Introduction
Cardiovascular disease is one of the most
significant contributors to morbidity and mortality rates in
children around the world. Damaged cardiac tissue is unable to
repair itself following injury, warranting the development of
alternative therapies. An increasing number of studies have found
that exosomes, a group of membrane vesicles with a certain volume
that are naturally derived from mammalian cells, may be essential
in cardiac cell communication and repair (1,2),
although their precise cellular origin and mechanism of action
remain to be fully elucidated.
Exosomes, which range between 30 and 100 nm in size,
are a type of extracellular vesicle; ultracentrifugation on a
linear sucrose gradient (2–0.25 M sucrose) revealed that they have
a density ranging between 1.13 and 1.19 g/ml (3,4).
They are set apart from other extracellular vesicles, including
apoptotic bodies and microvesicles, owing to their unique qualities
(5). Exosomes can transfer
molecules from one cell to another via membrane vesicle trafficking
and can perform the vital function of cell-to-cell communication in
physiological and pathophysiological conditions (6,7).
Exosomes have potential applications as novel bio-carriers for gene
and drug delivery (8), which
depend on their inner materials, including proteins, microRNAs
(miRNAs) and cytokines, and their cell types.
Wu et al reported that gastric cancer-derived
exosomes can promote tumour cell proliferation by stimulating the
nuclear factor-κB pathway (9).
Another study found that fibroblasts exhibited uptake of exosomes
derived from human amniotic epithelial cells (hAECs-Exo), and the
migration and proliferation functions of these exosomes were
promoted by hAECs-Exo via the activation of matrix
metalloproteinase-1 (10). These
studies focused on different exosomes, however, a number of
discussed the roles of CPC-derived exosomes and their mechanisms.
CPCs derived from adult hearts have gradually emerged as one of the
most promising stem cell types for cardioprotection and repair
through inducing differentiation and paracrine effects (11). Therefore, CPC-derived exosomes are
involved in treating cardiovascular diseases, including protecting
the ischemic myocardium from acute ischemia or reperfusion injury
(11), inhibiting cardiomyocyte
(CM) apoptosis and improving cardiac function following myocardial
infarction (2). However, further
investigations are required to better understand the underlying
mechanisms.
The Akt/mammalian target of rapamycin (mTOR)
signalling pathway is an important regulator in cell
differentiation, growth and apoptosis, angiogenesis, and protein
synthesis and degradation. Akt is a serine/threonine-specific
protein kinase that is involved in multiple cellular processes,
including glucose metabolism, apoptosis, cell proliferation,
transcription and cell migration, through different downstream
factors. The Akt kinase family contains three members with a
broadly similar structure: Akt1, Akt2 and Akt3. All three consist
of a conserved N-terminal pleckstrin homology domain, a central
catalytic domain and a C-terminal regulatory hydrophobic motif.
Although they have similar mechanisms of exhibiting regulation
function, they possess unique features (12). Akt1 and Akt2 are widely expressed
in various mammalian cells, whereas Akt3 exhibits a tissue-specific
expression. An early study demonstrated that cancer-derived
exosomes promote tumour cell proliferation via the activation of
Akt (13). mTOR, an important
regulator of the cell cycle and protein synthesis, is a critical
component in several signalling pathways, including
phosphoinositide 3-kinase (PI3K)/Akt. mTOR regulates cell growth by
accepting exogenous growth factors and insulin stimulation, which
affects factors including the feeling of changes in energy and
nutritional status. The present study focused on the roles of
CPC-derived exosomes in rat heart cell growth, and the
communication between CPC-derived exosomes and the Akt/mTOR
signalling pathway during this growth.
Materials and methods
Animals
All experiments were conducted in accordance with
the IRB of The Third Xiangya Hospital, Central South University
(Changsha, China; No. 2015-S001). The study was performed on 15
8-week-old male Sprague-Dawley (SD) rats purchased from Hunan
Silaike Jingda Experimental Animal Co., Ltd. (Changsha, China). The
rats, having a body weight of 200±10 g, were fed on a standard diet
with tap water and maintained in environmentally controlled rooms
at 22±2°C under a relative humidity of 50±10% with a 12/12 h
light-dark cycle.
Isolated CPCs
The isolated adult CMs were prepared from the hearts
of 2-month-old male SD rats. First, the rat heart tissue was
aseptically isolated on a clean bench, washed with sterile
phosphate-buffered saline (PBS) containing Heparin several times
and then placed in a Petri dish. The tissue was sliced with
scissors and a scalpel as finely as possible, and the tissue debris
was loaded into a 15-ml tube. Subsequently, 5 ml of type IV
collagenase digestion (1 mg/ml, containing DNase I) was added and
digested for 5 min at 37°C, three times in total. Following
standing for 5 min at 4°C or being briefly centrifuged for 3 min at
4°C (980 × g), the supernatant was discarded. The tissue block was
cleaned with PBS three times, resuspended in CEM (IMDM containing
20% FBS, 1% penicillin-streptomycin, 2 mM L-glutamine, 0.1 mM
2-hydroxy-1-ethanethiol) and inoculated in a 20-µg/ml
FN-coated petri dish; the medium was replaced once every 2–3 days.
Following 14 days of incubation, the dishes were gently washed
three times with PBS and then digested for 1–2 min with a 0.05%
trypsin solution preheated to 37°C; the cells were then collected.
Finally, the cells were transferred to the culture bottle, and the
medium was replaced once every 2–3 days.
Exosome isolation
CPC-exosome isolation was performed using
ExoQuick-TC™ Exosome Isolation reagent (System Biosciences, Palo
Alto, CA, USA), following the procedure outlined by the
manufacturer. To prepare the exosome media prior to ExoQuick
treatment, the concentration was increased from 50 to 130 µl
using an Amicon Ultra filter (EMD Millipore, Billerica, MA, USA)
with a 100,000-molecular weight cutoff (14).
Exosome labelling with
DioC18(3) (DiO)
The purified CPC-derived exosomes were labelled with
a DiO green fluorescent labelling kit (Yeasen Company, Shanghai,
China) in accordance with the manufacturer's protocol. The DiO
concentration for exosome labelling was 0.5 µM/µl of
exosomes from 1×104 cells. The labelled exosomes were
stained with DiO dye in 100 µl of dimethyl sulfoxide (DMSO)
diluted with 100 µl of Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 20
min at 37°C, and an equal volume of serum without exosomes was
added to terminate the labelling. H9C2 cardiomyocytes are a clonal
heart muscle cell line derived from embryonic rat hearts, which can
retain several cardiomyocyte phenotypes. In the present study, the
H9C2 cells were obtained from the Cell Bank of the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
incubated with the labelled CPC-exosomes for 12 h at 37°C and
washed with PBS. The uptake of labelled exosomes by the H9C2 cells
was detected using a fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Transmission electron microscopy
(TEM)
The ultrastructure of exosomes was observed using
TEM, according to the methods described in a previous study
(15). Briefly, a resuspended
pellet (3 ml) of exosomes was fixed with 2.5% glutaraldehyde,
post-fixed in buffered 1% osmium tetroxide with 1.5% potassium
ferrocyanide, embedded in 1% agar and processed according to the
standard EPON812 embedding procedure. The exosomes were visualised
in thin (60-nm) sections using TEM (FEI Company, Eindhoven, The
Netherlands) at 80 kV.
Methyl-thiazolyl-tetrazolium (MTT)
assay
The H9C2 cells were seeded at 1×104
cells/well in 96-well plates and treated with CPC-derived exosomes
at 0, 50, 100, 200 and 400 µg/ml, respectively. The cells
were grown at 37°C in a humidified incubator with 5% carbon dioxide
(CO2) for 12, 24 and 48 h. The medium was then replaced
with serum-free DMEM, and 20 µl of MTT solution was added.
Following further growth at 37°C in a humidified incubator with 5%
CO2 for 4 h, the supernatant was carefully discarded,
following which 150 µl of DMSO was added to each well.
Following vibrating for 10 min, the optical density of each well
was measured on an enzyme-linked immunosorbent detector at a
wavelength of 560 nm.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The H9C2 cells were seeded in 96-well plates at
1×104 cells per well and cultured to the normal growth
stage. Subsequently, the cells were treated with CPC-derived
exosomes for 24 and 48 h with final concentrations of 0, 200 and
400 µg/ml. The EdU solution (reagent A) was diluted with
1:1,000 cell medium to prepare the appropriate concentration of EdU
medium (50 µM). EDU medium (100 µl) was added to each
well, and the medium was discarded following 2 h of incubation.
Subsequently, 50 µl of fixative solution (4%
paraformaldehyde) was added to each well and incubated at room
temperature for 30 min. Following washing with PBS for 5 min, 100
µl of penetrant was added to each well, incubated for 10 min
with a decolourisation shaker and then washed once with PBS for 5
min. Following this, 100 µl of 1X Apollo was added to each
well and incubated with a decolourisation shaker in the dark at
room temperature for 30 min. Following incubation, 100 µl of
penetrant and 100 µl of methanol were added to each well and
washed. Reagent F was diluted with deionised water at a ratio of
1:100 to prepare an appropriate concentration of 1X Hoechst 33342
reaction solution, of which 100 µl was added to each well
and incubated for 30 min; subsequently, the reactant was washed.
The cells were observed immediately following staining under an
Olympus microscope (Olympus Corporation) equipped with a
Metamorph® image acquisition system (DP2-BSW software;
version 2.1 Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The H9C2 cells in different groups were collected at
each scheduled time point, and mRNA were extracted using an RNA
extraction kit (Omega Bio-Tek, Inc., Norcross, GA, USA). The mRNA
was reverse transcribed into cDNA following the RevertAid™ First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The real-time fluorescence RT-qPCR analysis was
accomplished using a SYBR Green PCR Master mix kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) containing 2 µl
cDNA and 0.5 µl each primer (10 µM), according to the
manufacturer's protocol, with the following thermal cycling
conditions for 40 cycles in total: 10 min at 95°C, 15 sec at 95°C
and 30 sec at 60°C. The primer sequences are listed in Table I. The signal of a gene was
standardised with β-actin using the following formulas: ΔCq=Cq
target−Cq reference; and ΔΔCq = mean value of ΔCq control − ΔCq
sample. Finally, the 2−ΔΔCq method (16) was used to calculate the
differences in mRNA transcription levels.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer
sequence |
|---|
| Rat-mTOR | F: 5′
CCTCGGCACATCACTCCCTT 3′ |
| R: 5′
GCTCCTACATTTCAGCACCCACT 3′ |
| Rat-Akt1 | F: 5′
TACCTGAAGCTACTGGGCAAGGG 3′ |
| R: 5′
CGGTCGTGGGTCTGGAATGAG 3′ |
| Rat-Akt2 | F: 5′
GATGGTAGCCAACAGTCTGAAGCA 3′ |
| R: 5′
CCCTTGCCGAGGAGTTTGAGATA 3′ |
| β-actin | F: 5′
AAGATCAAGATCATTGCTCCTCC 3′ |
| R: 5′
TAACAGTCCGCCTAGAAGCA 3′ |
Western blot analysis
The CPC-derived exosome samples, rat lymphocytes and
H9C2 cells from different groups were harvested and maintained on
ice for 10 min following being washed twice with ice-cold PBS.
Lysis buffer (80-µl; Beyotime Institute of Biotechnology,
Haimen China) containing 0.1% phenylmethylsulfonyl (CWBio, China)
was added to each well. The cell lysates were collected using a
scraper and centrifuged at 13,780 × g for 15 min (4°C), and the
supernatant was obtained. The protein concentration was detected
using an enhanced BCA protein assay kit (Beyotime Institute of
Biotechnology). Subsequently, 30 µg of extracted protein was
fractionated on 10–12% sodium dodecyl sulphatepolyacrylamide gels,
electrophoretically transferred onto 0.45-µm PVDF membranes
(EMD Millipore), and blocked with PBS containing 0.5% Triton-100
(CWBio) and 5% non-fat dry milk for 1 h. The membrane was then
incubated with a specific primary antibody at 4°C overnight, as
follows: Monoclonal anti-CD63 (1:500; cat no. ab108950; Abcam,
Cambridge, MA, USA), monoclonal anti-CD-81 (1:200; cat no. sc-9158;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), monoclonal
anti-Akt antibody (1:1,000; cat no. #9272; Cell Signaling
Technology, Inc., Danvers, MA, USA), monoclonal anti-phosphorylated
(p-)Akt antibody (1:2,000; cat no. #4060; Cell Signaling
Technology, Inc.), monoclonal anti-mTOR antibody (1:1,000; cat no.
#2983; Cell Signaling Technology, Inc.), monoclonal anti-p-mTOR
antibody (1:1,000; cat no. #5536; Cell Signaling Technology, Inc.)
and monoclonal anti-β-actin antibody (1:4,000; cat no. 60008-1;
ProteinTech Group, Inc., Chicago, IL, USA). This step was followed
by incubation with horseradish peroxidase-conjugated secondary
antibodies (1:6,000; goat anti-rabbit; cat no. SA00001-2;
ProteinTech Group, Inc.) for 1 h at room temperature. Ultimately,
the protein expression level was determined by enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
analyzed using Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Two-way analysis of variance was performed with
multiple comparisons and paired Student's t-tests with the
statistical software SPSS v22.0 (IBM Corp., Armonk, NY, USA). Data
are presented as the mean ± standard error of the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
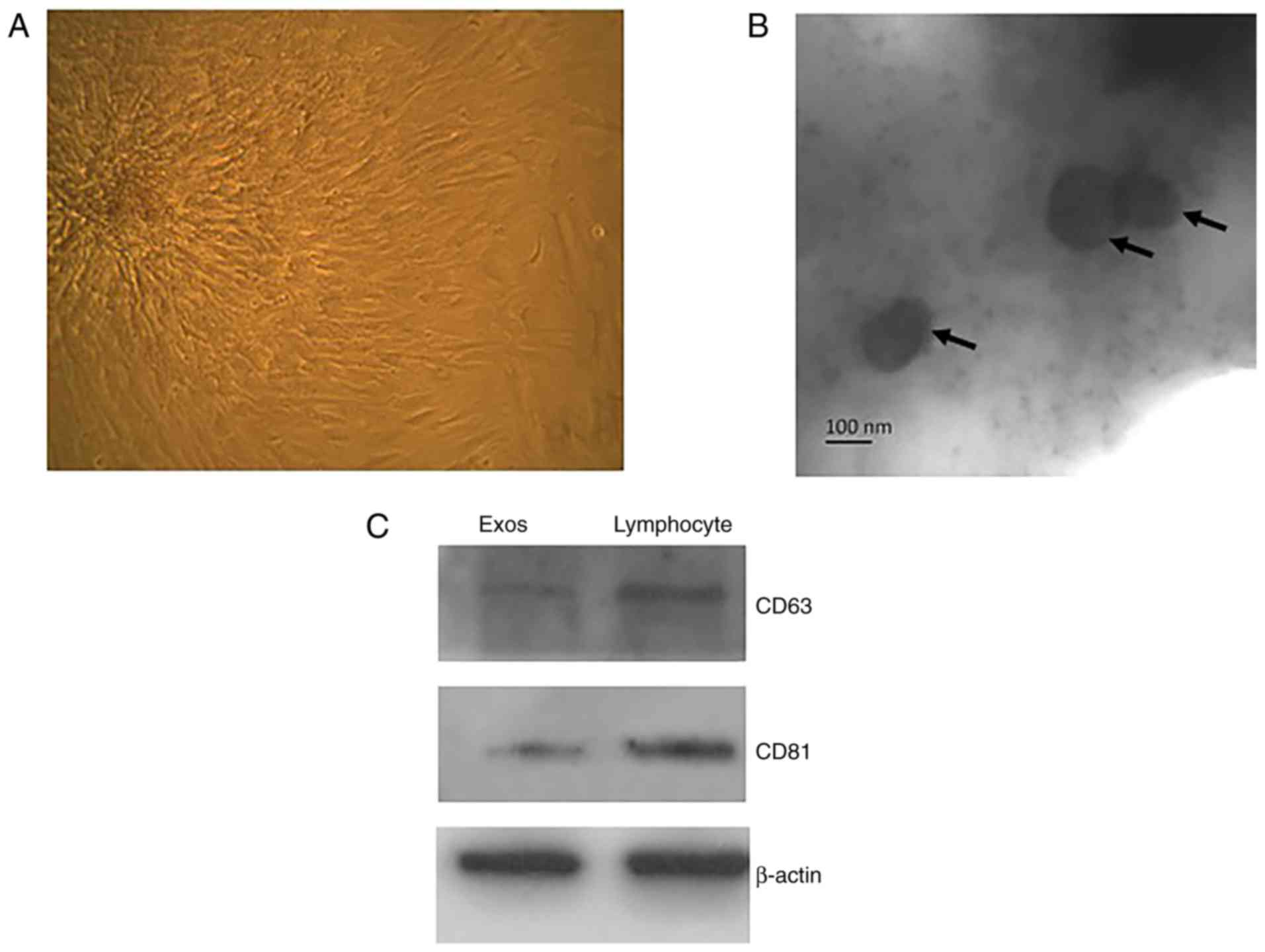
Identification of CPC-derived
exosomes
To extract the exosomes, the CPCs were first
isolated from the SD rat heart tissue (Fig. 1A). When the exosomes had been
prepared and purified using the ExoQuick-TC™ method, they were
observed by TEM, and the markers were characterised by western blot
analysis. As shown in previous studies, the exosomes were,
~35.61±3.89 nm in diameter (Fig.
1B). Comparing the CPC-derived exosomes with the lymphocyte
lysates, which have been shown to contain a large number of
exosomes, revealed that the tetraspanin molecules CD63 and CD81
were abundant in the former (Fig.
1C).
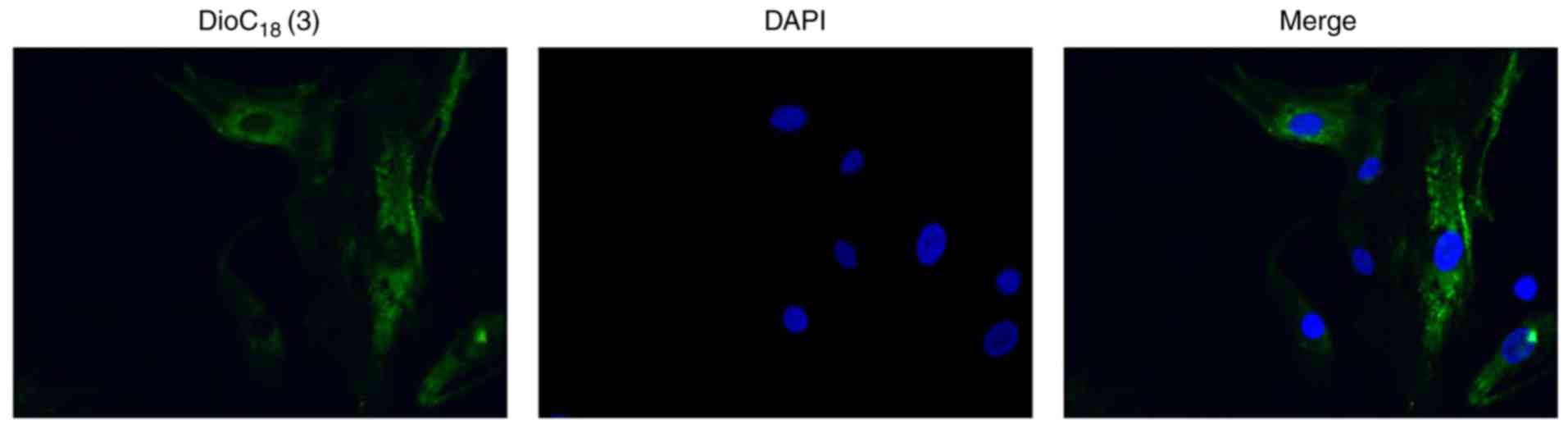
Exosome labelling and uptake of exosomes
by H9C2 cells
To determine whether CPC-derived exosomes were taken
up by H9C2 cells, the exosomes were labelled with DiO, a
fluorescent cell linker compound that is incorporated into the cell
membrane by selective partitioning. Following incubation of the
H9C2 cells with the exosomes labelled with DiO, green fluorescence
was observed in the cytoplasm of almost every H9C2 cell (Fig. 2), indicating that significant
quantities of CPC-derived exosomes had been taken up by the H9C2
cells.
CPC-derived exosomes promote H9C2 cell
growth in a time- and concentration-dependent manner
Exosomes are now considered to be an important
catalyst in cell proliferation and tissue repair. To investigate
the effect of CPC-derived exosomes on CMs, the present study
determined H9C2 cell growth via MTT and EdU assays with different
exosome concentrations and treatment durations. It was found that
the CPC-derived exosomes promoted H9C2 cell growth. In addition, at
a relatively low concentration, the higher the concentration, the
more marked the stimulation effect under the same treatment time.
In turn, the longer the treatment time, the more marked the
stimulation effect at the same concentration (Fig. 3A–D).
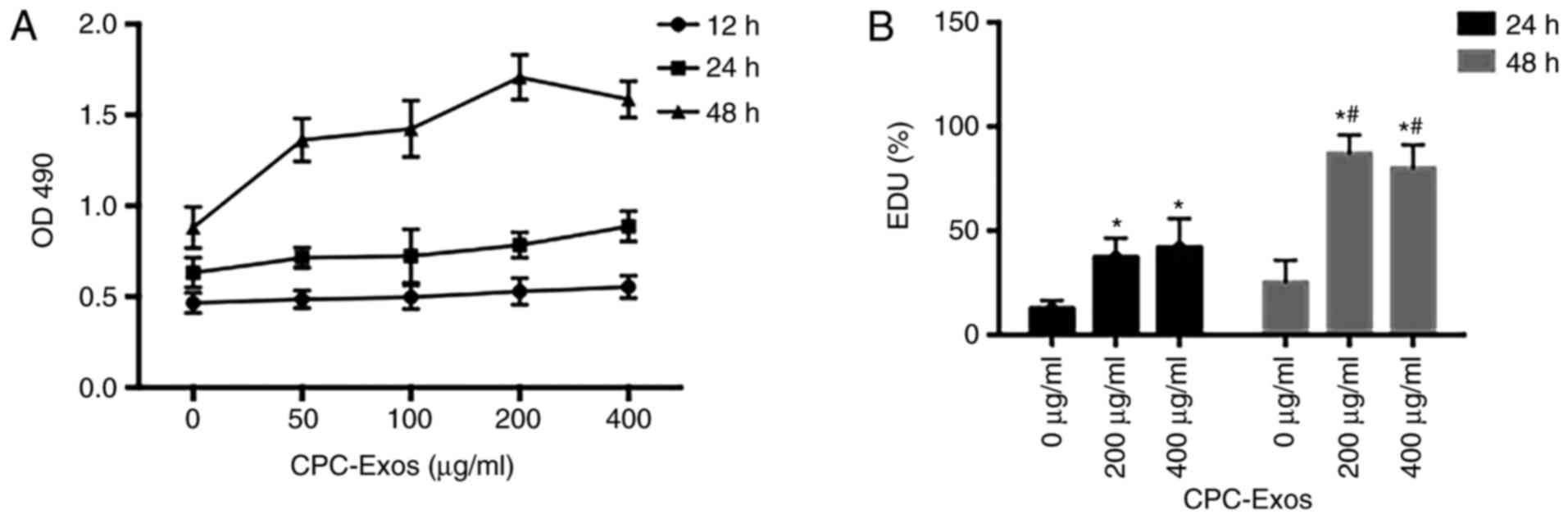 | Figure 3CPC-derived exosomes promote H9C2
cell growth in a time- and concentration-dependent manner. H9C2
cells were treated with CPC-exosomes at 0, 50, 100, 200 and 400
µg/ml respectively. (A) Following incubation for 12, 24 and
48 h, cell growth was examined using a methyl-thiazolyl-tetrazolium
assay. At the same concentration, cell growth in the 48 h group was
higher than that in the 24 h group, which was higher than that in
the 12 h group, and the differences were statistically significant
(P<0.05). At the same treatment duration of 48 h, cell growth
with 200 µg/ml CPC-derived exosomes was higher than that in
the other groups (P<0.05), and each group at 48 h was
significantly different compared with the control (0 µg/ml)
(P<0.05). In the 24 h group, activation of cell growth with 400
µg/ml of exosome was significantly higher (P<0.05),
compared with that in the control (0 µg/ml). Following
treatment with CPC-exosomes for 24 and 48 h with the different
final concentration of 0, 200 and 400 µg/ml, cell
proliferation was determined using the EdU method. (B) Compared
with the control (0 µg/ml), stimulation of cell
proliferation in the 200 and 400 µg/ml groups differed
significantly (*P<0.05, vs. control). At the same
concentration, activation in the 48 h groups differed significantly
(#P<0.05). CPC, cardiac progenitor cells; Exos,
exosomes; OD, optical density; EdU, 5-ethynyl-2′-deoxyuridine.
CPC-derived exosomes promote H9C2 cell growth in a time- and
concentration-dependent manner. Cell proliferation in the (C) 24 h
and (D) 48 h groups was observed using fluorescence microscope
following staining (magnification, ×100). When treated with the
same cardiac progenitor cell-derived exosomes, cell proliferation
was simulated as treatment time increased. EdU,
5-ethynyl-2′-deoxyuridine. |
CPC-derived exosomes stimulate the
expression and phosphorylation of Akt
The PI3K/Akt/mTOR signalling pathway is important
for cell proliferation. However, due to its frequent dysregulation,
Akt is typically accepted as a promising anticancer therapeutic
target (17). This signalling
pathway is activated by various extracellular growth factors,
including epidermal growth factor, insulin-like growth factor 1 and
insulin, and simulates downstream mTOR signalling (18). To investigate whether it can be
activated by CPC-derived exosomes in CMs, the H9C2 cells were
treated with 200 and 400 µg/ml of exosomes for 24 and 48 h,
respectively. Following this, the mRNA and protein expression
levels of Akt were analysed by RT-qPCR and western blot analyses.
In these experiments, it was found that the mRNA and protein
expression levels of Akt were increased; furthermore, the
phosphorylation was increased in the two groups, and stimulation in
the groups treated with 200 and 400 µg/ml of exosomes for 48
h was more marked than that in the groups treated with the same
exosome concentrations for 24 h. Compared with the groups treated
with 200 µg/ml of exosomes, the activation in the 400
µg/ml group was higher at 24 h (Fig. 4A–D).
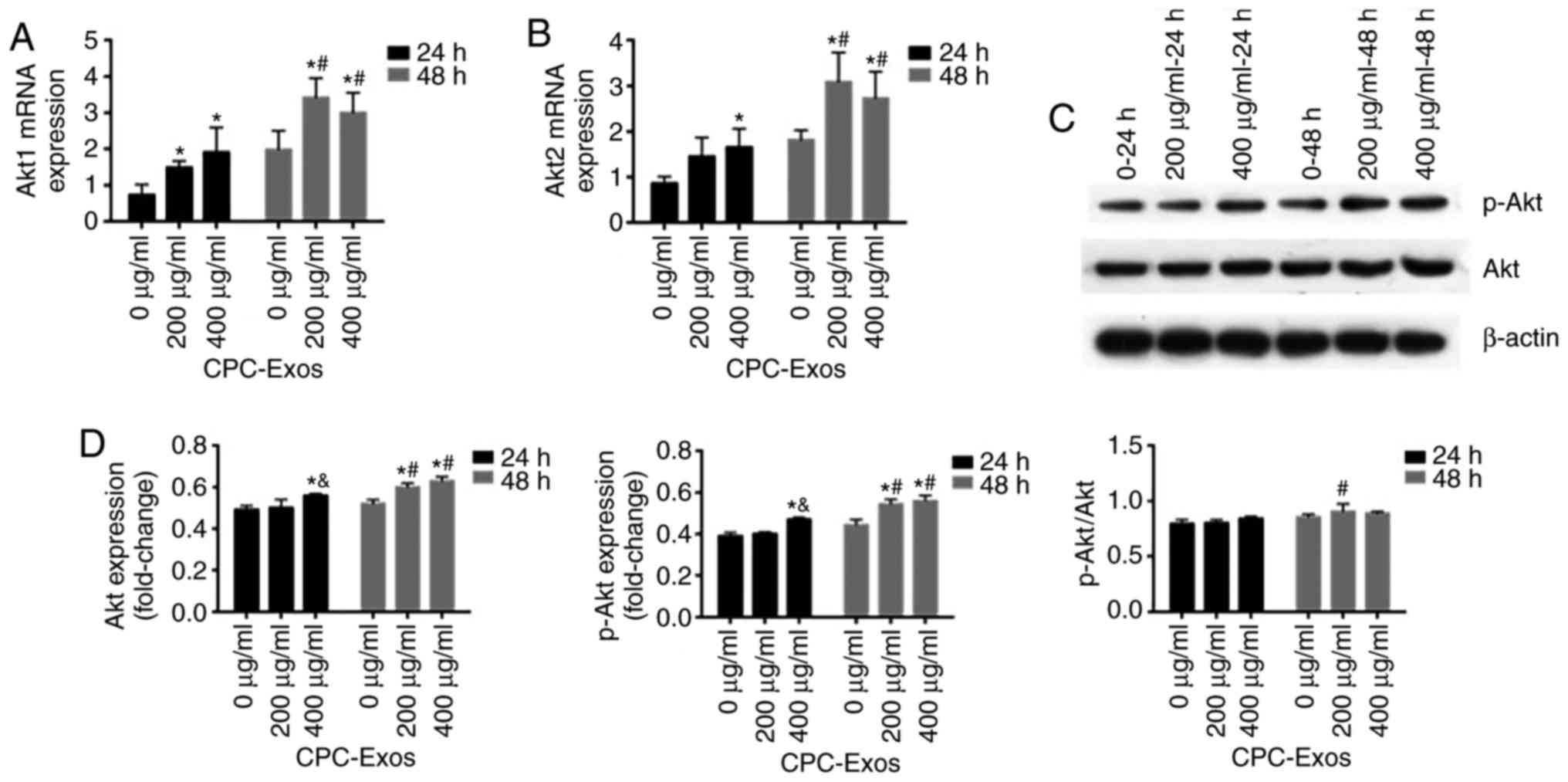 | Figure 4CPC-derived exosomes stimulate the
expression of Akt and its phosphorylation. H9C2 cells were treated
with CPC-exosomes with final concentrations of 0, 200 and 400
µg/ml. Cell lysates were harvested at 24 and 48 h, and
expression of Akt was determined by RT-qPCR and western blot
analyses. mRNA expression levels of (A) Akt1 and (B) Akt2 were
determined by RT-qPCR analysis (mean ± standard error of the mean,
n=3) and normalized to the internal control β-actin, which was
arbitrarily set to a value of 1.0. Compared with the control (0
µg/ml), expression levels of Akt1 and Akt2 in the 200 and
400 µg/ml groups were significantly higher
(*P<0.05). At the same concentration, expression
levels of Akt1 and Akt2 in the 48 h groups were significantly
higher than those in the 24 h group (#P<0.05). (C)
Samples were immunoblotted with β-actin to ensure equal protein
loading and (D) results are shown in graphs (mean ± standard error
of the mean, n=3). Compared with the control (0 µg/ml),
expression levels of Akt and p-Akt in the 200 µg/ml 48 h
group, 400 µg/ml 24 group and 400 µg/ml 48 h group
were significantly higher (*P<0.05). Compared with
the 200 µg/ml group, activation of Akt and p-Akt in the 24 h
group was significantly higher in the 400 µg/ml group
(&P<0.05). At the same concentration, the
expression of Akt and p-Akt in the 48 h group, and the ratio of
p-Akt/Akt in the 48 h group were significantly different
(#P<0.05). p-, phosphorylated; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CPC, cardiac
progenitor cells; Exos, exosomes. |
CPC-derived exosomes stimulate the
expression and phosphorylation of mTOR at a relatively low exosome
concentration
mTOR has been reported to regulate homeostasis by
directly influencing gene transcription, protein and lipid
synthesis, and organelle biogenesis and maintenance in response to
multiple extra- and intracellular signals, including growth factors
and nutrients. This serine/threonine kinase has long been known as
a critical regulator of cell proliferation. In the present study,
the expression of mTOR was determined by RT-qPCR and western blot
analyses, using H9C2 cells treated with 200 and 400 µg/ml of
exosomes for 24 and 48 h, respectively. Following exosome
treatment, the mRNA expression of mTOR was stimulated, and the
stimulation increased corresponding to an increase in treatment
time. The protein expression and phosphorylation of mTOR were
activated by exosomes, however, the results were not the same as
those for mRNA. In the 200 µg/ml group, the protein
expression and phosphorylation were increased in correspondence
with an increase in time, however, the opposite was true for
protein expression in the 400 µg/ml group. At the different
concentrations, the expression levels were higher in the 200
µg/ml groups than in the 400 µg/ml groups at 48 h
(Fig. 5A–C).
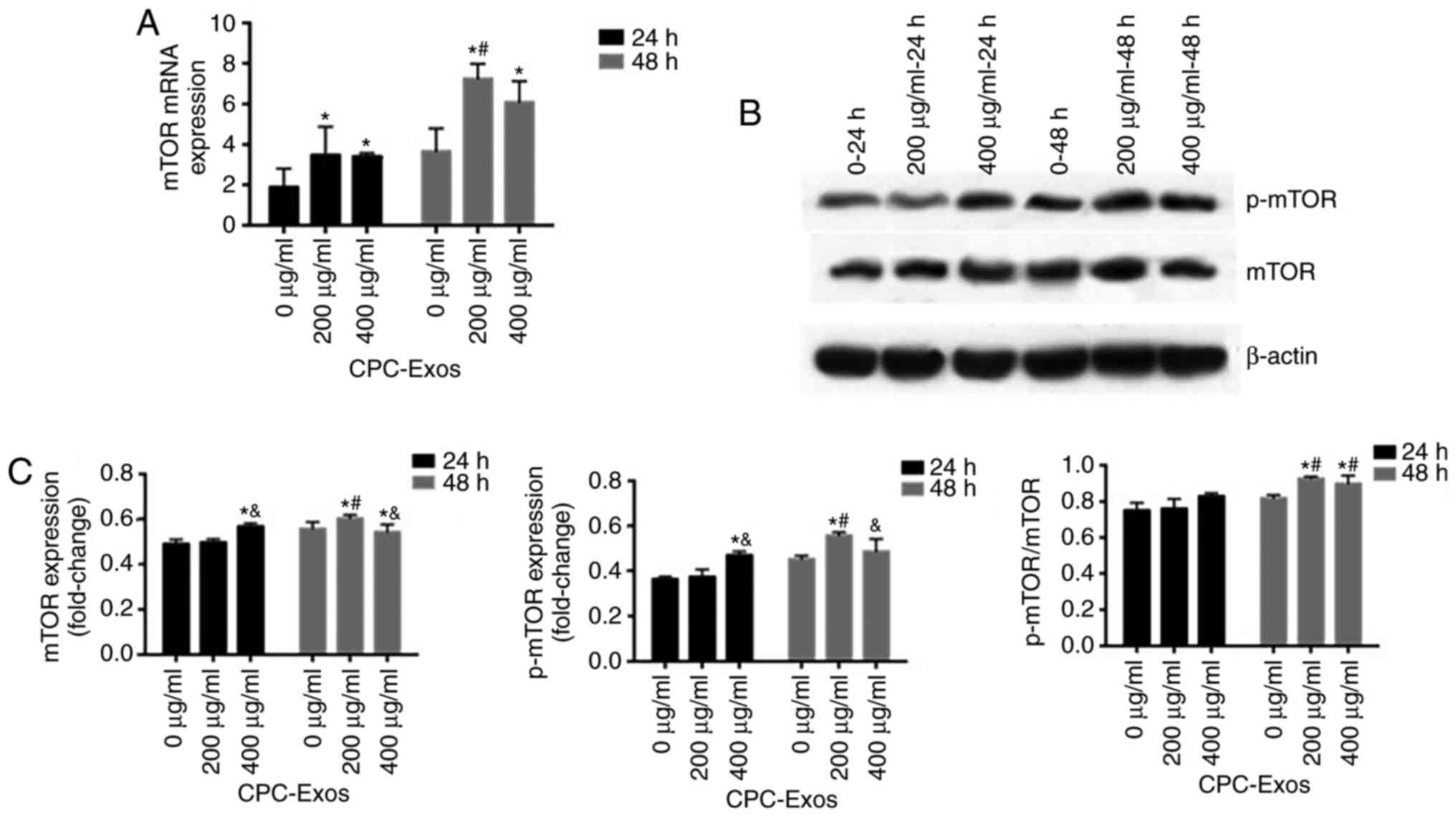 | Figure 5CPC-derived exosomes stimulate the
expression of mTOR and its phosphorylation in a relatively low
exosome concentration. H9C2 cells were treated with CPC-exosomes
with final concentrations of 0, 200 and 400 µg/ml. Cell
lysates were harvested at 24 and 48 h, and the expression of mTOR
was determined by RT-qPCR and western blot analyses. (A) mRNA
expression levels were determined by RT-qPCR analysis (mean ±
standard error of the mean, n=3) and normalized to the internal
control β-actin, which was arbitrarily set to a value of 1.0.
Compared with the control (0 µg/ml), expression levels of
mTOR in the 200 and 400 µg/ml groups were significantly
higher (*P<0.05). (B) Samples were immunoblotted with
an antibody to β-actin to ensure equal protein loading and (C)
results are shown in graphs (mean ± standard error of the mean,
n=3). Compared with the control (0 µg/ml), expression levels
of mTOR and p-mTOR in the 200 µg/ml 48 h group and 400
µg/ml group 24 and 48 h, and the ratio of p-mTOR/mTOR at 48
h were significantly higher (*P<0.05). Compared with
the 200 µg/ml 24 and 48 h groups, the expression levels of
mTOR and p-mTOR in the 400 µg/ml group were significantly
different (&P<0.05). At the same concentration at
different times, the expression levels of mTOR and p-mTOR and ratio
of p-mTOR/mTOR were significantly higher (#P<0.05).
mTOR, mammalian target of rapamycin; p-, phosphorylated; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; CPC,
cardiac progenitor cells; Exos, exosomes. |
Discussion
Cardiovascular disease remains a major contributor
to rates of morbidity and mortality worldwide. CPCs derived from
adult hearts appear to be a promising type of stem cell for
myocardial regeneration and repair. This assumption is based on the
hypothesis that CPCs can engraft, differentiate and replace damaged
cardiac tissues. Increasing evidence has revealed the therapeutic
benefits of CPC paracrine secretion (19). Following transplantation into an
injured heart, CPCs can contribute to myocardial repair through
direct and indirect mechanisms, including direct
transdifferentiation into CMs and vascular cells, secretion of
paracrine factors that may regulate the hyperplasia proliferation
of existing CMs, and cell fusion between transplanted cells and
existing CMs (20). Furthermore,
numerous studies have shown that transplanted CPCs can secrete a
number of functional factors to reduce tissue injury and/or enhance
tissue repair (2,11).
Exosomes are small membrane vesicles that are
actively released by cells in physiological and pathological states
(6,7). Exosomes contain various molecular
constituents of RNA and soluble proteins and may be involved in
cell-to-cell signalling. Exosomes deliver a cargo of RNA molecules,
including mRNA and miRNAs, which have multiple biological effects
and regulate gene expression within recipient cells (8). It is widely recognised that exosomes
can mediate between paracrine signals within the cardiovascular
system, for example, between endothelial cells and vascular smooth
muscle cells (VSMCs) (21),
between cardiac fibroblasts and CMs (22), and between VSMCs (23). Exosomes from the cardiovascular
system also exist in pericardial fluid (24) and in the circulation (25), revealing their potential role in
endocrine signalling.
In the present study, CPC-derived exosomes were
extracted to investigate whether they can affect H9C2 cell growth
to examine the associated signalling pathways. The results
demonstrated that the CPC-derived exosomes promoted H9C2 cell
growth in a time- and concentration-dependent manner. The H9C2
cells exhibited an increased growth capacity following treatment
with a higher concentration of CPC-derived exosomes or a longer
acting time. Zhang et al reported that exosomes derived from
H9C2 cells carry certain cardioprotective miRNAs, which repress
hypoxia-induced apoptosis. Among the hypoxia-induced exosomal
miRNAs, miR-152-3p and let-7i-5p exert an anti-apoptotic function
by targeting autophagy related 12 and Fas ligand, respectively
(26). Cui et al confirmed
that adipose-derived mesenchymal stem cell exosomes protect the
ischemic myocardium from ischemia/reperfusion injury via activation
of the Wnt/β-catenin signal pathway (27). Shao et al found that
MSC-derived exosomes (MSC-Exo) inhibit cardiac fibrosis and
inflammation, and improve cardiac function. The MSC-Exo facilitated
the proliferation of H9C2 cells, suppressed apoptosis induced by
H2O2 and inhibited the transformation of
fibroblast cells into myofibroblasts induced by transforming growth
factor-β (28). Xiao et al
revealed that CPC-derived exosomal miR-21 had an inhibitory
function in the apoptotic process by downregulating the expression
of programmed cell death 4 (PDCD4). Therefore, CPC-derived exosomes
protected CMs against oxidative stress-related apoptosis by
restoring the miR-21/PDCD4 pathway (29).
In the present study, it was found that CPC-derived
exosomes stimulated the expression and phosphorylation of Akt, with
the treatment concentration and time demonstrating an effect.
Furthermore, CPC-derived exosomes facilitated the expression and
phosphorylation of mTOR at a relatively low exosome concentration
(200 µg/ml). Therefore, the results showed that CPC-derived
exosomes may promote H9C2 cell growth via the activation of
Akt/mTOR in a relatively time- and concentration-dependent
manner.
The PI3K/AKT/mTOR intracellular signalling pathway
is important in regulating the cell cycle and is essential to
promote the growth, proliferation and differentiation of adult stem
cells, specifically (30). The
activation of Akt has vital effects on the CMs, including
increasing cell size, inhibiting apoptosis and altering glucose
metabolism (31). In addition,
several studies have revealed that the PI3K/Akt/mTOR signalling
pathway may contribute to cardioprotection, although the mechanisms
remain to be fully elucidated. Li et al found that
tanshinone IIA activated the PI3K/Akt/mTOR signalling pathway and
protected against myocardial ischemia reperfusion injury (32). Another finding suggested that
nerve growth factor exerts a cardioprotective effect by a variety
of mechanisms that restore autophagic flux and the attenuation of
protein ubiquitination through activation of the PI3K/AKT/mTOR
pathway (33). Zhang et al
provided evidence that sevoflurane-induced postconditioning, as a
mechanism of cardioprotection, was mediated by activation of the
PI3K/AKT/mTOR pathway, which included an anti-apoptotic effect on
CMs and the protection of mitochondria from injury (34). Wang et al found that basic
fibroblast growth factor had an effect on myocardial cell death
in vivo and in vitro and required activation of the
PI3K/Akt/mTOR signalling pathway (35).
A previous investigation indicated that jujuboside A
has a potential protective effect on isoproterenol (ISO)-induced
damage in H9C2 cells by accelerating activation of the
PI3K/Akt/mTOR signalling pathway (36). The PI3K/Akt/mTOR pathway is also
involved in promoting autophagy in H9C2 cells induced by
low-after-high glucose (37). In
addition, two studies have revealed that the PI3K/Akt/mTOR
signalling pathway is involved in cardioprotection by inhibiting CM
apoptosis and autophagy. Radix Paeoniae Rubra terpene glycosides
may protect the heart from ISO-induced myocardial ischemia by
improving cardiac energy metabolism and inhibiting CM apoptosis via
activation of the PI3K/AKT/mTOR signalling pathway (38). Apigenin may have a protective
effect against adriamycin-induced cardiotoxicity by inhibiting
apoptosis and autophagy via activation of the PI3K/AKT/mTOR pathway
(39). A previous study in mice
suggested that macrophage migration inhibitory factor facilitated
the survival of mouse cardiac stem cells, and the proliferation and
differentiation of endothelial cells via activation of the
PI3K/Akt/mTOR signalling pathway (40). These studies demonstrate that the
PI3K/AKT/mTOR pathway has an important regulatory function in
cardioprotection.
The results of the present study provide a novel
approach for investigating the role of CPCs and
cardiosphere-derived cells in heart disease research. CPCs are a
compounded group of cells distributed throughout cardiac tissue
that can be activated and can differentiate into new muscle or
vascular cells following stress or injury (20). The present study provided evidence
that CPC-derived exosomes promoted H9C2 cell growth. However,
further investigations are required to determine the exact
mechanism underlying the effect of CPC-derived exosomes in
cardioprotection, including the role of specific miRNAs transferred
in exosomes, the regulation effect of CPC-derived exosomes on
energy metabolism, and the apoptosis and autophagy of CMs.
Acknowledgments
The authors would like to thank the Center
Laboratory at The Third Xiangya Hospital of Central South
University for the provision of experimental equipment and
technical guidance necessary to complete the work.
Funding
This study was funded by the National Natural
Science Foundation (grant no. 81500225).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the experiments. SL
conducted the experiments. JJ, ZY and ZL participated in the
completion of the experiments. SL and XM analyzed the data. SL
wrote the paper. XL revised the manuscript. All the authors read
and approved the final paper.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
the IRB of The Third Xiangya Hospital, Central South University
(Changsha, China; no. 2015-S001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu B, Kim HW, Gong M, Wang J, Millard RW,
Wang Y, Ashraf M and Xu M: Exosomes secreted from GATA-4
overexpressing mesenchymal stem cells serve as a reservoir of
anti-apoptotic microRNAs for cardioprotection. Int J Cardiol.
182:349–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barile L, Lionetti V, Cervio E, Matteucci
M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T and
Vassalli G: Extracellular vesicles from human cardiac progenitor
cells inhibit cardiomyocyte apoptosis and improve cardiac function
after myocardial infarction. Cardiovasc Res. 103:530–541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sluijter JP, Verhage V, Deddens JC, van
den Akker F and Doevendans PA: Microvesicles and exosomes for
intracardiac communication. Cardiovasc Res. 102:302–311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waqas MY, Zhang Q, Ahmed N, Yang P, Xing
G, Akhtar M, Basit A, Liu T, Hong C, Arshad M, et al: Cellular
evidence of exosomes in the reproductive tract of Chinese
soft-shelled turtle pelodiscus sinensis. J Exp Zool A Ecol Genet
Physiol. 327:18–27. 2017.
|
|
5
|
Ailawadi S, Wang X, Gu H and Fan GC:
Pathologic function and therapeutic potential of exosomes in
cardiovascular disease. Biochim Biophys Acta. 1852:1–11. 2015.
View Article : Google Scholar
|
|
6
|
Kharaziha P, Ceder S, Li Q and Panaretakis
T: Tumor cell-derived exosomes: A message in a bottle. Biochim
Biophys Acta. 1826:103–111. 2012.PubMed/NCBI
|
|
7
|
Kalani A, Tyagi A and Tyagi N: Exosomes:
Mediators of neurode-generation, neuroprotection and therapeutics.
Mol Neurobiol. 49:590–600. 2014. View Article : Google Scholar
|
|
8
|
Jiang XC and Gao JQ: Exosomes as novel
bio-carriers for gene and drug delivery. Int J Pharm. 521:167–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q,
Guan H, Liu J, Shi J, Su L and Hu D: Exosomes derived from human
amniotic epithelial cells accelerate wound healing and inhibit scar
formation. J Mol Histol. 48:121–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Wang Y, Pan Y, Zhang L, Shen C,
Qin G, Ashraf M, Weintraub N, Ma G and Tang Y: Cardiac
progenitor-derived exosomes protect ischemic myocardium from acute
ischemia/reperfusion injury. Biochem Biophys Res Commun.
431:566–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Virtakoivu R, Pellinen T, Rantala JK,
Perälä M and Ivaska J: Distinct roles of AKT isoforms in regulating
β1-integrin activity, migration, and invasion in prostate cancer.
Mol Biol Cell. 23:3357–3369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, Jiang YH, Yang XH and Liu YP: Gastric cancer exosomes
promote tumour cell proliferation through PI3K/Akt and MAPK/ERK
activation. Dig Liver Dis. 41:875–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L,
Ferrara KW and Knowlton AA: Cardiac myocyte exosomes: Stability,
HSP60, and proteomics. Am J Physiol Heart Circ Physiol.
304:H954–H965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinescu ME, Gherghiceanu M, Suciu L and
Popescu LM: Telocytes in pleura: Two- and three-dimensional imaging
by transmission electron microscopy. Cell Tissue Res. 343:389–397.
2011. View Article : Google Scholar :
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Zhang Y, Zheng Y, Faheem A, Sun T, Li C,
Li Z, Zhao D, Wu C and Liu J: A novel AKT inhibitor, AZD5363,
inhibits phosphorylation of AKT downstream molecules, and activates
phosphorylation of mTOR and SMG-1 dependent on the liver cancer
cell type. Oncol Lett. 11:1685–1692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hasson SP, Rubinek T, Ryvo L and Wolf I:
Endocrine resistance in breast cancer: Focus on the
phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin
signaling pathway. Breast Care (Basel). 8:248–255. 2013. View Article : Google Scholar
|
|
19
|
Chimenti I, Smith RR, Li TS, Gerstenblith
G, Messina E, Giacomello A and Marbán E: Relative roles of direct
regeneration versus paracrine effects of human cardiosphere-derived
cells transplanted into infarcted mice. Circ Res. 106:971–980.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le T and Chong J: Cardiac progenitor cells
for heart repair. Cell Death Discov. 2:160522016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hergenreider E, Heydt S, Tréguer K,
Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS,
Yin X, Mayr M, et al: Atheroprotective communication between
endothelial cells and smooth muscle cells through miRNAs. Nat Cell
Biol. 14:249–256. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bang C, Batkai S, Dangwal S, Gupta SK,
Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al:
Cardiac fibroblast-derived microRNA passenger strand-enriched
exosomes mediate cardiomyocyte hypertrophy. J Clin Invest.
124:2136–2146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kapustin AN, Chatrou ML, Drozdov I, Zheng
Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT,
Alvarez-Hernandez D, et al: Vascular smooth muscle cell
calcification is mediated by regulated exosome secretion. Circ Res.
116:1312–1323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beltrami C, Besnier M, Shantikumar S,
Shearn AI, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E,
Angelini GD and Emanueli C: Human pericardial fluid contains
exosomes enriched with cardiovascular-expressed MicroRNAs and
promotes therapeutic angiogenesis. Mol Ther. 25:679–693. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pironti G, Strachan RT, Abraham D, Mon-Wei
Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ and Rockman HA:
Circulating exosomes induced by cardiac pressure overload contain
functional angiotensin II type 1 receptors. Circulation.
131:2120–2130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Ma J, Long K, Qiu W, Wang Y, Hu
Z, Liu C, Luo Y, Jiang A, Jin L, et al: Overexpression of exosomal
cardioprotective miRNAs mitigates hypoxia-induced H9c2 cells
apoptosis. Int J Mol Sci. 18:E7112017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui X, He Z, Liang Z, Chen Z, Wang H and
Zhang J: Exosomes from adipose-derived mesenchymal stem cells
protect ischemic myocardium from ischemia/reperfusion injury via
Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol.
70:225–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao L, Zhang Y, Lan B, Wang J, Zhang Z,
Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY and Li Y: MiRNA-sequence
indicates that mesenchymal stem cells and exosomes have similar
mechanism to enhance cardiac repair. Biomed Res Int.
2017:41507052017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao J, Pan Y, Li XH, Yang XY, Feng YL,
Tan HH, Jiang L, Feng J and Yu XY: Cardiac progenitor cell-derived
exosomes prevent cardiomyocytes apoptosis through exosomal miR-21
by targeting PDCD4. Cell Death Dis. 7:e22772016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peltier J, O'Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Latronico MV, Costinean S, Lavitrano ML,
Peschle C and Condorelli G: Regulation of cell size and contractile
function by AKT in cardiomyocytes. Ann N Y Acad Sci. 1015:250–260.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Shen L, Wang Z, Jiang HP and Liu LX:
Tanshinone IIA protects against myocardial ischemia reperfusion
injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed
Pharmacother. 84:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campenot RB: Local control of neurite
development by nerve growth factor. Proc Natl Acad Sci USA.
74:4516–4519. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Wang C, Yu S, Luo Z, Chen Y, Liu
Q, Hua F, Xu G and Yu P: Sevoflurane postconditioning protects rat
hearts against ischemia-reperfusion injury via the activation of
PI3K/AKT/mTOR signaling. Sci Rep. 4:73172014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han D, Wan C, Liu F, Xu X, Jiang L and Xu
J: Jujuboside a protects H9C2 cells from isoproterenol-induced
injury via activating PI3K/Akt/mTOR signaling pathway. Evid Based
Complement Alternat Med. 2016:95937162016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leithe E and Rivedal E: Ubiquitination and
down-regulation of gap junction protein connexin-43 in response to
12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem.
279:50089–50096. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ke Z, Wang G, Yang L, Qiu H, Wu H, Du M,
Chen J, Song J, Jia X and Feng L: Crude terpene glycoside component
from Radix paeoniae rubra protects against isoproterenol-induced
myocardial ischemic injury via activation of the PI3K/AKT/mTOR
signaling pathway. J Ethnopharmacol. 206:160–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu W, Sun H, Zha W, Cui W, Xu L, Min Q and
Wu J: Apigenin attenuates adriamycin-induced cardiomyocyte
apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement
Alternat Med. 2017:25906762017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui J, Zhang F, Wang Y, Liu J, Ming X, Hou
J, Lv B, Fang S and Yu B: Macrophage migration inhibitory factor
promotes cardiac stem cell proliferation and endothelial
differentiation through the activation of the PI3K/Akt/mTOR and
AMPK pathways. Int J Mol Med. 37:1299–1309. 2016. View Article : Google Scholar : PubMed/NCBI
|