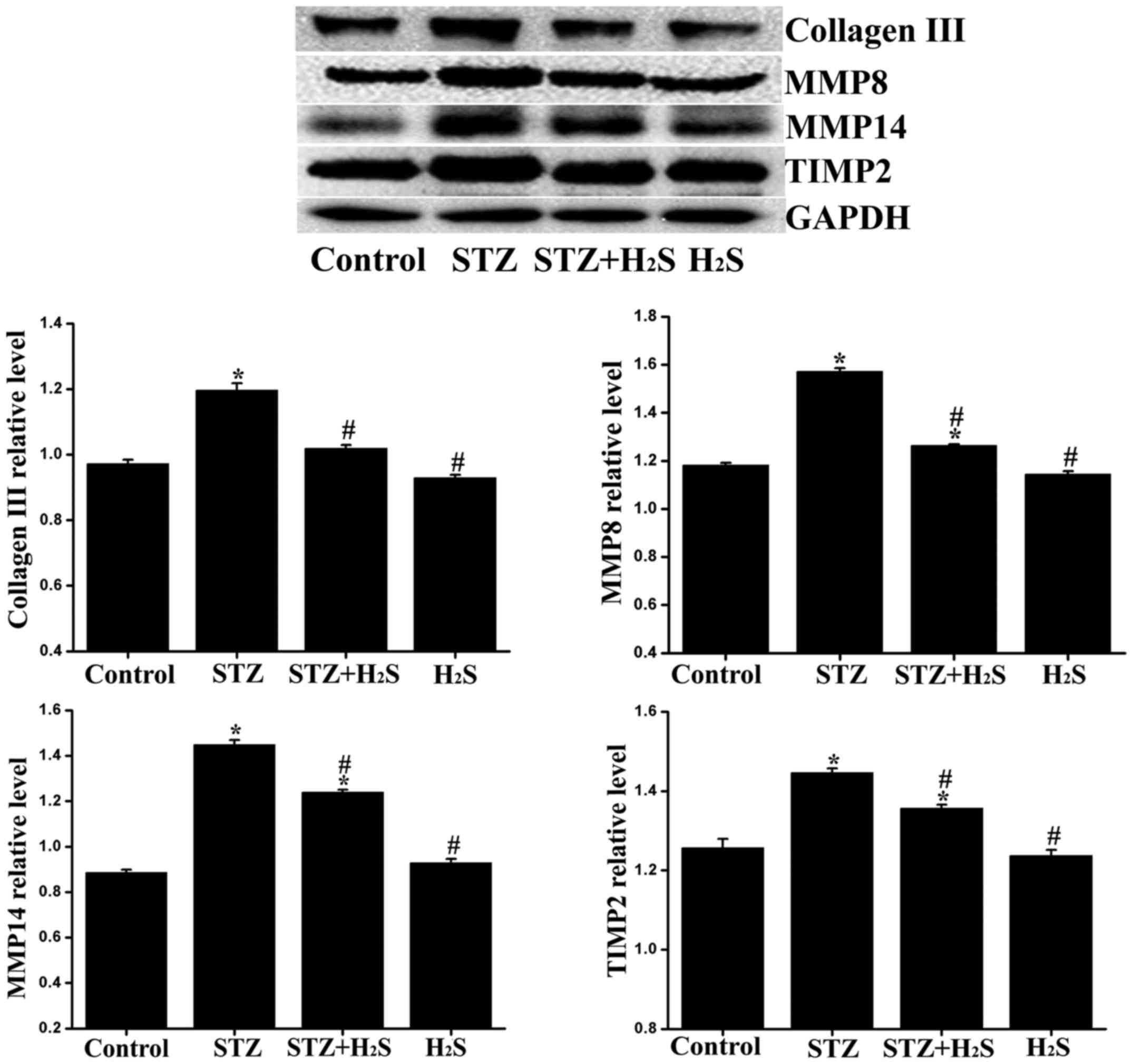
Effects of H2S on collagen
III, MMP8, MMP14 and TIMP2 expression in diabetic rats
As the balance of MMPs/TIMPs determines the ratio of
collagen synthesis and degradation, it may reflect the status of
fibrosis to a certain extent. Therefore, the expression of collagen
III, MMP8, MMP14 and TIMP2 was determined. Compared with the
control group, the expression levels of collagen III, MMP8, MMP14
and TIMP2 were significantly increased in the STZ group, and the
expression levels of TIMP2, MMP8 and MMP14 were significantly
increased in the STZ + H2S group. Compared with the STZ
group, the myocardial expression of collagen III, MMP8, MMP14 and
TIMP2 was significantly reduced in the STZ + H2S group.
No significant difference was observed in the expression levels of
collagen III, MMP8, MMP14 and TIMP2 between the control and
H2S groups (Fig.
4).
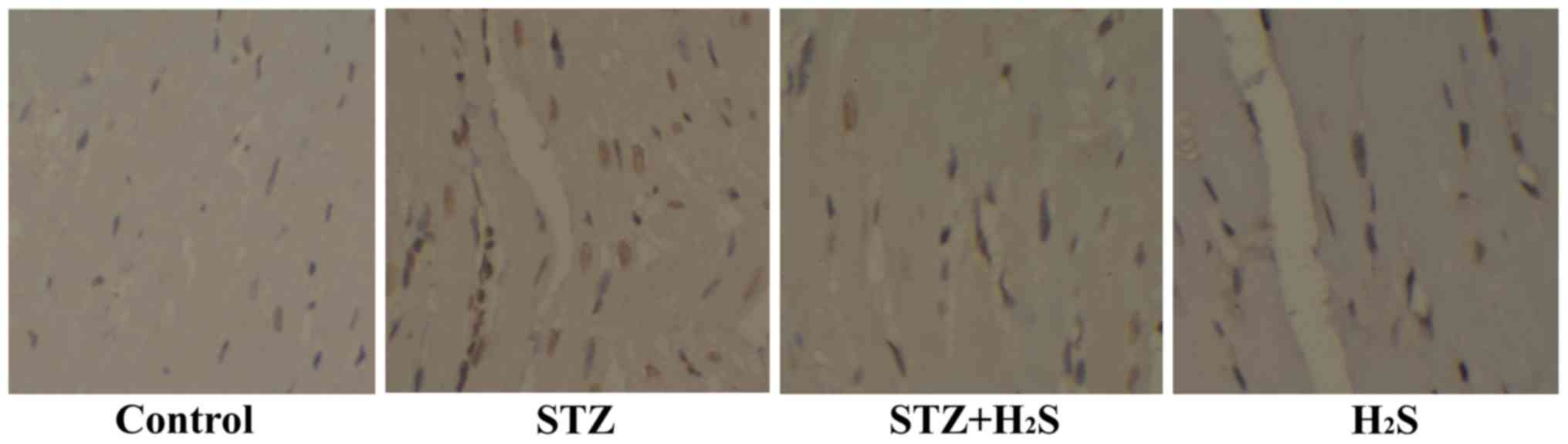
H2S reduces cardiomyocyte
apoptosis in diabetic rats
In the present study, the TUNEL assay was used to
detect apoptosis in heart tissue. The number of apoptotic cells was
obviously higher in the STZ group compared with that in the control
group. However, the number of TUNEL-positive cells was found to be
decreased in the STZ + H2S group. No significant
difference was observed between the control and H2S
groups (Fig. 5).
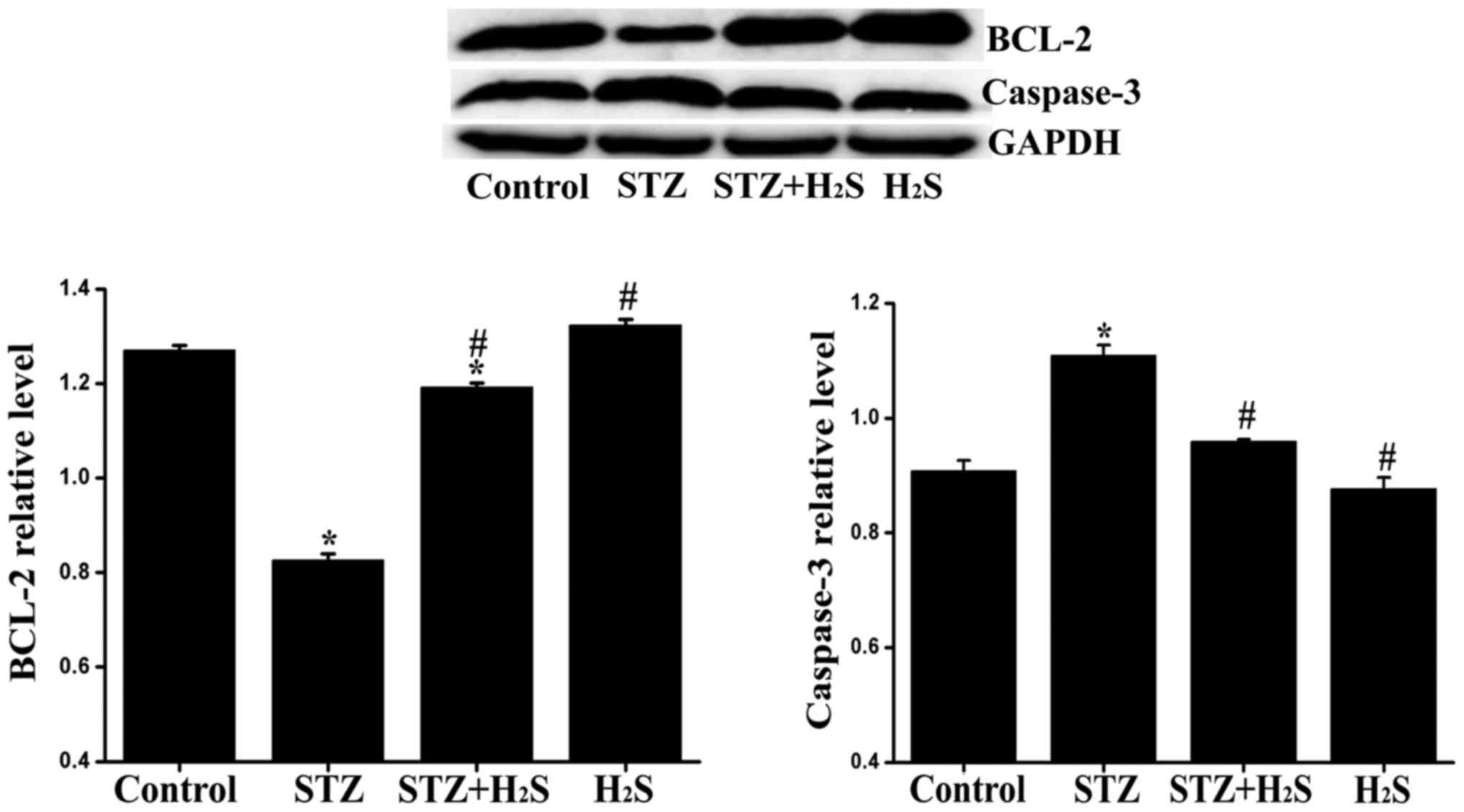
Effects of H2S on Bcl-2 and
caspase-3 expression in diabetic rats
It is widely accepted that Bcl-2 and caspase-3 are
closely associated with apoptosis. In our experiment, the
expression of Bcl-2 and caspase-3 was determined by western blot
analysis. Compared with the control group, the expression level of
caspase-3 was significantly increased in the STZ group, and the
expression level of Bcl-2 was significantly decreased in the STZ
and STZ + H2S groups. Compared with the STZ group, the
myocardial expression of caspase-3 was significantly reduced,
whereas that of Bcl-2 was significantly increased in the STZ +
H2S and H2S groups. No significant difference
was observed in the expression levels of Bcl-2 and caspase-3
between the control and H2S groups (Fig. 6).
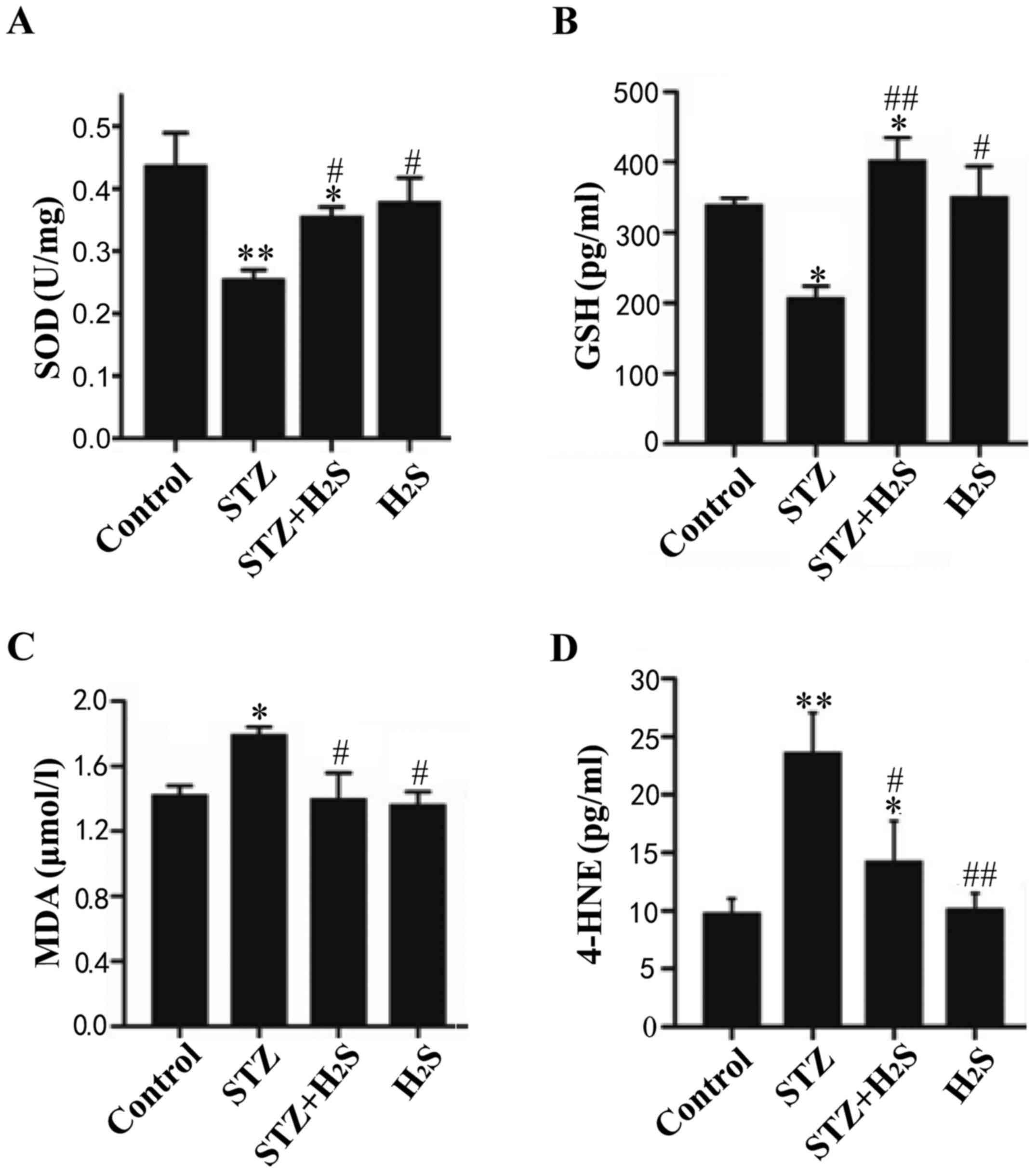
Protein expression levels of MDA, 4-HNE,
GSH and SOD
The levels of GSH, SOD, 4-HNE and MDA were measured
to assess oxidative damage in the myocardium and determine whether
H2S protects against this type of damage. Compared with
the control group, the expression levels of SOD and GSH were
significantly decreased in the STZ group, whereas the expression
level of SOD was significantly decreased and that of GSH was
significantly increased in the STZ + H2S group (Fig. 7A and B). Compared with the STZ
group, the myocardial expression of SOD and GSH was significantly
increased in the STZ + H2S and H2S groups. In
addition, the levels of MDA and 4-HNE in STZ group rats were
obviously higher compared with those in the control group. Compared
with the STZ group, the myocardial expression of MDA and 4-HNE was
significantly reduced in the STZ + H2S and
H2S groups (Fig. 7C and
D). No significant difference was observed in the expression
levels of SOD, GSH, MDA and 4-HNE between the control and
H2S groups.
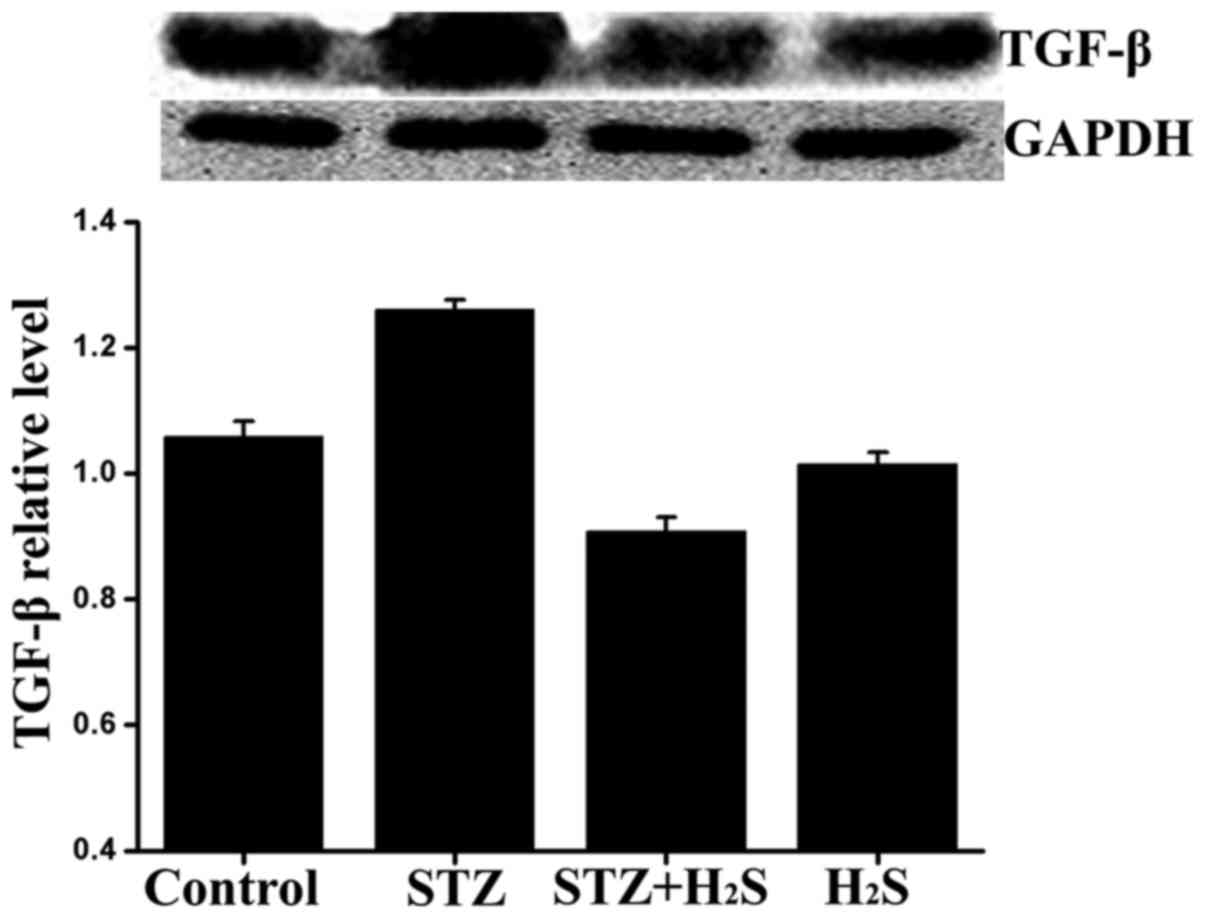
Effects of H2S on TGF-β
expression in diabetic rats
It is widely accepted that TGF-β is closely
associated with fibrogenesis. In our experiment, the expression of
TGF-β was determined by western blot analysis. Compared with the
control group, the expression level of TGF-β was significantly
higher in the STZ group and significantly lower in the STZ +
H2S group, while there was no obvious difference between
the control and H2S groups. Compared with the STZ group,
the myocardial expression of TGF-β was significantly reduced in the
STZ + H2S group (Fig.
8).
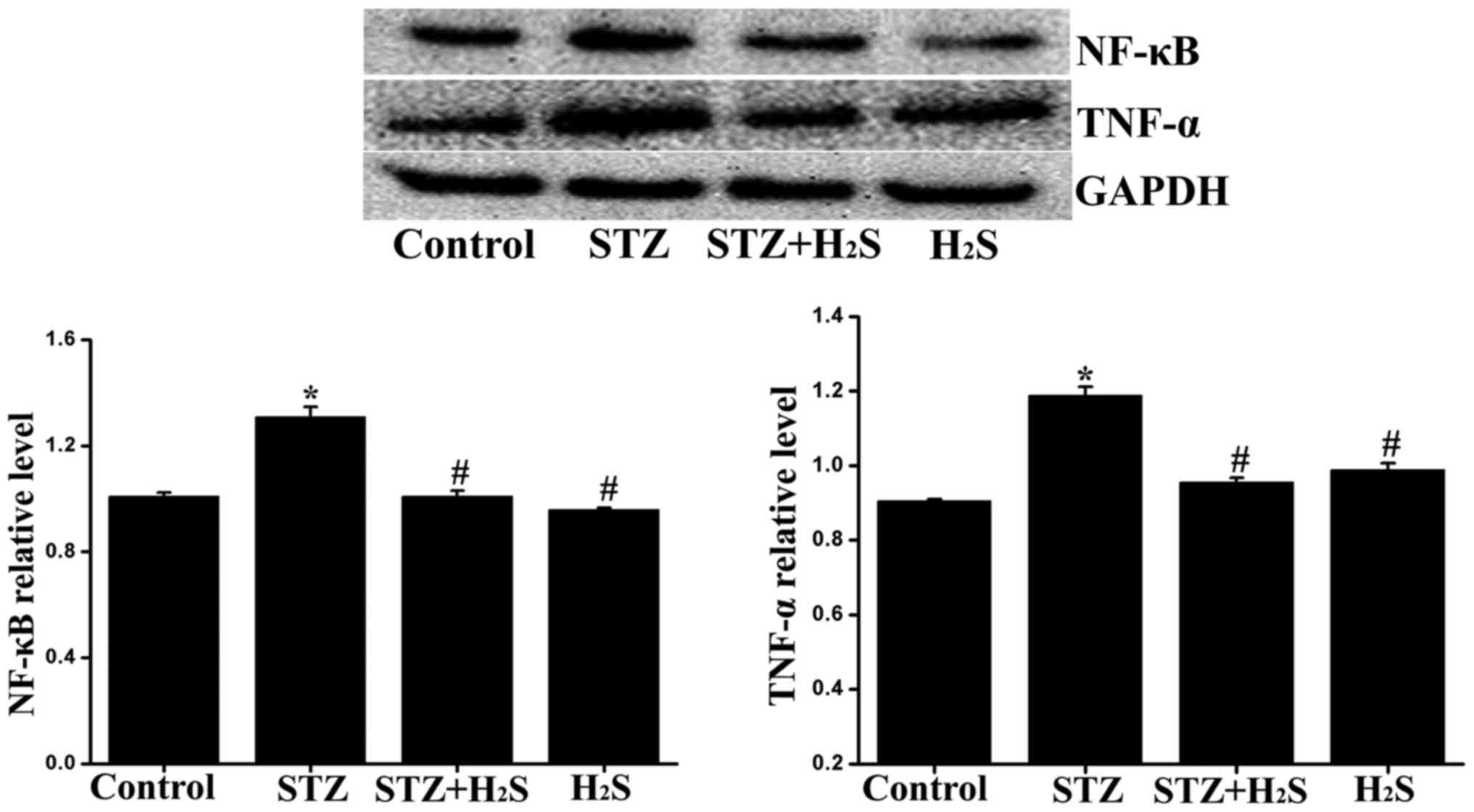
Effects of H2S on TNF-α and
NF-κB expression in diabetic rats
The expression levels of TNF-α and NF-κB were
measured to assess inflammatory response using western blot
analysis. Compared with the control group, the expression levels of
TNF-α and NF-κB were significantly increased in the STZ group, the
expression level of TNF-α was significantly decreased in the STZ +
H2S group, while there was no significant difference
between the control and H2S groups. Compared with the
STZ group, the myocardial expression of TNF-α and NF-κB were
significantly reduced in the STZ + H2S group (Fig. 9).
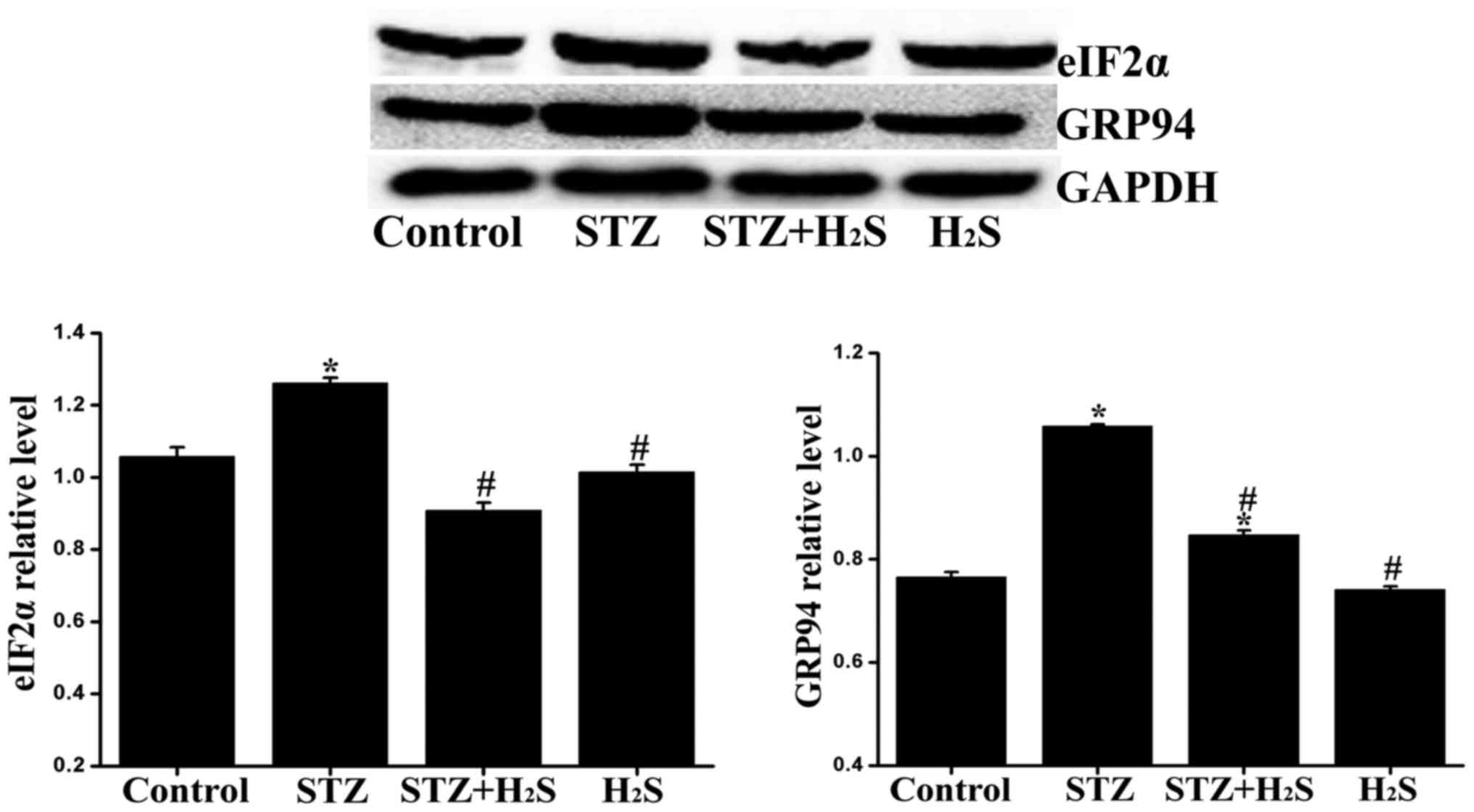
Effects of H2S on eIF2α and
GRP94 expression in diabetic rats
The expression of eIF2α and GRP94 was determined by
western blot analysis to assess ER stress in the myocardium.
Compared with the control group, the expression levels of eIF2α and
GRP94 were significantly increased in the STZ group and the
expression of GRP94 was significantly increased in the STZ +
H2S group. Compared with the STZ group, the myocardial
expression of eIF2α and GRP94 was significantly reduced in the STZ
+ H2S group. No significant difference was observed in
the expression levels of eIF2α and GRP94 between the control and
H2S groups (Fig.
10).
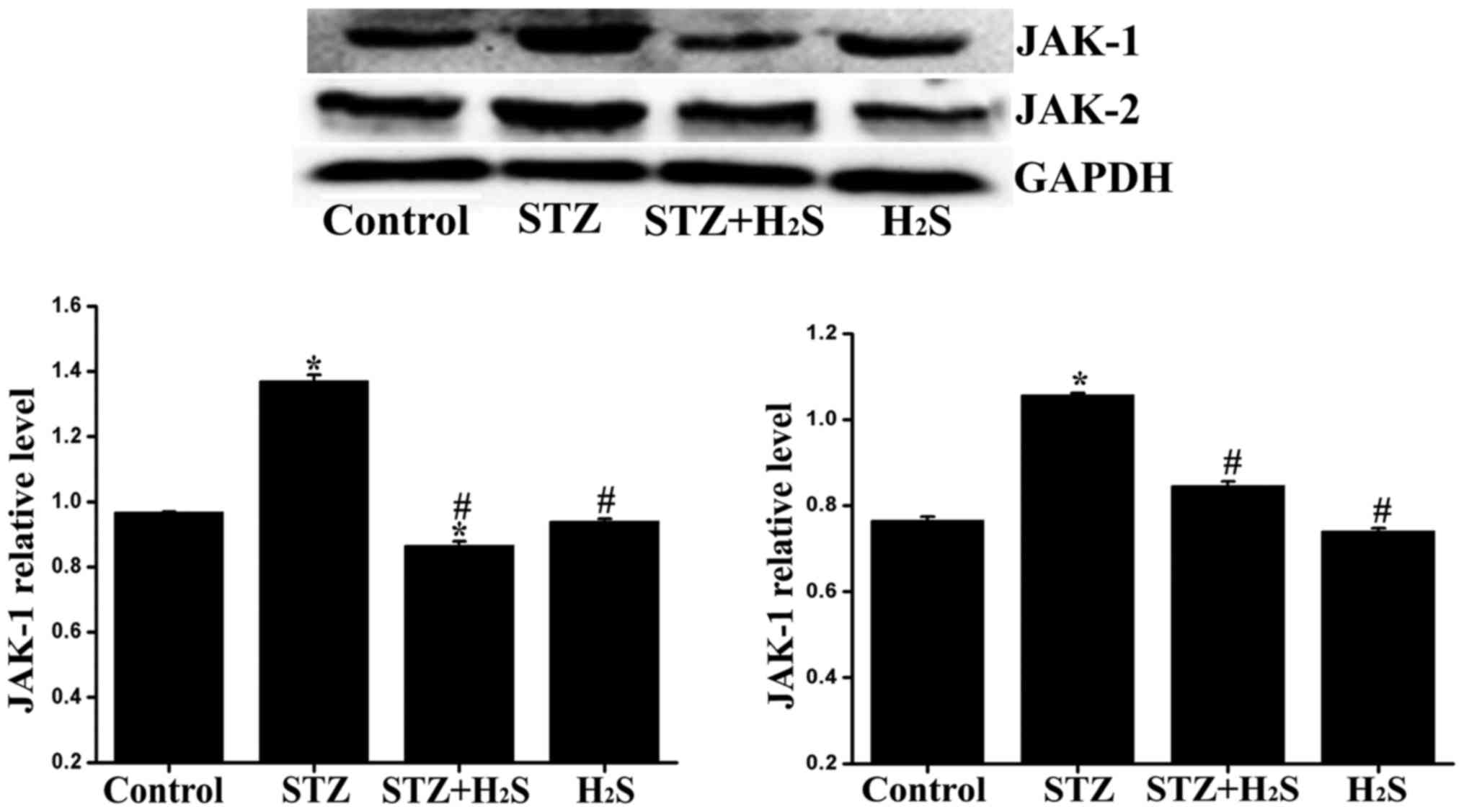
Administration of exogenous
H2S donor affects JAK-1/2 and STAT1/3/5/6 signaling
To further explore the potential signaling pathway
involved in diabetes, proteins associated with the JAK/STAT pathway
were detected by western blot analysis. Compared with the control
group, the expression level of JAK-1/2 in the STZ group was
markedly increased, the expression level of JAK-1 in the STZ +
H2S group was significantly decreased, and the
expression levels of JAK-2 in STZ + H2S group was
significantly increased. Compared with the STZ group, the
myocardial expression of JAK-1/2 exhibited a marked reduction in
the STZ + H2S group. No significant difference was
observed in the expression levels of JAK-1 and -2 between the
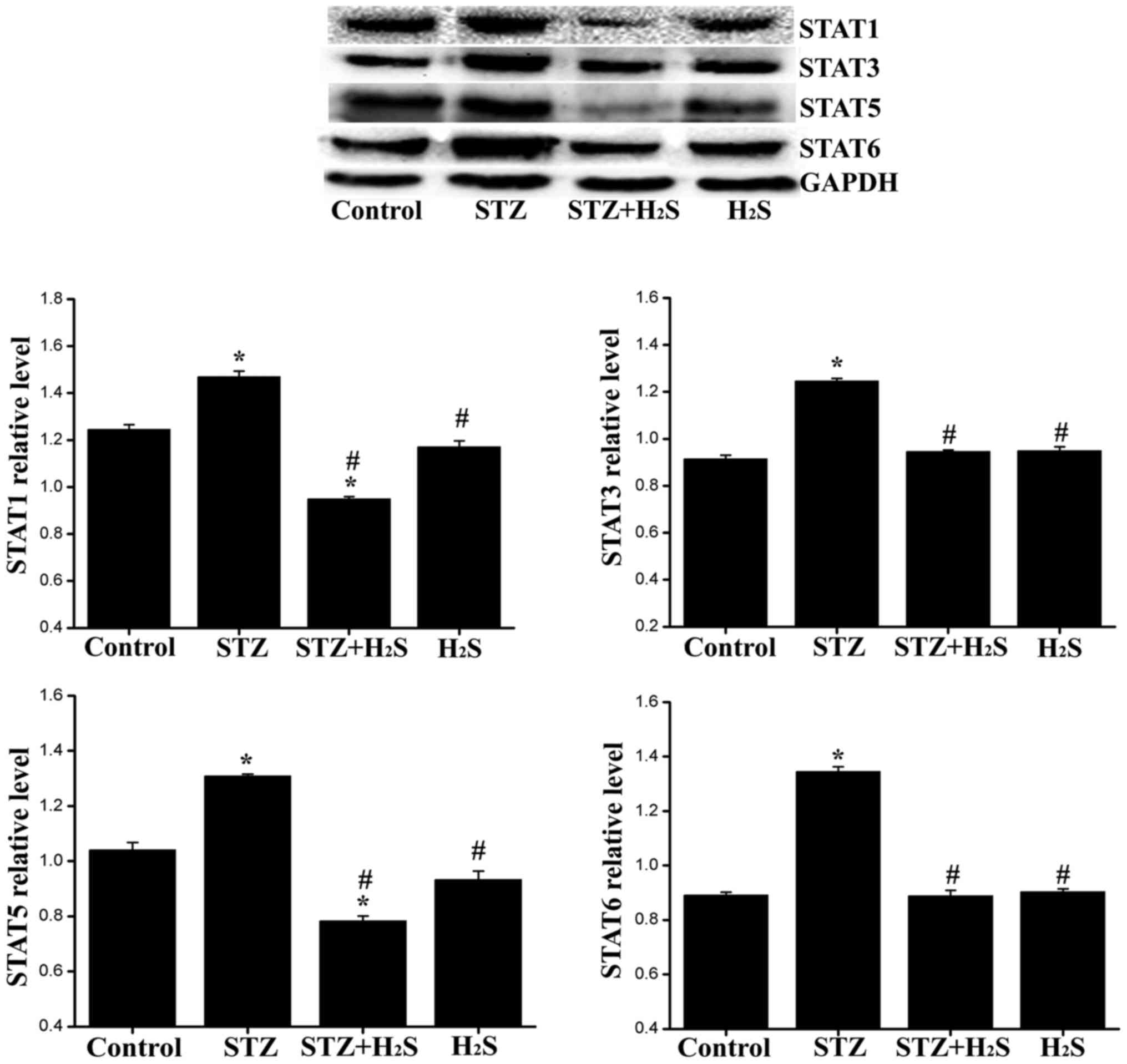
control and H2S groups (Fig. 11). Compared with the control
group, the expression levels of STAT1/3/5/6 in the STZ group were
significantly increased. Compared with the STZ group, the
myocardial expression of STAT1/3/5/6 was significantly reduced in
the STZ + H2S group. No significant difference was
observed in the expression levels of STAT1/3/5/6 between the
control and H2S groups (Fig. 12).
Discussion
Over the past two decades, the worldwide incidence
of diabetes mellitus has steadily increased as a consequence of
higher rates of obesity and changes in lifestyle. If unaddressed,
nearly half a billion individuals will suffer from diabetes
mellitus in 2030 (11). Diabetes
is associated with a number of fatal complications, such as DCM,
which accounts for most of the morbidity and mortality in this
population and represents a major global health concern (12).
Cardiac interstitial fibrosis, as a major
characteristic of DCM, results from the overproduction and
deposition of myocardial interstitial collagen, leading to
hypertrophy, myocardial stiffness, cardiac diastolic/systolic
dysfunction and, eventually, heart failure (13). The results of immunohistochemical
analysis revealed obvious interstitial fibrosis in the myocardium
of diabetic rats. Compared with the control group, the arrangement
of myocardial cells was markedly disordered, and collagen
deposition in cardiac extracellular matrix was increased in the STZ
group. The expression of collagen III in the myocardium was also
significantly increased in the STZ group. The results of H&E
and Masson's staining in the present study also demonstrated that
diabetes increased the relative disorganization of myocardial cells
and enhanced deposition of collagen in the STZ group, clearly
indicating that myocardial damage was caused by diabetes.
Furthermore, hyperglycemia induced an increase in the heart-to-body
weight (HW/BW) ratio. In addition, the results of the present study
demonstrated that there was a deregulation of MMPs/TIMPs expression
in the myocardial tissue of diabetic rats. All the abovementioned
results suggest that there was obvious cardiac interstitial
fibrosis in diabetic rats.
Numerous mechanisms may collectively contribute to
the development of DCM, including oxidative stress, insulin
resistance, myocardial inflammation and ER stress (14,15). However, the exact molecular
mechanisms that trigger and fuel these major pathological processes
are not entirely clear. An increasing number of clinical and
experimental studies indicate that sustained hyperglycemia results
in reduced antioxidant capacity and increased oxidative stress that
are involved in development of diabetes and its complications
(16–18). Inflammation is currently
recognized as a key contributor to the pathogenesis of diabetes and
its cardiovascular complications (19–21), and the diabetic myocardium
exhibits increased levels of proinflammatory cytokines, such as
TNF-α and IL-6 (22). Increased
oxidative stress is another common characteristic in models of DCM.
Oxidative stress indicates a severe imbalance between the
generation of reactive oxygen species (ROS) and their clearance by
antioxidant defense systems, which make the heart highly
susceptible to oxidative damage (23,24). ROS may induce lipid peroxidation
and result in the increased expression of MDA and 4-HNE. There are
natural detoxification molecules in the heart that reduce or
scavenge ROS, such as SOD and GSH. ROS, as the main inducer of
oxidative stress in vivo, may also result in cell apoptosis.
Accordingly, ROS and oxidative stress play an important role in the
occurrence and development of diabetic cardiovascular complications
(25,26). In the present study, oxidative
stress was evaluated through detecting the content of MDA, 4-HNE,
GSH and SOD in myocardial cells by ELISA. The expression of
collagen III, MMP8, MMP14, TIMP2, TGF-β, CSE, eIF2α, GRP94, Bcl-2,
caspase-3, TNF-α, NF-κB, JAK-1/2 and STAT1/3/5/6 was analyzed by
western blotting and the results demonstrated that the expression
of MDA, 4-HNE, TNF-α, NF-κB, TGF-β, eIF2α, GRP94 and caspase-3 was
significantly increased in the STZ group, and the expression of
SOD, GSH and Bcl-2 was markedly decreased in the STZ group. These
results suggest that there was significantly increased oxidative
stress in the myocardium of diabetic rats. Furthermore, there was
overactivation of ER stress pathways. Inflammatory factors, such as
TNF-α, NF-κB and TGF-β, were also significantly increased. In the
STZ group, the extent of myocardial tissue damage gradually
increased, along with a significantly increased apoptotic cell
rate, demonstrating that cells failed to repair themselves. The
result of the TUNEL assay revealed that, compared with the control
group, there was a significantly increased number of apoptotic
cells in the myocardium of diabetic rats. The expression of
caspase-3 was significantly upregulated in the myocardium of
diabetic rats, while that of Bcl-2 was significantly
downregulated.
ER stress is an adaptive response of cells to
ischemia, hypoxia and hyperglycemia (27,28). Oxidative stress may lead to ER
stress (29), and GRP94 and eIF2α
are classic markers of ER stress. Consistent with previous studies
(30,31), the expression of GRP94 and eIF2α
in the myocardium of diabetic rats were obviously upregulated in
the present study, suggesting the presence of excessive ER stress.
TGF-β1 is currently considered as one of the most important factors
promoting myocardial fibrosis and is considered to play an
important role in this process (32). It has been suggested that TGF-β1
plays a key role in myocardial interstitial fibrosis in DCM
(33). In our experiments, the
TGF-β1 expression in the myocardium of rats with DCM was
significantly higher compared with that in control rats, and was
positively correlated with the content of collagen; thus, TGF-β may
be involved in the regulation and crosstalk of oxidative stress, ER
stress, inflammation and apoptosis of cardiomyocytes.
The JAK/STAT signaling pathway is an important
cytokine signal transduction pathway and a pleiotropic cascade that
is crucial for cytokine and growth hormone receptor signaling, and
regulates diverse physiological and pathological processes,
including proliferation, differentiation, apoptosis and
inflammation (34). In the
present study, the expression of GRP94 and eIF2α in the myocardium
of diabetic rats were found to be obviously upregulated, suggesting
the presence of excessive ER stress. The activation of the JAK/STAT
signal transduction pathway may upregulate the expression of TGF-β
and type I and III collagens, leading to the occurrence of fibrosis
(35,36). It was recently demonstrated that
the exposure of glomerular mesangial cells to high glucose caused
the activation of JAK-2, STAT1, STAT3 and STAT5, along with an
increase in TGF-β1 and fibronectin synthesis (37). Conversely, it was reported that
inhibiting JAK/STAT signaling in mesangial cells cultured in high
glucose may decrease the synthesis of TGF-β1 and fibrin (38). The JAK/STATs signal transduction
pathway is closely associated with myocardial fibrosis and cardiac
hypertrophy caused by pressure overload, heart failure and cardiac
dysfunction induced by ischemia-reperfusion (8). In our study, the expression levels
of JAK-1, JAK-2 and STATs were significantly upregulated in rats of
the STZ group compared with control rats, and the relative contents
of JAK-1/2 and STATs were positively correlated with the content of
collagen and the expression of TGF-β1 in the myocardium. It is
suggested that high glucose or glycosylated products in diabetes
induce oxidative stress to produce inflammatory cytokines followed
by the activation of JAK/STAT and TGF-β signaling.
H2S is an endogenously produced gaseous
molecule that plays an important role in cellular signaling and
possesses potent anti-inflammatory, antioxidant, and other
regulatory properties (39,40). In addition, H2S has
been shown to exert potent cytoprotective effects against tissue
injury as well as antifibrotic effects, including prevention of
myocardial fibrosis (41). The
reported cytoprotective effects of H2S are partially
associated with its ability to neutralize ROS, reduce apoptotic
signaling, and reversibly modulate mitochondrial respiration
(42). However, the association
between H2S and JAK/STAT signaling remains unclear. Our
results demonstrated that the expression level of CSE was
significantly downregulated in rats of the DCM group compared with
control rats, suggesting that diabetes impairs the expression of
endogenous H2S/CSE. It was observed that, compared with
the rats in the STZ group, the expression of MDA, 4-HNE, TNF-α,
NF-κB, TGF-β, eIF2α, GRP94 and caspase-3 was significantly
downregulated and the expression of SOD, GSH and Bcl-2 was
significantly higher in the myocardium of rats treated with the
H2S donor NaHS. The levels of JAK-1/2 and STATs were
lower, the expression of TGF-β1 was downregulated, the content of
collagen in the myocardium decreased, and the level of myocardial
fibrosis was visibly reduced in the myocardium of rats in the STZ +
H2S group. H2S was able to attenuate matrix
deposition and myocardial fibrosis, reduce apoptosis, alleviate
inflammation, improve the deregulation of MMPs/TIMPs, and inhibit
excessive oxidative stress and ER stress in the myocardium of
diabetic rats. The protective mechanism of H2S against
diabetic myocardial fibrosis may be associated with the
downregulation of JAK/STAT and TGF-β1 signaling. These findings
indicate that H2S exerts a protective effect against
myocardial interstitial fibrosis in DCM by downregulating JAK/STAT
signaling.
Moreover, these results suggest that H2S,
acts as a scavenger of ROS, enhances the endogenous antioxidant
defenses and creates an environment resistant to oxidative stress
in the myocardium of diabetic rats. Another major finding of the
present study is that the JAK/STAT signaling pathway plays an
important role in mediating the cardioprotective effects of
H2S. To date, it has not been fully elucidated how
H2S exerts its beneficial effect against cardiac
fibrosis under diabetic conditions via the JAK/STAT signaling
pathway. However, as H2S attenuates diabetes-induced
oxidative damage and the subsequent cardiac fibrosis, it appears to
be a promising novel therapeutic strategy for the prevention and
treatment of DCM.
Glossary
Abbreviations
Abbreviations:
|
H2S
|
hydrogen sulfide
|
|
NaHS
|
sodium hydrosulfide
|
|
DCM
|
diabetic cardiomyopathy
|
|
CSE
|
cystathionine-γ-lyase
|
|
MDA
|
malondialdehyde
|
|
4-HNE
|
4-hydroxynonenal
|
|
GSH
|
glutathione
|
|
SOD
|
superoxide dismutase
|
|
STZ
|
streptozotocin
|
|
MMPs
|
matrix metalloproteinases
|
|
CRP
|
C-reactive protein
|
|
eIF2α
|
eukaryotic initiation factor 2α
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81202830 and
81270181).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bell DS: Diabetic cardiomyopathy. Diabetes
Care. 26:2949–2951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asbun J and Villarreal FJ: The
pathogenesis of myocardial fibrosis in the setting of diabetic
cardiomyopathy. J Am Coll Cardiol. 47:693–700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thandavarayan RA, Giridharan VV, Watanabe
K and Konishi T: Diabetic cardiomyopathy and oxidative stress: role
of antioxidants. Cardiovasc Hematol Agents Med Chem. 9:225–230.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar S, Prasad S and Sitasawad SL:
Multiple antioxidants improve cardiac complications and inhibit
cardiac cell death in streptozotocin-induced diabetic rats. PLoS
One. 8:e670092013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varga ZV, Giricz Z, Liaudet L, Haskó G,
Ferdinandy P and Pacher P: Interplay of oxidative,
nitrosative/nitrative stress, inflammation, cell death and
autophagy in diabetic cardiomyopathy. Biochim Biophys Acta.
1852:232–242. 2015. View Article : Google Scholar
|
|
8
|
Kiu H and Nicholson SE: Biology and
significance of the JAK/STAT signalling pathways. Growth Factors.
30:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura H: Production and physiological
effects of hydrogen sulfide. Antioxid Redox Signal. 20:783–793.
2014. View Article : Google Scholar :
|
|
10
|
Lavu M, Bhushan S and Lefer DJ: Hydrogen
sulfide-mediated cardioprotection: mechanisms and therapeutic
potential. Clin Sci (Lond). 120:219–229. 2011. View Article : Google Scholar
|
|
11
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Du J, Zhao YT, Zhang L, Lv G,
Zhuang S, Qin G and Zhao TC: Histone deacetylase (HDAC) inhibition
improves myocardial function and prevents cardiac remodeling in
diabetic mice. Cardiovasc Diabetol. 14:992015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falcão-Pires I and Leite-Moreira AF:
Diabetic cardiomyopathy: understanding the molecular and cellular
basis to progress in diagnosis and treatment. Heart Fail Rev.
17:325–344. 2012. View Article : Google Scholar
|
|
14
|
Yang L, Zhao D, Ren J and Yang J:
Endoplasmic reticulum stress and protein quality control in
diabetic cardiomyopathy. Biochim Biophys Acta. 1852:209–218. 2015.
View Article : Google Scholar
|
|
15
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat
Rev Endocrinol. 12:144–153. 2016. View Article : Google Scholar :
|
|
16
|
Sun X, Chen RC, Yang ZH, Sun GB, Wang M,
Ma XJ, Yang LJ and Sun XB: Taxifolin prevents diabetic
cardiomyopathy in vivo and in vitro by inhibition of oxidative
stress and cell apoptosis. Food Chem Toxicol. 63:221–232. 2014.
View Article : Google Scholar
|
|
17
|
Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G,
Wang L, Peng Y, Dong X, Huang H and Si L: Heme oxygenase-1 prevents
cardiac dysfunction in streptozotocin-diabetic mice by reducing
inflammation, oxidative stress, apoptosis and enhancing autophagy.
PLoS One. 8:e759272013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taye A, Abouzied MM and Mohafez OM: Tempol
ameliorates cardiac fibrosis in streptozotocin-induced diabetic
rats: role of oxidative stress in diabetic cardiomyopathy. Naunyn
Schmiedebergs Arch Pharmacol. 386:1071–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malfitano C, Alba Loureiro TC, Rodrigues
B, Sirvente R, Salemi VM, Rabechi NB, Lacchini S, Curi R and
Irigoyen MC: Hyperglycaemia protects the heart after myocardial
infarction: aspects of programmed cell survival and cell death. Eur
J Heart Fail. 12:659–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Linthout S, Riad A, Dhayat N,
Spillmann F, Du J, Dhayat S, Westermann D, Hilfiker-Kleiner D,
Noutsias M, Laufs U, et al: Anti-inflammatory effects of
atorvastatin improve left ventricular function in experimental
diabetic cardiomyopathy. Diabetologia. 50:1977–1986. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Westermann D, Walther T, Savvatis K,
Escher F, Sobirey M, Riad A, Bader M, Schultheiss HP and Tschöpe C:
Gene deletion of the kinin receptor B1 attenuates cardiac
inflammation and fibrosis during the development of experimental
diabetic cardiomyopathy. Diabetes. 58:1373–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westermann D, Van Linthout S, Dhayat S,
Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A,
Schultheiss HP, et al: Tumor necrosis factor-alpha antagonism
protects from myocardial inflammation and fibrosis in experimental
diabetic cardiomyopathy. Basic Res Cardiol. 102:500–507. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu W, Zha W, Guo S, Cheng H, Wu J and Liu
C: Flos Puerariae extract prevents myocardial apoptosis via
attenuation oxidative stress in streptozotocin-induced diabetic
mice. PLoS One. 9:e980442014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frustaci A, Kajstura J, Chimenti C,
Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B and Anversa P:
Myocardial cell death in human diabetes. Circ Res. 87:1123–1132.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yaras N, Sariahmetoglu M, Bilginoglu A,
Aydemir-Koksoy A, Onay-Besikci A, Turan B and Schulz R: Protective
action of doxycycline against diabetic cardiomyopathy in rats. Br J
Pharmacol. 155:1174–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tajiri S, Oyadomari S, Yano S, Morioka M,
Gotoh T, Hamada JI, Ushio Y and Mori M: Ischemia-induced neuronal
cell death is mediated by the endoplasmic reticulum stress pathway
involving CHOP. Cell Death Differ. 11:403–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams JA, Hou Y, Ni HM and Ding WX:
Role of intracellular calcium in proteasome inhibitor-induced
endoplasmic reticulum stress, autophagy, and cell death. Pharm Res.
30:2279–2289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang C, Koulajian K, Schuiki I, Zhang L,
Desai T, Ivovic A, Wang P, Robson-Doucette C, Wheeler MB, Minassian
B, et al: Glucose-induced beta cell dysfunction in vivo in rats:
link between oxidative stress and endoplasmic reticulum stress.
Diabetologia. 55:1366–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miki T, Miura T, Hotta H, Tanno M, Yano T,
Sato T, Terashima Y, Takada A, Ishikawa S and Shimamoto K:
Endoplasmic reticulum stress in diabetic hearts abolishes
erythropoietin-induced myocardial protection by impairment of
phospho-glycogen synthase kinase-3beta-mediated suppression of
mitochondrial permeability transition. Diabetes. 58:2863–2872.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeglinski MR, Roche P, Hnatowich M, Jassal
DS, Wigle JT, Czubryt MP and Dixon IM: TGFβ1 regulates Scleraxis
expression in primary cardiac myofibroblasts by a Smad-independent
mechanism. Am J Physiol Heart Circ Physiol. 310:H239–H249. 2016.
View Article : Google Scholar
|
|
33
|
Liu Y and Zhang J: Nox2 contributes to
cardiac fibrosis in diabetic cardiomyopathy in a transforming
growth factor-β dependent manner. Int J Clin Exp Pathol.
8:10908–10914. 2015.
|
|
34
|
Duhé RJ: Redox regulation of Janus kinase:
the elephant in the room. JAKSTAT. 2:e261412013.
|
|
35
|
Shi K, Jiang J, Ma T, Xie J, Duan L, Chen
R, Song P, Yu Z, Liu C, Zhu Q, et al: Dexamethasone attenuates
bleomycin-induced lung fibrosis in mice through TGF-β, Smad3 and
JAK-STAT pathway. Int J Clin Exp Med. 7:2645–2650. 2014.
|
|
36
|
Matsui F and Meldrum KK: The role of the
Janus kinase family/signal transducer and activator of
transcription signaling pathway in fibrotic renal disease. J Surg
Res. 178:339–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi Y, Zhang Y, Wang C, Du C, Zhao S, Qi
Z, Zhang Q and Duan H: Suppressor of cytokine signaling-1 reduces
high glucose-induced TGF-beta1 and fibronectin synthesis in human
mesangial cells. FEBS Lett. 582:3484–3488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boengler K, Hilfiker-Kleiner D, Drexler H,
Heusch G and Schulz R: The myocardial JAK/STAT pathway: from
protection to failure. Pharmacol Ther. 120:172–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calvert JW, Coetzee WA and Lefer DJ: Novel
insights into hydrogen sulfide - mediated cytoprotection. Antioxid
Redox Signal. 12:1203–1217. 2010. View Article : Google Scholar :
|
|
40
|
Xu W, Chen J, Lin J, Liu D, Mo L, Pan W,
Feng J, Wu W and Zheng D: Exogenous H2S protects H9c2
cardiac cells against high glucose-induced injury and inflammation
by inhibiting the activation of the NF-κB and IL-1β pathways. Int J
Mol Med. 35:177–186. 2015. View Article : Google Scholar
|
|
41
|
Xiao T, Luo J, Wu Z, Li F, Zeng O and Yang
J: Effects of hydrogen sulfide on myocardial fibrosis and
PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep.
13:1765–1773. 2016. View Article : Google Scholar
|
|
42
|
Zhou X and Lu X: Hydrogen sulfide inhibits
high-glucose-induced apoptosis in neonatal rat cardiomyocytes. Exp
Biol Med (Maywood). 238:370–374. 2013. View Article : Google Scholar
|