Introduction
Microglia function as macrophages in the central
nervous system (CNS), and serve critical roles in brain development
and maintenance. Microglial dysfunction caused by hyperactivity in
response to inflammatory signals is closely associated with the
onset and progression of various neurodegenerative diseases by
damaging brain neurons (1,2).
Pathogenic endotoxins, including lipopolysaccharide (LPS), which
are present in the outer membrane of Gram-negative bacteria, can
induce excessive activation of microglial cells through binding to
Toll-like receptor 4 (TLR4) (3,4).
TLR4 ultimately activates various downstream signal transduction
pathways, including the nuclear factor (NF)-κB signaling pathway,
thus leading to transcription of a series of pro-inflammatory genes
that induce neuroinflammation and neurodegeneration (5-7).
Overactivated microglial cells stimulated by LPS can induce
oxidative stress by increasing the generation of reactive oxygen
species (ROS), which further aggravates the inflammatory response
(8,9). Therefore, blocking excessive
activation of microglia is an important tool to delay the induction
and progression of numerous brain diseases.
Recent studies have reported that various natural
products, including flavonoids, which are a group of naturally
occurring polyphenol compounds found in plants, possess
anti-inflammatory effects by blocking the activation of microglial
cells (10-13). Isorhamnetin is a flavonoid present
in various plants, including Hippophae rhamnoides L. (sea
buckthorn) fruit and Oenanthe javanica (Blume) DC (water
dropwort) leaf, which has been reported to possess various
pharmacological effects. Previous studies have demonstrated that
isorhamnetin can protect against inflammatory and oxidative stress
responses in various in vitro and in vivo models
using LPS, inflammatory cytokines and ischemic injury (14-24). The anti-inflammatory effects of
isorhamnetin have been reported to be associated with inhibition of
NF-κB signaling activity (20,23,25-27). In addition, its antioxidant
effects can be achieved by blocking ROS production (15,21,22). However, the association between
TLRs and the anti-inflammatory action of isorhamnetin has yet to be
elucidated. Furthermore, to the best of our knowledge, studies on
the effects of isorhamnetin on microglia have also yet to be
conducted. Therefore, the present study aimed to examine the
anti-inflammatory and antioxidant potency of isorhamnetin, and to
determine the effects of isorhamnetin on activation of the TLR4
signaling pathway in LPS-stimulated BV2 microglia.
Materials and methods
Cell culture and LPS stimulation
The BV2 immortalized murine microglial cell line was
provided by Dr Il-Whan Choi (Department of Microbiology, College of
Medicine, Inje University, Busan, Korea). BV2 microglia were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; WelGENE,
Inc., Gyeongsan, Korea) containing 10% (v/v) fetal bovine serum
(WelGENE, Inc.), L-glutamine (2 mM), penicillin (100 U/ml) and 100
µg/ml streptomycin (WelGENE, Inc.) at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air. Isorhamnetin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) and was
adjusted to final concentrations using complete culture medium. The
final DMSO concentration was <0.05% in all experiments (i.e., a
non-cytotoxic range). To stimulate cells, the medium was replaced
with fresh DMEM and 100 ng/ml LPS (Sigma-Aldrich Chemical Co.) was
added in the presence or absence of isorhamnetin for the indicated
time periods.
Assessment of cell viability
Cell viability was measured based on the formation
of blue formazan, which is metabolized from colorless MTT by
mitochondrial dehydrogenases, enzymes that are only active in live
cells. Briefly, BV2 cells were seeded into 96-well plates at a
density of 1×104 cells/well. After 24 h of incubation,
cells were treated with various concentrations (0, 50, 100 and 200
µM) of isorhamnetin for 24 h, or were pretreated with
various concentrations of isorhamnetin for 1 h prior to LPS (100
ng/ml) treatment for 24 h at 37°C. Subsequently, the medium was
removed and MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to
each well. After 3 h at 37°C, the supernatant was replaced with
DMSO to dissolve blue formazan crystals in each well. After 10 min
at 37°C, optical density was measured at a wavelength of 540 nm
using an ELISA microplate reader (Dynex Technologies, Chantilly,
VA, USA). Growth inhibition was assessed as percentage viability
where vehicle (0.05% DMSO)-treated cells were taken as 100% viable
(28).
Measurement of pro-inflammatory mediators
and cytokines
Levels of nitric oxide (NO) production were
indirectly determined by measuring the stable NO catabolite,
nitrite, in the medium using the Griess reaction. Briefly, BV2
cells (5×105 cells/ml) were stimulated in 24-well plates
with or without various concentrations of isorhamnetin for 1 h
prior to LPS (100 ng/ml) treatment for 24 h. Subsequently, the
culture medium supernatant (100 µl) was mixed with the same
volume of Griess reagent (Sigma-Aldrich; Merck KGaA) and was
incubated at room temperature for 10 min. The optical density was
then measured at 540 nm using an ELISA microplate reader; the
concentration of nitrite was calculated according to a standard
curve generated from known concentrations of sodium nitrite. Levels
of prostaglandin E2 (PGE2) (cat. no. 514010;
Cayman Chemical Company, Ann Arbor, MI, USA), tumor necrosis factor
(TNF)-α (cat. no. MTA00B; R&D Systems, Inc., Minneapolis, MN,
USA), and interleukin (IL)-1β (cat. no. MLB00C; R&D Systems,
Inc.) in the culture medium were measured using commercial ELISA
kits according to the manufacturer’s protocols and as described
previously (10). Briefly, cells
were plated in 24-well plates (1.5×105 cells/well) and
pretreated with various concentrations of isorhamnetin for 1 h
prior to treatment with 100 ng/ml LPS for 24 h. A 100-ml aliquot of
the conditioned medium was collected to determine PGE2,
TNF-α and IL-1β concentrations by ELISA, according to the
recommended procedures. The cells were also treated with ISO (200
µM) alone or in combination with 15 µM
ethyl-(6R)-6-(N-(2-chloro-4-fluorophenyl)sulfamoyl)
cyclohex-1-ene-1-carboxylate (CLI-095; Invivogen Europe, Toulouse,
France), a TLR4 antagonist, for 1 h prior to treatment with LPS for
24 h.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
BV2 cells were pretreated with various
concentrations of isorhamnetin for 1 h, followed by treatment with
100 ng/ml LPS for 24 h. Total RNA was isolated from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer’s protocol,
and RNA levels were quantified. For mRNA expression analysis, cDNA
was synthesized from 1 µg total RNA using
AccuPower® RT PreMix (Bioneer Corporation, Daejeon,
Korea) containing Moloney murine leukemia virus reverse
transcriptase, according to the manufacturer’s protocol. The PCR
reactions were performed using AccuPower® PCR PreMix
(Bioneer Corporation) at 94°C for 5 min, followed by 27 cycles at
94°C for 30 sec, annealing [inducible nitric oxide synthase (iNOS),
52°C; cyclooxygenase (COX)-2, 57°C; TNF-α, 57°C; IL-1β, 57°C; and
GAPDH, 62°C] for 30 sec and 72°C for 30 sec, followed by a final
extension step at 72°C for 5 min. After amplification, the PCR
products were separated by 1% agarose gel electrophoresis and were
visualized using ethidium bromide (Sigma-Aldrich; Merck KGaA)
staining. GAPDH was used as an internal control. Bands were
semi-quantified using ImageJ (version 1.46; National Institutes of
Health, Bethesda, MD, USA), were normalized to GAPDH and the ratio
was determined. The PCR primers were as follows: iNOS forward,
5′-ATGTCCGAAGCAAACATCAC-3′ and reverse, 5′-TAA
TGTCCAGGAAGTAGGTG-3′; COX-2 forward, 5′-CAGC AAATCCTTGCTGTTCC-3′
and reverse, 5′-TGGGCAAAG AATGCAAACATC-3′; TNF-α forward,
5′-TCTCATCAGTT CTATGGCCC-3′ and reverse, 5′-GGGAGTAGACAA
GGTACAAC-3′; IL-1β forward, 5′-GGGCTGCTTCCAAA CCTTTG-3′ and
reverse, 5′-GCTTGGGATCCACACTC TCC-3′, and GAPDH forward,
5′-AGGCCGGTGCTGAGTA TGTC-3′ and reverse, 5′-TGCCT
GCTTCACCACCTTCT-3′.
Protein isolation and western blot
analysis
BV2 cells were pretreated with various
concentrations of isorhamnetin for 1 h, followed by treatment with
100 ng/ml LPS for 24 h. Alternatively, cells were treated with 100
ng/ml LPS for various durations. The cells were collected and
cellular proteins were prepared using lysis buffer [25 mM Tris-Cl
(pH 7.5), 250 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA),
1% Nonidet-P40, 1 mM phenylmethylsulfonyl fluoride and 5 mM
dithiothreitol], as described previously (29). Cytosolic and nuclear proteins were
isolated separately using an NE-PER Nuclear and Cytoplasmic
Extraction Reagents kit (Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer’s protocol. The insoluble materials
were discarded by centrifugation at 13,000 x g for 20 min at 4°C.
The protein concentrations in the cell lysates were determined
using a detergent-compatible protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer’s protocol.
For western blotting, equal amounts of protein (50 µg) were
separated by 8-10% SDS-PAGE and were transferred onto
polyvinylidene difluoride membranes (Schleicher and Schuell
Bioscience, Inc., Keene, NH, USA). Subsequently, these membranes
were blocked with 5% non-fat dry milk/Tris-buffered saline
containing 0.1% Triton X-100 (TBST) for 1 h at 25°C (room
temperature) and were incubated with specific primary antibodies
(Table I) at 4°C overnight. After
washing membranes with TBST, they were incubated with appropriate
horseradish-peroxidase (HRP)-conjugated secondary antibodies
[1:500; cat. no. sc 2004, goat anti-rabbit immunoglobulin
(Ig)G-HRP; sc 2005, goat anti-mouse IgG-HRP; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA] for 2 h at 25°C. Protein
bands were detected using an enhanced chemiluminescence kit (GE
Healthcare, Chicago, IL, USA), according to the manufacturer’s
protocol. The immunoreac-tive bands were detected and exposed to
X-ray film. Images of western blotting were also analyzed using
ImageJ.
 | Table IList of antibodies used for western
blot analysis in the present study. |
Table I
List of antibodies used for western
blot analysis in the present study.
| Antibody | Dilution | Product no. | Species of
origin | Supplier |
|---|
| iNOS | 1:1,000 | 610328 | Rabbit
polyclonal | BD Transduction
Laboratories; BD Biosciences, San Jose, CA, USA |
| COX-2 | 1:500 | 160126 | Rabbit
polyclonal | Cayman Chemical
Company, Ann Arbor, MI, USA |
| IL-1β | 1:1,000 | sc-7884 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA |
| TNF-α | 1:1,000 | 3707S | Rabbit
polyclonal | Cell Signaling
Technology, Inc., Danvers, MA, USA |
| NF-κB p65 | 1:1,000 | sc-71675 | Mouse
monoclonal | Santa Cruz
Biotechnology, Inc. |
| IκBα | 1:1,000 | sc-371 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. |
| p-IκBα | 1:1,000 | sc-8404 | Mouse
monoclonal | Santa Cruz
Biotechnology, Inc. |
| TLR4 | 1:1,000 | ab53629 | Goat
polyclonal | Abcam, Cambridge,
MA, USA |
| Myd88 | 1:1,000 | ab2064 | Rabbit
polyclonal | Abcam |
| Lamin B | 1:1,000 | sc-6216 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. |
| Actin | 1:1,000 | sc-1615 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. |
Immunofluorescence staining for nuclear
translocation of NF-κB and formation of LPS/TLR4 complexes
After cells (5×105 cells/ml) were
pretreated with or without 200 µM isorhamnetin for 1 h, they
were treated with 100 ng/ml LPS for 1 h. The cells were then washed
twice with PBS, fixed in 3.7% paraformaldehyde for 15 min at 25°C,
permeabilized with 0.2% Triton X-100 in PBS for 15 min, and blocked
for 10 min at 20°C with PBS containing 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA). The cells were then incubated with a
primary antibody against NF-κB p65 (dilution, 1:100; cat. no.
sc-71675; Santa Cruz Biotechnology, Inc.) at 4°C overnight,
followed by incubation with a fluorescein-conjugated anti-mouse
immunoglobulin G secondary antibody (dilution, 1:100; cat. no.
62-6511; Molecular Probes; Thermo Fisher Scientific, Inc.) in the
dark at 37°C for 40 min. In addition, BV2 cells were pretreated
with or without 200 µM isorhamnetin for 30 min, followed by
treatment with Alexa Fluor® (AF) 488-conjugated LPS
(AF-LPS; 100 ng/ml; Invitrogen; Thermo Fisher Scientific, Inc.) for
6 h, in order to analyze the formation of LPS/TLR4 complexes. Fixed
cells were also incubated with anti-TLR4 antibody (1:100; cat. no.
ab8376; Abcam, Cambridge, UK) at 4°C for 90 min and were then
incubated with AF 594-conjugated secondary antibody (1:100; cat.
no. A-11032; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Nuclei were sequentially stained with DAPI
(Sigma-Aldrich; Merck KGaA) solution (2.5 µg/ml). Slides
were mounted and fluorescence images were captured under a
fluorescence microscope (Zeiss AG, Oberkochen, Germany).
Measurement of TLR4 expression on the
cell surface
To investigate the effects of isorhamnetin on TLR4
expression on the cell surface, BV2 cells were pretreated with or
without 200 µM isorhamnetin for 30 min, followed by
treatment with 100 ng/ml AF-LPS for 6 h. Following treatment, cells
(5×105 cells/ml) were washed twice with PBS, harvested
with 0.005% EDTA and analyzed by flow cytometry. AF 488 was excited
using 488 argon-ion laser and detected on channel FL1 using a 530
nm emission filter. Fluorescence emission of samples was recorded
by flow cytometry (BD Biosciences, San Jose, CA, USA), as
previously described (30).
Detection of intracellular ROS
levels
Production of intracellular ROS was monitored using
5,6-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA;
Sigma-Aldrich; Merck KGaA), which is a cell-permeable fluorogenic
probe. Briefly, cells were treated with 100 ng/ml LPS for the
indicated time periods, or were pretreated with 200 µM
isorhamnetin or 10 mM N-acetyl cysteine (NAC; Sigma-Aldrich; Merck
KGaA) for 1 h followed by stimulation with or without 100 ng/ml LPS
for 1 h. These cells (5×105 cells/ml) were harvested and
stained with 10 µM DCF-DA in the dark at 37°C for 15 min.
After rinsing twice with PBS, cells were immediately analyzed by
flow cytometry with an excitation wavelength of 480 nm and an
emission wavelength of 525 nm. To observe the degree of ROS
production by fluorescence microscopy, the coverslips were placed
on a glass 6-well plate, and the cells (3×105 cells/ml)
were incubated for 24 h to attach to the coverslips. The cells were
treated with 100 ng/ml LPS for 1 h, or were pretreated with 200
µM isorhamnetin or 10 mM NAC for 1 h followed by stimulation
with or without 100 ng/ml LPS for 1 h. These cells were stained
with 10 µM DCF-DA at 37°C for 15 min, washed twice with PBS
and fixed with 4% paraformaldehyde (pH 7.4) for 20 min. Fixed cells
were washed twice with PBS and were then analyzed by fluorescence
microscopy.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism version 6.02 (GraphPad Software, Inc., La Jolla, CA, USA).
All data were collected from at least three independent experiments
and are presented as the means ± standard deviation. One-way
analysis of variance with Tukey’s multiple comparison post hoc test
was performed to analyze the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isorhamnetin suppresses LPS-induced
pro-inflammatory mediators and cytokines production in BV2
microglial cells
To determine the inhibitory effects of isorhamnetin
on LPS-induced production of NO and PGE2, which are
pro-inflammatory mediators, BV2 cells were pretreated with various
concentrations of isorhamnetin for 1 h and were then stimulated
with or without 100 ng/ml LPS for 24 h. Levels of NO and
PGE2 in culture supernatants were determined using the
Griess reaction assay and ELISA, respectively. As shown in Fig. 1A and B, LPS stimulation alone
markedly increased NO and PGE2 production, as compared
with in cells that were not stimulated by LPS. Conversely,
isorhamnetin significantly inhibited LPS-induced secretion of NO
and PGE2 in BV2 cells in a concentration-dependent
manner. The effects of isorhamnetin on the production of
pro-inflammatory cytokines, including TNF-α and IL-1β, were also
detected in LPS-stimulated BV2 cells. According to the results of
ELISA, as shown in Fig. 1C and D,
the production of these two cytokines was significantly increased
in the culture medium of LPS-stimulated BV2 cells; however,
isorhamnetin treatment decreased the production of these cytokines
in a concentration-dependent manner.
 | Figure 1Suppression of NO, PGE2,
TNF-α and IL-1β production by ISO in LPS-stimulated BV2 microglial
cells. Cells were pretreated with the indicated concentrations of
ISO for 1 h prior to incubation with 100 ng/ml LPS for 24 h. Levels
of (A) NO, (B) PGE2, (C) TNF-α and (D) IL-1β were
detected in the culture media by Griess assay and commercial ELISA
kits. Data are presented as the means ± standard deviation obtained
from three independent experiments. *P<0.05 compared
with the control group; #P<0.05 compared with the LPS
group. IL-1β, interleukin-1β; ISO, isorhamnetin; LPS,
lipopolysaccharide; NO, nitric oxide; PGE2,
prostaglandin E2; TNF-α, tumor necrosis factor-α. |
Isorhamnetin attenuates LPS-induced iNOS,
COX-2 and cytokine expression in BV2 microglial cells
The present study aimed to determine whether the
inhibitory effects of isorhamnetin on NO and PGE2
production were associated with regulation of iNOS and COX-2
expression. As shown in Fig. 2,
isorhamnetin inhibited the mRNA and protein expression levels of
iNOS and COX-2 in LPS-stimulated BV2 cells in a
concentration-dependent manner. In addition, isorhamnetin inhibited
LPS-induced increased expression of TNF-α and IL-1β in a
concentration-dependent manner (Fig.
2). These findings indicated that isorhamnetin may suppress NO,
PGE2 and cytokine production by reducing the expression
of their encoding genes.
 | Figure 2Inhibition of LPS-induced expression
of iNOS, COX-2, TNF-α and IL-1β by ISO in BV2 microglial cells. BV2
cells were pretreated with various concentrations of ISO for 1 h
followed by treatment with 100 ng/ml LPS for 24 h. (A) Total RNA
was isolated and RT-PCR was performed using the indicated primers.
(B) Total proteins were isolated and subjected to western blot
analyses. Experiments were repeated three times and similar results
were obtained. GAPDH and actin were used as the internal controls
for the RT-PCR and western blot analysis, respectively. Bands were
semi-quantified using ImageJ, normalized to (C) GAPDH and (D) actin
and ratios were determined. Data are presented as the means ±
standard deviation obtained from three independent experiments.
*P<0.05 compared with the control group;
#P<0.05 compared with the LPS group. COX-2,
cyclooxygenase 2; IL-1β, interleukin-1β; ISO, isorham-netin; LPS,
lipopolysaccharide; NO, nitric oxide; PGE2,
prostaglandin E2; RT-PCR, reverse
transcription-polymerase chain reaction; TNF-α, tumor necrosis
factor-α. |
Effect of isorhamnetin on the viability
of BV2 microglial cells
The MTT assay was performed to investigate whether
the inhibitory effects of isorhamnetin on production of
pro-inflammatory mediators and cytokines were caused by
cytotoxicity. As shown in Fig. 3,
the survival rate of BV2 cells was not significantly affected by
treatment with ≥200 µM isorhamnetin alone for 24 h. In
addition, no significant alteration in survival rate was detected
in BV2 cells treated with ≥200 µM isorhamnetin, even in the
presence of 100 ng/ml LPS.
Isorhamnetin alleviates LPS-induced NF-κB
nuclear translocation and inhibitor κB-α (IκBα) degradation in BV2
microglial cells
The present study aimed to determine whether
isorhamnetin could attenuate LPS-induced activation of NF-κB in BV2
cells. Immunoblotting data using cytoplasmic and nuclear extracts
revealed that pretreatment with isorhamnetin inhibited nuclear
accumulation of NF-κB p65 subunits in LPS-stimulated BV2 cells. In
addition, isorhamnetin attenuated the LPS-induced inhibition of
total IκBα protein expression and reduced phosphorylation of IκBα
(Fig. 4A and B). Consistent with
these results, immunocyto-chemical analysis indicated that the
fluorescence intensity of NF-κB p65 in the nucleus was increased in
LPS-stimulated cells. However, LPS-mediated nuclear translocation
of NF-κB was considerably blocked by pretreatment with isorhamnetin
(Fig. 4C), thus indicating that
isorhamnetin could attenuate transcriptional activation of
NF-κB.
Isorhamnetin inhibits LPS-induced TLR4
and Myd88 expression in BV2 microglial cells
To determine whether the anti-inflammatory effects
of isorhamnetin were associated with blockade of the TLR signaling
pathway, the expression levels of TLR4 and myeloid differentiation
factor 88 (Myd88) were investigated (Fig. 5). The results of immunoblotting
revealed that protein expression levels of TLR4 and Myd88 were
markedly increased by LPS treatment in a time-dependent manner
(Fig. 5A and C). However, when
cells were pretreated with isorhamnetin, the LPS-induced increase
in TLR4 and Myd88 expression was inhibited in a
concentration-dependent manner (Fig.
5B and D).
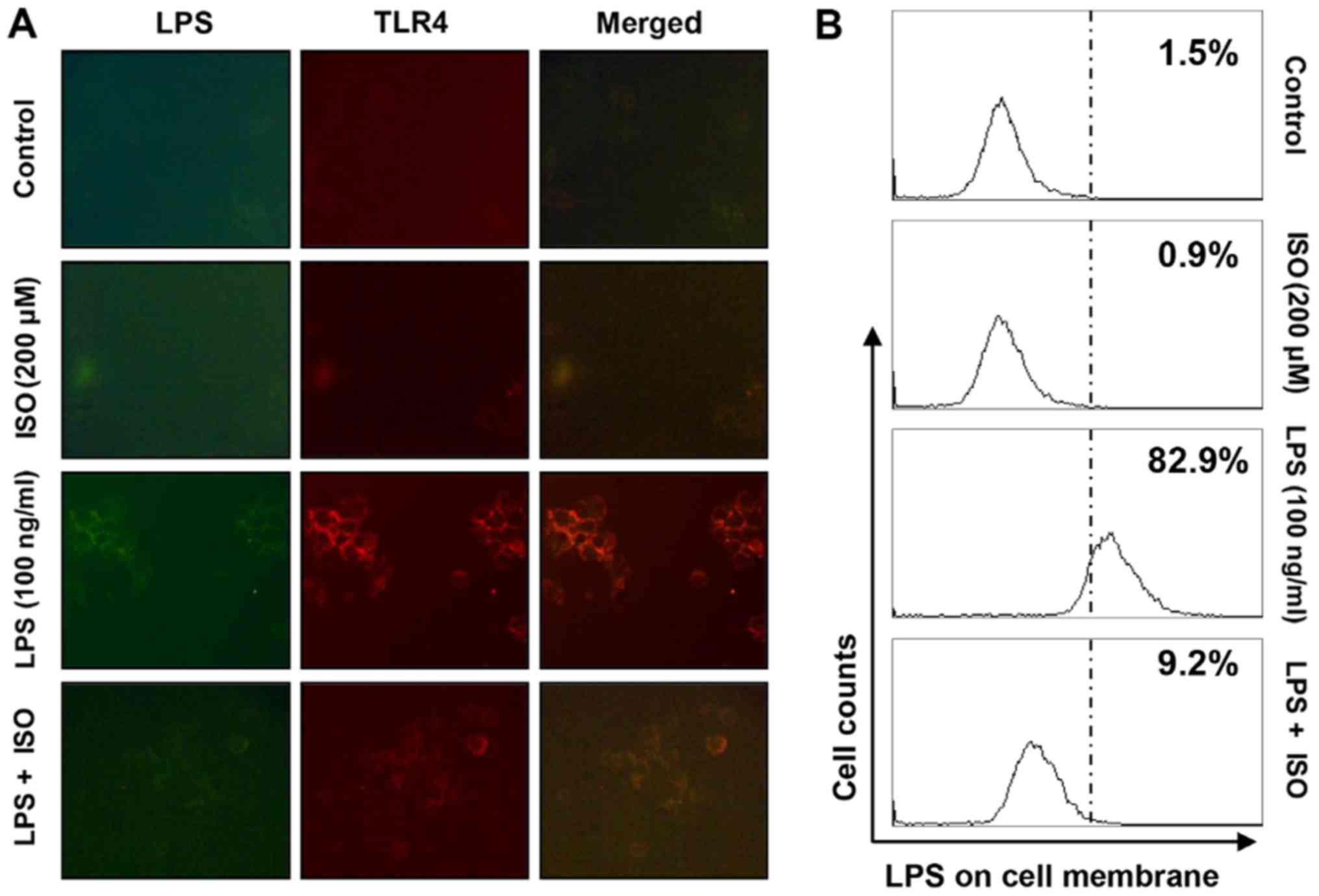
Isorhamnetin blocks LPS-mediated
interaction between LPS and TLR4 in BV2 microglial cells
The present study assessed whether isorhamnetin
could inhibit the interaction between LPS and TLR4 on the surface
of LPS-treated BV2 cells. As shown in Fig. 6A and B, fluorescence of LPS and
TLR4 was observed outside the cell membrane in BV2 cells treated
with AF-LPS. However, the fluorescence intensity of TLR4 and the
binding activity of LPS on the cell surface were markedly
attenuated in BV2 cells treated with LPS in the presence of
isorhamnetin.
Interception of TLR4 signaling increases
the anti-inflammatory efficacy of isorhamnetin on BV2 microglial
cells
To further determine whether blockade of TLR4
signaling is mediated by the anti-inflammatory action of
isorhamnetin, the present study examined the effects of the TLR4
antagonist, CLI-095, on isorhamnetin-induced inhibition of
inflammatory mediators. As shown in Fig. 7A and B, CLI-095 significantly
reduced the production of LPS-induced inflammatory mediators, such
as NO and PGE2, Furthermore, cotreatment with
isorhamnetin and CLI-095 synergistically inhibited LPS-induced
production of NO and PGE2 and blocked the transcription
of corresponding genes (Fig. 7C and
D). These results suggested that the inhibitory effects of
isorhamnetin on LPS-induced inflammation may be due to suppression
of the TLR4-mediated NF-κB signaling pathway in BV2 microglia.
Isorhamnetin reduces LPS-induced ROS
generation in BV2 microglial cells
The present study also examined the effects of
isorhamnetin on LPS-induced ROS production, in order to investigate
the antioxidant potential of isorhamnetin. Flow cytometry using the
fluorescent probe DCF-DA revealed that the levels of ROS were
gradually increased following treatment with LPS, peaking at 1 h;
thereafter, ROS levels were decreased (Fig. 8A). Conversely, treatment with
isorhamnetin alone did not induce ROS generation. Pretreatment with
isorhamnetin effectively attenuated the levels of ROS released by
LPS (Fig. 8B). The inhibitory
effects of isorhamnetin on ROS production were also observed under
fluorescence microscopy (Fig.
8C). In NAC-pretreated cells, which were used as a positive
control, the production of LPS-stimulated ROS was completely
blocked. These findings indicated that isorhamnetin had a strong
ROS-scavenging effect.
Discussion
The results of the present study demonstrated that
isorhamnetin inhibited LPS-induced inflammatory signaling in BV2
microglia, a brain microglial cell line. Similar to the results of
previous studies using macrophage and gingival fibroblast models
(14,31), the present results indicated that
isorhamnetin could significantly inhibit the increased production
of NO and PGE2 by LPS, in the absence of cytotoxicity,
which was associated with suppression of iNOS and COX-2 expression,
respectively. In addition, isorhamnetin reduced the release of
TNF-α and IL-1β by blocking their expression in LPS-stimulated
microglial cells; these findings are also similar to the results of
previous studies (17,26). These results suggested that
isorhamnetin may improve the inflammatory response by inhibiting
the expression of genes that regulate the production of
pro-inflammatory factors.
NF-κB is a key transcription factor that increases
the expression of pro-inflammatory enzymes and cytokines only if it
has migrated to the nucleus. NF-κB is usually located in the
cytoplasm in association with IκBα. When IκBα is phosphorylated and
degraded, NF-κB is isolated and translocated to the nucleus
(5-7). Therefore, this study aimed to
determine whether isorhamnetin could inhibit LPS-induced
degradation and phosphorylation of IκBα and nuclear translocation
of NF-κB. The results indicated that isorhamnetin could effectively
block nuclear expression of NF-κB (p65), and the degradation and
phosphorylation of IκBα in LPS-stimulated BV2 microglial cells.
These results suggested that isorhamnetin may reduce the expression
and production of pro-inflammatory mediators and cytokines by
inhibiting the NF-κB pathway in LPS-stimulated BV2 microglia. This
is in agreement with previous results observed in LPS-stimulated
macrophages and human umbilical vein endothelial cells (20,26,27).
Immune cells, including microglia, can recognize
pathogen-associated molecular patterns through TLR pattern
recognition receptors, which are expressed on the cell surface.
Among various TLRs, TLR4 is known to recruit adapter molecules,
including MyD88, LPS-binding protein and differentiation cluster
co-receptor, when immune cells are activated by LPS (1,2).
Upon activation of TLR4 by LPS, the TLR4-MyD88-mediated signal can
induce activation of mitogen-activated protein kinases (MAPKs),
which eventually promote the activation of NF-κB signaling,
resulting in the production of pro-inflammatory mediators and
cytokines (4,32). According to the results of Yang
et al (20), isorhamnetin
can significantly inhibit LPS-mediated activation of the MAPK c-Jun
N-terminal kinase in a macrophage model. The present study revealed
that isorhamnetin suppressed LPS-induced expression of TLR4 and
MyD88, and reduced the binding of TLR4 to LPS. These findings
indicated that isorhamnetin may inhibit the expression of
pro-inflammatory enzymes and cytokines by blocking the TLR4
signaling pathway, which is the early stage of intracellular
signaling in LPS-stimulated cells. This finding demonstrated that
isorhamnetin attenuated onset of the LPS-mediated intracellular
signaling pathway by suppressing activation of NF-κB and inhibiting
the binding of LPS to TLR4 in microglial cells. Therefore,
isorhamnetin may to inhibit NF-κB and MAPK signaling pathways by
exhibiting antagonistic effects on the binding of LPS to TLR4 in
BV2 microglial cells.
Alongside inflammatory insults, oxidative stress is
another major cause of CNS damage. Low levels of ROS serve an
important role as signaling molecules that regulate the immune
response to pathogens; however, overproduction of ROS contributes
to neurotoxicity (8,33-35). Previous studies have reported that
the LPS-induced inflammatory response in microglia is directly
associated with increased ROS production and that inhibition of the
inflammatory response is associated with blocking ROS production
(14,32,36,37). TLR4 signaling-mediated generation
of ROS by LPS accelerates the inflammatory response by activating
downstream signaling cascades containing NF-κB (38-40). Therefore, inhibiting ROS
production is an important strategy to suppress inflammatory
responses and oxidative stress. Previous studies using various
research models have demonstrated that isorhamnetin possesses
strong antioxidant efficacy. For example, the beneficial effects of
isorhamnetin on LPS-induced acute lung injury and collagen-induced
arthritis mouse models are directly associated with its antioxidant
effects (18,41). In addition, the protective effects
of isorhamnetin on oxidative stress-induced DNA damage and
apoptosis are associated with blocking of ROS production (22,26,42). These results are in agreement with
the antioxidant efficacy of isorhamnetin observed in the present
study, indicating that isorhamnetin may effectively block the
production of excessive ROS induced by LPS. To the best of our
knowledge, the present study is the first to report on the
inhibitory effects of isorhamnetin on ROS production in microglia;
however, additional studies are required to determine the direct
linkage between ROS production blockade and anti-inflammatory
efficacy.
In conclusion, the present study demonstrated that
isorhamnetin exerted potent anti-inflammatory effects on BV2
microglial cells. In LPS-stimulated BV2 cells, isorhamnetin was
able to reduce the production of pro-inflammatory mediators and
cytokines, which was associated with decreased expression of their
regulatory genes via the suppression of NF-κB activity.
Furthermore, isorhamnetin could block early intracellular signaling
cascades by antagonizing TLR4 or suppressing ROS accumulation.
Although the results of the current study may provide partial
understanding of the mechanism underlying the anti-inflammatory
effects of isorhamnetin, further studies are required to assess the
mechanical role of isorhamnetin in various oxidative stress- and
inflammation-mediated diseases.
Funding
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korean government (grant nos.
2018R1A2B2005705 and 2016R1A5A2007009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
SYK, HTP and YHC contributed to the conception and
design of the experiment. SYK, CYJ, CHK, YHY and GYK performed all
experiments and verified the analytical data. HMY and SHC
contributed to the statistical analysis and helped interpret the
results. YHC supervised the experiments in discussion with SYK,
HTP, YHC, CYJ, CHK, YHY and GYK wrote the manuscript. All authors
discussed the final results and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tremblay MÈ, Stevens B, Sierra A, Wake H,
Bessis A and Nimmerjahn A: The role of microglia in the healthy
brain. J Neurosci. 31:16064–16069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomez-Nicola D and Perry VH: Microglial
dynamics and role in the healthy and diseased brain: A paradigm of
functional plasticity. Neuroscientist. 21:169–184. 2015. View Article : Google Scholar :
|
|
3
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cherry JD, Olschowka JA and O’Banion MK:
Neuroinflammation and M2 microglia: The good, the bad, and the
inflamed. J Neuroinflammation. 11:982014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kopitar-Jerala N: Innate immune response
in brain, NF-Kappa B signaling and cystatins. Front Mol Neurosci.
8:732015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee MB, Lee JH, Hong SH, You JS, Nam ST,
Kim HW, Park YH, Lee D, Min KY, Park YM, et al: JQ1, a BET
inhibitor, controls TLR4-induced IL-10 production in regulatory B
cells by BRD4-NF-κB axis. BMB Rep. 50:640–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Bernhardi R, Eugenín-von Bernhardi L
and Eugenín J: Microglial cell dysregulation in brain aging and
neurodegeneration. Front Aging Neurosci. 7:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daulatzai MA: Fundamental role of
pan-inflammation and oxidative-nitrosative pathways in
neuropathogenesis of Alzheimer’s disease in focal cerebral ischemic
rats. Am J Neurodegener Dis. 5:102–130. 2016.
|
|
10
|
Choi HI, Choi JP, Seo J, Kim BJ, Rho M,
Han JK and Kim JG: Helicobacter pylori-derived extracellular
vesicles increased in the gastric juices of gastric adenocarcinoma
patients and induced inflammation mainly via specific targeting of
gastric epithelial cells. Exp Mol Med. 49:e3302017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Y, Chen J and Shen J: Herbal medicines
for ischemic stroke: Combating inflammation as therapeutic targets.
J Neuroimmune Pharmacol. 9:313–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Zhang X, Zhang C, Wang W, Chen R,
Jiao H, Li L, Zhang L and Cui L: Anti-inflammation of natural
components from medicinal plants at low concentrations in brain via
inhibiting neutrophil infiltration after stroke. Mediators Inflamm.
2016:95379012016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du L, Zhang Y, Chen Y, Zhu J, Yang Y and
Zhang HL: Role of microglia in neurological disorders and their
potentials as a therapeutic target. Mol Neurobiol. 54:7567–7584.
2017. View Article : Google Scholar
|
|
14
|
Qi F, Sun JH, Yan JQ, Li CM and Lv XC:
Anti-inflammatory effects of isorhamnetin on LPS-stimulated human
gingival fibroblasts by activating Nrf2 signaling pathway. Microb
Pathog. 120:37–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Gong HM, Zou HH, Liang L and Wu
XY: Isorhamnetin prevents H2O2-induced
oxidative stress in human retinal pigment epithelial cells. Mol Med
Rep. 17:648–652. 2018.
|
|
16
|
Ahn H and Lee GS: Isorhamnetin and
hyperoside derived from water dropwort inhibits inflammasome
activation. Phytomedicine. 24:77–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chi G, Zhong W, Liu Y, Lu G, Lü H, Wang D
and Sun F: Isorhamnetin protects mice from
lipopolysaccharide-induced acute lung injury via the inhibition of
inflammatory responses. Inflamm Res. 65:33–41. 2016. View Article : Google Scholar
|
|
18
|
Yang B, Li XP, Ni YF, Du HY, Wang R, Li
MJ, Wang WC, Li MM, Wang XH, Li L, et al: Protective effect of
isorhamnetin on lipopolysaccharide-induced acute lung injury in
mice. Inflammation. 39:129–137. 2016. View Article : Google Scholar
|
|
19
|
Zhao JJ, Song JQ, Pan SY and Wang K:
Treatment with isorhamnetin protects the brain against ischemic
injury in mice. Neurochem Res. 41:1939–1948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JH, Kim SC, Shin BY, Jin SH, Jo MJ,
Jegal KH, Kim YW, Lee JR, Ku SK, Cho IJ, et al: O-Methylated
flavonol isorhamnetin prevents acute inflammation through blocking
of NF-κB activation. Food Chem Toxicol. 59:362–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo S, Seo K, Ki SH and Shin SM:
Isorhamnetin inhibits reactive oxygen species-dependent hypoxia
inducible factor (HIF)-1α accumulation. Biol Pharm Bull.
39:1830–1838. 2016. View Article : Google Scholar
|
|
22
|
Choi YH: The cytoprotective effect of
isorhamnetin against oxidative stress is mediated by the
upregulation of the Nrf2-dependent HO-1 expression in C2C12
myoblasts through scavenging reactive oxygen species and ERK
inactivation. Gen Physiol Biophys. 35:145–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen TL, Zhu GL, Wang JA, Zhang GD, Liu
HF, Chen JR, Wang Y and He XL: Protective effects of isorhamnetin
on apoptosis and inflammation in TNF-α-induced HUVECs injury. Int J
Clin Exp Pathol. 8:2311–2320. 2015.
|
|
24
|
Seo K, Yang JH, Kim SC, Ku SK, Ki SH and
Shin SM: The antioxidant effects of isorhamnetin contribute to
inhibit COX-2 expression in response to inflammation: A potential
role of HO-1. Inflammation. 37:712–722. 2014. View Article : Google Scholar
|
|
25
|
Qin L, Liu Y, Hong JS and Crews FT: NADPH
oxidase and aging drive microglial activation, oxidative stress,
and dopaminergic neurodegeneration following systemic LPS
administration. Glia. 61:855–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Chi G, Shen B, Tian Y and Feng H:
Isorhamnetin ameliorates LPS-induced inflammatory response through
downregulation of NF-κB signaling. Inflammation. 39:1291–1301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim TH, Ku SK and Bae JS:
Anti-inflammatory activities of isorhamnetin-3-O-galactoside
against HMGB1-induced inflammatory responses in both HUVECs and
CLP-induced septic mice. J Cell Biochem. 114:336–345. 2013.
View Article : Google Scholar
|
|
28
|
Koh PO: Cerebral ischemic injury decreases
α-synuclein expression in brain tissue and glutamate-exposed HT22
cells. Lab Anim Res. 33:244–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park YS, Kwon YJ and Chun YJ: CYP1B1
Activates Wnt/β-catenin signaling through suppression of
Herc5-mediated ISGylation for protein degradation on β-catenin in
HeLa cells. Toxicol Res. 33:211–218, 20178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee IA, Hyam SR, Jang SE, Han MJ and Kim
DH: Ginsenoside Re ameliorates inflammation by inhibiting the
binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food
Chem. 60:9595–9602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You S, Nakanishi E, Kuwata H, Chen J,
Nakasone Y, He X, He J, Liu X, Zhang S, Zhang B, et al: Inhibitory
effects and molecular mechanisms of garlic organosulfur compounds
on the production of inflammatory mediators. Mol Nutr Food Res.
57:2049–2060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garcia G, Nanni S, Figueira I, Ivanov I,
McDougall GJ, Stewart D, Ferreira RB, Pinto P, Silva RF, Brites D,
et al: Bioaccessible (poly)phenol metabolites from raspberry
protect neural cells from oxidative stress and attenuate microglia
activation. Food Chem. 215:274–283. 2017. View Article : Google Scholar
|
|
33
|
Fetisova E, Chernyak B, Korshunova G,
Muntyan M and Skulachev V: Mitochondria-targeted antioxidants as a
prospective therapeutic strategy for multiple sclerosis. Curr Med
Chem. 24:2086–2114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Leden RE, Yauger YJ, Khayrullina G and
Byrnes KR: Central nervous system injury and nicotinamide adenine
dinucleotide phosphate oxidase: Oxidative stress and therapeutic
targets. J Neurotrauma. 34:755–764. 2017. View Article : Google Scholar :
|
|
35
|
Ohl K, Tenbrock K and Kipp M: Oxidative
stress in multiple sclerosis: Central and peripheral mode of
action. Exp Neurol. 277:58–67. 2016. View Article : Google Scholar
|
|
36
|
Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ,
Ham YW, Hellström M, Han SB, Kim HS, Park EK, et al: Inhibitory
effect of punicalagin on lipopolysaccharide-induced
neuroinflam-mation, oxidative stress and memory impairment via
inhibition of nuclear factor-kappaB. Neuropharmacology. 117:21–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iizumi T, Takahashi S, Mashima K, Minami
K, Izawa Y, Abe T, Hishiki T, Suematsu M, Kajimura M and Suzuki N:
A possible role of microglia-derived nitric oxide by
lipopolysaccharide in activation of astroglial pentose-phosphate
pathway via the Keap1/Nrf2 system. J Neuroinflammation. 13:992016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slusarczyk J, Trojan E, Glombik K,
Piotrowska A, Budziszewska B, Kubera M, Popiolek-Barczyk K, Lason
W, Mika J and Basta-Kaim A: Anti-inflammatory properties of
tianeptine on lipopolysaccharide-induced changes in microglial
cells involve toll-like receptor-related pathways. J Neurochem.
136:958–970. 2016. View Article : Google Scholar
|
|
39
|
Wang X, Wang C, Wang J, Zhao S, Zhang K,
Wang J, Zhang W, Wu C and Yang J: Pseudoginsenoside-F11 (PF11)
exerts anti-neuroinflammatory effects on LPS-activated microglial
cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt
signaling pathways. Neuropharmacology. 79:642–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng KW, Zhao MB, Ma ZZ, Jiang Y and Tu
PF: Protosappanin A inhibits oxidative and nitrative stress via
interfering the interaction of transmembrane protein CD14 with
Toll-like receptor-4 in lipopolysaccharide-induced BV-2 microglia.
Int Immunopharmacol. 14:558–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang X and Zhong W: Isorhamnetin
attenuates collagen-induced arthritis via modulating cytokines and
oxidative stress in mice. Int J Clin Exp Med. 8:16536–16542.
2015.PubMed/NCBI
|
|
42
|
Dong GZ, Lee JH, Ki SH, Yang JH, Cho IJ,
Kang SH, Zhao RJ, Kim SC and Kim YW: AMPK activation by
isorhamnetin protects hepatocytes against oxidative stress and
mitochondrial dysfunction. Eur J Pharmacol. 740:634–640. 2014.
View Article : Google Scholar : PubMed/NCBI
|