Introduction
Age-related hearing loss (ARHL), also known as
presbycusis, is a complex degenerative disease characterized by
declining auditory function, including increased hearing thresholds
and reduced frequency resolution (1). ARHL is one of the most prevalent and
chronic conditions of older populations, affecting tens of millions
of people worldwide (2), and may
cause social isolation, depression, and even dementia (3). In addition to degeneration of the
peripheral auditory organs, auditory cortex degeneration has been
demonstrated to serve a crucial role in ARHL pathogenesis (4,5);
however, the molecular mechanism is not well understood.
D-galactose (D-gal) is a reducing sugar and is
oxidized into aldehydes and H2O2 when present
at high levels (6). Chronic
systemic exposure of rats to D-gal has been used as an aging model
for many years and is widely used to study atherosclerosis
(7), reproductive system diseases
(8,9) and neurodegenerative diseases,
including Alzheimer’s disease (10) and ARHL (11,12).
Autophagy is a ubiquitous process that occurs in
plant, animal, and fungal cells (13), and three types of autophagy have
been described: Macroautophagy (hereafter referred to as
autophagy), microautophagy and chaperone-mediated autophagy
(14). In mammals, autophagy
maintains homeostasis by regulating the degradation of cellular
organelles and macromolecules and may be activated by starvation,
hypoxia and exposure to toxic agents (13,15–18). ULK1 is a serine/threonine kinase,
a homologue of yeast autophagy-related protein (Atg)1. In
combination with Atg13, FIP200 and Atg101, ULK1 initiates the
autophagy process (19). Notably,
ULK1 may be phosphorylated by mechanistic target of rapamycin
(mTOR) and 5′ AMP-activated protein kinase (AMPK) at different
sites and perform dual functions (20). mTOR is a well-known negative
regulator of autophagy, it phosphorylates ULK1 at Ser 757 when
nutrient levels are sufficient and represses its protein kinase
activity, thereby blocking the initiation of autophagy (21). However, in response to nutrient
deprivation, AMPK is activated and stimulates autophagy by directly
activating ULK1 via phosphorylation of Ser 317 and Ser 777
(20). In addition, AMPK promotes
activation of autophagy by inhibiting the activity of mTOR complex
1 through phosphorylation of either Raptor or tuberous sclerosis
complex 2, which subsequently suppresses the activity of mTOR
(22,23). In addition to changes in nutrient
levels, AMPK and mTOR may be activated by oxidative stress
(24,25), and studies have indicated that
AMPK and mTOR are essential modulators of the aging process
(26,27). Additionally, previous studies have
indicated that AMPK- and mTOR-dependent autophagy is involved in
neurodegenerative diseases (28–30). Therefore, it was hypothesized that
AMPK and mTOR are involved in the regulation of autophagy in the
degeneration of the auditory cortex in the present model.
Furthermore, Tsuchihashi et al (25) suggested that 4E binding protein 1
(4EBP1), a key substrate of mTOR, impairs autophagy independent of
mTOR, leading to premature senescence in auditory cells (25). However, there were no further
studies on this, and whether 4EBP1 regulates autophagy in the
degeneration of the auditory cortex is unknown. Thus, this issue
was investigated in the present study.
Autophagy has been demonstrated to serve important
roles in various diseases, including cancer, heart disease,
neurodegeneration, autoimmune diseases, aging and infection
(31–35). At the organismal and cellular
levels, autophagy has pro-death or pro-survival functions and has
different interactions with apoptosis depending on the context
(36–38). Nevertheless, to the best of our
knowledge, no studies have focused on the role of autophagy and its
interactions with apoptosis in central presbycusis.
Changes of autophagy in the
physiological/pathological processes of the auditory cortex are
unclear, and the role of autophagy, as well as its related
mechanisms, in the degeneration of the auditory cortex has not been
studied. Therefore, the present study assessed changes of autophagy
in the auditory cortex of naturally aging rats and mimetic aging
rats by 8 weeks of D-gal injection, and further investigated the
role of autophagy and its related mechanism in the degeneration of
the auditory cortex.
Materials and methods
Ethics statement
All animal procedures were performed in strict
accordance with the recommendations of the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(39). The experimental
procedures were performed with the approval of the Committee on the
Ethics of Animal Care and Use of Huazhong University of Science and
Technology (Wuhan, China; permit no. IACUC S512).
Animal procedures
A total of 180 male Sprague Dawley (SD) rats
(70.10±11.46 g, 3-weeks old) were obtained from the Experimental
Animal Centre of Tongji Medical College, Huazhong University of
Science and Technology. The rats were individually maintained in a
temperature-controlled (23±2°C with 50–60% relative humidity) room
with a 12-h light/dark cycle and free access to water and food.
Prior to treatment, the rats were allowed to acclimate for 1 week
and were then randomly divided into a control group (n=90) and a
mimetic aging group (n=90). The rats in the mimetic aging group
were treated with D-gal (500 mg/kg/day; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) via subcutaneous injection for 8 weeks. The
rats in the control group were injected with normal saline (NS; 500
mg/kg/day) on the same schedule. After the 8-week injection
protocol, the rats in the two groups were further divided into a
3-month-old subgroup (just after the last injection), a 9-month-old
subgroup (6 months after the last injection), and a 15-month-old
subgroup (12 months after the last injection), in which the rats
were sacrificed at 3, 9 and 15 months, respectively.
DNA extraction and quantification of the
mitochondrial (mt ) DNA 4,834-bp deletion
Total DNA of the auditory cortex was extracted using
a Genomic DNA Purification kit (Tiangen Biotech Co., Ltd., Beijing,
China), and TaqMan (Tiangen Biotech Co., Ltd.) quantitative
polymerase chain reaction (qPCR) analysis was used to determine the
proportion of the mtDNA common deletion (CD). The D-Loop region
copy was used as the conservative segment. The primers and probes
for the mtDNA CD and mtDNA D-loop are listed in Table I, as previously described
(40). PCR amplification was
performed using a LC-480 real-time PCR system (Roche Diagnostics,
Basel, Switzerland) in a 20-μl reaction volume. The cycling
conditions included an initial phase at 95°C for 30 sec, followed
by 40 cycles at 95°C for 5 sec and at 60°C for 30 sec. ΔCq
(Cqdeletion−CqD-loop) was used to reflect the
abundance of the mtDNA 4,834-bp deletion. The relative expression
indicating the factorial difference in the deletions between the NS
group and the D-gal-treated group was calculated using the
2−ΔΔCq method (41),
where ΔΔCq=ΔCqmtDNA deletion in D-gal-treated group −
ΔCqmtDNA deletion in NS group.
 | Table IProbes and primers used for
quantitative polymerase chain reaction analysis of mtDNA. |
Table I
Probes and primers used for
quantitative polymerase chain reaction analysis of mtDNA.
| Name | Probe/primer
direction | Sequence
(5′-3′) |
|---|
| D-Loop | Probe |
TTGGTTCATCGTCCATACGTTCCCCTTA |
| Forward primer |
GGTTCTTACTTCAGGGCCATCA |
| Reverse primer |
GATTAGACCCGTTACCATCGAGAT |
| Common
deletion | Probe |
TCACTTTAATCGCCACATCCATAACTGCTGT |
| Forward primer |
GATTAGACCCGTTACCATCGAGAT |
| Reverse primer |
CGAAGTAGATGATCCGTATGCTGTA |
Transmission electron microscopy
(TEM)
Following deep anesthesia, a total of 36 rats
(n=6/subgroup) were perfused transcardially with 2.5%
glutaraldehyde subsequent to brief perfusion with 0.9% sodium
chloride. Following perfusion, the brain was dissected from the
skull, and the auditory cortex was separated from the brain.
Subsequently, the auditory cortex was fixed with the 2.5%
glutaraldehyde at 4°C for 12 h, and further fixation was performed
using 1% osmium tetroxide at 4°C. After 2 h, the auditory cortex
was dehydrated using a series of ascending graded ethanol (50, 70,
80, 85, 90, 95 and 100%). Next, the auditory cortex was treated
with propylene oxide for ~0.5 h at room temperature and then
embedded in graded araldite (A3183; Sigma-Aldrich; Merck KGaA; 1/3
and 1/2, pure araldite) for 12 h per step at 37°C. Following this,
the embedded samples were placed in a 60°C incubator for 48 h.
Serial ultrathin sections (60–100 nm) were collected on copper
grids and stained at room temperature with uranyl acetate for 30
min and then lead citrate for 10 min. A Tecnai G220 TWIN
(FEI; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
transmission electron microscope was used to observe the
ultrastructure of the sections at ×1,700 and ×5,000
magnification.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) staining
Apoptosis was detected using TUNEL staining.
Following deep anesthesia, a total of 36 rats (n=6/subgroup) were
transcardially perfused with 0.9% sodium chloride and 4%
paraformaldehyde solution. Following the perfusion, separated brain
sections were fixed with 4% paraformaldehyde solution overnight at
4°C. Following rinsing with distilled water, brain sections were
dehydrated in a graded alcohol series (70, 80, 90, 95, 100, 100 and
100%), and immersed in paraffin after clearing in xylene. Serial
5-mm coronal sections containing the auditory cortex were
deparaffinized in xylene and rehydrated through graded
concentrations of ethanol (100, 100, 95, 90, 80 and 70%). Following
a 0.5-h incubation with proteinase K (20 μg/ml; Roche
Diagnostics) at 37°C, the sections were stained using an In
Situ Cell Death Detection kit (Roche Diagnostics), according to
the ratio 1:9 of solution A and B, and incubated at 37°C for 1 h. A
DAPI staining solution (1 μg/ml; Beyotime Institute of
Biotechnology, Haimen, China) was employed to counterstain the
nuclei at room temperature for 10 min. The labelled cells were
observed using a laser scanning confocal microscope (Nikon
Corporation, Tokyo, Japan), and six fields of view for each section
were observed.
RNA extraction and reverse transcription
(RT)-qPCR analysis
The auditory cortex tissues from 6 rats in each
subgroup were separated and prepared for RNA extraction using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). Following
this, an aliquot of the extracted RNA was immediately reverse
transcribed to cDNA using a PrimeScript RT Reagent kit (Takara Bio,
Inc., Otsu, Japan). qPCR was performed with a LightCycler 480
RT-PCR system (Roche Diagnostics) using SYBR-Green II PCR Master
Mix (Takara Bio, Inc.). The primers were designed by Takara Bio,
Inc., and are listed in Table
II. β-actin was included as an endogenous control. The
amplification conditions were as follows: 95°C for 5 min; 45 cycles
of 95°C for 10 sec, 60°C for 20 sec, and 72°C for 20 sec; followed
by 95°C for 5 sec and 65°C for 60 sec. The relative mRNA expression
levels in the control and mimetic aging groups were calculated
using the 2−ΔΔCq method.
 | Table IIPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Genes | Primer sequence
(5′-3′) | Accession no. |
|---|
| LC3 | F:
GTTAAGCCCCTACCAAGGCA | NM_022867.2 |
| R:
AGGGACTGTTTCCAGGGACT | |
| BECN1 | F:
GAATGGAGGGGTCTAAGGCG | NM_001034117.1 |
| R:
CTTCCTCCTGGCTCTCTCCT | |
| BCL2-associated X
protein | F:
GGGCCTTTTTGCTACAGGGT | NM_017059.2 |
| R:
TTCTTGGTGGATGCGTCCTG | |
| BCL-extra
large | F:
ATCTTGGCTTTGGATCCTGG | NM_031535.2 |
| R:
GGGGCTTCAGTCCTGTTCTC | |
| BCL2 | F:
TCGCGACTTTGCAGAGATGT | NM_016993.1 |
| R:
CAATCCTCCCCCAGTTCACC | |
| β-actin | F:
GCAGGAGTACGATGAGTCCG | NM_031144.3 |
| R:
ACGCAGCTCAGTAACAGTCC | |
Protein extraction and western
blotting
The protein expression levels were examined using
western blotting. A total of 36 rats (n=6/subgroup) were
sacrificed, and their auditory cortices were dissected and lysed in
radioimmunoprecipitation assay lysis solution (Beyotime Institute
of Biotechnology) containing phosphatase inhibitors and PMSF. A BCA
Protein Assay kit (Beyotime Institute of Biotechnology) was used to
determine the protein concentrations. The proteins were loaded onto
6 and 12% SDS-PAGE, separated, and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were incubated in blocking solution [5% non-fat milk (BD
Biosciences, Franklin Lakes, NJ, USA) in Tris-buffered saline] for
1 h at room temperature. Subsequently, the membranes were incubated
overnight at 4°C with the following primary antibodies: LC3B
(ab192890; 1:1,000; Abcam, Cambridge, MA, USA), Beclin 1 (BECN1;
ab217179; 1:1,000; Abcam), p62 (ab155686; 1:1,000; Abcam), cleaved
caspase 3 (CASP3; 9664; 1:500; Cell Signaling Technology, Inc.,
Danvers, MA, USA), B-cell lymphoma 2 (BCL2)-associated X protein
(BAX; 34260; 1:500; Signalway Antibody LLC, College Park, MD, USA),
BCL-extra large (BCL-xL; 21061; 1:500; Signalway Antibody LLC),
BCL2 (ab59348; 1:500; Abcam), phosphorylated (p)-AMPK (sc-33524;
1:1,000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-mTOR
(SAB4504476; 1:800; Sigma-Aldrich; Merck KGaA), p-ULK1 (14202;
1:1,000; Cell Signaling Technology, Inc.), Atg13 (ab201467;
1:1,000; Abcam), Atg5 (44254; 1:2,000; Signalway Antibody LLC),
Atg7 (38148; 1:500; Signalway Antibody LLC), p-4EBP1 (2855; 1:800;
Cell Signaling Technology, Inc.) and β-actin (ab8227; 1:3,000;
Abcam). The membranes were incubated with the corresponding
secondary antibodies (ANT019; ANT020; 1:4,000; AntGene Biotech Co.,
Ltd., Wuhan, China) for 60 min at room temperature. Enhanced
chemiluminescent Plus (Beyotime Institute of Biotechnology) was
used to visualize the membranes. The protein levels were normalized
to the level of β-actin in the corresponding lanes and ImageJ 10.0
software (National Institutes of Health, Bethesda, MD, USA) was
used for densitometry.
Immunofluorescence
The cleaved CASP3, LC3, BECN1, p62 and BCL2 protein
levels were also examined using immunofluorescence. Following deep
anesthesia, a total of 36 rats (n=6/subgroup) were transcardially
perfused, and separated brain sections were fixed with 4%
paraformaldehyde solution overnight at 4°C. Subsequent to rinsing
with distilled water, brain sections were dehydrated in graded
alcohol series (70, 80, 90, 95, 100, 100 and 100%), and immersed in
paraffin after clearing in xylene. A 5-um paraffin section was
deparaffinized in xylene and rehydrated through graded
concentrations of ethanol (100, 100, 95, 90, 80 and 70%). Following
10-min citrate solution antigen repair in boiling water, donkey
serum albumin (ANT051; AntGene Biotech Co., Ltd.) was used to block
nonspecific binding for 30 min at room temperature. Subsequently,
the sections were incubated overnight at 4°C with the
aforementioned primary antibodies diluted to 1:200. The sections
were then incubated with a secondary antibody (ANT024; 1:200;
AntGene Biotech Co., Ltd.) for 2 h after repeated washes with
Tris-buffered saline-Tween-20. Sections that were not incubated
with a primary antibody were used as the negative controls. A laser
scanning confocal microscope (Nikon Corporation) was used to
observe the staining at ×200 and ×600 magnifications.
Statistical analyses
The data were presented as the mean ± standard error
of the mean. The statistical analyses were performed using SPSS
20.0 software (IBM Corp., Armonk, NY, USA). Statistically
significant differences between the groups were determined using
one-way analysis of variance followed by Tukey’s post hoc test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
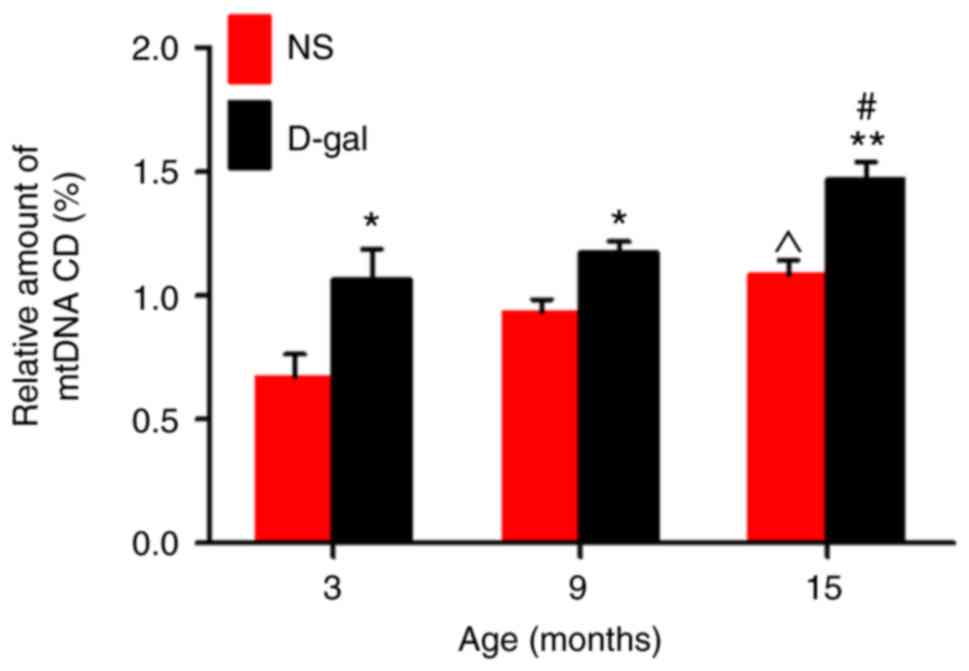
D-gal treatment elevates mtDNA CD in the
auditory cortex
CDs are also called mt 4,834-bp deletions and
correspond to mtDNA 4,977-bp deletions in humans (42). Previous research has indicated an
association between CD levels and presbycusis (43). TaqMan qPCR analysis demonstrated
that D-gal treatment induced significantly higher CD levels in the
D-gal groups at all ages compared with the levels in the
age-matched NS groups (Fig. 1).
In addition, the CDs accumulated with age in both the natural aging
group and the mimetic aging group.
D-gal treatment accelerates
neurodegeneration and activates apoptosis
Neurons in the auditory cortex in the NS group did
not display obvious ultrastructural changes in the 3- and
9-month-old rats (Fig. 2A and B).
However, in the 15-month-old rats in the NS group, swollen
mitochondria, disrupted myelin and lipofuscin were observed
(Fig. 2C). In the D-gal-treated
groups, normal nuclei, mitochondria, Golgi apparatus and rough
endoplasmic reticulum were observed in the 3-month-old subgroup
(Fig. 2D), whereas in the
9-month-old D-gal-treated rats, irregular nuclei, condensed
chromatin, accumulated lipofuscin, swollen mitochondria and
disrupted myelin were identified (Fig. 2E). Additionally, these changes
were more pronounced in the 15-month-old D-gal-treated rats
(Fig. 2F). Furthermore, more
autophagosomes and auto-lysosomes were observed in the 15-month-old
NS group and the 3- and 9-month-old D-gal subgroups, while few
autophagosome and auto-lysosomes were identified in the 3- and
9-month-old NS subgroups and 15-month-old D-gal subgroup,
particularly in the 15-month-old D-gal subgroup.
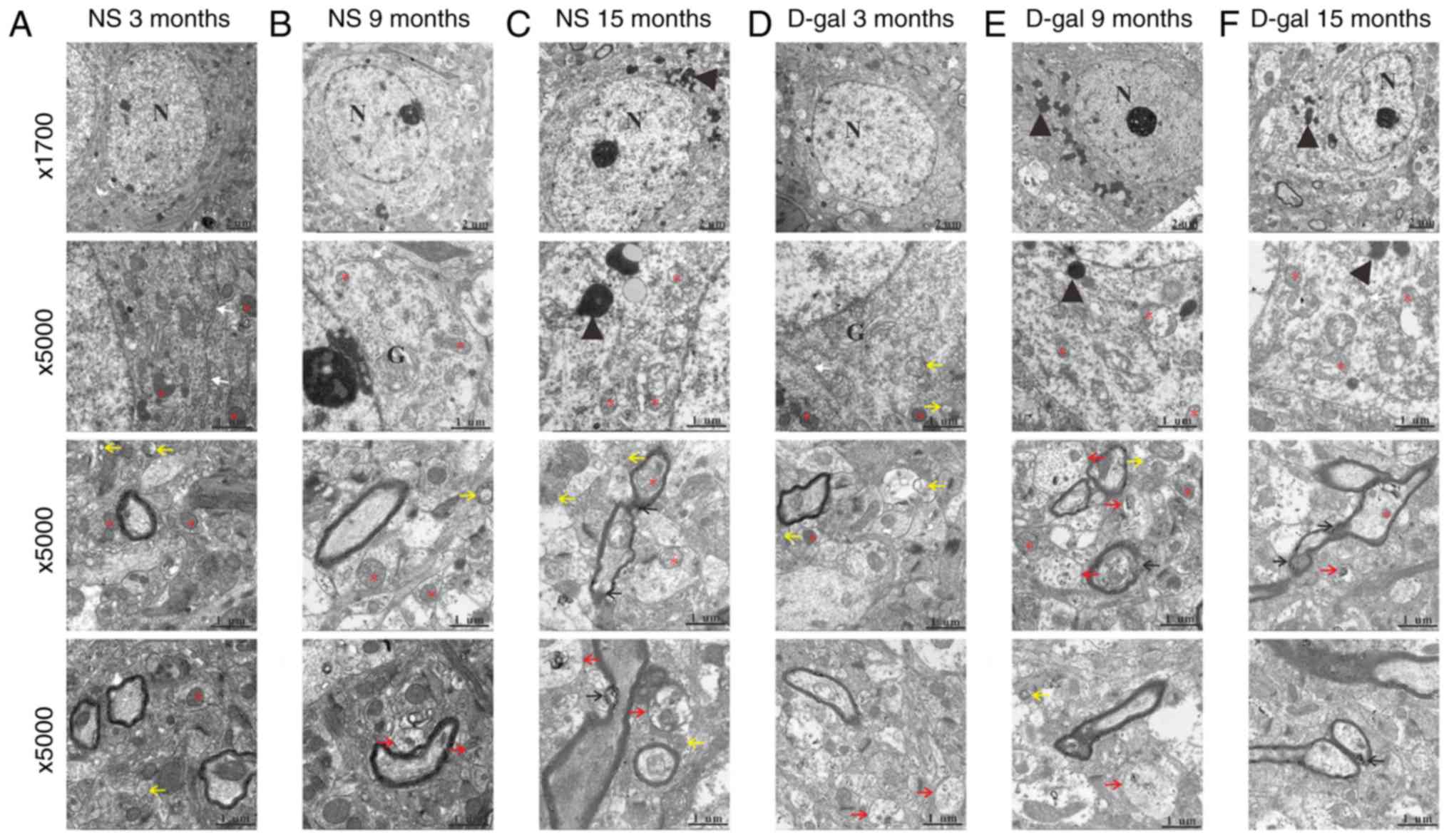 | Figure 2Ultrastructural morphology changes
with aging in the NS and D-gal groups. Transmission electron
microscopy demonstrated ultrastructural changes in the auditory
cortex of the NS and D-gal groups at different ages. Transmission
electron microscopy images of the NS groups at (A) 3, (B) 9 and (C)
15 months, and the D-gal groups at (D) 3, (E) 9 and (F) 15 months.
The red asterisks indicate the mitochondria; the black arrows
indicate the disrupted myelin; the yellow arrows indicate the
autophagosomes; the red arrows indicate the auto-lysosomes; the
white arrows indicate the endoplasmic reticulum; and the black
arrowheads indicate the lipofuscin. Scale bar, 2 mm at
magnification, ×1,700 and 1 mm at magnification, ×5,000. Sections
were stained with uranyl acetate and lead citrate. N, nucleus; G,
Golgi apparatus; NS, normal saline; D-gal, D-galactose. |
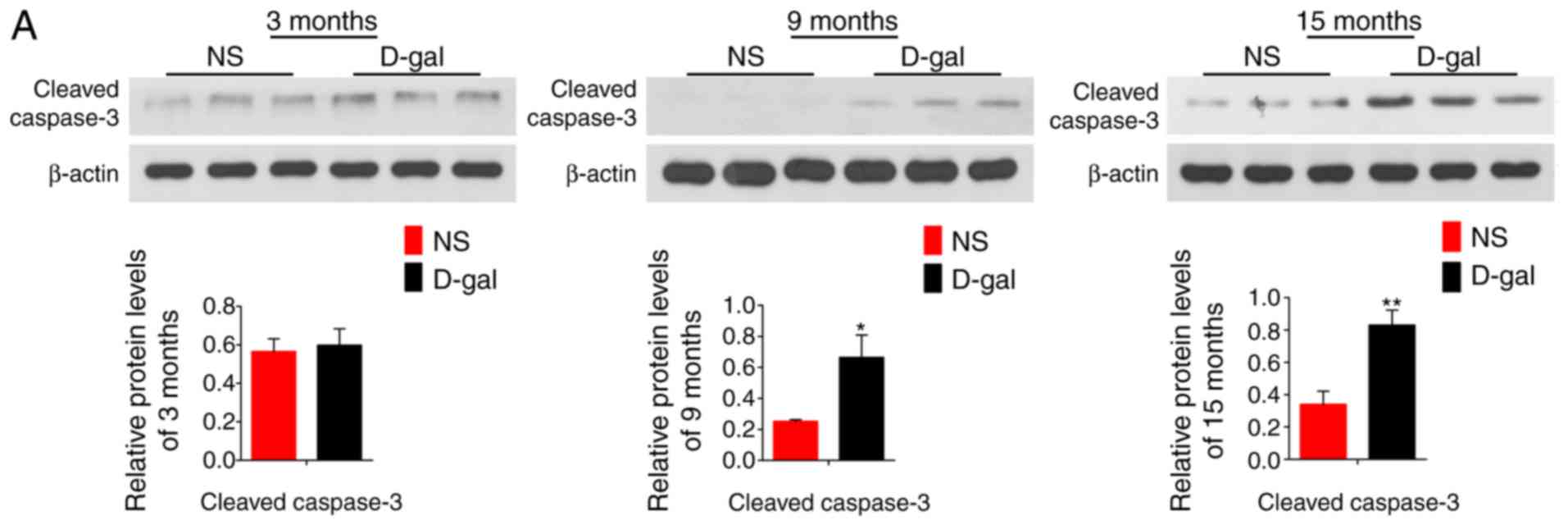
The detected protein level of cleaved CASP3 by
western blotting and immunofluorescence assays, as well as the
results of TUNEL staining (Fig.
3), revealed that apoptosis levels were significantly increased
in the 9- and 15-month-old D-gal groups compared with the levels in
the respective NS control groups, and this level increased with age
in both groups.
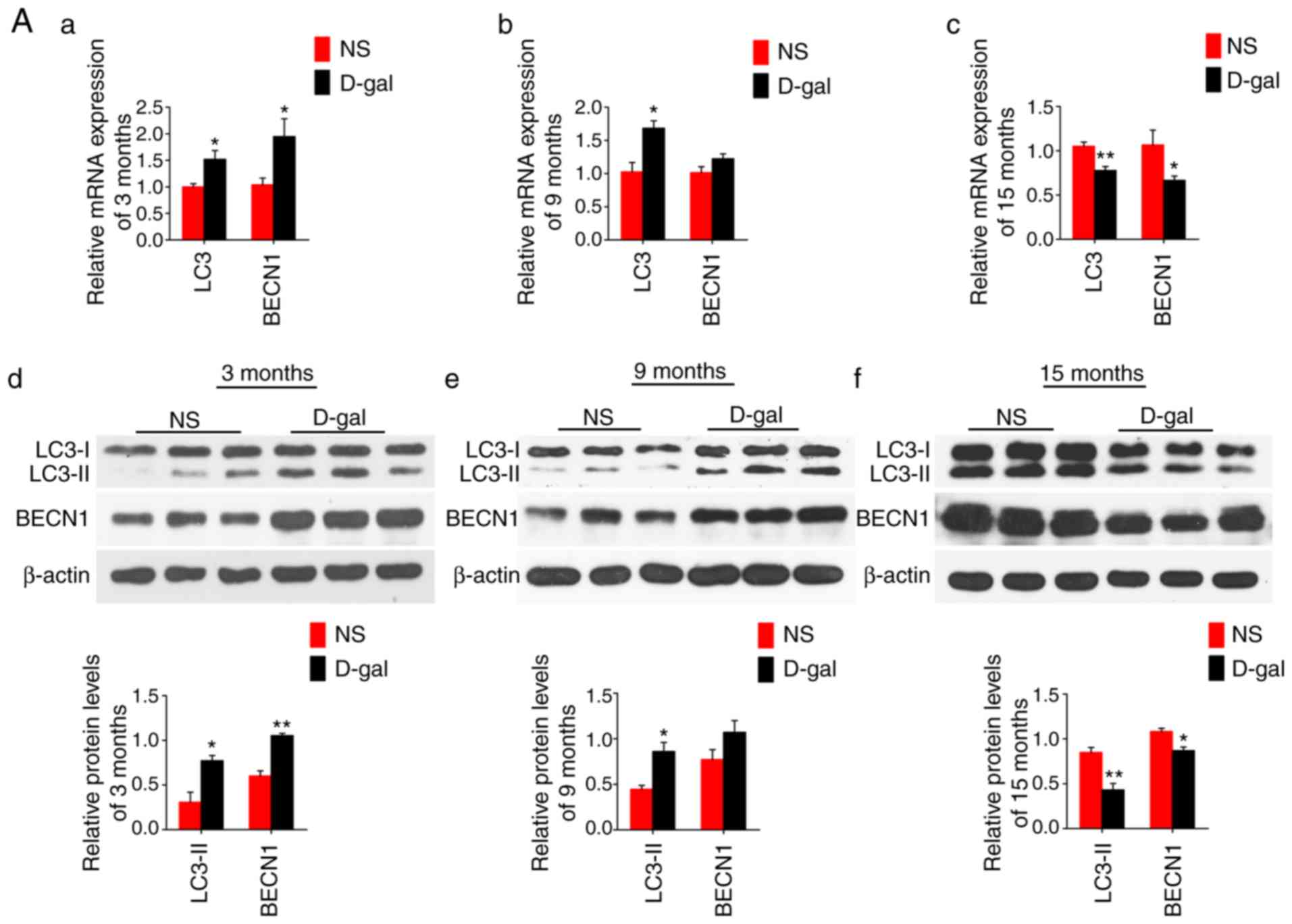
D-gal treatment enhances autophagy
activity and p62 degradation
The mRNA levels of LC3 and BECN1 were increased at 3
and 9 months in the D-gal-treated groups; however, these levels
were decreased at 15 months compared with those in the age-matched
NS control groups (Fig. 4A a–c).
The same trend was observed for the protein expression levels of
LC3 and BECN1 (Fig. 4A d–f). The
immunofluorescence images demonstrating the protein levels of LC3
and BECN1 in the auditory cortex with aging are shown in Fig. 4B. It was demonstrated that
autophagy was increased from 3 months to 15 months in the NS group,
while it decreased with aging in the D-gal group.
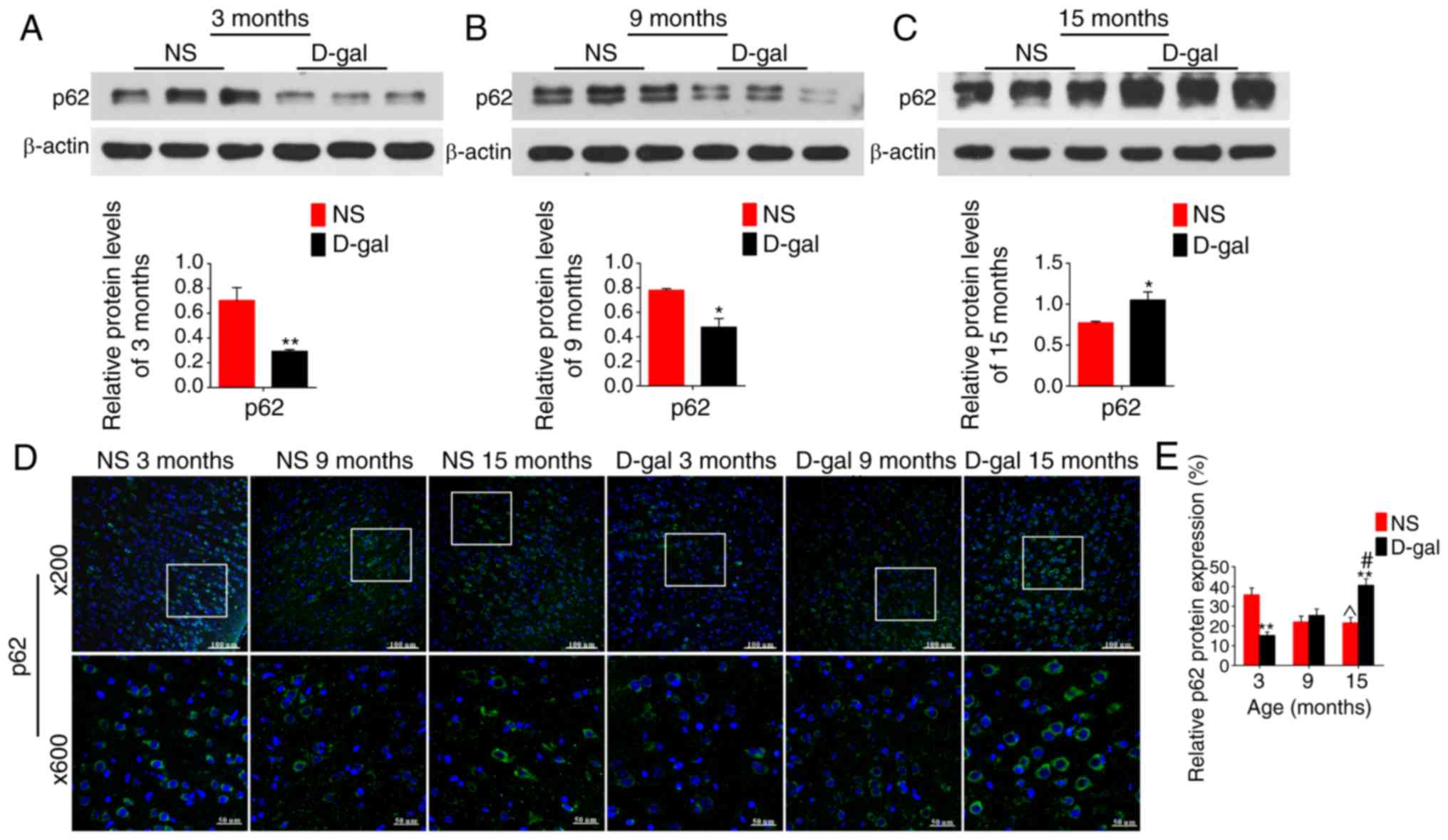
Furthermore, in addition to LC3 and BECN1, p62
protein expression levels were detected using western blotting and
immunofluorescence assays. p62 expression levels were significantly
decreased in the 3-month-old D-gal subgroup compared with the
levels in the age-matched NS group, while these levels were
significantly increased at 15 months compared with those in the NS
groups (Fig. 5). Additionally,
these autophagy-related proteins demonstrated approximately
equivalent levels in the 9-month-old D-gal induced mimetic aging
group and 15-month-old NS group.
Apoptosis and autophagy levels in the
auditory cortex change with aging
By investigating the levels of CD, and cell
apoptosis and neuronal degeneration at 3, 9 and 15 months in the
two groups, the present results demonstrated that the aging level
in the 15-month-old rats in the NS group was higher than those in
9-month-old NS subgroup. Additionally, the present results
indicated that 9-month-old rats in the D-gal-treated group
presented an approximately similar life status compared with that
of the 15-month-old rats in NS group, which was further confirmed
by immunofluorescence analyses of LC3 and BECN1. Furthermore,
neuronal degeneration and cell apoptosis were more apparent in the
15-month-old D-gal subgroup than the 9-month-old D-gal subgroup.
The present results revealed that the function of D-gal exposure
that accelerated the aging in rats was confirmed in the present
study, which is consistent with previously research (6,44).
The lifespan of the majority of SD rats is 2.5–3 years, and 15
months is equivalent to the late adult period of rats and roughly
equivalent to 40-year-old humans (45). In other words, rats in the
15-month-old NS subgroup and 9-month-old D-gal subgroup were
classified as late adults; 15-month-old rats in the D-gal-induced
mimetic aging group demonstrated more severe aging than that of
other subgroups and should be classified in the old period.
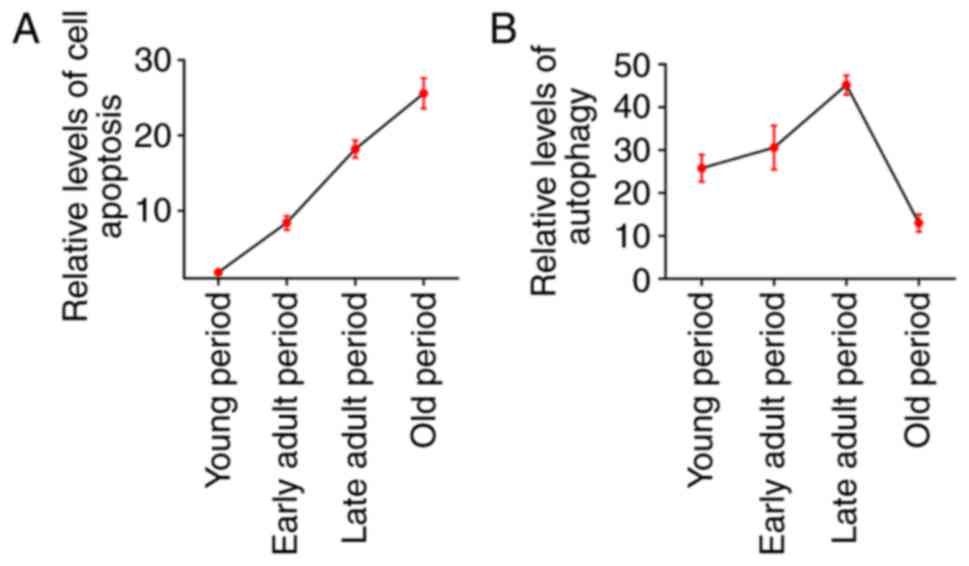
Based on the present results, it was demonstrated
that cell apoptosis level increased with aging in the auditory
cortex of SD rats, while the level of autophagy showed an
increasing trend from young to adult rats and decreased at an old
age (Fig. 6).
D-gal treatment initially increases
anti-apoptotic proteins, including BCL2 and BCL-xL, and then
impairs them
Previous research has suggested that autophagy has
an anti-apoptotic effect in injured spinal cord neurons (46). To investigate the role of
autophagy in the present study, in addition to the detection of
apoptosis level by TUNEL analysis and cleaved CASP3, the changes in
the BCL2 family proteins (BCL2, BCL-xL and BAX) were also assessed.
mRNA and protein expression levels of anti-apoptotic proteins, BCL2
and BCL-xL, as well as the ratio of BCL2/BAX and BCL-xL/BAX were
significantly increased at 3 months in the mimetic aging groups but
decreased at 15 months compared with those in the age-matched NS
control groups. However, the mRNA and protein levels of
pro-apoptotic protein, BAX, demonstrated no significant changes at
3 months in the mimetic aging groups, but increased significantly
at 9 and 15 months compared with those in the age-matched NS
control groups (Fig. 7A). The
immunofluorescence images demonstrated that BCL2 was increased from
3 to 15 months in the NS group, while it decreased with aging in
the D-gal group (Fig. 7B).
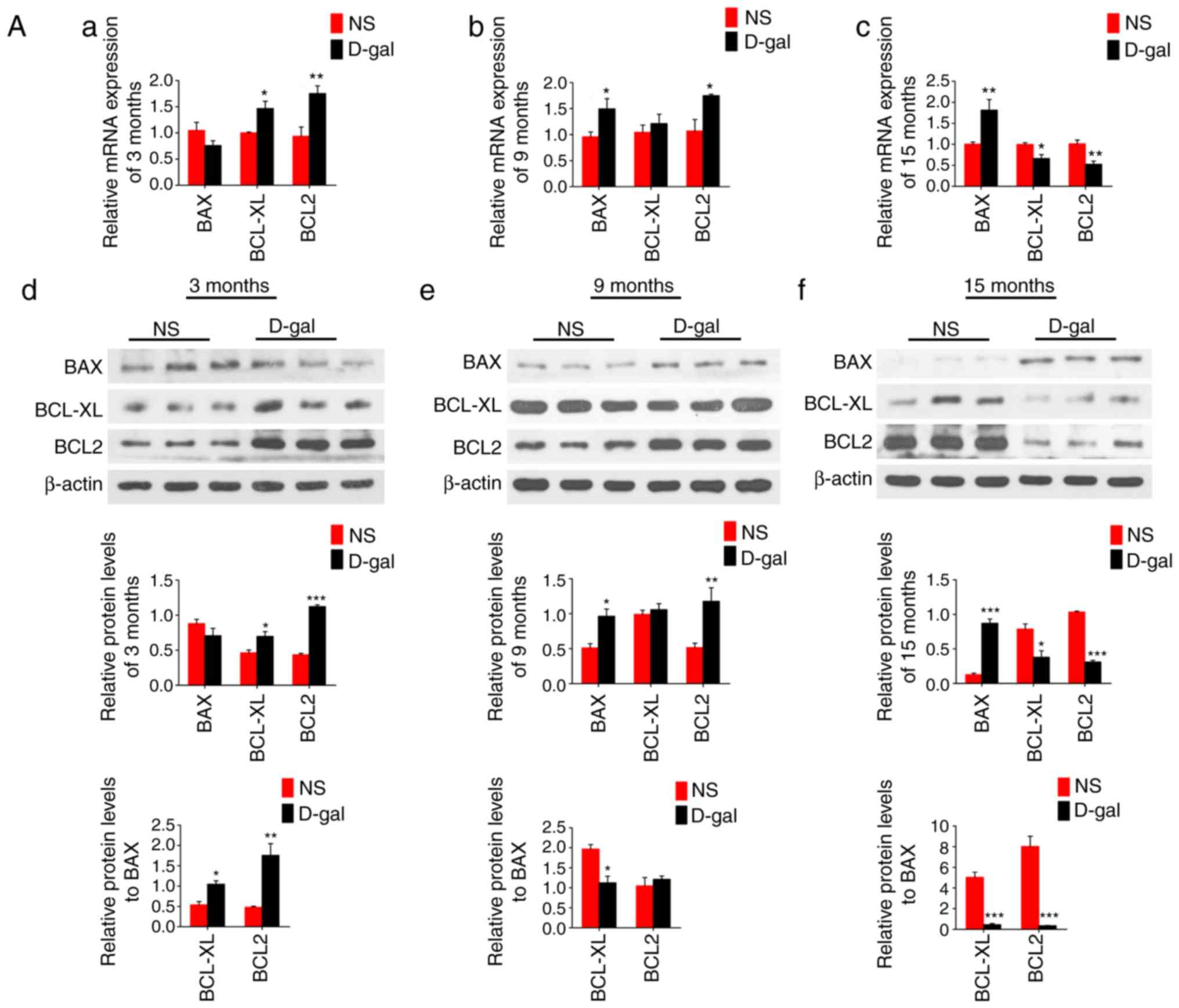 | Figure 7mRNA and protein expression levels of
BCL2, BCL-xL and BAX in the NS natural aging group and
D-gal-induced mimetic aging group. (Aa-c) Reverse
transcription-quantitative polymerase chain reaction and (Ad-f)
western blotting were used to study the mRNA and protein expression
levels in the D-gal group and NS group, respectively. β-actin was
used as a loading control. (B) Visualization of changes of BCL2
protein expression with aging was demonstrated using
immunofluorescence, and the nuclei were stained with DAPI. Data are
expressed as mean ± standard error of the mean.
*P<0.05, **P<0.01 and
***P<0.001 vs. the age-matched NS subgroup;
^P<0.01 vs. the 3-month-old NS subgroup; #P<0.001
vs. the 3-month-old D-gal subgroup. Scale bar, 100 mm at
magnification, ×200 and 50 mm at magnification, ×600. NS, normal
saline; D-gal, D-galactose; BCL2, B-cell lymphoma 2; BCL-xL, B-cell
lymphoma-extra large; BAX, B-cell lymphoma 2-associated X
protein. |
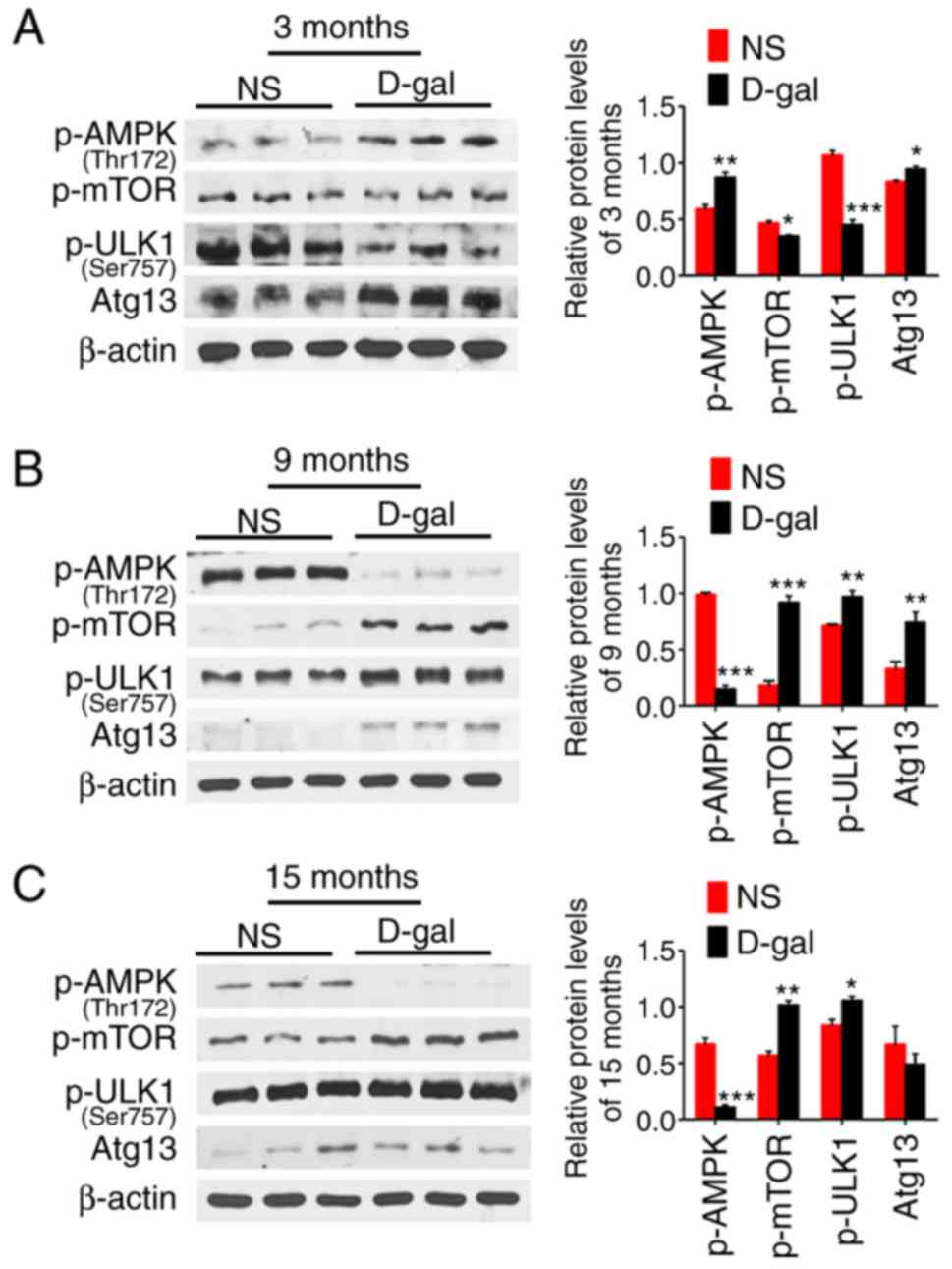
D-gal treatment induces activation of the
AMPK-mTOR-ULK1 signaling pathway in the auditory cortex
To investigate the hypotheses regarding the
activation and inhibition of autophagy, the protein expression
levels of the AMPK-mTOR-ULK1 signaling pathway in the auditory
cortex were detected using western blotting. Representative western
blots demonstrated that the expression of p-AMPK was significantly
increased in a compensatory manner at 3 months, but significantly
decreased at 9 and 15 months in the D-gal groups compared with its
expression in the age-matched NS group. However, p-mTOR and p-ULK1
demonstrated an inverse trend with p-AMPK. Additionally, the
autophagy-related protein Atg13 was significantly increased at 3
and 9 months in the D-gal group compared with the levels in the
age-matched NS group (Fig. 8),
which was consistent with the expression results for LC3-II and
BECN1.
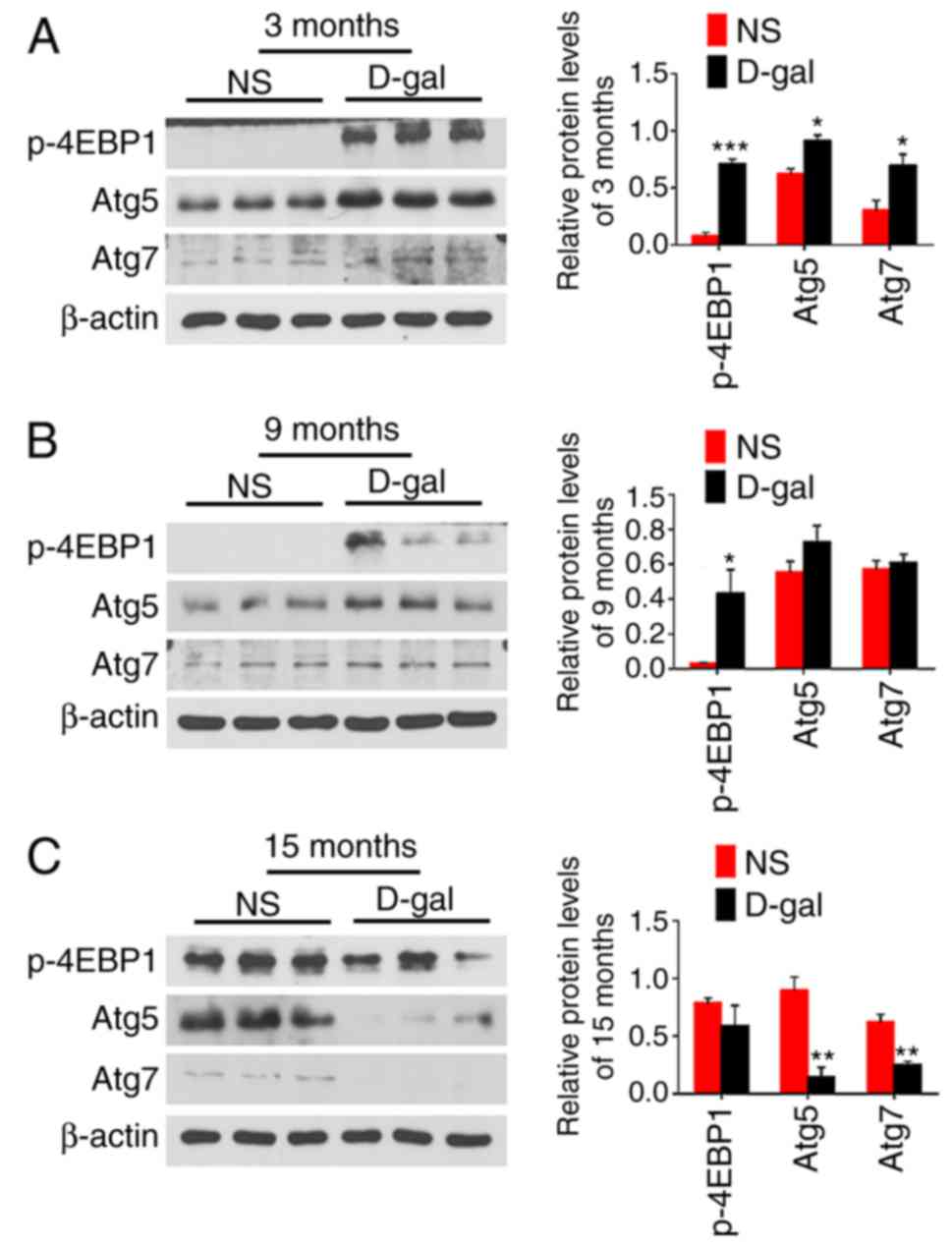
The 4EBP1 phosphorylation level was also
investigated. As demonstrated in Fig.
9, the expression level of p-4EBP1 was significantly increased
at 3 and 9 months in the D-gal groups compared with that in the
age-matched NS groups. However, the autophagy-related proteins Atg5
and Atg7 were significantly increased at 3 months, while
significantly decreased at 15 months in D-gal groups compared with
the levels in the age-matched NS group. These results suggest that
there is no obvious relevance between 4EBP1 activity and autophagy
regulation. Based on these findings, it was hypothesized that
p-4EBP1 was not involved in the inhibition of autophagy in the
present model. Additionally, the potential relationships among
AMPK, mTOR, p-ULK1 (Ser 757), autophagy and apoptosis in the
present model are presented in Fig.
10.
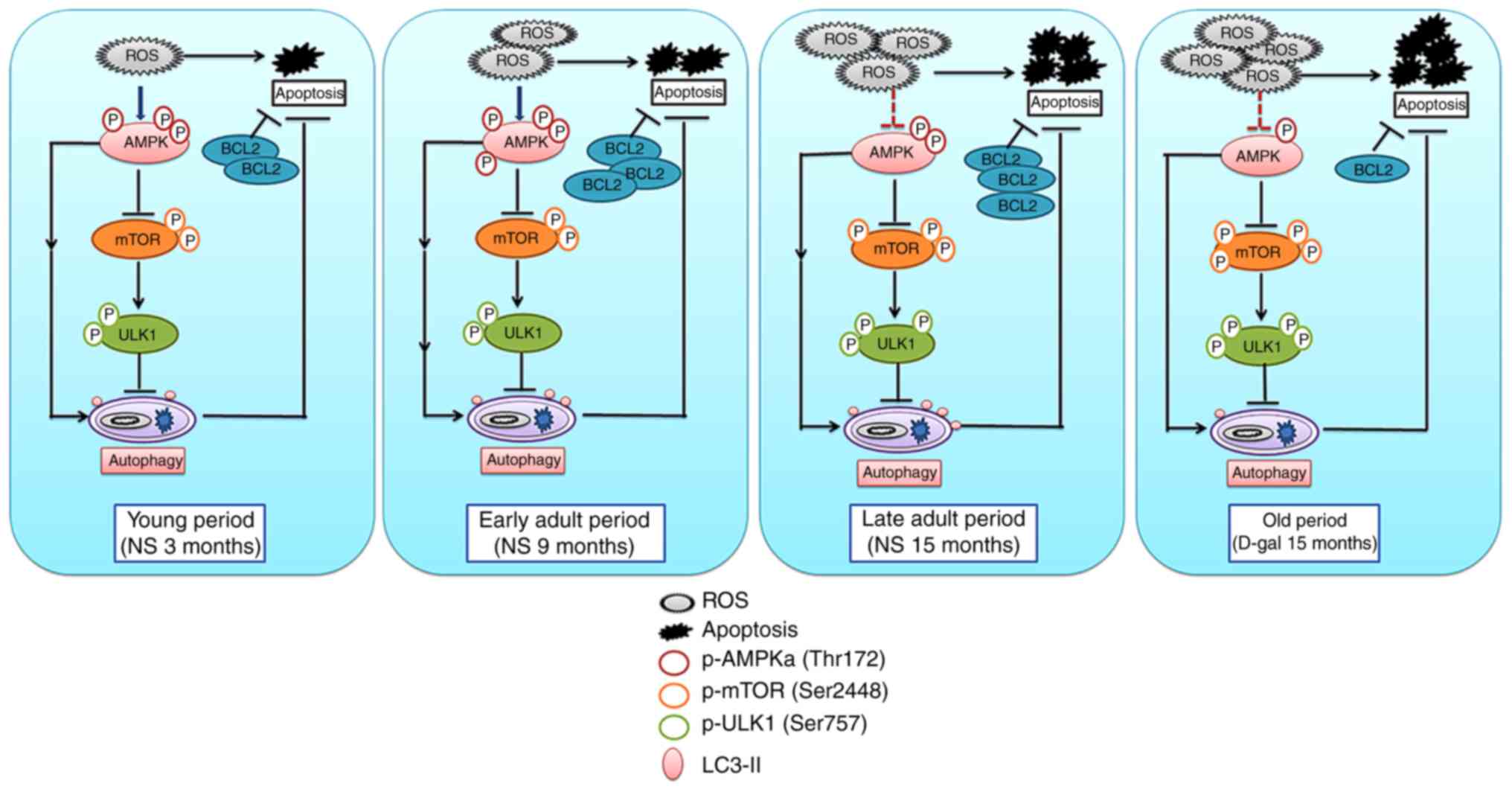 | Figure 10Schematic of the interrelationships
among p-AMPK, p-mTOR, p-ULK1, autophagy and apoptosis. Accumulated
ROS results in the phosphorylation and activation of AMPK.
Activated AMPK suppresses mTOR, thereby activating autophagy and
exerting an anti-apoptotic function. Additionally, AMPK may
directly activate autophagy through other mechanisms. However,
excessive ROS and metabolites lead to impairment of AMPK activity,
and increased activity of mTOR phosphorylates ULK1 at Ser 757,
leading to the suppression of autophagy initiation. Autophagy and
BCL2 cooperate against apoptosis and delay the process of
senescence. AMPK, 5′ AMP-activated protein kinase; mTOR,
mechanistic target of rapamycin; p, phosphorylated; ROS, reactive
oxygen species; BCL2, B-cell lymphoma 2; NS, normal saline; D-gal,
D-galactose. |
Discussion
Autophagy is a cellular catabolic process that is
essential for survival, differentiation, development and
homeostasis (47). A large body
of evidence indicates that autophagy declines with aging (48,49). However, age-related increases of
autophagy in rat nucleus pulposus and pancreatic islet cells have
also been reported (50,51). Recent research has suggested that
autophagy increases from the perinatal period to adulthood and then
declines after the age of 12 months in the inner ear of mice
(52). However, no studies have
examined autophagy in the auditory cortex and the changes with age.
In the present study, 3-, 9- and 15-month-old rats in the natural
aging and D-gal induced mimetic aging groups were observed. The
present results demonstrated that autophagy-related proteins, LC3
and BECN1, were increased from 3 months to 15 months in the natural
aging group and decreased at 15 months in the mimetic aging group,
while the degradation of p62 demonstrated an inverse trend. These
results suggest that autophagy level is time-dependent and
increases from young to adult rats and decreases at an old age in
the auditory cortex of SD rats.
Ultrastructural morphology of neurons and cell
apoptosis level in the mimetic aging group demonstrated no
significant differences with those of the age-matched NS group,
while the autophagy level increased. However, at 15 months, it was
observed that autophagy in the mimetic aging group was
significantly decreased compared to that in the age-matched natural
aging group, and neuron degeneration and cell apoptosis were more
severe than that in the NS group, as expected. It was concluded
that autophagy serves a protective role in the degeneration of the
auditory cortex, and a compensatory increase in autophagy at 3
months protected the auditory cortex from D-gal-induced premature
senescence. Deficient or impaired autophagy lost its protective
function, leading to the acceleration of apoptosis and finally
senescence.
The protective role of autophagy was further
demonstrated by investigating anti-apoptotic protein levels of BCL2
and BCL-xL in the present study. It was demonstrated that autophagy
had a similar trend to that of BCL2 and BCL-xL in natural aging and
mimetic aging rats, and it was concluded that autophagy cooperated
with BCL2 and BCL-xL to exert anti-apoptotic effects in the present
rat model. However, BCL2 and BCL-xL have been reported to suppress
autophagy by binding BECN1 directly via its BH3 domain, preventing
activation of autophagy (53,54); however, relevant evidence showing
that autophagy was inhibited by BCL2 and BCL-xL was not identified
in the present study. In addition, Pattingre et al
(55) demonstrated that BCL2
interacts with BECN1 to maintain autophagy at levels that are
compatible with cell survival rather than cell death, and
Al-Shenawy (56) found a positive
correlation between BECN1 and BCL-XL/BCL2 expression in studies on
autophagy-related marker, BECN1, and its relationship with
apoptotic markers in chronic hepatitis and hepatocellular
carcinoma. Hence, the present study assumed that BCL2 and BCL-xL
acted as anti-apoptotic proteins rather than inhibitors of
autophagy.
Activation of AMPK was significantly increased in
the mimetic aging group at 3 months, and p-mTOR and p-ULK1 (Ser
757) levels decreased, which could account for the rise of
autophagy at 3 months in the mimetic aging group. However, an
accordant decrease of autophagy at 9 months in the D-gal group was
not identified, while AMPK activity was notably decreased and
p-mTOR and p-ULK1 were increased. It was hypothesized that the loss
of activation by AMPK and increased inhibition by mTOR failed to
suppress autophagy activity until 15 months. As a major inhibitor
of autophagy, high mTOR activity prevents ULK1 activation by
phosphorylating it at Ser 757, disrupting the interaction between
ULK1 and AMPK, and finally suppressing autophagy (20). Previous research has indicated
that increased 4EBP1 activity results in the impairment of
autophagy in H2O2-induced premature
senescence in auditory cells (25). However, by detecting 4EBP1
activity and autophagy-related proteins, Atg5 and Atg7, the present
study demonstrated that 4EBP1 phosphorylation was increased at 3
and 9 months in the D-gal group compared with that in the
age-matched NS group, and no significant difference was observed at
15 months. Atg5 and Atg7 were increased at 3 months and decreased
at 15 months, indicating that 4EBP1 activity may be not involved in
the regulation of autophagy in the present model. Additionally, the
present results revealed that 4EBP1 phosphorylation may be
independent of mTOR, which is consistent with previous studies
(25,57). We hypothesized that 4EBP1 may have
different functions in tissues and cells, and the specific role of
4EBP1 in regulating autophagy and its related mechanisms require
further study. The present results demonstrated that AMPK and mTOR,
as well as ULK1, are involved in the regulation of autophagy;
however, the relationships between AMPK and autophagy, and mTOR and
autophagy, need to be clarified. Research has demonstrated that
death-associated protein 1 is a novel mTOR substrate and has an
inhibitory role in autophagy (58).
In summary, by studying autophagy and apoptosis, as
well as the AMPK-mTOR-ULK1 signaling pathway, in naturally aging
rats and mimetic aging rats at 3, 9 and 15 months, the present
study demonstrated that autophagy increased in a time-dependent
manner (from young to adult ) and then decreased at old age.
Furthermore, autophagy acts in a compensatory manner to block
apoptosis and maintain homeostasis in the body, whereas the loss of
AMPK activity and increased inhibition of mTOR signaling impaired
autophagy. This may be one mechanism underlying the degeneration of
the auditory cortex and may be partially responsible for the
pathogenesis of ARHL. Although the molecular mechanisms involved in
ARHL have yet to be fully elucidated, the present results provide
important insights into the role of autophagy during D-gal-induced
premature senescence of the auditory cortex. Based on these
results, the activation of AMPK and the suppression of mTOR
signaling may upregulate autophagy, which may delay the
neurodegenerative process. However, further studies are required to
elucidate the mechanisms of AMPK-mTOR-ULK1 signaling and other
pathways that regulate autophagy, as well as the connections
between autophagy and apoptosis, to determine how senescence and
autophagy affect auditory cortex function and contribute to the
pathology of hearing impairments.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81230021) and the
Major State Basic Research Development Program of China (973
program; grant no. 2011CB504504).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao F, Wang G, Ma W, Ren F, Li M, Dong Y,
Liu C, Liu B, Bai X, Zhao B and Edden RA: Decreased auditory GABA+
concentrations in presbycusis demonstrated by edited magnetic
resonance spectroscopy. Neuroimage. 106:311–316. 2015. View Article : Google Scholar :
|
|
2
|
Yamasoba T, Lin FR, Someya S, Kashio A,
Sakamoto T and Kondo K: Current concepts in age-related hearing
loss: Epidemiology and mechanistic pathways. Hear Res. 303:30–38.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menardo J, Tang Y, Ladrech S, Lenoir M,
Casas F, Michel C, Bourien J, Ruel J, Rebillard G, Maurice T, et
al: Oxidative stress, inflammation, and autophagic stress as the
key mechanisms of premature age-related hearing loss in SAMP8 mouse
Cochlea. Antioxid Redox Signal. 16:263–274. 2012. View Article : Google Scholar
|
|
4
|
Mazelová J, Popelar J and Syka J: Auditory
function in presbycusis: Peripheral vs. central changes. Exp
Gerontol. 38:87–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gates GA, Feeney MP and Mills D:
Cross-sectional age-changes of hearing in the elderly. Ear Hear.
29:865–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Cho JY and Kim WK:
Anti-inflammation effect of Exercise and Korean red ginseng in
aging model rats with diet-induced atherosclerosis. Nut Res Pract.
8:284–291. 2014. View Article : Google Scholar
|
|
8
|
Ahangarpour A, Lamoochi Z, Fathi Moghaddam
H and Mansouri SM: Effects of Portulaca oleracea ethanolic extract
on reproductive system of aging female mice. Int J Reprod Biomed.
14:205–212. 2016.
|
|
9
|
Liao CH, Chen BH, Chiang HS, Chen CW, Chen
MF, Ke CC, Wang YY, Lin WN, Wang CC and Lin YH: Optimizing a Male
reproductive aging mouse model by D-galactose injection. Int J Mol
Sci. 17:E982016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao J, Zhou R, You X, Luo F, He H, Chang
X, Zhu L, Ding X and Yan T: Salidroside suppresses inflammation in
a D-galactose-induced rat model of Alzheimer’s disease via
SIRT1/NF-kappaB pathway. Metab Brain Dis. 31:771–778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Y, Hu YJ, Chen B, Peng W, Sun Y,
Yang Y, Zhao XY, Fan GR, Huang X and Kong WJ: Mitochondrial
transcription factor A overexpression and base excision repair
deficiency in the inner ear of rats with D-galactose-induced aging.
FEBS J. 278:2500–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Sun Y, Hu YJ, Yang Y, Yao LL, Zhou
XX, Wang H, Zhang R, Huang X and Kong WJ: Increased p66Shc in the
inner ear of D-galactose-induced aging mice with accumulation of
mitochondrial DNA 3873-bp deletion: p66Shc and mtDNA damage in the
inner ear during aging. PLoS One. 7:e504832012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia G and Sowers JR: Autophagy: A
housekeeper in cardiorenal metabolic health and disease. Biochim
Biophys Acta. 1852:219–224. 2015. View Article : Google Scholar :
|
|
15
|
Kim I and Lemasters JJ: Mitochondrial
degradation by autophagy (mitophagy) in GFP-LC3 transgenic
hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol.
300:C308–C317. 2011. View Article : Google Scholar :
|
|
16
|
Lee JA: Neuronal autophagy: A housekeeper
or a fighter in neuronal cell survival? Exp Neurobiol. 21:1–8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen HM and Codogno P: Autophagy is a
survival force via suppression of necrotic cell death. Exp Cell
Res. 318:1304–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loos B, Engelbrecht AM, Lockshin RA,
Klionsky DJ and Zakeri Z: The variability of autophagy and cell
death susceptibility: Unanswered questions. Autophagy. 9:1270–1285.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shang L and Wang X: AMPK and mTOR
coordinate the regulation of Ulk1 and mammalian autophagy
initiation. Autophagy. 7:924–926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Cell Biol. 13:132–141. 2011.
|
|
21
|
Hosokawa N, Hara T, Kaizuka T, Kishi C,
Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, et
al: Nutrient-dependent mTORC1 association with the
ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell.
20:1981–1991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perl A: Oxidative stress in the pathology
and treatment of systemic lupus erythematosus. Nat Rev Rheumatol.
9:674–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuchihashi NA, Hayashi K, Dan K, Goto F,
Nomura Y, Fujioka M, Kanzaki S, Komune S and Ogawa K: Autophagy
through 4EBP1 and AMPK regulates oxidative stress-induced premature
senescence in auditory cells. Oncotarget. 6:3644–3655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salminen A and Kaarniranta K:
AMP-activated protein kinase (AMPK) controls the aging process via
an integrated signaling network. Ageing Res Rev. 11:230–241. 2012.
View Article : Google Scholar
|
|
27
|
Johnson SC, Rabinovitch PS and Kaeberlein
M: mTOR is a key modulator of ageing and age-related disease.
Nature. 493:338–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crino PB: The mTOR signalling cascade:
Paving new roads to cure neurological disease. Nat Rev Neurol.
12:379–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song JX, Sun YR, Peluso I, Zeng Y, Yu X,
Lu JH, Xu Z, Wang MZ, Liu LF, Huang YY, et al: A novel curcumin
analog binds to and activates TFEB in vitro and in vivo independent
of MTOR inhibition. Autophagy. 12:1372–1389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meijer AJ and Codogno P: AMP-activated
protein kinase and autophagy. Autophagy. 3:238–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang YH, Al-Aidaroos AQ, Yuen HF, Zhang
SD, Shen HM, Rozycka E, McCrudden CM, Tergaonkar V, Gupta A, Lin
YB, et al: A role of autophagy in PTP4A3-driven cancer progression.
Autophagy. 10:1787–1800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee E, Koo Y, Ng A, Wei Y, Luby-Phelps K,
Juraszek A, Xavier RJ, Cleaver O, Levine B and Amatruda JF:
Autophagy is essential for cardiac morphogenesis during vertebrate
development. Autophagy. 10:572–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Komatsu M, Waguri S, Chiba T, Murata S,
Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E and
Tanaka K: Loss of autophagy in the central nervous system causes
neurodegeneration in mice. Nature. 441:880–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gianchecchi E, Delfino DV and Fierabracci
A: Recent insights on the putative role of autophagy in autoimmune
diseases. Autoimmun Rev. 13:231–241. 2014. View Article : Google Scholar
|
|
35
|
Munch D, Rodriguez E, Bressendorff S, Park
OK, Hofius D and Petersen M: Autophagy deficiency leads to
accumulation of ubiquitinated proteins, ER stress, and cell death
in Arabidopsis. Autophagy. 10:1579–1587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pattingre S, Bauvy C, Carpentier S, Levade
T, Levine B and Codogno P: Role of JNK1-dependent Bcl-2
phosphorylation in ceramide-induced macroautophagy. J Biol Chem.
284:2719–2728. 2009. View Article : Google Scholar :
|
|
37
|
Ito H, Daido S, Kanzawa T, Kondo S and
Kondo Y: Radiation-induced autophagy is associated with LC3 and its
inhibition sensitizes malignant glioma cells. Int J Oncol.
26:1401–1410. 2005.PubMed/NCBI
|
|
38
|
Saita S, Shirane M and Nakayama KI:
Selective escape of proteins from the mitochondria during
mitophagy. Nat Commun. 4:14102013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
National Research Council Committee for
the Update of the Guide for the C and Use of Laboratory A: The
National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. th (ed). National Academies Press (US), National Academy
of Sciences; Washington (DC): 2011
|
|
40
|
Nicklas JA, Brooks EM, Hunter TC, Single R
and Branda RF: Development of a quantitative PCR (TaqMan) assay for
relative mitochondrial DNA copy number and the common mitochondrial
DNA deletion in the rat. Environ Mol Mutagen. 44:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
42
|
Edris W, Burgett B, Stine OC and Filburn
CR: Detection and quantitation by competitive PCR of an
age-associated increase in a 4.8-kb deletion in rat mitochondrial
DNA. Mutat Res. 316:69–78. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai U, Seidman MD, Hinojosa R and Quirk
WS: Mitochondrial DNA deletions associated with aging and possibly
presbycusis: A human archival temporal bone study. Am J Otol.
18:449–453. 1997.PubMed/NCBI
|
|
44
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: Comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andreollo NA, Santos EF, Araújo MR and
Lopes LR: Rat’s age versus human’s age: What is the relationship?
Arq Bras Cir Dig. 25:49–51. 2012.In English, Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang ZY, Lin JH, Muharram A and Liu WG:
Beclin-1-mediated autophagy protects spinal cord neurons against
mechanical injury-induced apoptosis. Apoptosis. 19:933–945. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cuervo AM, Bergamini E, Brunk UT, Dröge W,
Ffrench M and Terman A: Autophagy and aging: The importance of
maintaining ‘clean’ cells. Autophagy. 1:131–140. 2005. View Article : Google Scholar
|
|
49
|
Ott C, König J, Höhn A, Jung T and Grune
T: Macroautophagy is impaired in old murine brain tissue as well as
in senescent human fibroblasts. Redox Biol. 10:266–273. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ye W, Xu K, Huang D, Liang A, Peng Y, Zhu
W and Li C: Age-related increases of macroautophagy and
chaperone-mediated autophagy in rat nucleus pulposus. Connect
Tissue Res. 52:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang S, Sun QQ, Xiang B and Li XJ:
Pancreatic islet cell autophagy during aging in rats. Clin Invest
Med. 36:E72–E80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Iriarte Rodríguez R, Pulido S,
Rodríguez-de la Rosa L, Magariños M and Varela-Nieto I:
Age-regulated function of autophagy in the mouse inner ear. Hear
Res. 330:39–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Decuypere JP, Parys JB and Bultynck G:
Regulation of the autophagic bcl-2/beclin 1 interaction. Cells.
1:284–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Levine B, Sinha SC and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al-Shenawy HA: Expression of Beclin-1, an
autophagy-related marker, in chronic hepatitis and hepatocellular
carcinoma and its relation with apoptotic markers. APMIS.
124:229–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chao SK, Lin J, Brouwer-Visser J, Smith AB
III, Horwitz SB and McDaid HM: Resistance to discodermolide, a
microtubule-stabilizing agent and senescence inducer, is
4E-BP1-dependent. Proc Natl Acad Sci USA. 108:391–396. 2011.
View Article : Google Scholar
|
|
58
|
Koren I, Reem E and Kimchi A: DAP1, a
novel substrate of mTOR, negatively regulates autophagy. Curr Biol.
20:1093–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|