Introduction
Diabetes, which partly results from either a loss of
β-cell mass [type 1 diabetes mellitus (T1DM)] or insulin resistance
(T2DM), has a considerably high rate of morbidity worldwide
(1,2). For patients with T2DM, although
their blood insulin concentrations are presumably high, prolonged
disease may ultimately lead to the development of insulin-deficient
diabetes, T1DM. Clinical studies on T2DM patients have indicated
that the condition is associated with a decreased β-cell mass and
increased β-cell apoptosis (3).
Of note, in elderly T2DM patients, islet regeneration capability
was impaired with age, and thus, such patients have to rely on
exogenous insulin injection to maintain blood sugar homeostasis.
Although islet transplantation may be performed for severe cases of
T1DM, it is limited by the shortage of appropriate organ donors and
the long-term prescription of immunosuppressant drugs. Previous
studies have demonstrated that in response to physiological and
pathophysiological changes, islet β-cells exhibited a compensatory
capacity throughout adulthood (4,5).
Indeed, after partial pancreatectomy (PPx) in rodents as a classic
model of pancreatic regeneration, islet regeneration was observed
to occur via the replication of pre-existing differentiated cells,
the hypertrophy of β-cells and the differentiation of whole new
pancreatic lobes (6). Therefore,
it appears that the successful induction of insulin secretion in
aged mice may be achieved by PPx surgery, despite the acute loss of
islet β-cells in the short term. Of note, the study by Tschen et
al (7), revealed that the
β-cell proliferation capability declined with age and that this
decline was regulated by the Bmi1/p16INK4a pathway.
Consistently, Rankin and Kushner (8) reported that basal β-cell
proliferation was severely decreased with advanced age and that in
a mouse model, PPx failed to increase β-cell replication in aged
mice. p16INK4a, as a negative regulator of the cell
cycle, is differentially expressed in aging tissues and has been
reported to restrict islet growth (9,10).
Therefore, to investigate the mechanisms of mangiferin-induced
islet regeneration in aged mice, the present study focused on
p16INK4a.
Previous studies on mangiferin, a traditional
Chinese medicine isolated from the leaves of Mangiferina
indica (mango), have identified antitumor (11), antiviral (12), antioxidant (13) and immunomodulatory activities
(14). In addition, studies on
the anti-diabetic effect of mangiferin revealed that it markedly
lowered blood glucose levels in streptozotocin (STZ)-induced
diabetic rats (15,16). It was also reported to exert
beneficial effects on hyperlipidemia in T2DM (17). Furthermore, mangiferin
significantly prevented the progression of diabetic nephropathy and
improved renal function (18,19). These anti-diabetic effects of
mangiferin may be due to the stimulation of peripheral glucose
utilization (20). A previous
study by our group on mangiferin clearly indicated that mangiferin
treatment markedly induced islet regeneration in young, partially
pancreatectomized mice (21).
Therefore, to further elucidate the anti-diabetic effect of
mangiferin, the present study investigated whether mangiferin
induces islet regeneration in aged mice, and evaluated the
underlying mechanisms, including the potential regulation of cell
cycle regulatory proteins.
Materials and methods
Study design
A total of 90 male C57BL/6J mice (age, 12 months,
26±2 g) were purchased from the Affiliated Animal Institute of
Sichuan Academy of Medical Science & Sichuan Provincial
People's Hospital (Chengdu, China). Mice were maintained in a 12-h
light/dark cycle in an atmosphere of 0.03% CO2 with free
access to water and food. All mice were raised under specific
pathogen-free conditions with a controlled temperature (23±2°C).
Mice were randomly divided into three groups (n=30 in each group):
i) A sham group, in which mice received a sham operation; ii) a PPx
control group, in which mice were administered normal saline after
PPx surgery; and iii) a mangiferin group, in which mice were
intraperitoneally (i.p.) administered 90 mg/kg mangiferin for 28
consecutive days after PPx surgery (17,22). Mangiferin (purity, >98%) was
purchased from Desite Co. (Chengdu, China). The animal procedures
were approved by the Ethics Committee of Sichuan Academy of Medical
Science & Sichuan Provincial People's Hospital (Chengdu,
China).
Animal surgery
All animals were fasted for 12 h prior to and 5 h
after surgery, after which the animals were given free access to a
standard diet and water. Mice were anesthetized by i.p. injection
of 50 mg/kg pentobarbital (Beijing Propbs Biotechnology, Beijing,
China), and the spleen and entire splenic portion of the pancreas
were then surgically removed, while the mesenteric pancreas between
the portal vein and duodenum was left intact. For the sham
operation, the spleen was removed, while the pancreas was left
intact. All mice were labeled with 0.8 mg/ml 5-bromo-2-deoxyuridine
(BrdU; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), continuously
provided in drinking water after the PPx surgery.
Biochemical measurements
After the animals were fasted for 8 h, their blood
glucose levels were measured with a SureStep Blood Glucose meter
(LifeScan, Milpitas, CA, USA). An intravenous glucose tolerance
test (IVGTT) was performed by tail vein injection of 1 g/kg
D-glucose (Sigma-Aldrich; Merck KGaA) on days 14 and 28. Plasma
insulin levels were determined by using an ultrasensitive mouse
insulin ELISA kit (cat. no. 80-INSMS-E01; ALPCO, Salem, NH, USA)
and plasma glucagon levels were determined by using a glucagon
ELISA kit (cat. no. 81518; Crystal Chem Inc., Downers Grove, IL,
USA).
Immunoblotting and cyclin D kinase (Cdk)4
kinase assay
After sacrificing the mice with 150 mg/kg
pentobarbital, the remaining pancreas was perfused with collagenase
P (Roche, Indianapolis, IN, USA), and the islets were then isolated
as previously described (23).
The islet tissues were lysed and total protein was quantified as
previously described (24),
followed by loading onto a NuPage Novex 10% Bis-Tris gel (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for electrophoresis.
After electrophoresis, the proteins were transferred onto
polyvinylidene fluoride membranes (Pall Corp., Port Washington, NY,
USA). The membranes were blocked and subsequently incubated with
primary antibodies at 4°C overnight, followed by incubation with
horseradish peroxidase (HRP)-conjugated secondary antibodies at
room temperature for 2 h. Chemiluminescence detection was performed
with Immobilon Western Chemiluminescent HRP Substrate (EMD
Millipore, Billerica, MA, USA) and measured directly with a
BioSpectrum Imaging System (UVP, Upland, CA, USA). The following
primary antibodies were used: Rabbit polyclonal anti-caspase-3
(cat. no. sc-7148; 1:1,000 dilution), rabbit poly-clonal
anti-B-cell lymphoma 2-associated X protein (Bax; cat. no. sc-493;
1:500 dilution), mouse monoclonal anti-BH3 domain interacting death
agonist (Bid; cat. no. sc-135847; 1:1,000 dilution), rabbit
polyclonal anti-poly(ADP ribose) polymerase (PARP; cat. no.
sc-7150; 1:1,000 dilution), mouse monoclonal anti-cyclin D1 (cat.
no. sc-450; 1:1,000 dilution), rabbit polyclonal anti-cyclin D2
(cat. no. sc-450; 1:1,000 dilution), rabbit polyclonal anti-cyclin
D3 (cat. no. sc-182; 1:1,000 dilution), rabbit polyclonal anti-cdk4
(cat. no. sc-260; 1:1,000 dilution), mouse monoclonal
anti-phospho-signal transducer and activator of transcription 3
(p-STAT3; cat. no. sc-8059; 1:500 dilution), mouse monoclonal
anti-STAT3 (cat. no. sc-8019; 1:1,000 dilution), rabbit poly-clonal
anti-retinoblastoma (Rb; cat. no. sc-7905; 1:1,000 dilution),
rabbit polyclonal anti-insulin (cat. no. sc-7953; 1:1,000
dilution), rabbit polyclonal anti-glucokinase (GCK; cat. no.
sc-7908; 1:1,000 dilution), rabbit polyclonal anti-insulin promoter
factor 1 (PDX-1; cat. no. sc-25403; 1:1,000 dilution) and mouse
monoclonal anti-β-actin (cat. no. sc-47778; 1:5,000 dilution) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA);
rabbit monoclonal anti-p16INK4a (cat. no. ab108349;
1:500 dilution), rabbit monoclonal anti-p27Kip1 (cat.
no. ab32034; 1:1,000 dilution), rabbit poly-clonal anti-phospho-Rb
(anti-phospho-S780, cat. no. ab47763; 1:1,000 dilution) and rabbit
polyclonal anti-glucose transporter 2 (GLUT-2; cat. no. ab54460;
1:1,000 dilution) antibodies were from Abcam (Cambridge, MA, USA).
Rabbit polyclonal anti-cleaved caspase-3 (cat. no. 9661; 1:1,000
dilution) was purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The HRP-conjugated goat anti-mouse polyclonal
immunoglobulin (Ig)G (cat. no. 115-035-003) and HRP-conjugated goat
anti-rabbit polyclonal IgG (cat. no. 111-035-003) were purchased
from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) and
diluted at 1:5,000. β-actin served as the loading control and its
levels were used for normalization.
The Cdk4 kinase assay was performed based on a
previously described protocol (25). The amount of
32P-labeled glutathione S-transferase-Rb (cat. no.
SRP5124, Sigma-Aldrich; Merck KGaA) was evaluated by
autoradiography and quantified by analysis with a PhosphorImager
and an ImageQuant (Molecular Dynamics, Sunnyvale, CA, USA).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
For analysis of neurogenin (Ngn)3 mRNA abundance,
RNA was extracted from the remnant pancreas, and for analysis of
other genes, RNA was extracted from the islets. Total RNA from the
islets and the remnant pancreas was isolated using an RNeasy micro
kit (Qiagen, Valencia, CA, USA) and reverse transcribed as
previously described (26). The
primer sequences and probes were as previously described (21,23). The RNA and primer were heated to
72°C and slowly cooled prior to reverse transcription at 42°C for 1
h. When cooled to room temperature, the reaction was diluted to 100
µl with RNase-free water. qPCR was performed with SYBR-Green
PCR Master mix in a total reaction volume of 20 µl using the
following amplification steps: Initial denaturation at 95°C for 10
min; followed by 40 cycles of denaturation at 95°C for 15 sec; and
then elongation at 55°C for 30 sec. The expression levels were
normalized to those of the internal standard GAPDH. Real-time qPCR
was performed on an ABI 7900 system using an Applied Biosystems
Power SYBR Green PCR Master Mix kit (Thermo Fisher Scientific,
Inc.). The copy number was calculated using the 2−ΔΔCq
method. The Cq value for GAPDH was used to normalize the samples
(27).
Histological staining
Pancreas tissues were fixed in 10% formalin (cat.
no. HT-5011; Sigma-Aldrich, Merck KGaA) for 24 h at room
temperature, dehydrated, embedded in paraffin (cat. no. A601888;
Sangon Biological, Shanghai, China) and cut into 5 µm
sections. For immunohistochemical staining, rat monoclonal BrdU
antibody (1:100 dilution, cat. no. ab6326; Abcam), rabbit
polyclonal Ki67 antibody (1:100 dilution, cat. no. PA1-21520;
Thermo Fisher Scientific, Inc.), rabbit polyclonal proliferating
cell nuclear antigen (PCNA) antibody (1:100 dilution, cat. no.
ab18197; Abcam), and guinea pig polyclonal insulin antibody (1:100
dilution, cat. no. A0564; Dako, Glostrup, Denmark) were used
according to a previously described protocol (25). The primary antibodies were
detected with corresponding secondary antibodies (1:5,000 dilution;
Jackson Immunoresearch Laboratories Inc.), including horseradish
peroxidase (HRP)-conjugated goat anti-rat IgG polyclonal antibody
(cat. no. 112-035-003), HRP-conjugated goat anti-rabbit polyclonal
IgG (cat. no. 111-035-003), Alkaline Phosphatase (AP)-conjugated
goat anti-guinea pig IgG polyclonal antibody (cat. no.
106-055-003), FITC-conjugated goat anti-guinea pig IgG polyclonal
antibody (cat. no. 106-095-003), Alex Fluor 647-conjugated goat
anti-guinea pig IgG polyclonal antibody (cat. no. 106-005-603),
Cy5-conjugated goat anti-rabbit IgG polyclonal antibody (cat. no.
111-175-144). The secondary antibodies were incubated with the
section for 2 h at room temperature. For terminal deoxynucleotidyl
transferase deoxyuridinetriphosphate nick end labeling (TUNEL)
staining, a Promega Fluorometric DeadEnd kit (Promega Corp.,
Madison, WI, USA) was used following the manufacturer's
protocols.
Images of the islets were captured with a Nikon 80i
microscope (Nikon Corp., Tokyo, Japan). All of the microscopy
imaging and quantification procedures were performed by two
technicians who were blinded to the experimental conditions of each
sample. The numbers of β-cells, BrdU(+) β-cells, Ki67(+) β-cells,
PCNA(+) β-cells and TUNEL(+) β-cells were manually counted and
checked with Image-Pro Plus 6.3 software (Media Cybernetics, Silver
Spring, MD, USA). At least 10 islets containing at least 1,000
β-cells were counted per mouse, and at least 10 consecutive
sections from eight mice per group were stained. The 1st and 10th
section were selected at a 50-µm distance. Islet diameters
were determined with an intraocular calibrated grid. Islet size was
also measured in images of insulin-stained islets converted to gray
scale at a magnification of ×400.
Analysis of relative β-cell volume was performed via
point counting morphometry using a 56-point grid. An average of
10,000 points/mouse were counted. The islet β-cell mass was
calculated by multiplying the relative β-cell volume by the total
weight of the remnant pancreas.
Islet isolation and primary culture of
islet β-cells
Male adult (age, 3 months, 22–24 g, n=20) and aged
(age, 12 months, 26–28 g, n=90) mice were anesthetized and
sacrificed by i.p. injection of 150 mg/kg pentobarbital. The
pancreas was subsequently perfused with 1 ml collagenase P (1.0
mg/ml) and digested at 37°C for 15 min. Isolated cells were then
centrifuged at 250 × g for 2 min at 4°C. The supernatant was
discarded and the cells were re-suspended in 10 ml Hank's balanced
salt solution. The intact re-suspended islets were handpicked using
a pipette under a dissection scope, and primary β-cell cultures
were prepared from the handpicked islets. Subsequently, 400-500
islets were incubated in 1 ml 1X accutase solution (cat. no. A6964;
Sigma-Aldrich; Merck KGaA) for 15 min at 37°C. The digestion was
stopped by adding cell culture medium, and the cells were collected
by centrifugation at 300 × g for 5 min at 4°C. Subsequently, the
islet cells were cultured in RPMI-1640 medium (Gibco, Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco,
Thermo Fisher Scientific, Inc.), 50 µM β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA), 1 mM sodium pyruvate (Sigma-Aldrich;
Merck KGaA), 2 mM L-glutamine (Sigma-Aldrich; Merck KGaA) and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich;
Merck KGaA) at 37°C in a humidified atmosphere containing 5%
CO2.
p16INK4a overexpression and
knockdown
The PCR product of the p16INK4a gene was
cloned into pcDNA3 plasmids (Addgene, Cambridge, MA, USA) under the
control of a cytomegalovirus promoter, and the plasmids were
transfected into the primary cultured islet cells in the presence
of Lipofectamine LTX (Thermo Fisher Scientific, Inc.). Small
interfering RNA (siRNA) specific for p16INK4a pool (cat.
no. 12578) and non-targeting siRNA controls were obtained from GE
Dharmacon (Lafayette, CO, USA). Transfection with the siRNAs was
performed using Dharmafect (GE Dharmacon).
In vitro proliferation assay
Islet cells at the logarithmic growth phase were
seeded in a 24-well plate (2×105 cells per well) and
incubated at 37°C for 24 h. Mangiferin was then added and the cells
were incubated for another 24 h. Subsequently, 10 µl of a 5
mg/ml solution of MTT was added to each well, followed by
incubation at 37°C for 4 h. Subsequently, the medium was removed
and the plates were thoroughly agitated for 1 h. Finally,
termination buffer was added to each well, and the absorbance at
570 nm was measured with a spectrophotometer (Model 3550 Microplate
Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
proliferation rates were calculated from the optical density (OD)
according to the following formula: Cell proliferation (%) = [OD
570 nm (drug)/OD 570 nm (control)] × 100%.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean from at least three independent experiments. Statistical
significance between multiple groups was determined by analysis of
variance followed by a Bonferroni post hoc test, and between 2
groups using Student's t-test using GraphPad Prism (version 5.0 for
windows) statistical software package (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference between groups.
Results
Mangiferin promotes homeostasis in aged
mice
According to a previous study by our group,
treatment with mangiferin (90 mg/kg) maintained glucose homeostasis
in adult mice by islet regeneration induced by mangiferin (21). Therefore, the present study
investigated the regulatory role of mangiferin in aged mice. As
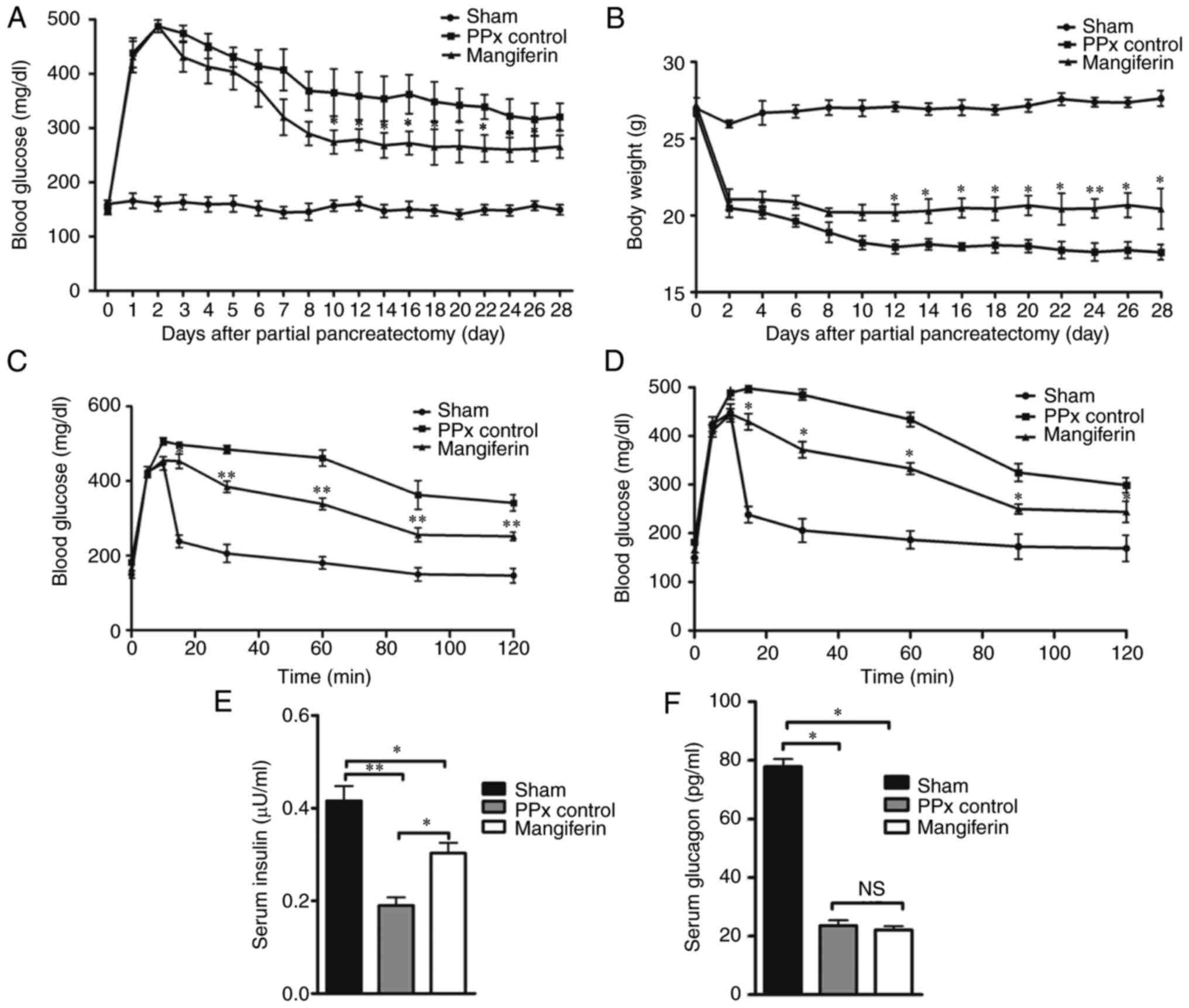
presented in Fig. 1A, compared
with the mice in the sham group, the PPx control mice exhibited
significantly increased blood glucose levels. In turn, mangiferin
treatment markedly reduced the blood glucose levels in PPx mice.
Although the mice that received mangiferin treatment did not
maintain normal blood glucose to the same extent as the
sham-operated mice, a time-dependent decrease in blood glucose
levels was observed from day 3 to day 10. Subsequently, the body
weight of the mice in each group was assessed, revealing that the
body weight of the mangiferin-treated mice were higher when
compared with that of the PPx control mice (Fig. 1B). Further analysis by IVGTT
clearly substantiated the hypothesis that islet regeneration was
induced by mangiferin. According to the IVGTT data on days 14
(Fig. 1C) and 28 (Fig. 1D), mangiferin partially recovered
the impaired glucose tolerance of the PPx mice on day 14 and
maintained the partially recovered glucose tolerance on day 28.
Furthermore, the serum insulin levels (Fig. 1E) and glucagon levels (Fig. 1F) were measured, and it was
observed that mangiferin-treated aged mice had elevated levels of
insulin secretion and had no effect on glucagon secretion compared
with those in the PPx group. These experiments illustrated that
mangiferin treatment maintained homeostasis in aged mice after
PPx.
Mangiferin induces islet β-cell
proliferation and hyperplasia in aged mice
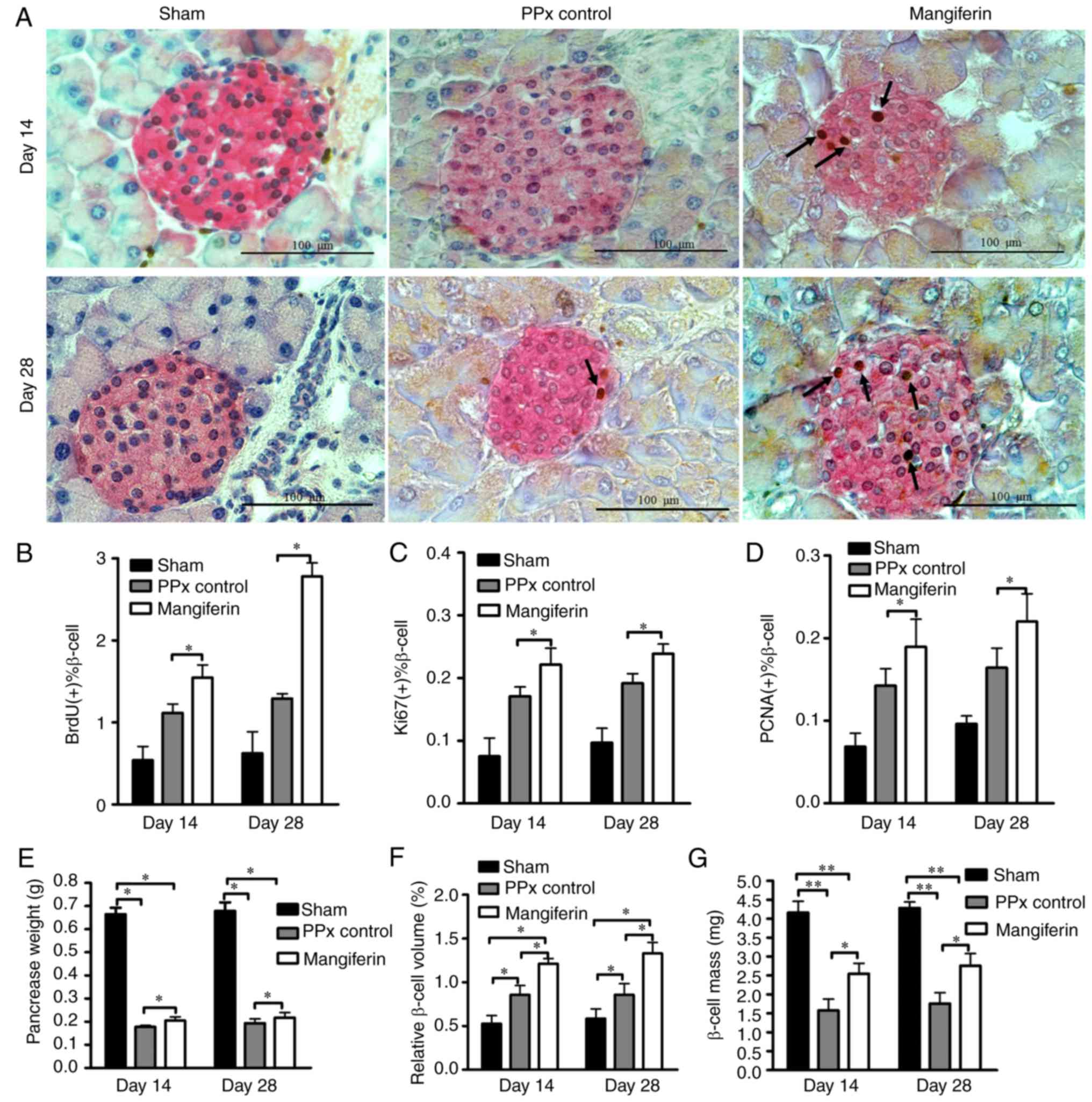
To further explore the mechanisms via which
mangiferin promotes homeostasis, the remaining pancreas tissues
were stained with specific antibodies for proliferating cells, and
the proliferation rates were subsequently quantified.
Representative immunohistochemical staining images of the islet
β-cells and proliferating cells are provided in Fig. 2A. It was evident that the islets
of mangiferin-treated mice had more proliferating cells (indicated
by arrows) on day 14 than those of the PPx mice. Of note, with
prolonged treatment with mangiferin for 28 days, an increased
quantity of BrdU-labeled proliferating cells was present, which
provided evidence that mangiferin induces islet regeneration in a
time-dependent manner. To further validate this hypothesis, cell
proliferation was assessed with two endogenous markers, PCNA and
Ki67. According to the levels of proliferating cells indicated by
BrdU-labeling (Fig. 2B), Ki67
labeling (Fig. 2C) and PCNA
labeling (Fig. 2D), mangiferin
induced robust cell proliferation in the islets of aged mice.
Although it has been reported that pancreatic duct epithelial cells
may be considered as progenitor cells that contribute to
neogenesis, no proliferation of the duct cells was observed (data
not shown). Therefore, it appears that mangiferin induced islet
regeneration in aged mice mainly via inducing the proliferation of
β-cells. However, whether those proliferated cells resulted in
islet hyperplasia remains elusive. To investigate this, the
remaining pancreas was subsequently weighed (Fig. 2E) to calculate the relative β-cell
volume (Fig. 2F) and β-cell mass
(Fig. 2G). It was revealed that
the relative β-cell volume and β-cell mass of mangiferin-treated
mice were significantly increased relative to those in PPx control
mice, which is consistent with the results on β-cell
proliferation.
Mangiferin inhibits β-cell apoptosis in
aged mice
Previous clinical studies on T2DM patients clearly
demonstrated that their β-cell mass was decreased due to increased
β-cell apoptosis (3,28). Therefore, therapeutic approaches
comprising the inhibition of β-cell apoptosis may not only palliate
hyperglycemia, but reverse and prevent the progression of the
disease, and thus, the present study further focused on the effect
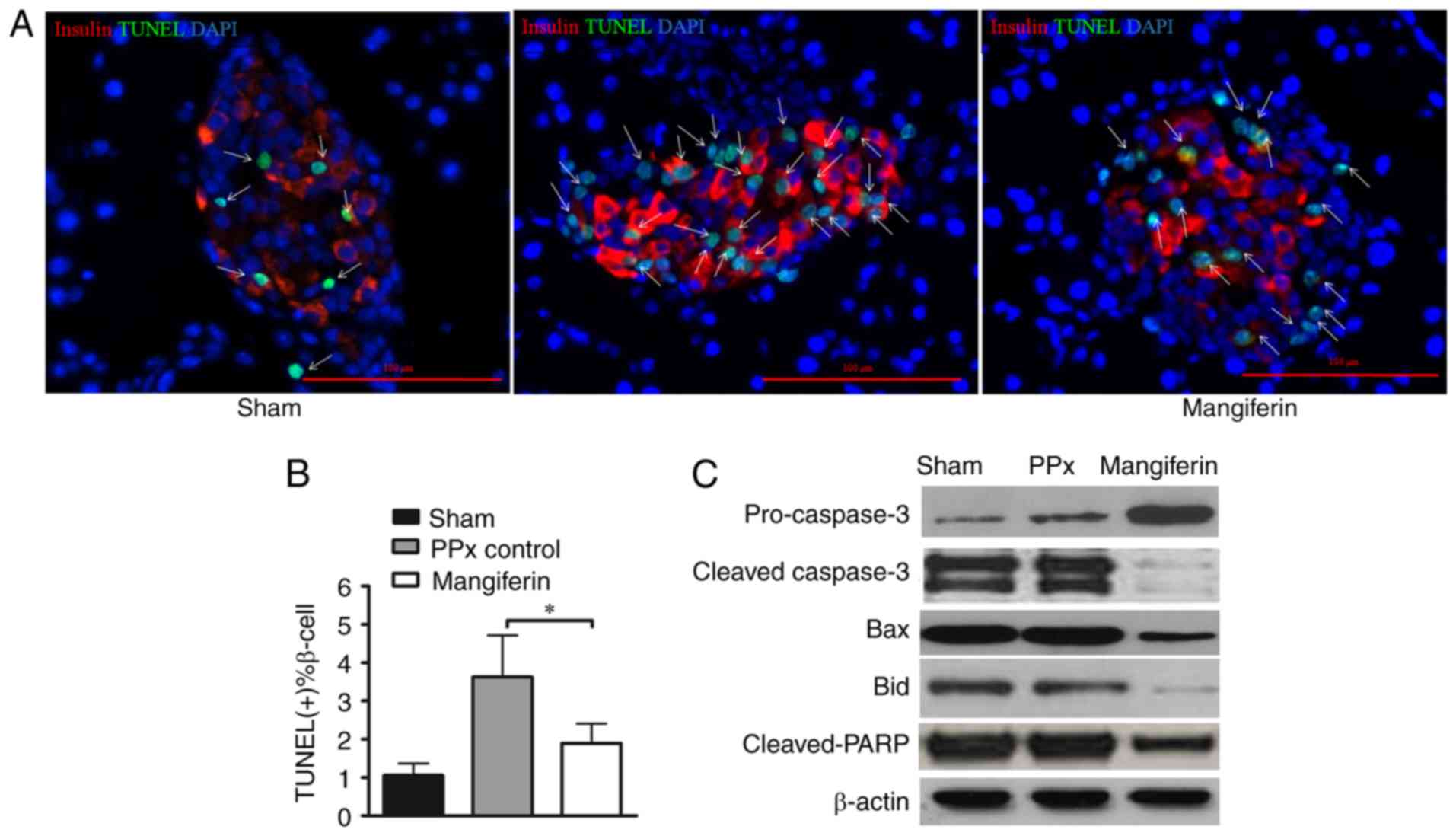
of mangiferin on β-cell apoptosis. A previous study by our group
illustrated that mangiferin inhibited β-cell apoptosis in young
mice (21). In the present study,
the remnant pancreas was stained using an in situ TUNEL
apoptosis detection kit. Of note, the islets of PPx mice lacking
mangiferin treatment exhibited more apoptotic cells (green cells
indicated by arrows; Fig. 3A)
than those of mice treated with mangiferin. Consistent with these
qualitative observations, calculation of the percentages of
apoptotic cells (TUNEL-positive islet β-cell percentage) revealed
that mangiferin treatment markedly inhibited apoptosis in the aged
mice (Fig. 3B). To further
validate that mangiferin inhibited cell apoptosis in the islets of
the aged mice, key proteins in the caspase pathway were analyzed
(Fig. 3C). Activation of caspases
has a central role in apoptosis, and caspase-3 serves as the
convergence point of different apoptotic signaling pathways
(29). As presented in Fig. 3C, caspase-3 was de-activated upon
mangiferin treatment with increased pro-caspase-3. Bid induces a
conformational change of Bax (30), and Bax induces the release of
cytochrome c from the mitochondria during apoptosis
(31). The present results
suggested that mangiferin-treated cells had lower expression levels
of Bax and Bid compared with those in the control, suggesting that
more apoptotic events were inhibited by mangiferin. Furthermore,
decreased cleavage of PARP was identified in the mangiferin-treated
group. Therefore, changes in the apoptotic tendency of β-cells,
along with enhanced proliferation capability, likely contributed to
the increased levels of insulin secretion in vivo.
Mangiferin inhibits islet β-cell
senescence in aged mice
A study by Krishnamurthy et al (9) indicated that p16INK4a
restricted proliferation and regeneration in the islets of aged
mice, while mice lacking p16INK4a exhibited enhanced
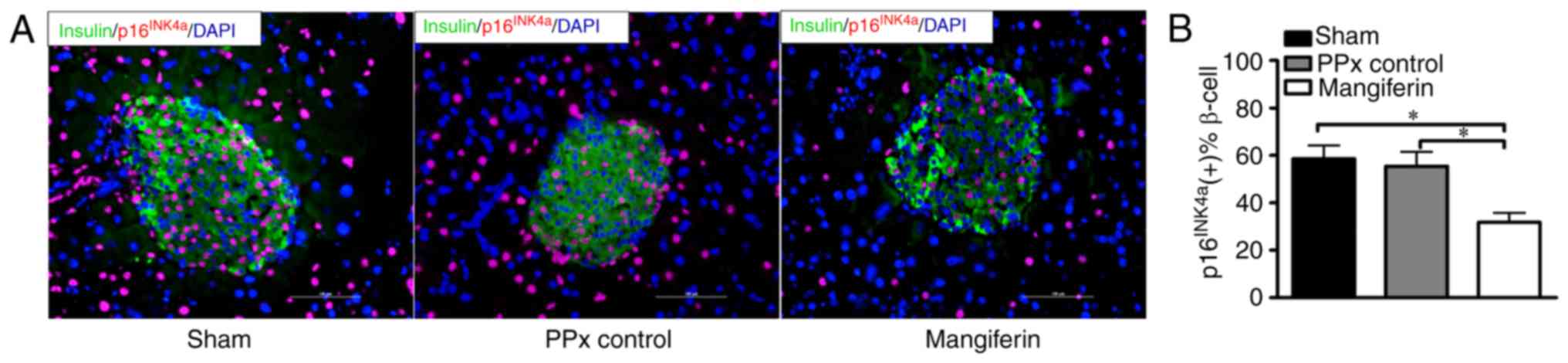
islet proliferation rates. To assess whether mangiferin treatment
prevents β-cell senescence, in situ fluorescence staining of
p16INK4a was performed. Representative images of
p16INK4a immunofluorescent staining are presented in
Fig. 4A. Quantification of the
staining clearly indicated that >50% of β-cells were labeled
with p16INK4a after PPx and also in the sham operated
group, indicating that PPx did not induce marked senescence
compared with the sham group. In contrast to the PPx controls, the
islets of mangiferin-treated mice exhibited positive staining for
active p16INK4a at a rate of ~35%, indicating that
mangiferin markedly inhibits β-cell senescence (Fig. 4B). It was therefore concluded that
mangiferin treatment inhibits β-cell senescence in aged mice.
Mangiferin regulates cell cycle
proteins
A plethora of literature suggests that cyclin D/Cdk4
complexes have a critical role in the cell cycle regeneration of
β-cells (32–34), and a previous study by our group
indicated that mangiferin induced β-cell regeneration via the
regulation of cell cycle complexes (21). Therefore, to investigate whether
cyclin D-Cdk4 complexes may be regulated by mangiferin in aged
mice, the expression of cyclin D1, -D2 and -D3, as well as Cdk4,
p16INK4a and p27Kip1 was assessed at the
protein (Fig. 5A) and mRNA
(Fig. 5B) level. As expected,
mangiferin greatly increased the transcription and translation of
cyclin D1 and -D2, as well as Cdk4. Furthermore, the expression
levels of p16INK4a and p27Kip1 were
significantly reduced by mangiferin in the aged mice.
Quantification of the western blot results indicated that the
expression and phosphorylation levels of STAT3 were markedly
elevated in the mangiferin-treated mice relative to those in the
untreated PPx controls, and the total STAT3 protein levels of PPx
control was also significantly higher than those of the sham group.
Notably, PPx could induce the increased expression of STAT3, and
mangiferin treatment could induce the phosphorylation and
activation of STAT3 (Fig. 5C and
D).
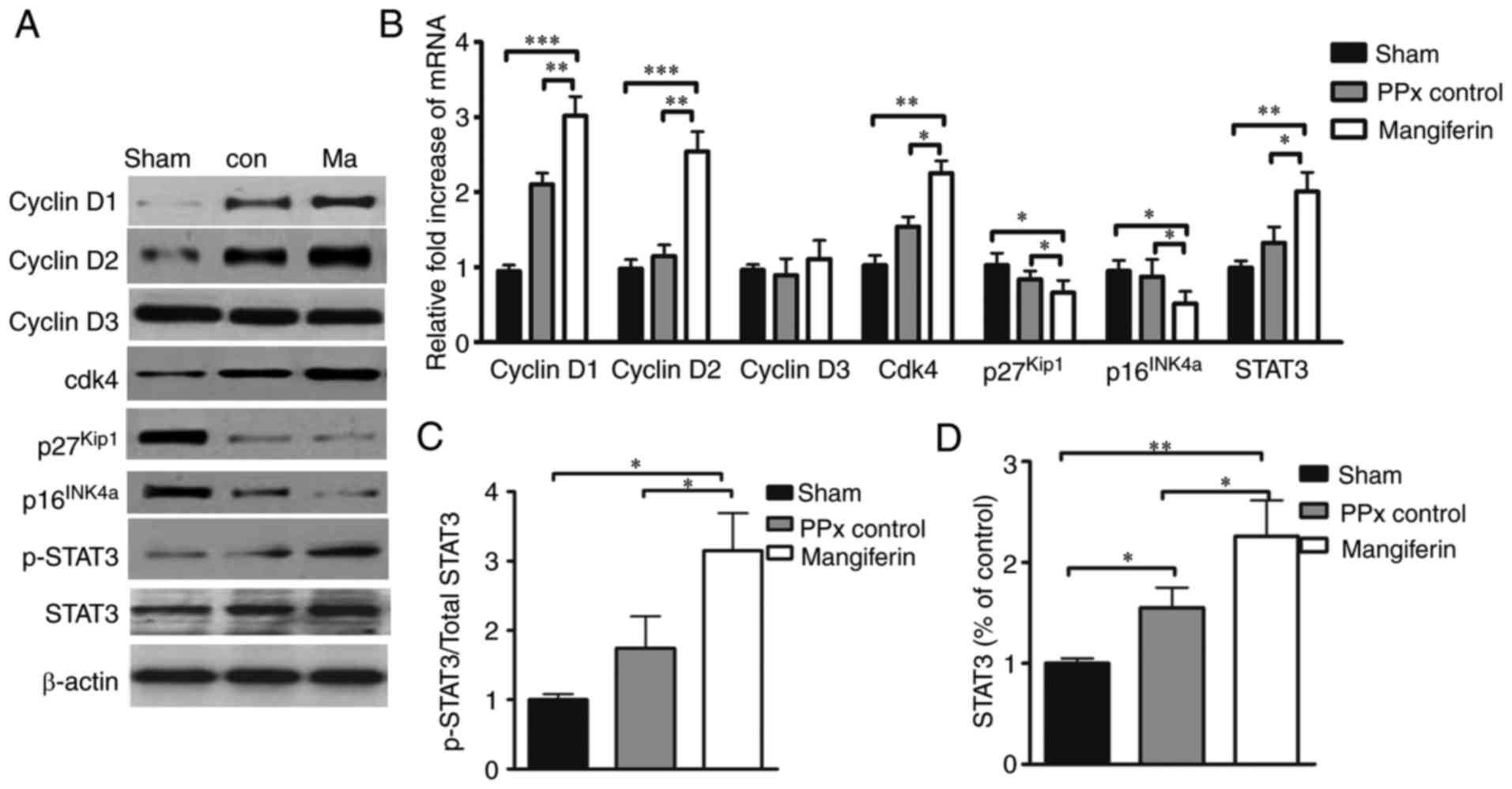 | Figure 5Mangiferin regulates cell
cycle-regulatory proteins. (A) Representative western blots of
cyclin D1, D2 and D3, as well as Cdk4, p16INK4a,
p27Kip1, STAT3 and phospho-STAT3. (B) Reverse
transcription-quantitative polymerase chain reaction analysis of
cell cycle regulators. (C and D) Quantified levels of (C)
phospho-STAT3 vs. total STAT3 ratio and (D) total STAT3 determined
by grey-value scan of the blots. Values are expressed as the mean ±
standard error of the mean of at least three independent
experiments. *P<0.05, **P<0.01 and
***P<0.001. STAT3, signal transducer and activator of
transcription; Cdk, cyclin D kinase; PPx, partial pancreatectomy;
con, control; Ma, mangiferin-treated group. |
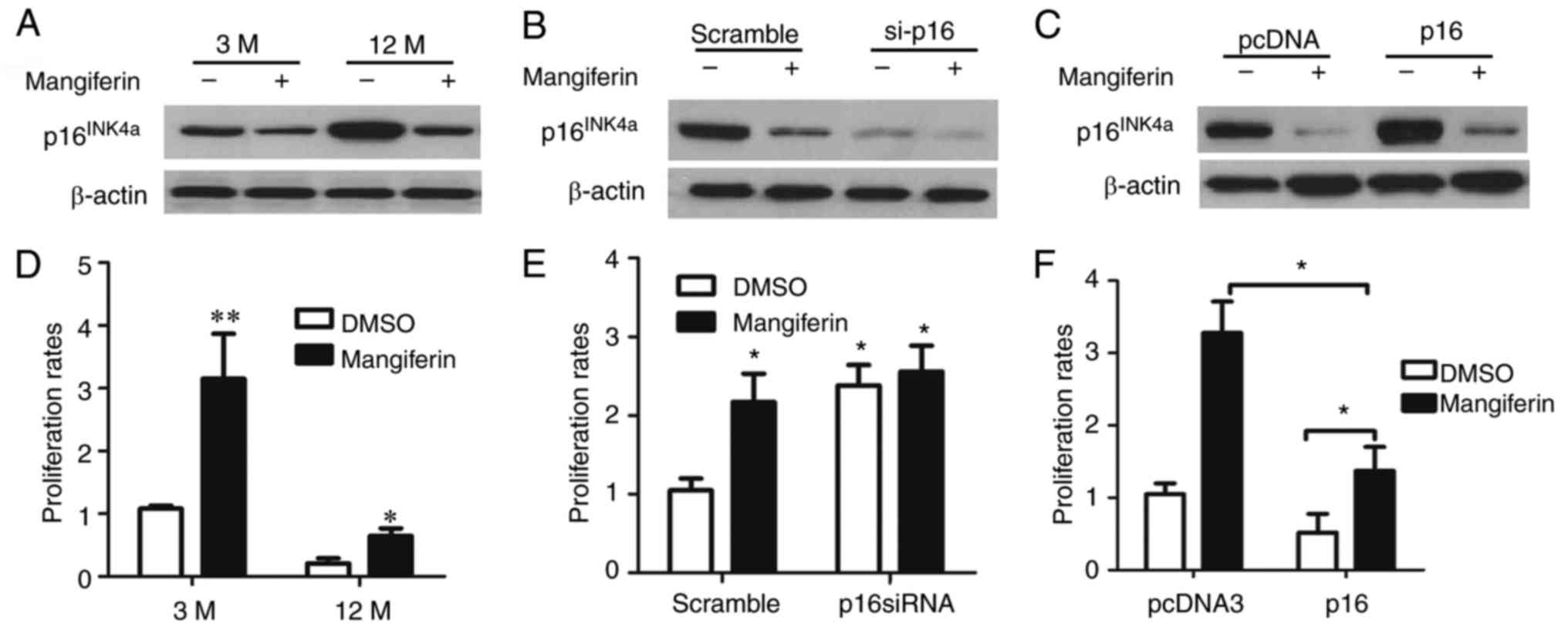
To confirm the effects of mangiferin on β-cell
proliferation via the regulation of p16INK4a, islet
cells of adult and aged mice were isolated and cultured in the
presence of mangiferin or vehicle (dimethyl sulfoxide) (Fig. 6A); furthermore, the
p16INK4a gene was knocked down in the isolated islet
cells of the aged mice (Fig. 6B)
and p16INK4a was overexpressed in the isolated islet
cells of the adult mice (Fig.
6C). Of note, mangiferin induced the proliferation of the islet
cells from the adult and the aged mice (Fig. 6D). Furthermore,
p16INK4a silencing in islet cells from aged mice led to
elevated proliferation rates, regardless of whether the islets were
treated with or without mangiferin (Fig. 6E). In addition, mangiferin
treatment of the isolated islet cells of the adult mice with
over-expression of p16INK4a significantly increased the
proliferation rate (Fig. 6F).
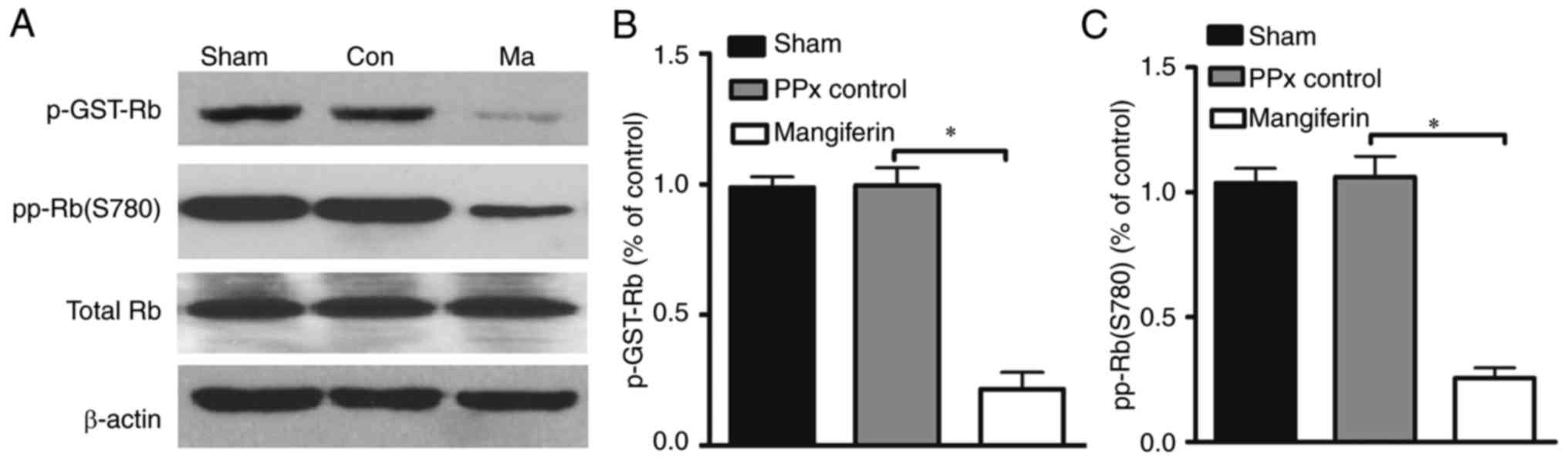
Furthermore, to address whether the activity of Cdk4
may be regulated by mangiferin, a Cdk4 kinase assay was performed.
Previous studies demonstrated that Rb is an important substrate of
the cyclin D1/Cdk4 complex, and that its activation is closely
associated with senescence (35).
In the Cdk4 assay, increased radioactive labeling of the substrate
is considered to indicate greater enzymatic activity of Cdk4. As
presented in Fig. 7A and B, the
in vitro kinase assay indicated lower levels of labeled
phosphorylated Rb in the mangiferin-treated group when compared
with that in the control group. In addition, in vivo
analysis of Rb phosphorylation on serine 780 revealed that
mangiferin treatment greatly reduced the phosphorylation of Rb,
suggesting that the enhanced Cdk4 activity resulted in decreased Rb
activity (Fig. 7C). Collectively,
these results indicate that mangiferin may induce β-cell
proliferation through the regulation of cell cycle proteins.
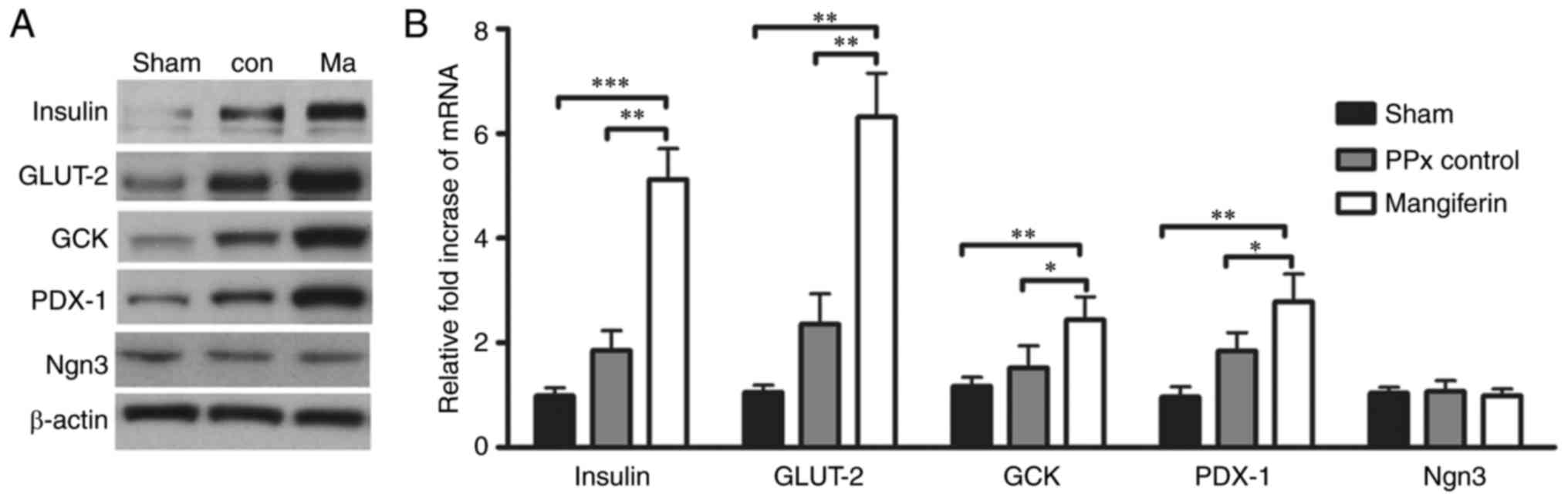
Mangiferin regulates insulin
secretion-associated genes
To elucidate the mechanism underlying
mangiferin-induced insulin secretion in aged mice, it was assessed
whether mangiferin regulates β-cell-specific genes, including
insulin, GCK, GLUT-2 and PDX-1, as critical genes for β-cell
function. As expected, a significant increase in the protein
expression levels of insulin, GLUT-2, GCK and PDX-1 was detected in
the islets of the mangiferin-treated mice (Fig. 8A). Accordingly, the mRNA levels of
these genes were also elevated (Fig.
8B). Notably, mangiferin treatment led to upregulation of the
expression of PDX-1, the upstream gene of insulin. However, no
changes in the transcription or translation levels of Ngn3 were
observed in mangiferin-treated mice when compared with those in the
controls (Fig. 8), suggesting
that no neogenesis occurred in the aged mice. Therefore, it was
concluded that mangiferin treatment markedly contributes to insulin
secretion through the regulation of insulin-associated genes.
Discussion
To the best of our knowledge, the present study was
the first to report that islet regeneration was present in
mangiferin-treated aged mice. Furthermore, along with the increased
proliferation of islet β-cells, comparatively reduced blood glucose
levels, enhanced glucose tolerance and slightly but gradually
increased body weight were identified in the mangiferin-treated
aged mice. The islet cells were labelled with the proliferation
markers BrdU, Ki67 and PCNA, and staining was quantified to
evaluate the rate of islet regeneration. All three different
markers were increased in the mangiferin-treated group, indicating
that the drug induced the proliferation of islet β-cells.
Furthermore, as negative regulators of the cell cycle have a
critical and fundamental role in the re-entry of islet β-cells into
the cell cycle, cell cycle-associated genes and proteins were
analyzed in the present study, and the results indicated that
mangiferin induced the activation of cyclin D/Cdk4 complexes and
inhibited the expression of p16Ink4a in aged mice
following PPx. In addition, genes associated with β-cell function
were significantly upregulated by mangiferin. Taken together, these
results clearly indicated that mangiferin treatment induced islet
regeneration and maintained homeostasis in the aged mice with PPx
by modulating cell cycle regulators and insulin
secretion-associated genes.
Previous clinical studies demonstrated that, due to
the increased rate of β-cell apoptosis, the β-cell mass was
decreased in T2DM patients (3).
Thus, therapeutic approaches that inhibit apoptosis and increase
the β-cell mass may be effective novel strategies for the treatment
of T2DM, particularly for patients of advanced age. In the present
study, a gradual control of homeostasis was observed in the mice
treated with mangiferin, suggesting that the mice still possessed
islet regeneration capacity, which led to the gradual recovery of
blood glucose levels. By genetic lineage tracing, Dor et al
(36) determined that terminally
differentiated β-cells retained a significant proliferative
capacity in vivo. Their study also suggested that
pre-existing β-cells, rather than pluripotent stem cells, were the
major source of new β-cells during adult life and after PPx in mice
(36). Teta et al
(37) also demonstrated that the
growth and regeneration of adult β-cells did not involve any
specialized progenitors. Thus, the present study evaluated the
proliferation rates of islet β-cells in the different groups of
mice, and the results indicated that the mice treated with
mangiferin possessed a higher islet regeneration capability. As
mentioned above, adaptive β-cell proliferation is severely
restricted with advanced age; therefore, β-cell proliferation was
observed and quantified using three different proliferation
markers, BrdU, Ki67 and PCNA according to previously reported
procedures (38,39). By immunohistochemical staining for
BrdU, Ki67 and PCNA, the proliferated cells were labeled to
visualize islet regeneration that was stimulated by mangiferin in
the aged mice after PPx. It is widely acknowledged that cell
proliferation depends on various cell cycle check point proteins
(40). To elucidate the mechanism
of mangiferin-induced regeneration of islet cells, it was assessed
whether mangiferin regulates cell cycle proteins. From the results,
it was obvious that various cell cycle proteins were regulated by
mangiferin, and as the proliferation of islet cells is controlled
by cell cycle proteins, it was suggested that mangiferin treatment
induced islet regeneration in aged mice by modulating cell cycle
regulators and also insulin secretion-associated genes. The results
of the labeling with the three different markers of cell
proliferation clearly indicated that mangiferin treatment was able
to ameliorate the diabetic condition by inducing islet β-cell
proliferation. This also implied that, for human patients of
advanced age who have little regenerative capacity with regard to
β-cell mass, mangiferin may be a promising therapeutic. As a
decrease in β-cell mass is a primary pathogenic factor in T2DM, the
present study also assessed the β-cell mass in the mice by
determining the remnant pancreas weight, relative β-cell volume and
relative β-cell mass. As expected, the β-cell mass of the
mangiferin-treated mice exhibited a comparative increase relative
to that in the untreated PPx control group, indicating that
mangiferin induced hyperplasia of the pancreas. In studies on T2DM
patients, Butler et al (3,
41) reported that a major defect
leading to the decrease in β-cell mass was an increased rate of
β-cell apoptosis. Therefore, therapeutic approaches designed to
inhibit apoptosis may ameliorate of halt the progression of T2DM,
and the present results regarding apoptosis strongly suggest that
mangiferin inhibits β-cell apoptosis.
The present study addressed the important issue of
whether islets in elderly individuals still possess proliferation
capacity using a mouse model. In previous studies on rodents, the
plasticity of the β-cell mass was correlated with β-cell
proliferation (42). The results
of the present study clearly indicated the proliferation potential
of islets in aged mice. However, the mechanisms underlying the
proliferation of β-cells during islet regeneration require further
investigation. Studies by Krishnamurthy et al (9,10)
suggested that β-cell mass expansion may be regulated by
p16Ink4a, as a negative regulator of cyclin D/Cdk4. In
addition, in transgenic mice overexpressing p16Ink4a,
reduced islet cell proliferation and a reduction in the
regenerative capacity of islets was observed following STZ-induced
degeneration of the islet β-cell mass (8). Senescence and apoptosis rely on
telomere shortening and p16INK4a activation (43). Based on the results of a previous
study by our group, indicating that mangiferin regulates cell cycle
proteins (21), the hypothesis
that mangiferin administration induces islet regeneration in aged
mice through the regulation of cell cycle proteins was proposed. In
the present study, p16INK4a, a marker of aging, was
demonstrated to be downregulated in mangiferin-treated mice,
suggesting that the anti-aging effect of mangiferin occurred via
the regulation of p16Ink4a. Compared with the sham
group, PPx did not induce a marked increase of senescence. However,
mangiferin could inhibit senescence of aged mice. It has been
reported that senescence requires activation of Rb and the
expression of their regulators, most prominently
p16INK4a. As p16INK4a targets Rb, an in
vivo phosphorylation assay of Rb was performed, and decreased
activation of Rb was identified in the mangiferin-treated mice.
These pre-clinical data on mangiferin therapy may aid in
elucidating the regulation of β-cell proliferation and β-cell mass
in T2DM.
It has been widely acknowledged that apoptosis,
necrosis and autophagy are three types of programmed cell death
(PCD) (44). Fehsel et al
(45) demonstrated that islet
cells undergo necrosis instead of apoptosis after the injection of
STZ or nitric oxide (NO). Hoorens et al (46) indicated that interleukin-1 also
mediated islet necrosis. These effects have been attributed to the
induction of NO synthase in β-cells and subsequent generation of
toxic NO levels. These studies provided evidence that necrosis
occurs in islets. However, an overwhelming amount of studies have
indicated that apoptosis is the major pathway for islet cell death,
particularly for islets of aged mice (7,8)
and p16INK4a has been indicated to induce senescence of
islets in aged mice (9).
Furthermore, numerous studies reported that hyperglycemia induces
apoptosis of islets (8,41,47,48). Based on these previous studies,
with regard to PCD of islets, apoptosis may be the predominant type
of cell death, particularly for aged islets. In the study by
Hoorens et al (46),
Hoechst 33342 and PI were used to stain the cells, and the
double-positive cells were regarded as necrotic cells. A limitation
of the present study is that no Hoechst 3342 plus PI staining,
sorting of the cells by flow cytometry and quantification of the
RIP1 expression levels was performed. However, according to
previous studies on aged islets, the major cell death pathway is
apoptosis and in addition, the western blot results of the present
study on pro-caspase-3, cleaved caspase-3, Bax, Bid and cleaved
PARP indicated the activation of the caspase-dependent apoptotic
pathway in aged islets. However, in the present study, mangiferin
increased the expression levels of pro-caspase-3 and decreased the
cleavage of caspase-3 and PARP. In addition, mangiferin treatment
could also inhibit the expression of Bax and Bid. Therefore, the
conclusion is drawn that mangiferin inhibits islet cell apoptosis
as one of the mechanisms for the decreased blood glucose levels in
PPx mice after mangiferin treatment.
A study by Xu et al (49) demonstrated that during islet
regeneration after injury, Ngn3, a basic helix-loop-helix
transcription factor, was activated in duct-associated stem or
progenitor cells of islet β-cells. It was therefore suggested that
Ngn3 is critical for the development of endocrine cells in the
islets and for islet neogenesis. In addition, this previous study
demonstrated that β-cell neogenesis was activated when the β-cell
mass was reduced as a compensatory mechanism (49). A previous study by our group
reported that mangiferin activates Ngn3 in young PPx mice, and
subsequently induces neogenesis in pancreatic duct cells/progenitor
cells (21). However, in the
present study, no proliferating cells were observed in the duct. Of
note, no change in the expression levels of Ngn3 was identified in
the islets of the mangiferin-treated mice when compared with those
in the controls. This indicated that with age, not only the islet
regeneration capacity had declined, but that the neogenic ability
of islets was also lost.
Taken together, the results of the present study
were consistent with the role of mangiferin in modulating β-cell
proliferation and stimulating insulin secretion; the
mangiferin-treated aged mice exhibited reduced hyperglycemia and
glucose intolerance, increased serum insulin levels, and an
expanded β-cell mass attributed to increased β-cell proliferation
and decreased apoptosis. Investigation into the mechanisms of
mangiferin-induced islet regeneration revealed that mangiferin
modulated cell cycle regulators and insulin secretion-associated
genes. However, these experiments provided preliminary data based
on aged rodents, and thus, the applicability to humans remains
elusive. However, mangiferin may be a promising potential novel
therapeutic for the treatment of diabetes.
Acknowledgments
The authors would like to thank Dr Shunyao Liao at
the Institute of Organ Transplantation, Sichuan Academy of Medical
Science & Sichuan Provincial People's Hospital (Chengdu, China)
for providing help with histological staining and Dr Lingling Wei
at the Institute of Organ Transplantation, Sichuan Academy of
Medical Science & Sichuan Provincial People's Hospital
(Chengdu, China) for providing help with islet isolation.
Notes
[1]
Funding
This study was supported by the Sichuan Health and
Family Planning Commission (grant no. 16ZD0253), Sichuan Provincial
People's Hospital and a Sichuan Scientific Research Grant for
Returned Overseas Chinese Scholars to YW. The study was also
supported by the National Key Speciality Construction Project of
Clinical Pharmacy (grant no. 30305030698).
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
[3] Authors'
contributions
HW, XH and TL performed the partial pancreatectomy
operation, islet isolation and culture and the PCR experiments. YL
and GH performed the western blotting and MS analyzed the data. SD
and HY revised the manuscript. RT and YW designed the experiment
and wrote the manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the ethics
committee of Sichuan Academy of Medical Science & Sichuan
Provincial People's Hospital (Chengdu, China).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Dean PG, Kudva YC and Stegall MD:
Long-term benefits of pancreas transplantation. Curr Opin Organ
Transplant. 13:85–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shapiro AM, Ricordi C, Hering BJ,
Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD,
Berney T, Brennan DC, et al: International trial of the Edmonton
protocol for islet transplantation. N Engl J Med. 355:1318–1330.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butler AE, Janson J, Bonner-Weir S, Ritzel
R, Rizza RA and Butler PC: Beta-cell deficit and increased
beta-cell apoptosis in humans with type 2 diabetes. Diabetes.
52:102–110. 2003. View Article : Google Scholar
|
|
4
|
Bock T, Pakkenberg B and Buschard K:
Increased islet volume but unchanged islet number in ob/ob mice.
Diabetes. 52:1716–1722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonner-Weir S: Islet growth and
development in the adult. J Mol Endocrinol. 24:297–302. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonner-Weir S and Sharma A: Are there
pancreatic progenitor cells from which new islets form after birth?
Nat Clin Pract Endocrinol Metab. 2:240–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tschen SI, Dhawan S, Gurlo T and Bhushan
A: Age-dependent decline in beta-cell proliferation restricts the
capacity of beta-cell regeneration in mice. Diabetes. 58:1312–1320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rankin MM and Kushner JA: Adaptive
beta-cell proliferation is severely restricted with advanced age.
Diabetes. 58:1365–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krishnamurthy J, Ramsey MR, Ligon KL,
Torrice C, Koh A, Bonner-Weir S and Sharpless NE:
p16INK4a induces an age-dependent decline in islet
regenerative potential. Nature. 443:453–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishnamurthy J, Torrice C, Ramsey MR,
Kovalev GI, Al-Regaiey K, Su L and Sharpless NE: Ink4a/Arf
expression is a biomarker of aging. J Clin Invest. 114:1299–1307.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guha S, Ghosal S and Chattopadhyay U:
Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a
naturally occurring glucosylxanthone. Chemotherapy. 42:443–451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dar A, Faizi S, Naqvi S, Roome T,
Zikr-ur-Rehman S, Ali M, Firdous S and Moin ST: Analgesic and
antioxidant activity of mangiferin and its derivatives: The
structure activity relationship. Biol Pharm Bull. 28:596–600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prabhu S, Narayan S and Devi CS: Mechanism
of protective action of mangiferin on suppression of inflammatory
response and lysosomal instability in rat model of myocardial
infarction. Phytother Res. 23:756–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muruganandan S, Srinivasan K, Gupta S,
Gupta PK and Lal J: Effect of mangiferin on hyperglycemia and
atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol.
97:497–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miura T, Ichiki H, Hashimoto I, Iwamoto N,
Kato M, Kubo M, Ishihara E, Komatsu Y, Okada M, Ishida T and
Tanigawa K: Antidiabetic activity of a xanthone compound,
mangiferin. Phytomedicine. 8:85–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miura T, Iwamoto N, Kato M, Ichiki H, Kubo
M, Komatsu Y, Ishida T, Okada M and Tanigawa K: The suppressive
effect of mangiferin with exercise on blood lipids in type 2
diabetes. Biol Pharm Bull. 24:1091–1092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Cui X, Sun X, Li X, Zhu Q and Li W:
Mangiferin prevents diabetic nephropathy progression in
streptozotocin-induced diabetic rats. Phytother Res. 24:893–899.
2010.
|
|
19
|
Liu YW, Zhu X, Zhang L, Lu Q, Wang JY,
Zhang F, Guo H, Yin JL and Yin XX: Up-regulation of glyoxalase 1 by
mangiferin prevents diabetic nephropathy progression in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 721:355–364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bwititi P, Musabayane CT and Nhachi CF:
Effects of Opuntia megacantha on blood glucose and kidney function
in streptozotocin diabetic rats. J Ethnopharmacol. 69:247–252.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HL, Li CY, Zhang B, Liu YD, Lu BM,
Shi Z, An N, Zhao LK, Zhang JJ, Bao JK and Wang Y: Mangiferin
facilitates islet regeneration and β-cell proliferation through
upregulation of cell cycle and β-cell regeneration regulators. Int
J Mol Sci. 15:9016–9035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miura T, Ichiki H, Iwamoto N, Kato M, Kubo
M, Sasaki H, Okada M, Ishida T, Seino Y and Tanigawa K:
Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides
and active components, mangiferin and its glucoside. Biol Pharm
Bull. 24:1009–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Liu Y, Wang H, Li C, Qi P and Bao
J: Agaricus bisporus lectins mediates islet β-cell proliferation
through regulation of cell cycle proteins. Exp Biol Med.
237:287–296. 2012. View Article : Google Scholar
|
|
24
|
Li C, Chen J, Lu B, Shi Z, Wang H, Zhang
B, Zhao K, Qi W, Bao J and Wang Y: Molecular switch role of Akt in
Polygonatum odoratum lectin-induced apoptosis and autophagy in
human non-small cell lung cancer A549 cells. PLoS One.
9:e1015262014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wang H, Liu Y, Li C, Qi P and Bao
J: Antihyperglycemic effect of ginsenoside Rh2 by inducing islet
β-cell regeneration in mice. Horm Metab Res. 44:33–40. 2012.
View Article : Google Scholar
|
|
26
|
Huang G, Lv J, Li T, Huai G, Li X, Xiang
S, Wang L, Qin Z, Pang J, Zou B and Wang Y: Notoginsenoside R1
ameliorates podocyte injury in rats with diabetic nephropathy by
activating the PI3K/Akt signaling pathway. Int J Mol Med.
38:1179–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
28
|
Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB,
Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al: Selective
beta-cell loss and alpha-cell expansion in patients with type 2
diabetes mellitus in Korea. J Clin Endocrinol Metab. 88:2300–2308.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Desagher S, Osen-Sand A, Nichols A, Eskes
R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou
JC: Bid-induced conformational change of Bax is responsible for
mitochondrial cytochrome c release during apoptosis. J Cell Biol.
144:891–901. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Shimoda M, Chen J, Matsumoto S and
Grayburn PA: Transient overexpression of cyclin D2/CDK4/GLP1 genes
induces proliferation and differentiation of adult pancreatic
progenitors and mediates islet regeneration. Cell Cycle.
11:695–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fiaschi-Taesch N, Bigatel TA, Sicari B,
Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K,
Cozar-Castellano I and Stewart AF: Survey of the human pancreatic
beta-cell G1/S proteome reveals a potential therapeutic role for
cdk-6 and cyclin D1 in enhancing human beta-cell replication and
function in vivo. Diabetes. 58:882–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takasawa S, Ikeda T and Akiyama T: Cyclin
D1 activation through ATF-2 in Reg-induced pancreatic beta-cell
regeneration. FEBS letters. 580:585–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Narita M, Nunez S, Heard E, Narita M, Lin
AW, Hearn SA, Spector DL, Hannon GJ and Lowe SW: Rb-mediated
hetero-chromatin formation and silencing of E2F target genes during
cellular senescence. Cell. 113:703–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dor Y, Brown J, Martinez OI and Melton DA:
Adult pancreatic beta-cells are formed by self-duplication rather
than stem-cell differentiation. Nature. 429:41–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teta M, Rankin MM, Long SY, Stein GM and
Kushner JA: Growth and regeneration of adult beta cells does not
involve specialized progenitors. Dev Cell. 12:817–826. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kee N, Sivalingam S, Boonstra R and
Wojtowicz JM: The utility of Ki-67 and BrdU as proliferative
markers of adult neurogenesis. J Neurosci Methods. 115:97–105.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muskhelishvili L, Latendresse JR, Kodell
RL and Henderson EB: Evaluation of cell proliferation in rat
tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in
situ hybridization for histone mRNA. J Histochem Cytochem.
51:1681–1688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Golias CH, Charalabopoulos A and
Charalabopoulos K: Cell proliferation and cell cycle control: A
mini review. Int J Clin Pract. 58:1134–1141. 2004. View Article : Google Scholar
|
|
41
|
Butler AE, Janson J, Soeller WC and Butler
PC: Increased beta-cell apoptosis prevents adaptive increase in
beta-cell mass in mouse model of type 2 diabetes: Evidence for role
of islet amyloid formation rather than direct action of amyloid.
Diabetes. 52:2304–2314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanley SC, Austin E, Assouline-Thomas B,
Kapeluto J, Blaichman J, Moosavi M, Petropavlovskaia M and
Rosenberg L: {beta}-Cell mass dynamics and islet cell plasticity in
human type 2 diabetes. Endocrinology. 151:1462–1472. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Gu X, Su IH, Bottino R, Contreras
JL, Tarakhovsky A and Kim SK: Polycomb protein Ezh2 regulates
pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes
mellitus. Genes Dev. 23:975–985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fehsel K, Kolb-Bachofen V and Kröncke KD:
Necrosis is the predominant type of islet cell death during
development of insulin-dependent diabetes mellitus in BB rats. Lab
Invest. 83:549–559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoorens A, Stangé G, Pavlovic D and
Pipeleers D: Distinction between interleukin-1-induced necrosis and
apoptosis of islet cells. Diabetes. 50:551–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maedler K, Schumann DM, Schulthess F,
Oberholzer J, Bosco D, Berney T and Donath MY: Aging correlates
with decreased beta-cell proliferative capacity and enhanced
sensitivity to apoptosis: A potential role for Fas and pancreatic
duodenal homeobox-1. Diabetes. 55:2455–2462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Donath MY, Gross DJ, Cerasi E and Kaiser
N: Hyperglycemia-induced beta-cell apoptosis in pancreatic islets
of Psammomys obesus during development of diabetes. Diabetes.
48:738–744. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu X, D'Hoker J, Stangé G, Bonné S, De Leu
N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et
al: Beta cells can be generated from endogenous progenitors in
injured adult mouse pancreas. Cell. 132:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|