Introduction
Cardiovascular disease is the second leading cause
of death among the ten leading chronic diseases in Taiwan according
to the 2017 annual report of the Ministry of Health and Welfare,
Taiwan, R.O.C. (1). A total of
20,812 people died, and the death rate was 88.5 per 100,000
population, increased by 8.1% from 2015 to 2016 (1). Cardiovascular disease includes
coronary heart disease (CHD), peripheral arterial disease, aortic
disease and stroke, and many risk factors are associated with the
lesions (2,3). The drug treatments can greatly
improve cardio-vascular disease (4). Importantly, traditional Chinese
medicine, dietary foods and supplements may prevent or help in
fighting heart disease (5,6).
Ginger (Zingiber officinale Roscoe) is a
natural herb that is widely used for medicinal and culinary
purposes (7,8). Ginger exerts many health benefits
and may be used to treat ailments, including cramps, arthritis and
disorders of the gastrointestinal tract, such as constipation,
dyspepsia, diarrhea, nausea and vomiting (8). In addition, ginger is recommended by
traditional healers to treat cardiomyopathy, high blood pressure
and palpitations (7,9,10).
The main bioactive constituents of ginger are gingerol, shogaol,
zingerone and paradol (11,12). Furthermore, the main aromatic
components of ginger are zingiberol, gingediol,
monoacyldigalactosyl-glycerol, iarylheptanoids and phytosterols
(13). 6-Gingerol has numerous
biological activities, including antioxidant, antitumor and
anti-inflammatory effects (14–16). The pharmacological effects of
6-gingerol ameliorate hyperlipidemia by decreasing serum
cholesterol and serum triglyceride levels (17). 6-shogaol is a dehydrated form of
6-gingerol, which is isolated from the dried or cooked rhizomes of
ginger (18,19). In a previous study, ginger crude
extract (GCE) was reported to exhibit hypotensive,
endothelium-independent vasodilatory and cardiosuppressive
properties, via its specific inhibitory action at voltage-dependent
calcium channels (20). The
present study aimed to investigate the relaxant effects of GCE on
porcine coronary arteries in vivo.
Materials and methods
Reagents and chemicals
DL-homocysteine (Hcy), 1H-[1,2,4]
oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), bradykinin,
1,1-diphenyl-2-picrylhydrazyl (DPPH), dimethyl sulfoxide,
propranolol, n-butanol and other chemicals were high-grade
products purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). NG-nitro-L-arginine
(L-NNA) and glibenclamide (Glib) were obtained from MP
Biomedicals, LLC (Santa Ana, CA, USA). KH solution was composed of
70.2 mM NaCl, 4.2 mM KCl, 2.8 mM CaCl2, 2.7 mM
MgSO4, 21.0 mM NaHCO3, 0.2 mM
KH2PO4 and 9.8 mM glucose, and the pH was
adjusted to 7.4.
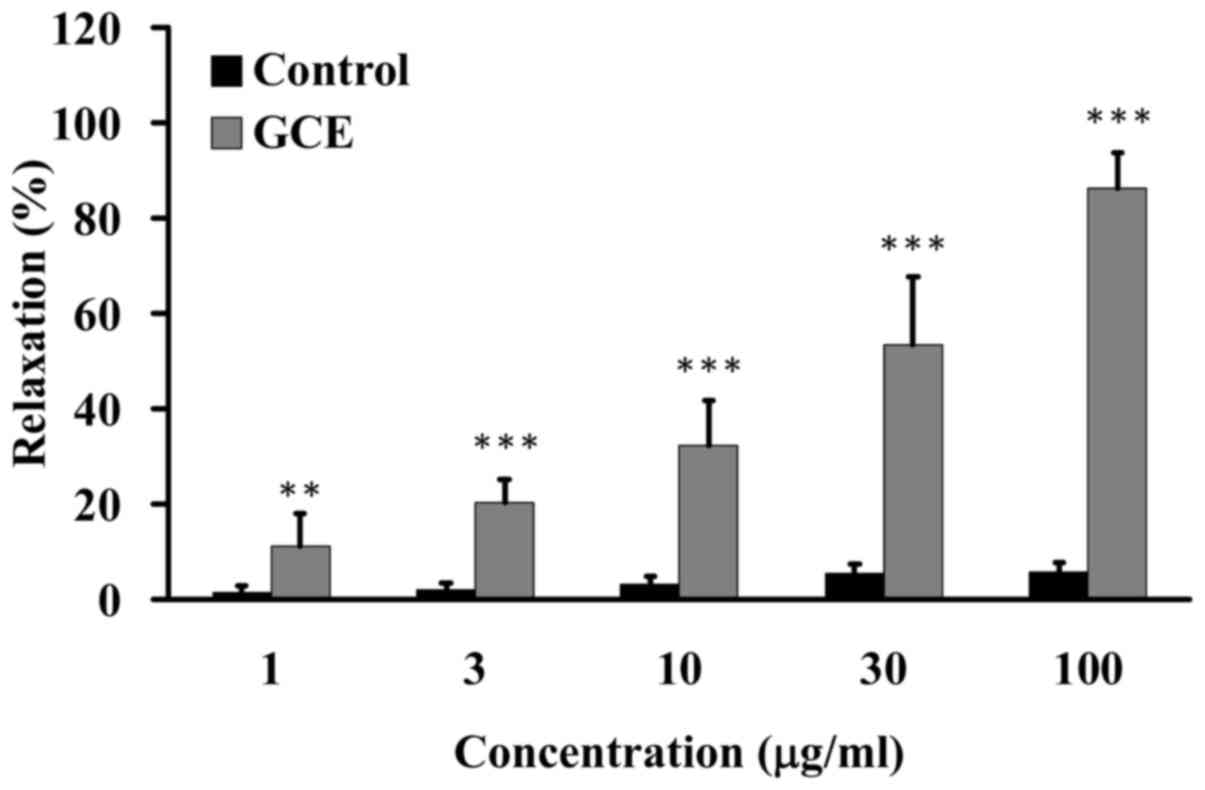
Ginger extraction
A total of 600 g fresh ginger rhizome was soaked in
2.5 l ethanol. The extracts were refluxed at 78°C for 2 h; this was
repeated three times. Subsequently, the filtrate was concentrated
in a rotary evaporator. The weight of extracts was ~34.2 g (yield,
5.7%). The residue was then suspended in 50 ml water and extracted
with 50 ml chloroform twice, after which the chloroform partition
was evaporated to obtain 8.4 g residue (GCE). The aqueous phase was
partitioned with n-butanol. The n-butanol partition
was evaporated to obtain 6.3 g residue (ginger n-butanol
extract, GNE). The water extract underwent reverse osmosis to
obtain 19.5 g residue (ginger water extract, GWE); this process is
summarized in Fig. 1. The stock
solution of ginger extraction was prepared by dimethyl sulfoxide to
dilute for further experiments.
Coronary artery ring preparation
Porcine hearts were freshly obtained from the local
abattoir, immersed in cold 0.9% NaCl at 4°C and were transported to
the research laboratory. Excess connective tissue was removed and
the arteries were cut into 5-mm rings. Endothelium-intact and
-denuded porcine coronary artery rings were prepared, and the rings
were then mounted with two stainless steel hooks in 10 ml KH
solution-filled organ baths. KH solution was kept in oxygenated
conditions (95% O2 and 5% CO2) at 37°C and
was replaced every 15 min to maintain continuous equilibration. The
rings were perfused with 30 mM KCl in the organ bath until tonic
phase contraction was achieved, as previously described (21) before pretreatment with 100
µM L-NNA, 10 µM ODQ, 10 µg/ml indomethacin, 20
µM propranolol, 1 µM Glib, 100 µM Hcy, 30 mM
bradykinin and 77.5 mM H2O2, respectively,
for indicated period of time.
Isometric tension of porcine coronary
arteries
The porcine coronary arteries were harvested, cut
into numerous 5-mm rings, and were maintained in 5 ml organ baths
containing 95% O2 and 5% CO2 at 37°C. Ginger
extracts were individually added to the 5-mm rings for 30 min and
relaxation was observed. Alterations in tension were recorded using
a Grass Force displacement transducer (model FT03; Grass; Natus
Medical Incorporated, Pleasanton, CA, USA).
DPPH radical scavenging assay
The DPPH radical scavenging assay was performed
according to the method described by Sakanashi et al
(21). Briefly, in each well of a
96-well plate, 50 µl sample extract was added to 150
µl 0.25 mM DPPH methanolic solution. After mixing
thoroughly, the reactants were incubated in the dark for 30 min at
room temperature. The control was prepared by mixing 50 µl
methanol with 150 µl DPPH. The absorbance was detected at
517 nm using a spectrophotometer. Samples were measured in
triplicate.
Lucigenin-enhanced chemiluminescence
assay
The levels of superoxide anion produced by
endothelial cells of the porcine arteries were detected using the
lucigenin-enhanced chemiluminescence method, as previously
described by Sun et al (22). Briefly, the samples of GCE, GNE
and GWE were mixed with 5 µM lucigenin for 6 min. Time-based
reading was recorded in a 5 min period using a luminometer. The
area of each vessel segment was measured using a caliper and was
used to normalize the data for each sample.
Protein preparation
Following treatment with or without GCE, GWE and
GNE, porcine coronary artery endothelial cells were collected as
previously described (23) and
mixed with protein lysis buffer [50 mM Tris-HCl (pH 7.4), 1 mM NaF,
150 mM NaCl, 1 mM EGTA, 1 mM phenylmethane-sulfonyl fluoride, 1%
NP-40 and 10 µg/ml leupeptin] on ice. The samples were
homogenized for 20 sec, incubated for 20 min on ice and centrifuged
at 15,000 × g for 30 min at room temperature. The supernatants were
then transferred into new tubes for protein quantification, as
previously described (24,25).
Western blot analysis
A total of 50 µg protein was loaded and
separated by 10% SDS-PAGE. The samples in the gels were then
transferred onto polyvinylidene difluoride membranes. The membranes
were incubated with 0.1% PBS-Tween containing 5% non-fat milk for
30 min at room temperature, and were then hybridized with
cyclooxygenase-2 (COX-2; cat. no. GTX100656; 1:1,000 dilution;
GenTex, Hsinchu, Taiwan), inducible nitric oxide synthase (iNOS;
cat. no. GTX130246; 1:1,000 dilution; GenTex), endothelial nitric
oxide synthase (eNOS; cat. no. 3GTX129843; 1:1,000 dilution;
GenTex) and β-actin (cat. no. GTX109639; 1:5,000 dilution; GenTex)
primary antibodies. Subsequently, membranes were incubated with
horseradish peroxidase-conjugated rabbit IgG antibody (cat. no.
GTX213110-01; 1:10,000 dilution; GenTex) at room temperature for 1
h and were then visualized using Immobilon Western HRP substrate
kit (EMD Millipore, Billerica, MA, USA). Densitometric analysis of
each band was performed utilizing National Institutes of Health
(NIH) ImageJ 1.47 software (NIH, Bethesda, MD, USA).
Statistical analysis
Data are presented as the means ± standard deviation
from at least three separate experiments. Statistical data were
analyzed using one-way ANOVA with post hoc Dunnett's test for
comparing groups to the control by SPSS version 13.0 for Windows
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significantly difference.
Results
Ginger extracts (GCE, GNE and GWE)
preparation
The three varieties of ginger extract (GCE, GNE and
GWE) were prepared according to the diagram presented in Fig. 1. These three ginger extracts were
used in the present study to explore their effects on the
vasorelaxation of porcine coronary artery rings.
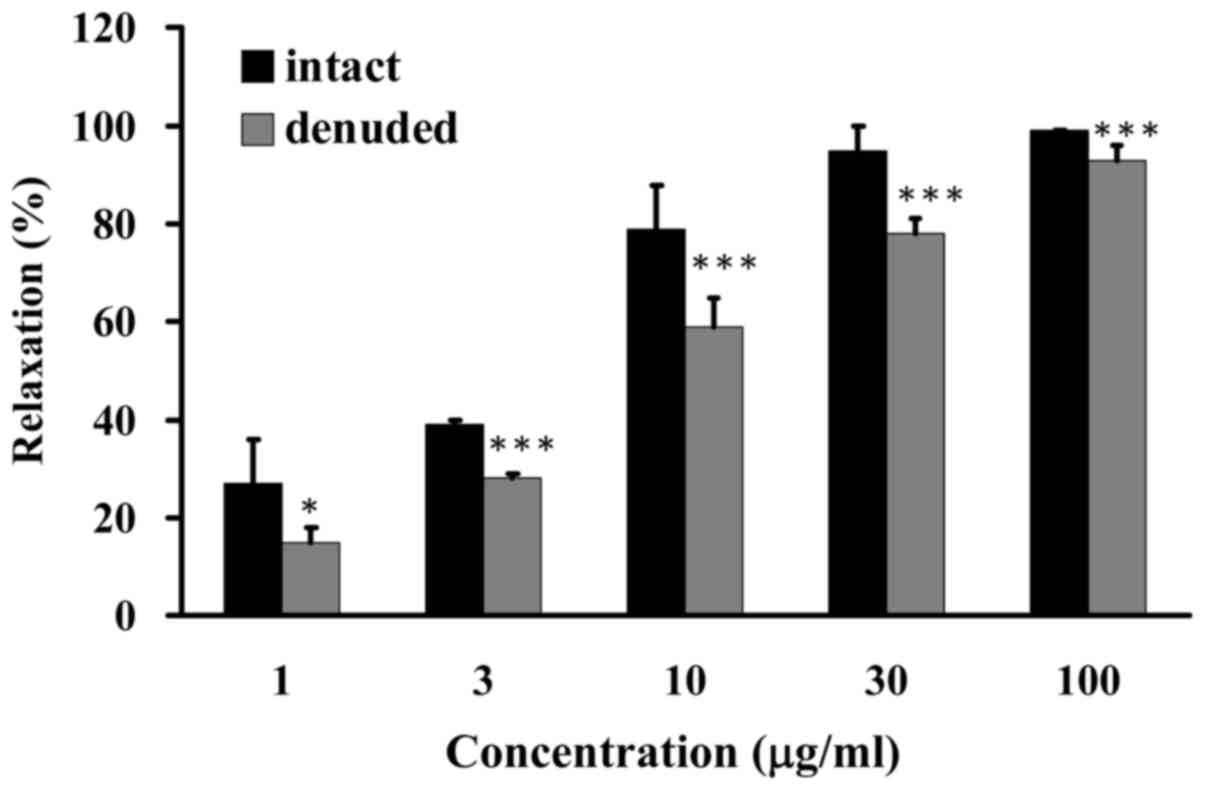
GCE relaxes porcine coronary
arteries
Porcine coronary arteries were suspended in an organ
bath. Various amounts of GCE (1, 3, 10, 30 and 100 µg/ml)
were added to the porcine coronary arteries; water was used as a
vehicle control. A dose-dependent increase in relaxation was
observed in response to GCE (Fig.
2).
GCE induces endothelium-dependent
relaxation of porcine coronary arteries
The results of the present study indicated that GCE
induced endothelium-dependent vasorelaxation. Endothelium-intact
and -denuded porcine coronary artery rings were incubated with
various amounts of GCE (1, 3, 10, 30 and 100 µg/ml). GCE was
able to reduce KCl-induced contraction and increase vasorelaxation
from 27 to 99% in the endothelium-intact porcine coronary artery
rings, whereas GCE exerted mild effects on the vasorelaxation of
denuded porcine coronary artery rings (from 15 to 93%) (Fig. 3). Based on these data, it was
suggested that endothelium-dependent relaxation was increased in
porcine coronary arteries following GCE exposure.
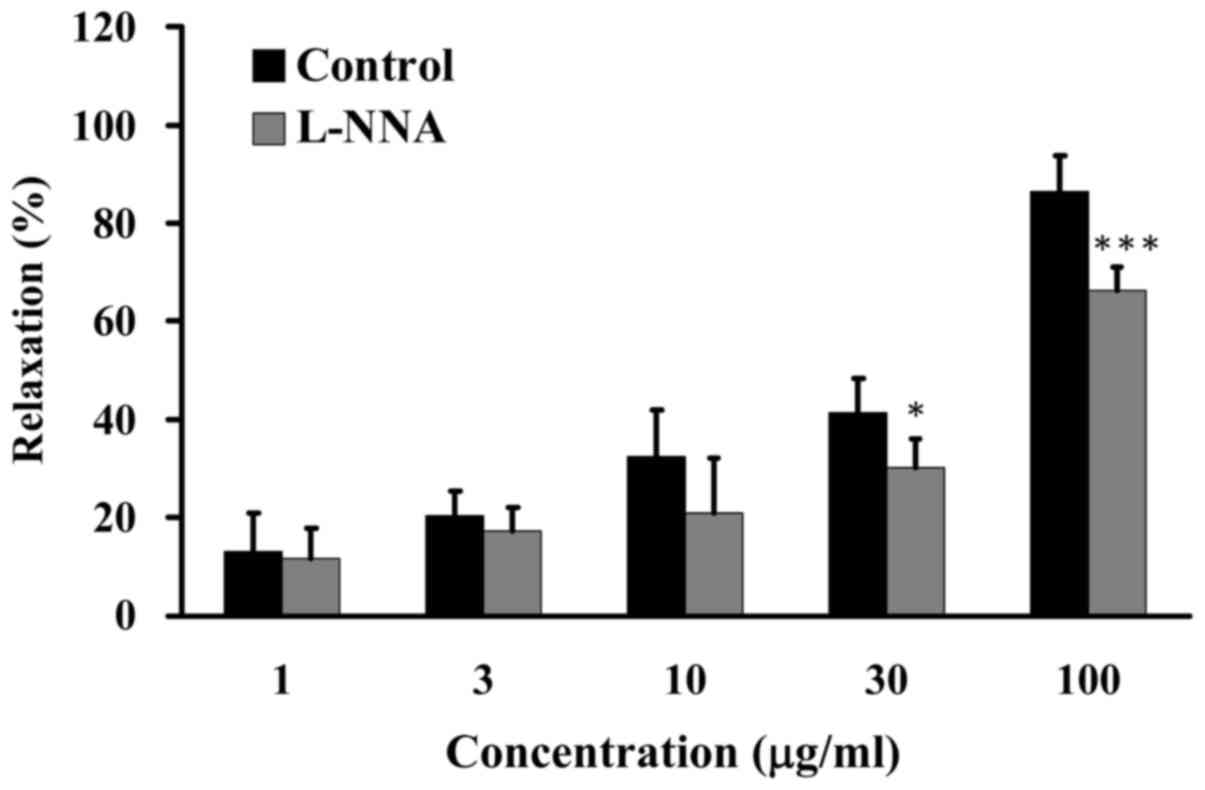
The NOS signaling pathway is involved in
GCE-induced relaxation
Numerous in vitro and in vivo studies
have reported that endothelium-dependent relaxation and
vasodilatation persist in the presence of NOS inhibitors, including
L-arginine analogues, such as L-NNA (23,26). Porcine coronary artery rings were
pretreated in the absence (control) or presence of 100 µM
L-NNA for 20 min, and were then incubated with 30 mM KCl
to induce contraction until the tonic phase (27). Relaxation was examined in the
presence of various concentrations of GCE (1, 3, 10, 30 and 100
µg/ml) in an organ bath. GCE induced relaxation of porcine
coronary artery rings from 13 to 86% without L-NNA pretreatment.
Conversely, GCE (100 µg/ml) induced relaxation of porcine
coronary artery rings to 66%, in the presence of L-NNA
(Fig. 4). These results revealed
that GCE-induced relaxation of porcine coronary arteries may be
mediated by the NOS signaling pathway.
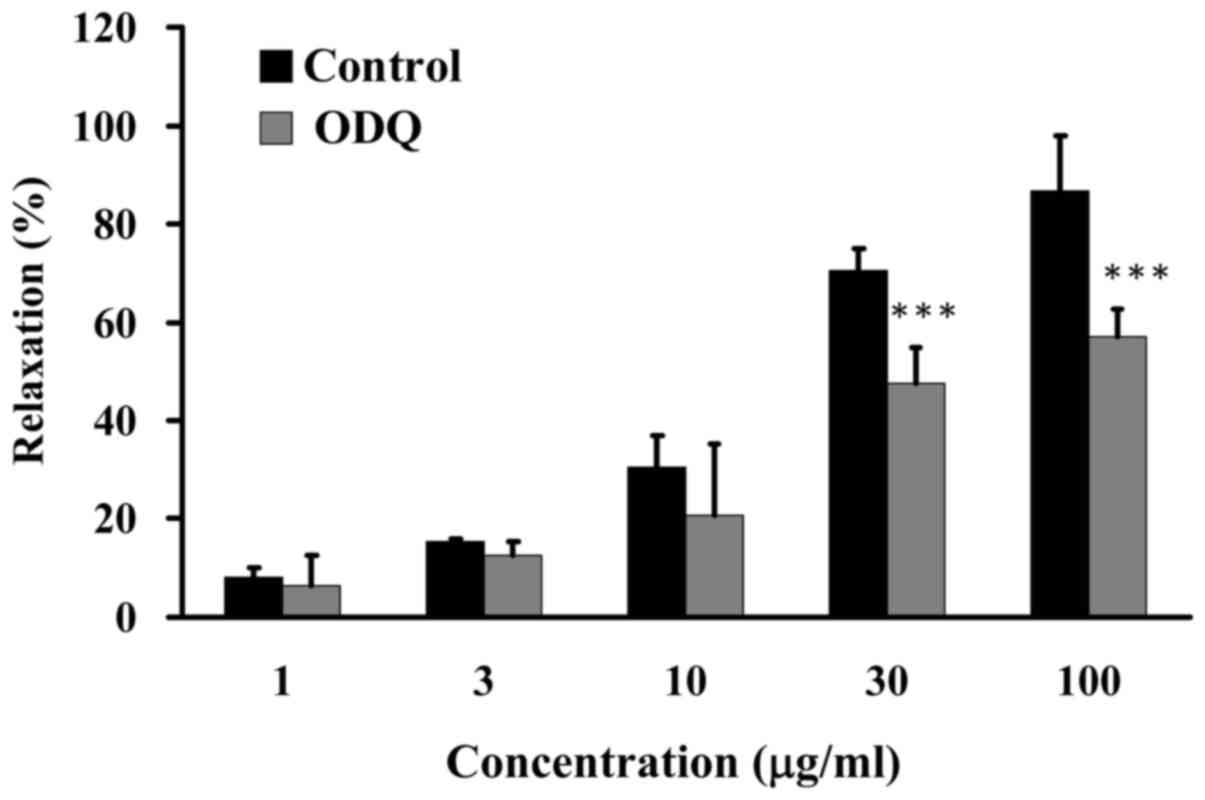
GCE improves relaxation via NO-activated
soluble guanylate cyclase (sGC)
The present study determined the effects of GCE on
sGC-induced relaxation. Porcine coronary artery rings were
pretreated in the absence (control) or presence of 10 µM ODQ
for 20 min, and were then incubated with 30 mM KCl to induce
contraction until the tonic phase. Relaxation was examined in the
presence of various concentrations of GCE (1, 3, 10, 30 and 100
µg/ml) in an organ bath. GCE induced an increase in
relaxation from 8 to 87% in porcine coronary artery rings following
pretreatment without 10 µM ODQ. Conversely, relaxation of
porcine coronary artery rings was significantly reduced following
pretreatment with 10 µM ODQ and treatment with GCE at 30 and
100 µg/ml (Fig. 5). These
results indicated that NO is a vital factor in GCE-induced
relaxation of porcine coronary arteries.
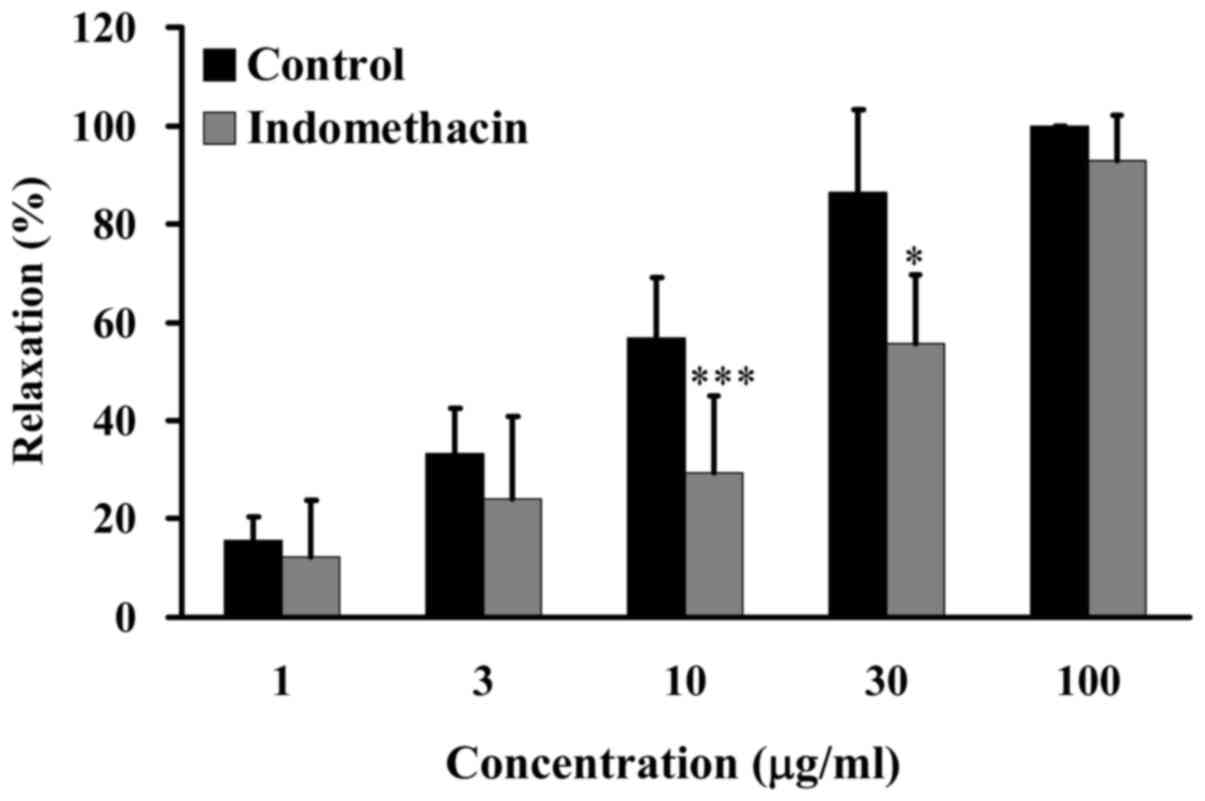
GCE improves relaxation via COX
The present study further examined the effects of
GCE on relaxation following treatment with indomethacin, which is
an inhibitor of COX. Porcine coronary artery rings were pretreated
in the absence (control) or presence of 1 µg/ml indomethacin
for 20 min, and were then incubated with 30 mM KCl to induce
contraction until the tonic phase. Relaxation was examined in the
presence of various concentrations of GCE (1, 3, 10, 30 and 100
µg/ml) in an organ bath. GCE induced an increase in
relaxation from 15 to 100% in porcine coronary artery rings without
1 µg/ml indomethacin treatment. Conversely, following
pretreatment with 1 µg/ml indomethacin and treatment with
low concentrations of GCE (3–30 µg/ml), relaxation of
porcine coronary artery rings was significantly attenuated
(Fig. 6). These results suggested
that GCE attenuated relaxation induced by arachidonic acid.
GCE-induced relaxation of porcine coronary arteries may be through
COX pathway.
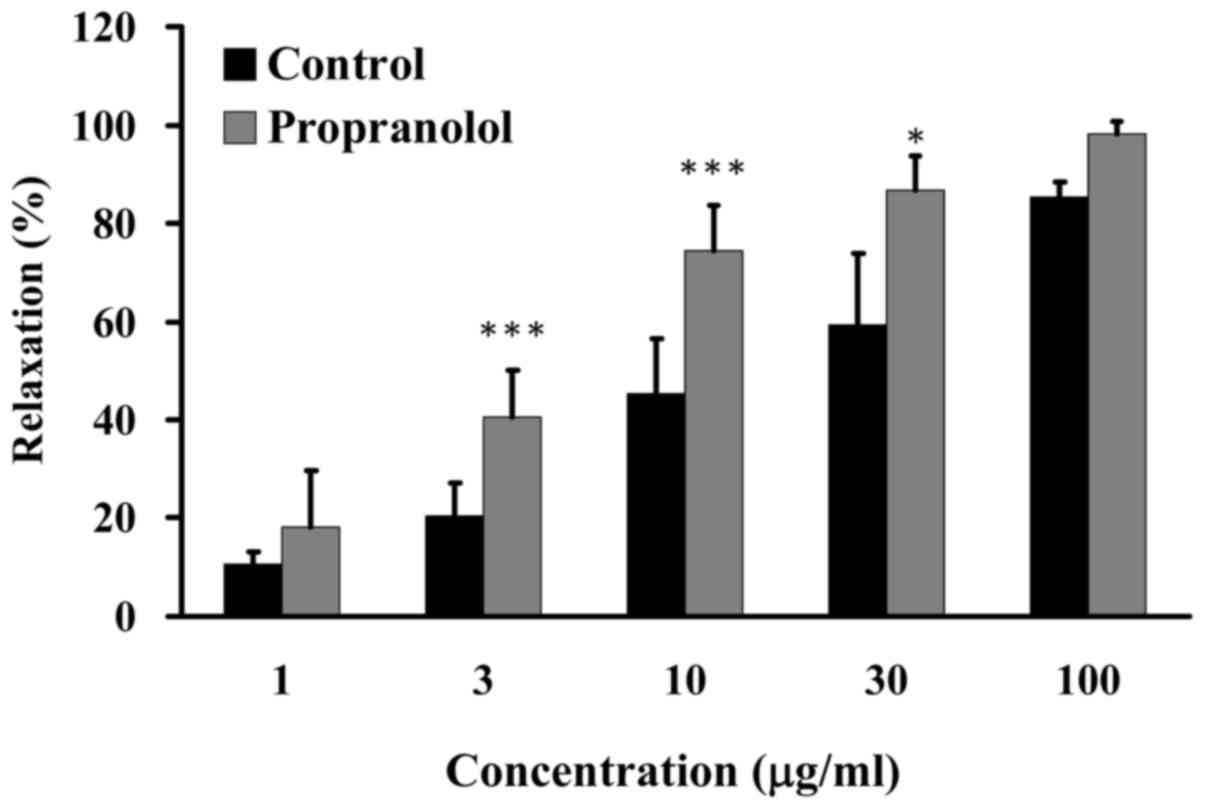
GCE has no effect on relaxation induced
by β1-adrenergic receptor blocker
β-blockers have been widely used in the treatment of
numerous cardiovascular diseases, particularly hypertension and
atherosclerosis (28). Some
β1-adrenergic receptor blockers cause vasodilation by increasing NO
(29). The present study examined
the effects of GCE on relaxation induced by propranolol, which is a
β-blocker. Porcine coronary artery rings were pretreated in the
absence (control) or presence of 20 µM propranolol for 20
min, and were then incubated with 30 mM KCl to induce contraction
until the tonic phase. Relaxation was examined in the presence of
various amounts of GCE (1, 3, 10, 30 and 100 µg/ml) in an
organ bath. GCE at 3–30 µg/ml induced an increase in
relaxation from 11 to 91% in porcine coronary artery rings without
pretreatment with 20 µM propranolol (Fig. 7). These results indicated that GCE
exhibited no apparent effect on propranolol-induced relaxation.
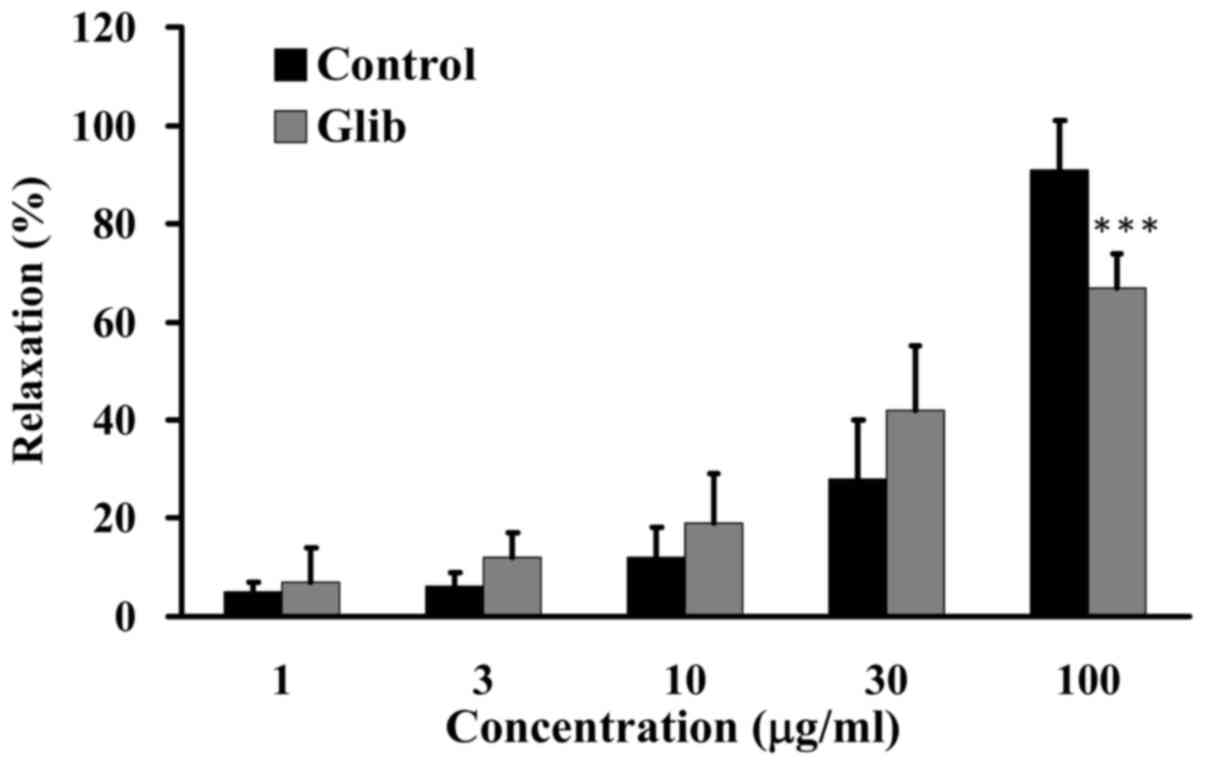
ATP-sensitive potassium (KATP)
channel blocker exerts no effects on GCE-induced relaxation
KATP channels are activated and opened by
declining intracellular ATP levels and elevated cAMP concentration,
which leads to hyperpolarization of endothelial cells and the
promotion of NO formation in vitro (30,31). It has been suggested that
endothelial cell hyperpolarization may contribute to vascular
relaxation. KATP channels are inhibited by sulfonylurea
agents, including Glib (31,32). The present study examined the
effects of Glib, a KATP channel blocker, on GCE-induced
relaxation. Porcine coronary artery rings were pretreated in the
absence (control) or presence of 1 µM Glib for 60 min, and
were then incubated with 30 mM KCl to induce contraction until the
tonic phase. Relaxation was examined in the presence of various
amounts of GCE (1, 3, 10, 30 and 100 µg/ml) in an organ
bath. GCE induced an increase in relaxation from 15 to 76% in the
porcine coronary artery rings without 1 µM Glib pretreatment
(Fig. 8). These results suggested
that Glib had no effect on GCE-induced relaxation.
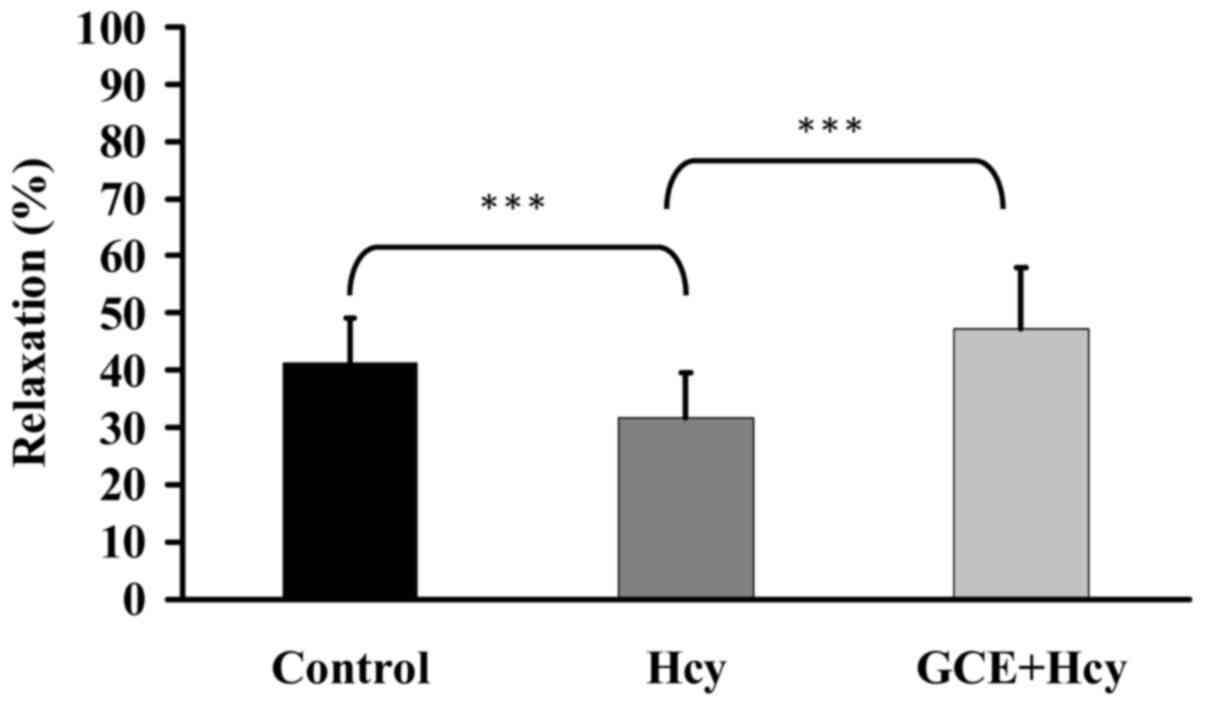
GCE prevents Hcy-induced endothelial
vasomotor dysfunction
The present study investigated the effects of GCE on
Hcy-induced endothelial cell damage. Porcine coronary artery rings
were incubated with 30 µg/ml GCE for 15 min, and were then
treated with 100 µM Hcy for 30 min. Porcine coronary artery
rings were placed in an organ bath containing 30 mM KCl to induce
contraction until the tonic phase. Relaxation was examined
following the addition of 30 mM bradykinin into the organ bath. Hcy
reduced relaxation, whereas GCE significantly prevented Hcy-induced
endothelial dysfunction (Fig. 9).
These results indicated that GCE may improve Hcy-induced
endothelial cell damage.
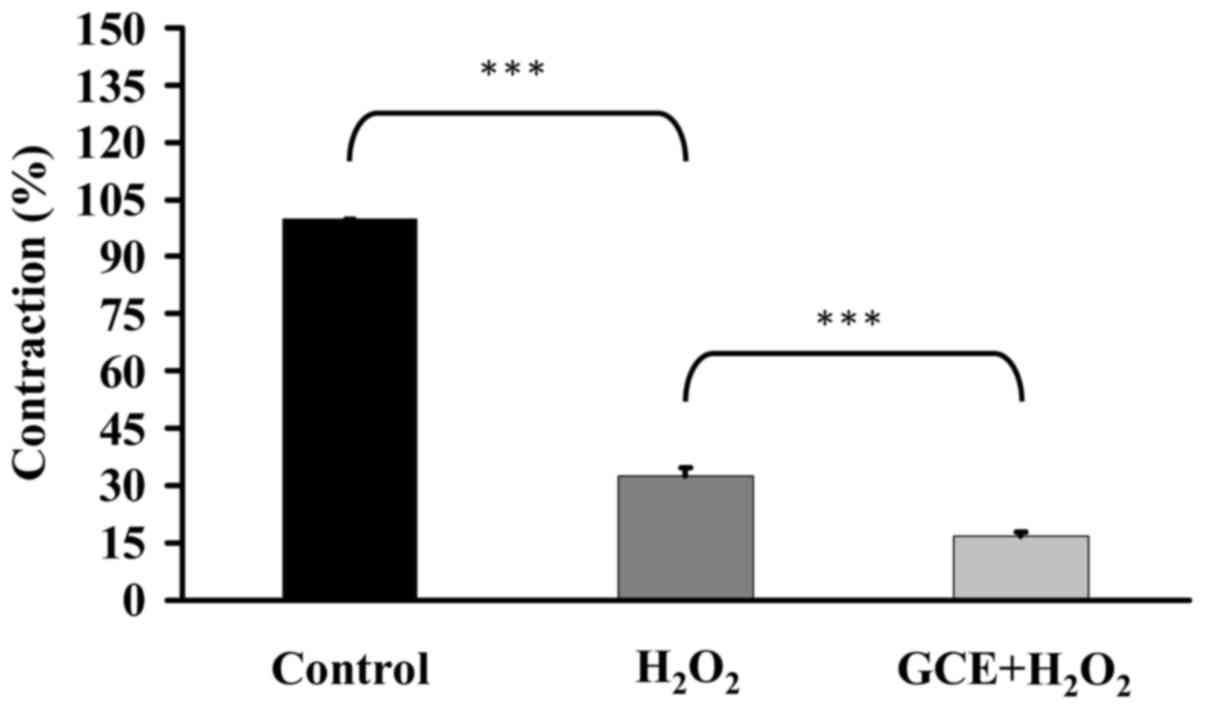
GCE prevents hydrogen peroxide
(H2O2)-induced endothelial cell damage
The present study clarified the effects of GCE on
H2O2-induced endothelial cell damage. Porcine
coronary artery rings were incubated with 30 µg/ml GCE for
15 min, and were then placed in an organ bath containing 30 mM KCl
to induce contraction until the tonic phase. Rings were treated
with 77.5 mM H2O2 for 15 min and contraction
was examined. H2O2 induced endothelial
contraction, whereas GCE significantly prevented
H2O2-induced endothelial dysfunction
(Fig. 10). These data revealed
that GCE may attenuate H2O2-induced
endothelial cell injury.
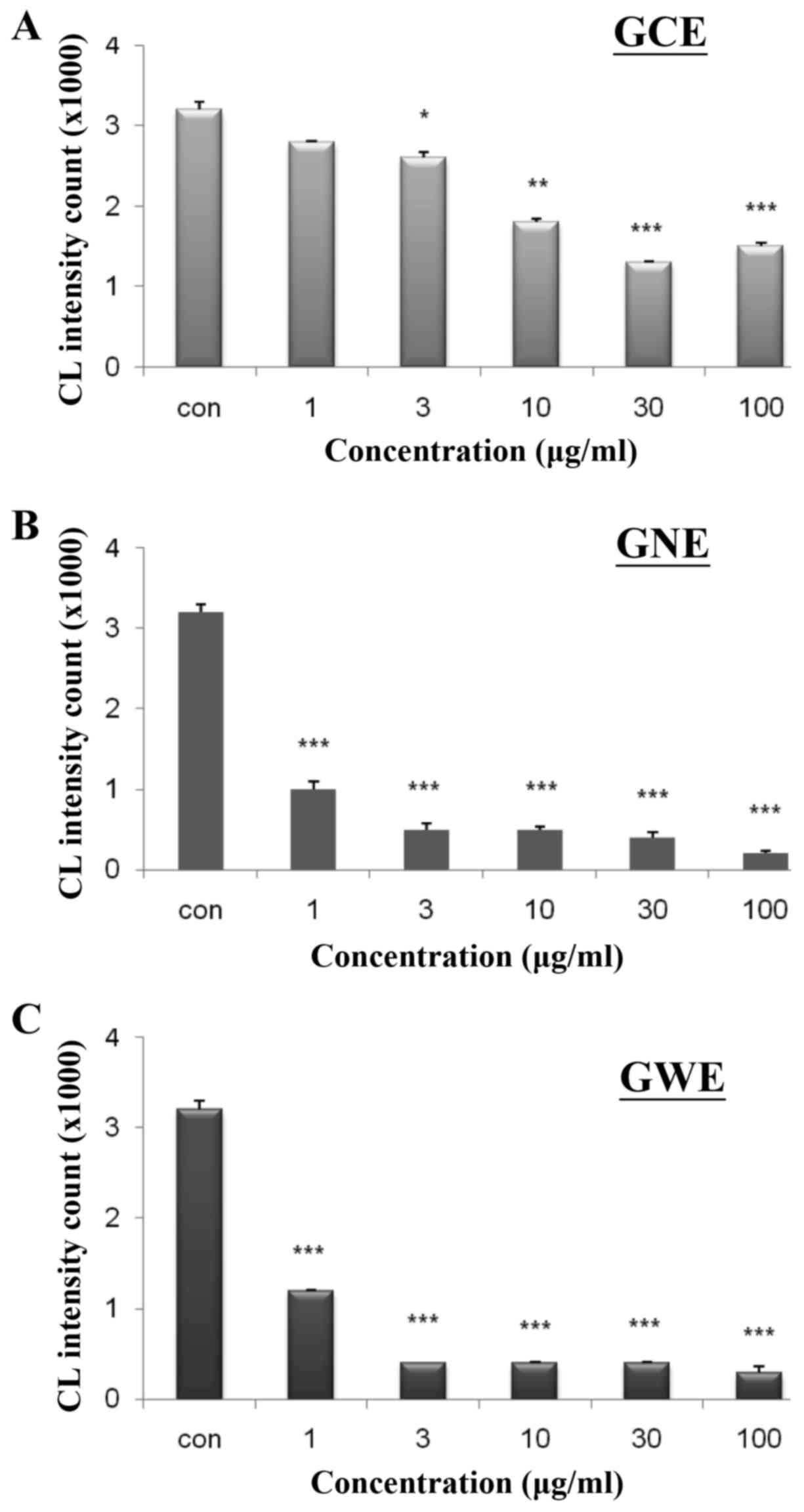
Ginger extracts possess antioxidant
abilities
Reactive oxygen species (ROS) are produced under
oxidative stress and adverse cellular environments (33). Vitamins E and C, β-carotene,
flavonoids and polyphenols have previously been demonstrated to
possess free radical-scavenging abilities (34). In the present study, the
antioxidant properties of ginger extracts were individually
determined according to DPPH and lucigenin-enhanced
chemiluminescence assays. DPPH absorbance decreased from 0.70 to
0.24, as GCE concentration increased from 62.5 to 1,000
µg/ml (Fig. 11A). The
rate of inhibition was increased from 40 to 85% in a dose-dependent
manner. DPPH absorbance decreased from 0.79 to 0.39, as GNE
concentration increased from 62.5 to 1,000 µg/ml. The rate
of inhibition was increased from 37 to 78% in a dose-dependent
manner (Fig. 11B). DPPH
absorbance decreased from 0.73 to 0.66, as GWE concentration
increased from 62.5 to 1,000 µg/ml. The rate of inhibition
was increased from 42 to 48% (Fig.
11C). These findings indicated that GCE possesses a stronger
ability to reduce free radical levels. To determine whether ginger
extracts possess H2O2-scavenging abilities, a
lucigenin-enhanced chemiluminescence assay was conducted. Various
concentrations of GCE, GNE and GWE were used to evaluate their
ability to remove H2O2. The
H2O2-scavenging ability was increased from 10
to 52% in response to GCE (Fig.
12A). The H2O2-scavenging ability was
increased from 68 to 94% in response to GNE (Fig. 12B) and from 63 to 90% in response
to GWE (Fig. 12C). These
findings indicated that GCE may possess a stronger antioxidant
ability to scavenge free radicals.
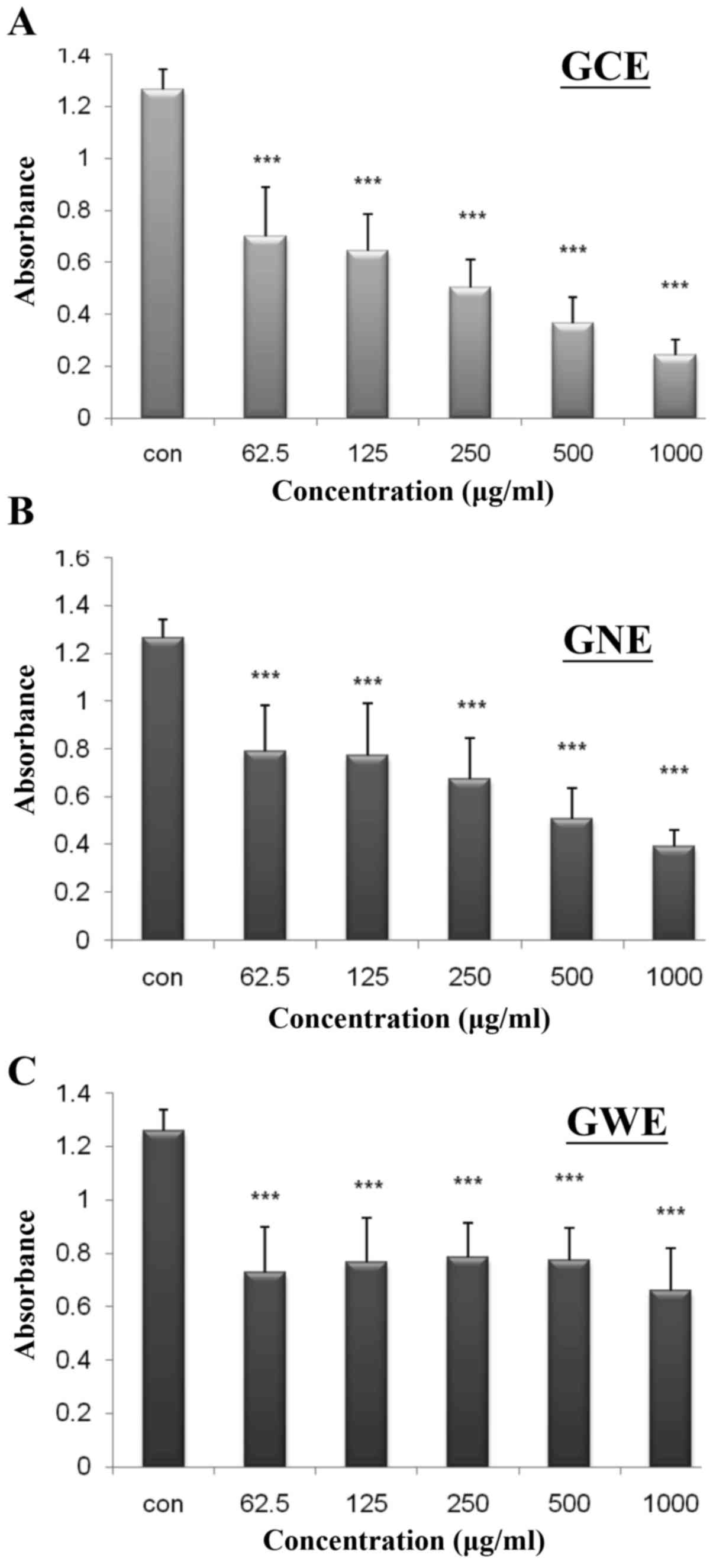 | Figure 11DPPH-scavenging activities of three
varieties of ginger extract. DPPH was mixed with various amounts
(62.5, 125, 250, 500 and 1,000 µg/ml) of (A) GCE, (B) GNE
and (C) GWE. Data are presented as absorbance read at 517 nm.
Values are expressed as the means ± standard deviation.
***P<0.001 vs. the control group (n=6). DPPH,
1,1-diphenyl-2-picrylhydrazyl; GCE, ginger crude extract; GNE,
ginger n-butanol extract; GWE, ginger water extract. |
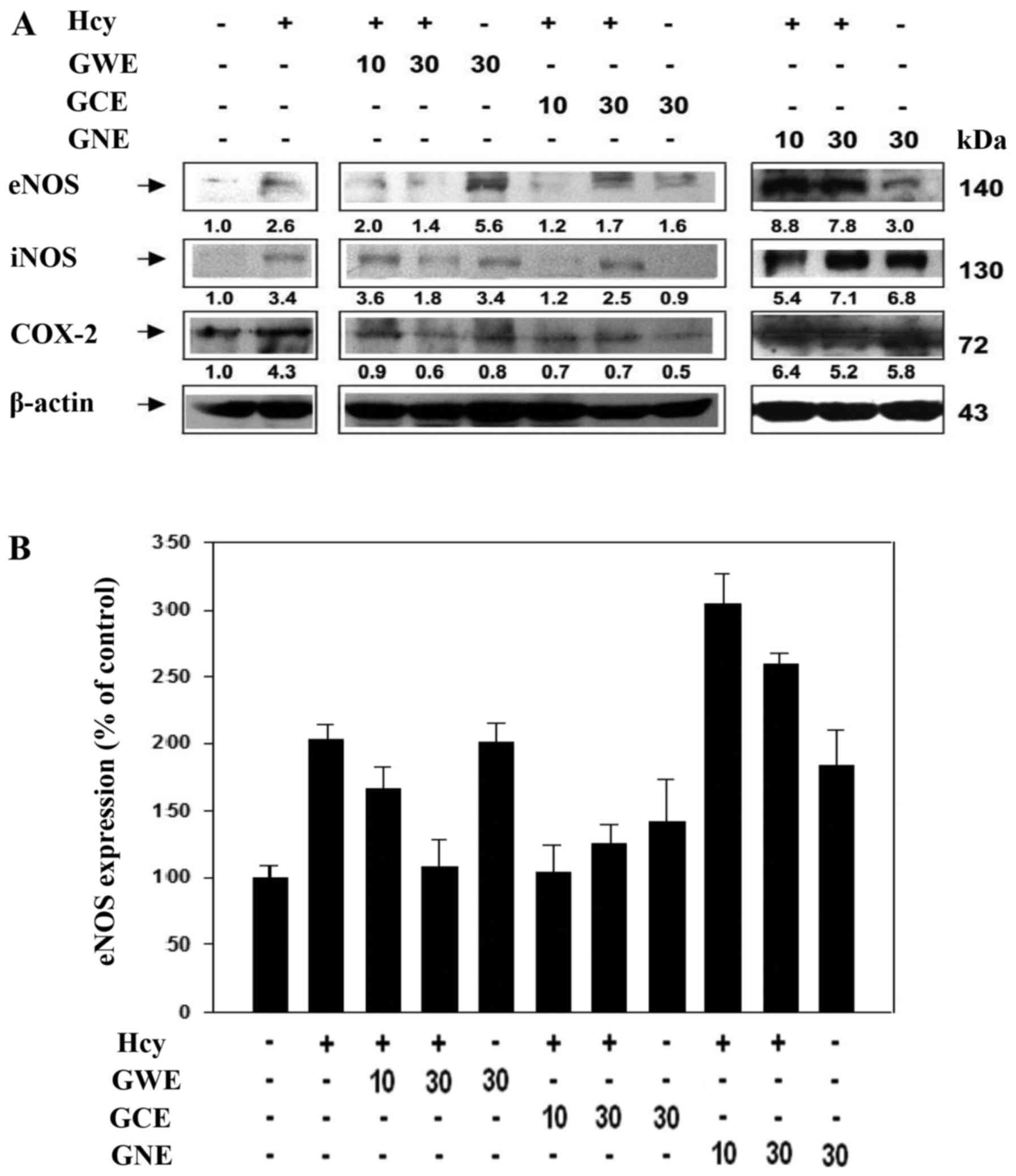
GCE exerts strong vasoprotective
effects
The present study investigated the effects of ginger
extracts on Hcy-induced endothelial cell damage by analyzing the
protein expression levels of endothelial NOS (eNOS), iNOS and
COX-2. Hcy increased eNOS, iNOS and COX-2 expression. In the
absence of Hcy, GWE induced eNOS, maintained iNOS and reduced COX-2
expression (Fig. 13A).
Conversely, low concentration (10 µg/ml) of GWE slightly
reduced eNOS, slightly induced iNOS and reduced COX-2 expression in
the presence of Hcy. A high concentration (30 µg/ml) of GWE
markedly reduced the expression levels of eNOS, iNOS and COX-2 in
the presence of Hcy. GCE markedly reduced eNOS, iNOS and COX-2
expression in the presence of Hcy, whereas GNE markedly induced
eNOS, iNOS and COX-2 expression in the presence of Hcy. These
findings indicated that GCE exerts stronger vasoprotective effects.
In addition, eNOS expression was quantified from western blot
analysis (Fig. 13B). These data
suggested that GCE, not GWE or GNE, possesses a strong
vasoprotective effect.
Discussion
Numerous phytochemicals used in traditional Chinese
medicine have beneficial health effects on blood pressure and
endothelial function (19,35).
Ginger, which is a spice used to enhance the flavor of foods, has
been used for centuries in the Taiwanese, Chinese, Indian, Arabic,
Tibetan, Unani and Siddha systems of traditional medicine (7–9).
It has previously been reported that ginger possesses various
beneficial pharmacological effects, including hypoglycemic,
insulinotropic and hypolipidemic activities, in humans and animals
(13–16). Ginger, and its extracts, have also
been reported to possess anticancer, analgesic and antioxidant
pharmacological activities (11–13). The present study demonstrated that
GCE exerts strong vasoprotective effects and exhibits free
radical-scavenging abilities in porcine coronary arteries in
vivo.
Ginger has been used to treat cardiovascular
diseases for a long time, and it is known to exert diuretic and
blood pressure-lowering functions (7,9,10).
In the present study, GCE relaxed porcine coronary arteries in a
dose-dependent manner (Fig. 2).
In rats, ginger has been reported to exhibit hypotensive,
endothelium-dependent and -independent vasodilatory effects
(36). Distinct receptors on the
surface of the aorta and coronary arteries result in varying
responses to stimulants. For example, epinephrine induces
vasoconstriction of the aorta, but vasodilation of the coronary
arteries (37). The present
results indicated that GCE may relax KCl-induced contraction of
endothelium-intact porcine coronary artery rings, whereas GCE only
exerted a mild effect on relaxation of endothelium-denuded porcine
coronary artery rings (Fig. 3).
These data suggested that GCE may induce endothelium-dependent
relaxation of porcine coronary arteries. NO is a major mediator of
endothelium-dependent arterial relaxation.
Vasodilators, including NO, prostaglandin
I2 and endothelium-derived hyperpolarizing factor,
contribute to endothelium-dependent relaxation (38). The present results indicated that
GCE-induced endothelium-dependent relaxation was markedly inhibited
by L-NNA, an endothelial NOS inhibitor (Fig. 4). NO activates sGC, which is
responsible for the enzymatic conversion of GTP to cyclic GMP
(cGMP). An increase in cGMP has been reported to mediate relaxation
of coronary arteries. ODQ, which is a potent inhibitor of
NO-activated sGC, inhibits NO-stimulated activity (39). In the present study, GCE-induced
relaxation was significantly attenuated in the porcine coronary
artery rings in response to pretreatment with ODQ (Fig. 5). These results indicated that the
NO signaling pathway may be involved in GCE-induced relaxation of
porcine coronary arteries. Arachidonic acid causes
endothelium-dependent relaxation of coronary arteries (40). COX converts arachidonic acid into
prostaglandin G2 (41). The
present results indicated that GCE -induced relaxation was
significantly attenuated in porcine coronary artery rings in
response to pretreatment with indomethacin (Fig. 6). These data suggested that COX
may be involved in GCE-induced relaxation of porcine coronary
arteries.
Elevated Hcy levels in the blood
(hyperhomocysteinemia) induce endothelial cell injury and are
correlated with the occurrence of blood clots, which in turn may
lead to atherogenesis. Hcy is a possible risk factor for coronary
artery disease (42).
Ilkhanizadeh et al (43)
demonstrated that ginger extract may significantly reduce cardiac
structural abnormalities in diabetic rats, and these effects were
associated with improvements in serum apolipoprotein, leptin,
cathepsin G and Hcy levels. The present results suggested that Hcy
reduced relaxation, whereas GCE significantly prevented Hcy-induced
endothelial dysfunction.
ROS are well-known mediators of vascular damage.
H2O2 induces contraction in isolated canine
basilar arteries (44). The
present study revealed that GCE improved
H2O2-induced endothelial cell injury, and
possessed a stronger antioxidant ability to scavenge free radicals,
compared with GNE and GWE. Dugasani et al (14) demonstrated that [6]-gingerol,
[8]-gingerol, [10]-gingerol and [6]-shogaol exhibited substantial
scavenging activities with half maximal inhibitory concentration
(IC50) values of 26.3, 19.47, 10.47 and 8.05 µM
against DPPH radical, IC50 values of 4.05, 2.5, 1.68 and
0.85 µM against super-oxide radical and IC50
values of 4.62, 1.97, 1.35 and 0.72 µM against hydroxyl
radical, respectively. 6-Shogaol exhibited the most potent
antioxidant and anti-inflammatory properties. In addition, elevated
Hcy levels in the blood are associated with atherogenesis. It has
been reported that Hcy increases the mRNA expression levels of eNOS
and upregulates iNOS expression, thus resulting in COX-2
production, which eventually leads to the inflammatory response
(45,46). The present study examined the
effects of ginger extracts on Hcy-induced endothelial cell damage
and on the protein expression levels of eNOS, iNOS and COX-2. Hcy
increased eNOS, iNOS and COX-2 expression, whereas GCE markedly
reduced eNOS, iNOS and COX-2 expression in the presence of Hcy
(Fig. 13). These results
indicated that GCE may exert a strong vasoprotective effect.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that GCE may induce
relaxant and vasoprotective effects on porcine coronary arteries,
and may possess free radical-scavenging activities. Therefore, GCE
may be considered a potential cardioprotective factor in the
context of human diseases.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Ministry of Health and Welfare, Republic
of China (Taiwan). https://www.mohw.gov.tw/cp-3425-33347-2.html.
2017
|
|
2
|
Li H, Sun K, Zhao R, Hu J, Hao Z, Wang F,
Lu Y, Liu F and Zhang Y: Inflammatory biomarkers of coronary heart
disease. Front Biosci (Schol Ed). 10:185–196. 2018. View Article : Google Scholar
|
|
3
|
Antonopoulos AS, Papanikolaou E, Vogiatzi
G, Oikonomou E and Tousoulis D: Anti-inflammatory agents in
peripheral arterial disease. Curr Opin Pharmacol. 39:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salvo F, Bezin J, Bosco-Levy P, Letinier
L, Blin P, Pariente A and Moore N: Pharmacological treatments of
cardiovascular diseases: Evidence from real-life studies. Pharmacol
Res. 118:43–52. 2017. View Article : Google Scholar
|
|
5
|
Brown AC: An overview of herb and dietary
supplement efficacy, safety and government regulations in the
United States with suggested improvements. Part 1 of 5 series. Food
Chem Toxicol. 107:449–471. 2017. View Article : Google Scholar
|
|
6
|
Aggarwal M, Aggarwal B and Rao J:
Integrative medicine for cardiovascular disease and prevention. Med
Clin North Am. 101:895–923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla Y and Singh M: Cancer preventive
properties of ginger: a brief review. Food Chem Toxicol.
45:683–690. 2007. View Article : Google Scholar
|
|
8
|
Baliga MS, Haniadka R, Pereira MM,
Thilakchand KR, Rao S and Arora R: Radioprotective effects of
Zingiber officinale Roscoe (ginger): past, present and future. Food
Funct. 3:714–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borrelli F, Capasso R, Aviello G, Pittler
MH and Izzo AA: Effectiveness and safety of ginger in the treatment
of pregnancy-induced nausea and vomiting. Obstet Gynecol.
105:849–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghayur MN, Gilani AH, Afridi MB and
Houghton PJ: Cardio-vascular effects of ginger aqueous extract and
its phenolic constituents are mediated through multiple pathways.
Vascul Pharmacol. 43:234–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Connell DW and McLachla R: Natural pungent
compounds: IV. Examination of the gingerols, shogaols, paradols and
related compounds by thin-layer and gas chromatography. J
Chromatogr A. 67:29–35. 1972. View Article : Google Scholar
|
|
12
|
Jolad SD, Lantz RC, Chen GJ, Bates RB and
Timmermann BN: Commercially processed dry ginger (Zingiber
officinale): composition and effects on LPS-stimulated
PGE2 production. Phytochemistry. 66:1614–1635. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Macleod AJ and Pieris NM: Volatile aroma
constituents of Sri Lankan ginger. Phytochemistry. 23:353–359.
1984. View Article : Google Scholar
|
|
14
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of [6]-gingerol, [8]-gingerol,
[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–520. 2010.
View Article : Google Scholar
|
|
15
|
Mashhadi NS, Ghiasvand R, Askari G, Hariri
M, Darvishi L and Mofid MR: Anti-oxidative and anti-inflammatory
effects of ginger in health and physical activity: review of
current evidence. Int J Prev Med. 4(Suppl 1): S36–S42.
2013.PubMed/NCBI
|
|
16
|
Weng CJ, Chou CP, Ho CT and Yen GC:
Molecular mechanism inhibiting human hepatocarcinoma cell invasion
by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 56:1304–1314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao Y, Yu Y, Li C, Yu J, Zong RR and Pei
CG: Synergistic effect of quercetin and 6-gingerol treatment in
streptozotocin induced type 2 diabetic rats and poloxamer P-407
induced hyperlipidemia. Rsc Adv. 6:12235–12242. 2016. View Article : Google Scholar
|
|
18
|
Ok S and Jeong WS: Optimization of
extraction conditions for the 6-shogaol-rich extract from ginger
(Zingiber officinale Roscoe). Prev Nutr Food Sci. 17:166–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang LC and Yu YL: Dietary components as
epigenetic-regulating agents against cancer. Biomedicine (Taipei).
6:22016. View Article : Google Scholar
|
|
20
|
Gilani AH, Mandukhail SU, Iqbal J,
Yasinzai M, Aziz N, Khan A and Najeeb-ur-Rehman: Antispasmodic and
vasodilator activities of Morinda citrifolia root extract are
mediated through blockade of voltage dependent calcium channels.
BMC Complement Altern Med. 10:22010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakanashi M, Matsuzaki T and Aniya Y:
Nitroglycerin relaxes coronary artery of the pig with no change in
glutathione content or glutathione S-transferase activity. Br J
Pharmacol. 103:1905–1908. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun B, Wang W and Salvaterra PM:
Functional analysis and tissue-specific expression of Drosophila
Na+, K+-ATPase subunits. J Neurochem.
71:142–151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen RA, Plane F, Najibi S, Huk I,
Malinski T and Garland CJ: Nitric oxide is the mediator of both
endothelium-dependent relaxation and hyperpolarization of the
rabbit carotid artery. Proc Natl Acad Sci USA. 94:4193–4198. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu CC, Yang SH, Hsia SM, Wu CH and Yen GC:
Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis
and liver fibrosis in vitro. J Funct Foods. 20:20–30. 2016.
View Article : Google Scholar
|
|
25
|
Lee CF, Yang JS, Tsai FJ, Chiang NN, Lu
CC, Huang YS, Chen C and Chen FA: Kaempferol induces
ATM/p53-mediated death receptor and mitochondrial apoptosis in
human umbilical vein endothelial cells. Int J Oncol. 48:2007–2014.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Randriamboavonjy V, Busse R and Fleming I:
20-HETE-induced contraction of small coronary arteries depends on
the activation of Rho-kinase. Hypertension. 41:801–806. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Randriamboavonjy V, Kiss L, Falck JR,
Busse R and Fleming I: The synthesis of 20-HETE in small porcine
coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc
Res. 65:487–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomiyama H and Yamashina A: Beta-blockers
in the management of hypertension and/or chronic kidney disease.
Int J Hypertens. 2014:9192562014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta S and Wright HM: Nebivolol: a highly
selective beta1-adrenergic receptor blocker that causes
vasodilation by increasing nitric oxide. Cardiovasc Ther.
26:189–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flagg TP, Enkvetchakul D, Koster JC and
Nichols CG: Muscle KATP channels: recent insights to
energy sensing and myoprotection. Physiol Rev. 90:799–829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quast U, Stephan D, Bieger S and Russ U:
The impact of ATP-sensitive K+ channel subtype
selectivity of insulin secretagogues for the coronary vasculature
and the myocardium. Diabetes. 53(Suppl 3): S156–S164. 2004.
View Article : Google Scholar
|
|
32
|
Drain P, Li L and Wang J: KATP
channel inhibition by ATP requires distinct functional domains of
the cytoplasmic C terminus of the pore-forming subunit. Proc Natl
Acad Sci USA. 95:13953–13958. 1998. View Article : Google Scholar
|
|
33
|
Valavanidis A, Vlahogianni T, Dassenakis M
and Scoullos M: Molecular biomarkers of oxidative stress in aquatic
organisms in relation to toxic environmental pollutants. Ecotoxicol
Environ Saf. 64:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang F, Zhao S, Li F, Zhang B, Qu Y, Sun
T, Luo T and Li D: Investigation of antioxidant interactions
between Radix Astragali and Cimicifuga foetida and identification
of synergistic antioxidant compounds. PLoS One. 9:e872212014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Padma VV: An overview of targeted cancer
therapy. Biomedicine (Taipei). 5:192015. View Article : Google Scholar
|
|
36
|
Tesfamariam B and Halpern W:
Endothelium-dependent and endothelium-independent vasodilation in
resistance arteries from hypertensive rats. Hypertension.
11:440–444. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nabel EG, Ganz P, Gordon JB, Alexander RW
and Selwyn AP: Dilation of normal and constriction of
atherosclerotic coronary arteries caused by the cold pressor test.
Circulation. 77:43–52. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang J, Zheng JP, Li Y, Gan Z, Jiang Y,
Huang D, Li H, Liu Z and Ke Y: Differential contribution of
endothelium-derived relaxing factors to vascular reactivity in
conduit and resistance arteries from normotensive and hypertensive
rats. Clin Exp Hypertens. 38:393–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghalayini IF: Nitric oxide-cyclic GMP
pathway with some emphasis on cavernosal contractility. Int J Impot
Res. 16:459–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pratt PF, Rosolowsky M and Campbell WB:
Mediators of arachidonic acid-induced relaxation of bovine coronary
artery. Hypertension. 28:76–82. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Seibold SA, Rieke CJ, Song I,
Cukier RI and Smith WL: Prostaglandin endoperoxide H synthases:
peroxidase hydro-peroxide specificity and cyclooxygenase
activation. J Biol Chem. 282:18233–18244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Steed MM and Tyagi SC: Mechanisms of
cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox
Signal. 15:1927–1943. 2011. View Article : Google Scholar :
|
|
43
|
Ilkhanizadeh B, Shirpoor A, Khadem Ansari
MH, Nemati S and Rasmi Y: Protective Effects of ginger (Zingiber
officinale) extract against diabetes-induced heart abnormality in
rats. Diabetes Metab J. 40:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
45
|
Förstermann U and Li H: Therapeutic effect
of enhancing endothelial nitric oxide synthase (eNOS) expression
and preventing eNOS uncoupling. Br J Pharmacol. 164:213–223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsumoto T, Goulopoulou S, Taguchi K,
Tostes RC and Kobayashi T: Constrictor prostanoids and uridine
adenosine tetraphosphate: vascular mediators and therapeutic
targets in hypertension and diabetes. Br J Pharmacol.
172:3980–4001. 2015. View Article : Google Scholar : PubMed/NCBI
|